Summary
Objective
Although severe hypoglycaemia (SH) can lead to adverse health outcomes, little is known about its occurrence and re‐occurrence among youth with type 1 or type 2 diabetes.
Methods
This study included 2740 participants aged <20 years at diabetes diagnosis and 5‐14 years diabetes duration from the SEARCH for Diabetes in Youth Cohort Study. Participants reported SH events in the past 6 months. Differences in SH events by demographic and clinical factors were tested using logistic regression models.
Results
Severe hypoglycaemia in the past 6 months was more common among youth with type 1 (7.0%, 168 of 2399) than with type 2 diabetes (2.6%, nine of 341) (P < 0.002). The median number of SH events per youth who had at least one SH event in the past 6 months was 1 for both type 1 type 2 diabetes. For youth with type 1 diabetes, those who reported SH events were older, were more likely to have obesity or to be physically active, and had lower HbA1c. After adjustments, one unit increase in HbA1c was associated with 16% lower likelihood (OR 0.84, 95% CI 0.75, 0.94) and being physically active was associated with an 87% higher likelihood (OR 1.87, 95% CI 1.23, 2.86) of reporting a SH event. There were too few SH events among youth with type 2 diabetes to analyse further.
Conclusions
In youth with diabetes, SH was common even within a short 6‐month window. Better understanding the causes of SH may help prevent them from occurring.
Keywords: hypoglycaemic events, type 1 diabetes, type 2 diabetes, youth
1. INTRODUCTION
Severe hypoglycaemia (SH) is an acute complication of diabetes that can lead to adverse consequences among children including seizures, hospitalizations, potential structural changes in the brain and death.1 In spite of advances in pharmacotherapy (including insulin analogues) and technologies (such as continuous glucose monitoring devices), hypoglycaemia remains common among children with diabetes. Compared to adults, children and adolescents with diabetes are at increased risk of hypoglycaemia events, at least in part due to variable eating patterns, inaccurate insulin dosing, erratic physical activity and difficulty recognizing symptoms of hypoglycaemia.1 Young adults in their early 20s may also be at an increased risk for hypoglycaemia events for similar reasons as children and adolescents.
There is a wide range in reported frequency of hypoglycaemia events. SH is defined by the American Diabetes Association and The Endocrine Society as “an event requiring assistance of another person to actively administer carbohydrates, glucagon, or take other corrective actions”.1 However, this definition is somewhat subjective, particularly when discussing care of young children. In recent years, there has been a wide range in reporting SH events among children and adolescents with type 1 diabetes, from 15 SH events per 100 patient‐years2 to 3.6 per 100 patient‐years.3 In a small study of 74 youth with type 1 diabetes, 44% reported at least one SH event in the past year.4 Increased risk of SH among youth with type 1 diabetes has been associated with lower HbA1c, younger age, higher insulin dose, male sex and lower parental socio‐economic status.5 However, there are few studies that have focused on adolescents or young adults.
There is mixed evidence on whether the incidence of hypoglycaemia is decreasing. A recent study in Germany and Austria among youth (<20 years) with type 1 diabetes found a decrease in hypoglycaemia events from 20.1 per 100 patient‐years in 1995 to 3.6 per 100 patient‐years in 2012.4 In contrast, the Danish Registry of Childhood and Adolescent Diabetes (DanDiabKid) Study found no significant change in hypoglycaemia events in those with type 1 diabetes from 1998 to 2009.2 There are no studies reporting the frequency of SH events among youth with type 2 diabetes.
The SEARCH for Diabetes in Youth Cohort Study is one of the few studies of youth‐onset diabetes that includes both type 1 diabetes and type 2 diabetes and collects data on SH events. This presents a unique opportunity to examine and compare the occurrence of SH events in youth with type 1 diabetes and those with type 2 diabetes. We report on the prevalence of SH events in a 6‐month period among youth with either type 1 diabetes or type 2 diabetes and the characteristics of participants who experienced events compared to those who did not.
2. METHODS
2.1. Study population
Participants include children and adolescents (<20 years of age at diabetes diagnosis) identified from a population‐based SEARCH for Diabetes in Youth Registry Study6 between 2002 and 2008 at five US sites (South Carolina; Cincinnati, Ohio and surrounding counties; Seattle, Washington and surrounding counties; Kaiser Permanente Southern California members in seven counties; Colorado and southwestern Native American sites). Participants who had at least 5 years of diabetes duration and were aged ≥10 years were recruited for a follow‐up visit from 2012 to 2015 (mean of 7.9 standard deviation [SD] 1.9) years from diabetes diagnosis in the SEARCH for Diabetes in Youth Cohort Study.7 The study was approved by Institutional Review Boards with jurisdiction. Parents and young adults provided consent and adolescents provided assent.
2.2. Outcomes
Our primary outcomes, SH occurrence and frequency, were determined by self‐report. Participants were asked, “In the past 6 months, have/has (you or your child) had any severe hypoglycaemia, that is, very low blood sugar that required him/her to get help?” Participants who answered “yes” were then asked how many times severe hypoglycaemia occurred, whether they received a glucagon injection, called 911, went to the emergency room or had to stay in the hospital overnight. SH was categorized as zero events vs one or more events in the past 6 months.
