Summary
Aims
Reduced heart rate variability (HRV) and increased heart rate (HR) are associated with cardiovascular (CV) mortality. In the Liraglutide Effect and Action in Diabetes outcome trial, it was demonstrated a lower rate of CV events in type 2 diabetes (T2D) patients treated with liraglutide compared to placebo. We aimed to investigate the effects of liraglutide compared with glimepiride treatment in T2D patients on the CV risk parameters HR and HRV.
Methods
This was a post hoc study whereas sixty‐two T2D individuals (45 males) were randomized to once daily 1.8 mg liraglutide or once daily 4 mg glimepiride, both in combination with 1 g metformin. HR and measurement of sympathetic activity, that is standard deviation (SD) of beat‐to‐beat (NN) intervals (SDNN), was assessed by 24‐hour Holter monitoring system. Parasympathetic activity was analysed by root mean square of successive differences (RMSSD) in NN intervals and high‐frequency (HF), low‐frequency (LF) and very low‐frequency power.
Results
Baseline clinical characteristics for liraglutide (n = 33) and glimepiride (n = 29) groups were well matched. There was a persistent increase in diurnal HR followed by a significantly increased HR at daytime 5.4 beats per minute, P = 0.011 in the liraglutide‐treated group. There was no treatment change between groups in SDNN and RMSSD, or in HF and LF frequency power analysis.
Conclusions
Liraglutide treatment increased diurnal variation in hourly mean HR followed by an increase in mean daytime HR, independently of changes in sympathetic or parasympathetic activity.
Keywords: automatic nervous system, cardiac autonomic neuropathy, glimepiride, heart rate variability, liraglutide, type 2 diabetes mellitus
1. INTRODUCTION
Individuals with type 2 diabetes (T2D) have an increased risk of cardiovascular (CV) complications compared with the general population in which a multitude of risk factors for CV events are involved.1 Multifactorial intervention against CV risk factors such as hyperlipidaemia, hypertension and hyperglycaemia has a sustained beneficial effect on CV complications.2
One overlooked diabetes complication is cardiovascular autonomic neuropathy (CAN).3 Micro‐ and macrovascular complications resulting from CAN involve damage