2.3. Covariates
Socio‐demographic characteristics included participant’s age at the time of the follow‐up study visit, sex, self‐reported race/ethnicity, highest level of parental education, household income and health insurance status. Self‐reported race/ethnicity was grouped as Hispanic (regardless of race), non‐Hispanic White, non‐Hispanic Black and non‐Hispanic other. Highest level of education by any parent or guardian was categorized as less than high school, high school graduate, some college and college degree or higher. Household income was categorized as <$25 000, $25 000 to $49 999, $50 000 to $74 999, and $75 000 or higher. Health insurance was categorized as private, Medicare or Medicaid, other, or none.
Clinical measures obtained at the initial visit and follow‐up visit included HbA1c and body mass index (BMI). BMI was categorized based on BMI z‐score and percentile for youth <18 years or by categories for young adults (≥18 years and older) as follows: obesity ≥95th percentile or >30 kg/m2; overweight 85‐95th percentile or 25‐30 kg/m2; and underweight or normal <85th percentile or <25 kg/m2.
Current diabetes treatment was based on self‐report at the cohort visit. Current insulin regimens were categorized as insulin pump, basal insulin (glargine, detemir or degludec) and rapid‐acting insulin, basal insulin and any other combination (at least one other insulin aside from rapid‐acting insulin), and any insulin ≤2 times a day. Participants were also asked about the use of continuous glucose monitoring (yes or no).
Smoking status was categorized as never, former or current smoker based on self‐report. Being physically active was defined as self‐report of vigorous physical activity (sweating or breathing hard) for at least 20 minutes for at least three out of the past 7 days, dichotomized as yes or no.8
2.4. Statistical analysis
Analyses were stratified by diabetes type (type 1 or type 2 diabetes) as determined by the health care provider around the time of diagnosis. Analyses were also done by aetiologic type as determined by the presence or absence of diabetes auto‐antibodies and insulin sensitivity score.9 Demographics, behavioural (smoking and physical activity) and clinical (insulin use, BMI, diabetes duration and HbA1c) characteristics were described overall and contrasted by occurrence of a SH event using Wilcoxon two‐sample tests for continuous variables and chi‐square tests for categorical variables. For categorical variables with very small sample sizes, typically variables among the group with type 2 diabetes, Fisher’s exact tests were used, since these tests are typically more robust to deviations from underlying assumptions (eg, normality for continuous outcomes and cell counts ≥5 for categorical outcomes.). Prevalence of SH with 95% confidence intervals was obtained using the score confidence interval approach.10 Associations between socio‐demographic and clinical characteristics, including HbA1c and insulin regimen, with SH were tested using logistic regressions, adjusted for age at the follow‐up visit, race/ethnicity and sex.
Results of participants’ characteristics by report of SH events are only presented for type 1 diabetes due to small number of youth and young adults with type 2 diabetes who reported any SH events (n = 9).
3. RESULTS
The prevalence of the occurrence of one or more SH events was higher among youth and young adults with type 1 diabetes (7.0%, 95% CI 6.8%‐8.1%) compared to those with type 2 diabetes (2.6%, 95% CI 1.4%‐4.9%; Table 1). Characterizing participants by aetiologic diabetes type9 did not change the results (data not shown). The demographic and clinical characteristics of adolescents and young adults in the SEARCH cohort differ by diabetes type (Table 1). Compared to youth and young adults with type 1 diabetes, those with type 2 diabetes were older at the follow‐up visit (22.3 vs 17.3 years, P < 0.0001), were more likely to be female (63.6% vs 50.4%, P < 0.0001) and less likely to be non‐Hispanic White (22.3% vs 74.7%, P < 0.0001). Few participants with type 1 diabetes reported not having health insurance (3.3%) compared to 20.2% of participants with type 2 diabetes. Participants with type 1 diabetes were more likely to report never smoking (69.4%) and being physically active (57.4%) compared to participants with type 2 diabetes (41.5% and 39.3%, respectively). Participants with type 2 diabetes were almost six times more likely to have obesity both at time of diagnosis and at follow‐up visit compared to participants with type 1 diabetes. Diabetes treatment regimen also differed by diabetes type.
Table 1.
Characteristics of adolescents and young adults with diabetes in the SEARCH for Diabetes in Youth Cohort Study by diabetes type
| Variables | All (N = 2740) | Type 1 diabetes (N = 2399) | Type 2 diabetes (n = 341) | P‐value |
|---|---|---|---|---|
| Prevalence of SH event, % | 6.5 | 7.0 | 2.6 | <0.002 |
| Age at follow‐up visit, mean years (SD) | 17.9 (4.8) | 17.3 (4.7) | 22.3 (3.5) | <0.0001 |
| Female, % | 52.0 | 50.4 | 63.6 | <0.0001 |
| Male, % | 48.0 | 49.6 | 36.4 | |
| Race/Ethnicity, % | ||||
| Hispanic | 13.5 | 12.0 | 23.8 | <0.0001 |
| Non‐Hispanic Black | 15.0 | 10.9 | 44.0 | |
| Non‐Hispanic White | 68.1 | 74.7 | 22.3 | |
| Non‐Hispanic other | 3.4 | 2.5 | 10.0 | |
| Highest parental education, % | ||||
| <High school | 5.0 | 3.8 | 13.5 | <0.0001 |
| High school graduate | 14.6 | 11.7 | 35.6 | |
| Some college or higher | 32.7 | 32.8 | 32.2 | |
| College graduate or higher | 47.7 | 51.7 | 18.7 | |
| Health insurance, % | ||||
| None | 5.3 | 3.3 | 20.2 | <0.0001 |
| Other | 5.3 | 5.1 | 6.8 | |
| Medicare/Medicaid | 23.4 | 21.4 | 38.2 | |
| Private | 66.0 | 70.2 | 34.8 | |
| Smoking status, % | ||||
| Never | 65.8 | 69.4 | 41.5 | <0.0001 |
| Former | 19.3 | 17.9 | 27.9 | |
| Current | 15.0 | 12.6 | 30.6 | |
| Physical activitya,% | ||||
| Yes | 55.0 | 57.4 | 39.3 | <0.0001 |
| No | 45.0 | 42.6 | 60.7 | |
| Age at diagnosis, mean years (SD) | 9.8 (4.5) | 9.2 (4.3) | 14.1 (2.6) | <0.0001 |
| Duration of diabetes, mean years (SD) | 8.0 (2.0) | 8.0 (1.9) | 8.1 (2.1) | 0.43 |
| Insulin sensitivity score at time of diagnosis, mean (SD) | 10.1 (4.0) | 10.9 (3.4) | 4.5 (2.6) | <0.0001 |
| Insulin sensitivity score at follow‐up visit, mean (SD) | 6.5 (2.9) | 7.0 (2.7) | 3.4 (2.0) | <0.0001 |
| BMI category at time of diagnosisb, % | ||||
| Normal or underweight | 58.9 | 66.7 | 5.3 | <0.0001 |
| Overweight | 18.4 | 19.5 | 10.6 | |
| Obesity | 22.7 | 13.8 | 84.1 | |
| BMI category at follow‐up visitb, % | ||||
| Normal or underweight | 54.5 | 61.0 | 9.6 | 0.0001 |
| Overweight | 24.2 | 25.3 | 16.8 | |
| Obesity | 21.3 | 13.7 | 73.6 | |
| HbA1c at time of diagnosis, mean % (SD) | 7.6 (1.6) | 7.7 (1.5) | 7.1 (2.0) | <0.0001 |
| Mean mmol/mol | 60 | 61 | 54 | |
| HbA1c at follow‐up visit, mean % (SD) | 9.1 (2.0) | 9.1 (1.9) | 9.0 (3.0) | 0.07 |
| Mean mmol/mol | 76 | 76 | 75 | |
| Use of oral hypoglycaemia medication, % | 10.7 | 3.9 | 78.8 | <0.001 |
| Use of sulfonylurea, % | 7.6 | 0.5 | 7.6 | <0.001 |
| Use of GLP‐1 analogues | 0.5 | 0.23 | 2.3 | <0.001 |
| Use of insulinc, % | 95.8 | 98.7 | 66.8 | <0.001 |
| Use of continuous glucose monitors (CGM), % | 19.3 | 18.3 | 28.7 | 0.0001 |
| Insulin regimend, % | ||||
| Pump | 52.6 | 55.6 | 7.1 | <0.0001 |
| Basal insulin +short/rapid insulin (three or more times per day) | 18.7 | 19.3 | 10.3 | |
| Basal insulin +any other combination | 17.0 | 16.4 | 25.6 | |
| Any insulin injection regimen excluding basal insulin | 11.7 | 8.8 | 57.1 | |
BMI, body mass index; SD, standard deviation; SH, severe hypoglycaemia.
Physical activity defined as self‐report of vigorous physical activity (sweating or breathing hard) for at least 20 min for at least three out of the past 7 d.
BMI category based on BMI z‐score and CDC growth charts for youth <18 y old. Normal or underweight defined as BMI z‐score <85th percentile or BMI <25 kg/m2; overweight defined as BMI z‐score 85‐<95th percentile or BMI ≥25 and <30 kg/m2; obesity defined as BMI z‐score ≥95th percentile or BMI ≥30 kg/m2.
Insulin use may be in combination with oral diabetes medications.
Among participants who report insulin use (type 1 diabetes n = 2368; type 2 diabetes n = 161).
Figure 1 presents the number of hypoglycaemia events, hospital stays, emergency room visits and paramedic calls among those with type 1 or type 2 diabetes who reported at least one SH event. The mean number of events was similar for participants with type 1 vs type 2 diabetes (P = 0.89). Among individuals with type 1 diabetes who reported any SH event, the number of events reported in the past 6 months ranged from 1 to 72 with a median of 1 and mean of 4.1 (SD 8.1). For type 2 diabetes, the number of events ranged from 1 to 15 with a median of 1 and mean of 3.6 (SD 4.9). In general, few of the reported events required paramedic calls (39.9% for type 1 diabetes, 14.3% for type 2 diabetes, P‐value 0.87), emergency room visits (28.4% for type 1 diabetes, 28.6% for type 2 diabetes, P‐value 0.84) or hospital stays (8.3% for type 1 diabetes, 0% for type 2 diabetes, P‐value 0.34).
Figure 1.
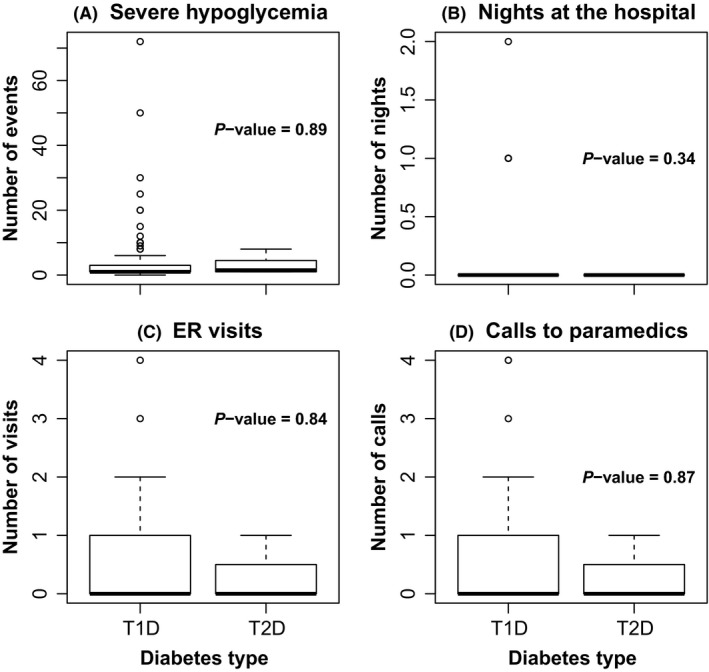
Box plots of (A) number of severe hypoglycaemia (SH) events, (B) paramedic calls, (C) emergency room (ER) visits and (D) hospital stays among those who report SH events in the past 6 mo among children, adolescents and young adults with type 1 and type 2 diabetes in the SEARCH for Diabetes in Youth Cohort Study, 2010‐2015
Among participants with type 1 diabetes, those who reported SH events were older both at the follow‐up visit (18.4 years vs 17.2 years, P‐value 0.007) and at diagnosis of diabetes (10.0 years vs 9.2 years, P = 0.02), and tended to be male (56.6% vs 49.1%, P‐value 0.07) compared to participants who did not report SH events (Table 2). The distribution of race/ethnicity, highest parental education and health insurance status was similar for participants who did or did not report SH events. Youth that reported a SH event were more likely to report being physically active compared to those who did not report a SH event (70.3% vs 54.2%, P‐value = 0.0003). While there was no significant difference at the time of diagnosis in overweight and obesity status or HbA1c levels between participants who reported SH events and those who did not, there were differences at the follow‐up visit. Participants who reported SH events were more likely to be overweight or have obesity compared to those who reported no events (P‐value = 0.03). Participants reporting SH events had lower mean HbA1c values at the follow‐up visit as compared to those who reported no SH event (8.7% vs 9.2%, P‐value 0.001). There was no significant difference between the two groups by use of continuous glucose monitoring (CGM) or by insulin regimen.
Table 2.
Demographic and clinical characteristics by occurrence of serve hypoglycaemia events among youth and young adults with type 1 diabetes in the SEARCH for Diabetes in Youth Cohort Study, 2010‐2015
| Characteristics | 0 events (n = 2231) | 1 or more events (n = 168) | P‐value |
|---|---|---|---|
| Age at follow‐up visit, mean years (SD) | 17.2 (4.6) | 18.4 (5.4) | 0.007 |
| Female, % | 50.9 | 43.5 | 0.07 |
| Male, % | 49.1 | 56.6 | |
| Race/ethnicity, % | |||
| Hispanic | 10.8 | 12.5 | 0.58 |
| Non‐Hispanic Black | 12.2 | 8.9 | |
| Non‐Hispanic White | 74.5 | 76.2 | |
| Non‐Hispanic other | 2.5 | 2.4 | |
| Highest parental education, % | |||
| <High school | 3.9 | 2.4 | 0.82 |
| High school graduate | 11.8 | 10.8 | |
| Some college or higher | 32.7 | 34.3 | |
| College graduate or higher | 51.6 | 52.4 | |
| Health insurance, % | |||
| None | 1.4 | 1.8 | 0.52 |
| Other | 2.2 | 3.6 | |
| Medicare/Medicaid | 17.7 | 18.1 | |
| Private | 78.8 | 76.5 | |
| Smoking status, % | |||
| Never | 66.0 | 59.1 | 0.28 |
| Former | 19.2 | 22.0 | |
| Current | 14.9 | 18.2 | |
| Physical activitya,% | |||
| No | 45.8 | 29.7 | 0.0003 |
| Yes | 54.2 | 70.3 | |
| Age at diabetes diagnosis, mean years (SD) | 9.2 (4.3) | 10.0 (4.9) | 0.02 |
| Duration of diabetes, mean years (SD) | 7.9 | 8.3 | 0.06 |
| BMI category at baseline visitb, % | |||
| Normal or underweight | 67.2 | 64.1 | 0.64 |
| Overweight | 19.3 | 20.3 | |
| Obesity | 13.5 | 15.7 | |
| BMI category at follow‐up visitb, % | |||
| Normal or underweight | 61.6 | 52.8 | 0.03 |
| Overweight | 25.1 | 27.0 | |
| Obesity | 13.2 | 20.1 | |
| HbA1c at baseline visit, mean % (SD) | 7.7 (1.5) | 7.9 (1.7) | 0.13 |
| Mean mmol/mol | 61 | 63 | |
| HbA1c at follow‐up visit, mean % (SD) | 9.2 (1.9) | 8.7 (1.8) | 0.001 |
| Mean mmol/mol | 77 | 72 | |
| Use of continuous glucose monitors (CGM), % | 18.1 | 21.1 | 0.35 |
| Insulin regimen, % | |||
| Pump | 55.9 | 51.2 | 0.23 |
| Basal insulin + short/rapid insulin (three or more times per day) | 19.4 | 17.3 | |
| Basal insulin + any other combination | 16.2 | 19.6 | |
| Any insulin injection regimen excluding basal insulin | 8.5 | 11.9 | |
BMI, body mass index; SD, standard deviation; SH, severe hypoglycaemia.
Physical activity defined as self‐report of vigorous physical activity (sweating or breathing hard) for at least 20 min for at least three out of the past 7 d.
BMI category based on BMI z‐score and CDC growth charts for youth <18 y old. Normal or underweight defined as BMI z‐score <85th percentile or BMI <25 kg/m2; overweight defined as BMI z‐score 85‐<95th percentile or BMI ≥25 and <30 kg/m2; obesity defined as BMI z‐score ≥95th percentile or BMI ≥30 kg/m2.
After adjusting for demographic and clinical characteristics, youth and young adults that reported being physically active were significantly more likely to have experienced SH events than those who did not report being physically active (OR 1.87 [95% CI 1.23, 2.86]; Table 3). In the adjusted model, each unit increase in HbA1c level was associated with a 16% reduction in the odds of having SH events (OR 0.84 [95% CI 0.75, 0.94]). Younger age at follow‐up visit was also associated with a 15% reduction per year of age in the odds of having SH events after adjustments (OR 0.85 [95% CI 0.77, 0.95]).
Table 3.
Odds ratios (95% confidence interval) for occurrence of severe hypoglycaemia events adjusting for demographic and clinical characteristics among youth and young adults with type 1 diabetes, SEARCH for Diabetes in Youth Cohort 2012‐2015
| Variables | Levels |
Model 1 OR (95% CI) |
Model 2 OR (95% CI) |
Model 3 OR (95% CI) |
Model 4 OR (95% CI) |
|---|---|---|---|---|---|
| Sex | Male (reference female) | 1.36 (0.96, 1.94) | 1.35 (0.95, 1.92) | 1.10 (0.75, 1.63) | 1.10 (0.75, 1.63) |
| Race/ethnicity | Non‐Hispanic White (reference all others) | 1.10 (0.72, 1.66) | 1.30 (0.82, 2.06) | 1.18 (0.7, 2.0) | 1.18 (0.7, 2.0) |
| Age at follow‐up visit (per 1 y increase in age) | 1.04 (1.0, 1.08) | 0.85 (0.77, 0.95) | 0.85 (0.77, 0.95) | ||
| Age at diabetes diagnosis (per 1 y increase in age) | 0.97 (0.88, 1.08) | 0.99 (0.89, 1.11) | |||
| HbA1c at follow‐up visit (per 1 unit increase in HbA1c) | 0.85 (0.77, 0.95) | 0.84 (0.75, 0.94) | |||
| Physical activitya | Yes (no reference) | 1.87 (1.23, 2.86) | |||
| Smoking | Past/current (never reference) | 1.18 (0.7, 2.0) |
Model 1 includes sex and race.
Model 2: Model 1 covariates plus age at the follow‐up visit and clinic (South Carolina, Colorado, Ohio, Washington, California).
Model 3: Model 2 covariates plus age at diabetes diagnosis and HbA1c at follow‐up visit.
Model 4: Model 3 covariates plus physical activity and smoking status.
Physical activity defined as self‐report of vigorous physical activity (sweating or breathing hard) for at least 20 min for at least three out of the past 7 d.
4. DISCUSSION
Our findings showed that 7 out of 100 youth with type 1 diabetes reported at least one episode of SH event that required assistance in the past 6 months. While SH events were more common among youth with type 1 diabetes compared to those with type 2 diabetes, almost three out of 100 youth with type 2 diabetes also reported SH events over a 6‐month timeframe. Recurrent SH events among youth who reported at least one SH event were common, with an average of four events in 6 months for both groups. Direct comparisons of these results to those from previous studies are not straightforward. In this study, we present prevalence of occurrence of any SH in the past 6 months prior to the follow‐up visit as opposed to calculating number of events per patient year. Given this caveat, it is difficult to know whether our results are in line with recent reports which range from 3.6 to 15 events per 100 patient‐years.2, 3 However, our results are lower than reports from the early 1990s of 44% reporting events in the past year.4
Studies of hypoglycaemia events among adults report rates of occurrence (per population or patient‐years) or report events based on administrative data from emergency departments or hospitals11, 12 and a few include self‐reports of events.13, 14 The incidence of hypoglycaemia in the emergency department decreased in the US from 2006 to 2011 in adults 45 years and older from 1.3 per 100 to 1.0 per 100 adults.11 The incidence of hypoglycaemia in emergency department visits in the US was 1.4 per 100 adult among adults age 18‐44 with diagnosed diabetes in 2011.11 This is lower than the per cent occurrence we observed for SH events, possibility due to inclusion of an older age group or possibly indicating an underestimate of SH events when only based on ER or hospital admissions. Moreover, this study focused on emergency departments and did not distinguish between type 1 and type 2 diabetes. A recent study based in England found an increase in hospitalizations for hypoglycaemia among individuals with either type 1 or type 2 diabetes from 1998 to 2008.12 Among adults ages 18‐44, the annual incidence in 2013 of hypoglycaemia hospitalizations was 7.7/1000 people with type 1 diabetes and 0.7/1000 people with type 2 diabetes.12 While these studies report incidence of hypoglycaemia captured in ED or hospitalizations, fewer have examined the incidence of hypoglycaemia events that occurred outside these settings. Among adult patients in Minnesota, SH in the past 6 months was reported by 28.3% of adults with type 1 diabetes and 16.9% of adults with type 2 diabetes.14 A study of adults with type 2 diabetes in Turkey that used a similar measure of SH as the SEARCH Study found that 15.5% reported an SH event in the past year.13
We explored factors associated with increased risk of SH. A previous study of youth with type 1 diabetes found increased risk of SH associated with lower HbA1c, younger age, higher insulin dose, male sex and lower parental socio‐economic status.5 While we found lower HbA1c levels to be associated with SH, we did not find a statistically significant association with insulin regimen, sex or parental education. We also found that participants who reported SH tended to be young adults as opposed to those who did not report SH.
We found a significant association between physical activity and SH. Youth and young adults with type 1 diabetes who reported being physical active were more likely to report occurrence of SH events in the past 6 months. In this study, we do not have any information on the temporal association between specific SH events and physical activity. For example, we do not know whether the SH event occurred due to vigorous physical activity, particularly since the report of physical activity was for the past week. Therefore, we cannot conclude that physical activity led to the SH event. There are many benefits of regular physical activity including lowering HbA1c levels and maintaining a healthy body weight among individuals with diabetes.15, 16 Individuals taking insulin who are physically active may experience hypoglycaemia and may have to monitor glucose levels more closely.15, 16 Youth and young adults who exercise regularly may benefit from additional glycemic and nutritional recommendations and education around these recommendations.17, 18 Some studies suggest hypoglycaemia experienced during physical activity leads to a reduced awareness of future hypoglycaemia events.19 However, we have no information as to what education the individuals in this study have received about their self‐management around exercise, nor do we know whether the SH event was related to exercise.
While we observed an association between individuals being overweight or having obesity and reports of SH, this association was not significant after adjusting for demographic and clinical characteristics. A recent study of adults using insulin with type 1 and type 2 diabetes found that higher BMI was associated with SH among adults with type 1 diabetes but lower BMI was associated with SH events among adults with type 2 diabetes.20 The ACCORD trial of individuals with type 2 diabetes and over 40 years of age also reported a positive association between BMI and risk of SH events.21
We did not observe any significant association of occurrence of SH events by use of CGM or by insulin regimen. However, this could be due to the small sample size in the group reporting SH events. A recent study among children with type 1 diabetes in Denmark, Iceland, Sweden and Norway found that individuals who reported using insulin pumps experienced lower incidence of SH.22 As new technologies, such as the hybrid closed‐loop23 and CGM become more widely available, we expect that the prevalence of SH will decrease. It will be important to monitor trends in SH by socio‐demographic variables including health insurance, as the uptake of new technologies tends to be faster in individuals with private health insurance. This could result in greater disparities in care and outcomes, including frequency of SH.
We also found no significant association between SH and parental education or health insurance, both markers of socio‐economic status. This is consistent with the findings of a recent systematic review which found that neither health insurance nor education was consistently associated with either an increase or decrease in risk of hypoglycaemia among both children and adults with type 1 diabetes.24
At the national level, there is increased interest in understanding and preventing hypoglycaemic events among persons with diabetes. The release of the National Action Plan to Prevent Adverse Drug Events in the fall of 2014 (http://www.health.gov/hai/ade.asp#final) highlighted hypoglycaemic agents as one of its three initial target areas. The primary adverse event of interest with these agents is hypoglycaemia.25 While the action plan focuses on the risk of hypoglycaemia events and prevention strategies among the older diabetes population, youth with diabetes are also at risk. Furthermore, the prevalence and risk factors associated with hypoglycaemia events is likely to differ by age group.
Few studies have looked at risk factors associated with SH among youth or young adults. Among adults with type 2 diabetes, lower health literacy was associated with self‐report of hypoglycaemia events.26 We do not have a measure of health literacy in SEARCH, and this may be an area for further research. However, we did not observe an association of SH events with parental education. In a study of adults with type 1 or type 2 diabetes in a managed care network, hypoglycaemia events based on emergency department or inpatient diagnoses differed by race/ethnicity with African Americans having higher rates compared to individuals from other racial/ethnic groups.27 We did not observe significant differences by race/ethnicity among individuals with type 1 diabetes in our study. However, we were not able to look at characteristics of youth and young adults with type 2 diabetes due to the small sample size.
The main limitation of this study was the reliance on self‐report of SH events and behavioural characteristics. However, previous studies among type 1 diabetes suggest high accuracy of self‐reported SH events28, 29; comparing prospectively and retrospectively collected self‐reported SH for a 1‐year period, there was 90% agreement.29 The Diabetes Control and Complications Trail/Epidemiology of Diabetes Interventions and Complications study used similar question to assess SH with good reliability, but with a shorter look‐back window of only 3 months.28 A more accurate method of monitoring SH events would be to use a real‐time reporting method, a shorter look‐back window or medical records to validate the reports of SH. Another limitation of this study is the fact that the cohort was not designed specifically to examine the occurrence of SH events. Therefore, it is possible that the study is underpowered for assessing some specific factors related to SH events. However, we did observe a significant association between HbA1c and severe hypoglycaemia. Further, while this cohort was not designed to be nationally representative, the study participants were derived from a population of youth and adolescents that is similar to the youth population of the United States in terms of age, sex, race/ethnicity, household income and parental education.7, 30
Severe hypoglycaemia events occur in youth and young adults with both type 1 and type 2 diabetes. This is the first study that we are aware of that includes reports of the occurrence of SH events among adolescents with type 2 diabetes. This study provides some insight into possible associated clinical characteristics, such as lower HbA1c levels and being physically active. Our findings offer insight into the complexity of balancing glucose control and avoidance of SH. Prevention efforts may include increased education/awareness of patients and their caregivers to prevent the occurrence and re‐occurrence of SH.
CONFLICT OF INTEREST
Nothing to declare.
AUTHOR CONTRIBUTION
SHS participated in the study design, wrote and revised the manuscript and contributed to the discussion. JD participated in the study design, data analysis, revision of the manuscript and contributed to the discussion. GI, RB, LH, EMD, VZ, DD, JML and CP participated in the study design, data collection, revision of the manuscript and contributed to the discussion.
DISCLAIMER
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
ETHICAL APPROVAL
Local institutional review board approval was obtained for each centre. Written informed consent was obtained from participants age 18 and older, while assent with parental written informed consent was obtained for participants younger than 18 years.
ACKNOWLEDGEMENTS
The SEARCH for Diabetes in Youth Study is indebted to many youth and their families, and their health care providers, whose participation made this study possible.
Saydah S, Imperatore G, Divers J, et al. Occurrence of severe hypoglycaemic events among US youth and young adults with type 1 or type 2 diabetes. Endocrinol Diab Metab. 2019;2:e00057 10.1002/edm2.57
Funding information
Grant Support: SEARCH for Diabetes in Youth is funded by the Centers for Disease Control and Prevention (PA numbers 00097, DP‐05‐069, and DP‐10‐001) and supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Site Contract Numbers: Kaiser Permanente Southern California (U48/CCU919219, U01 DP000246, and U18DP002714), University of Colorado Denver (U48/CCU819241‐3, U01 DP000247, and U18DP000247‐06A1), Children’s Hospital Medical Center (Cincinnati) (U48/CCU519239, U01 DP000248, and 1U18DP002709), University of North Carolina at Chapel Hill (U48/CCU419249, U01 DP000254, and U18DP002708), University of Washington School of Medicine (U58/CCU019235‐4, U01 DP000244, and U18DP002710‐01), Wake Forest University School of Medicine (U48/CCU919219, U01 DP000250, and 200‐2010‐35171). Role of the Sponsors: Dr. Saydah (CDC) is a participating member of the study Steering Committee and the Writing Group for this manuscript because of the cooperative funding agreement. She was involved in the design of the study but not the conduct of the study; she was not involved in the collection, management and analysis of the data, but was involved in interpretation of the data; she was involved in the preparation, review and approval of the manuscript and the decision to submit the manuscript for publication.
DATA ACCESSIBILITY
The data are fully accessible to the researchers and are stored in a secure location at Wake Forest University. Further information on data availability to researchers can be found at https://www.searchfordiabetes.org/dspHome.cfm.
REFERENCES
- 1. Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36:1384‐1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johansen A, Kanijo B, Fredheim S, et al. Danish Society for Diabetes in C: prevalence and predictors of severe hypoglycemia in Danish children and adolescents with diabetes. Pediatr Diabetes. 2015;16:354‐360. [DOI] [PubMed] [Google Scholar]
- 3. Karges B, Rosenbauer J, Kapellen T, et al. Hemoglobin A1c levels and risk of severe hypoglycemia in children and young adults with type 1 diabetes from Germany and Austria: a trend analysis in a cohort of 37,539 patients between 1995 and 2012. PLoS Medicine. 2014;11:e1001742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Limbert C, Schwingshandl J, Haas J, Roth R, Borkenstein M. Severe hypoglycemia in children and adolescents with IDDM: frequency and associated factors. J Diabet Complications. 1993;7:216‐220. [PubMed] [Google Scholar]
- 5. Bulsara MK, Holman CD, Davis EA, Jones TW. The impact of a decade of changing treatment on rates of severe hypoglycemia in a population‐based cohort of children with type 1 diabetes. Diabetes Care. 2004;27:2293‐2298. [DOI] [PubMed] [Google Scholar]
- 6. Hamman RF, Bell RA, Dabelea D, et al. The SEARCH for Diabetes in Youth Study: rationale, findings, and future directions. Diabetes Care. 2014;37:3336‐3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dabelea D, Stafford JM, Mayer‐Davis EJ, et al. Search for Diabetes in Youth Research Group: association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. JAMA. 2017;317:825‐835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lobelo F, Liese AD, Liu J, et al. Physical activity and electronic media use in the SEARCH for diabetes in youth case‐control study. Pediatrics. 2010;125:e1364‐1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dabelea D, Pihoker C, Talton JW, et al. Study SfDiY: etiological approach to characterization of diabetes type: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2011;34:1628‐1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Agresti A, Coull BA. Approximate is better than “exact” for interval estimation of binomial proportions. Am Stat. 1998;52:119‐126. [Google Scholar]
- 11. Wang J, Geiss L, Williams DE, Gregg EW. Trends in emergency department visits for hypoglycemia and hyperglycemia crisis among adults with diabetes in the United States, 2006–2011. Diabetes. 2014;63;1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhong VW, Juhaeri J, Cole SR, et al. Incidence and Trends in hypoglycemia hospitalizations in adults with type 1 and type 2 diabetes in England, 1998–2013: a retrospective cohort study. Diabetes Care. 2017;40:1651‐1660. [DOI] [PubMed] [Google Scholar]
- 13. Buyukkaya Besen D, Arda Surucu H, Kosar C. Self‐reported frequency, severity of, and awareness of hypoglycemia in type 2 diabetes patients in Turkey. PeerJ. 2016;4:e2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McCoy RG, Van Houten HK, Ziegenfuss JY, Shah ND, Wermers RA, Smith SA. Self‐report of hypoglycemia and health‐related quality of life in patients with type 1 and type 2 diabetes. Endocr Pract. 2013;19:792‐799. [DOI] [PubMed] [Google Scholar]
- 15. American Diabetes Association . Standards of medical care in diabetes‐ 2018: glycemic targets. Diabetes Care. 2018;41:S55‐S64. [DOI] [PubMed] [Google Scholar]
- 16. Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39:2065‐2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Riddell MC, Milliken J. Preventing exercise‐induced hypoglycemia in type 1 diabetes using real‐time continuous glucose monitoring and a new carbohydrate intake algorithm: an observational field study. Diabetes Technol Ther. 2011;13:819‐825. [DOI] [PubMed] [Google Scholar]
- 18. Robertson K, Adolfsson P, Scheiner G, Hanas R, Riddell MC. Exercise in children and adolescents with diabetes. Pediatr Diabetes. 2009;10(Suppl 12):154‐168. [DOI] [PubMed] [Google Scholar]
- 19. Cade WT, Khoury N, Nelson S, et al. Hypoglycemia during moderate intensity exercise reduces counterregulatory responses to subsequent hypoglycemia. Physiol Rep. 2016;4(17):pii: e12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Malkani S, Kotwal A. Frequency and predictors of self‐reported hypoglycemia in insulin‐treated diabetes. J Diabetes Res. 2017;2017:7425925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miller ME, Bonds DE, Gerstein HC, et al. The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340:b5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Birkebaek NH, Drivvoll AK, Aakeson K, et al. Incidence of severe hypoglycemia in children with type 1 diabetes in the Nordic countries in the period 2008–2012: association with hemoglobin A 1c and treatment modality. BMJ Open Diabetes Res Care. 2017;5:e000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Breton MD, Chernavvsky DR, Forlenza GP, et al. Closed‐Loop control during intense prolonged outdoor exercise in adolescents with type 1 diabetes: the artificial pancreas ski study. Diabetes Care. 2017;40:1644‐1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Linder L, Rathmann W, Rosenbauer J. Inequalities in glycemic control, hypoglycemia and diabetic ketoacidosis according to socio‐economic status and level of deprivation in type 1 diabetes mellitus: a systematic review. Diabet Med. 2018;35:12‐32. [DOI] [PubMed] [Google Scholar]
- 25. U.S. Department of Health and Human Services Office of Disease Prevention and Health Promotion . National Action Plan for Adverse Drug Event Prevention. Washington, DC: Office of Disease Prevention and Health Promotion; 2014. [Google Scholar]
- 26. Sarkar U, Karter AJ, Liu JY, Moffet HH, Adler NE, Schillinger D. Hypoglycemia is more common among type 2 diabetes patients with limited health literacy: the Diabetes Study of Northern California (DISTANCE). J Gen Intern Med. 2010;25:962‐968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Karter AJ, Lipska KJ, O'Connor PJ, et al. Group S‐DS: high rates of severe hypoglycemia among African American patients with diabetes: the surveillance, prevention, and management of diabetes mellitus (SUPREME‐DM) network. J Diabet Complications. 2017;31:869‐873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gubitosi‐Klug RA, Braffett BH, White NH, et al. Complications Trial /Epidemiology of Diabetes I, Complications Research G: risk of severe hypoglycemia in type 1 diabetes over 30 years of follow‐up in the DCCT/EDIC Study. Diabetes Care. 2017;40:1010‐1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pedersen‐Bjergaard U, Pramming S, Thorsteinsson B. Recall of severe hypoglycaemia and self‐estimated state of awareness in type 1 diabetes. Diabetes Metab Res Rev. 2003;19:232‐240. [DOI] [PubMed] [Google Scholar]
- 30. Dabelea D, Mayer‐Davis EJ, Saydah S, et al. Search for Diabetes in Youth Study Group: prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311:1778‐1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are fully accessible to the researchers and are stored in a secure location at Wake Forest University. Further information on data availability to researchers can be found at https://www.searchfordiabetes.org/dspHome.cfm.


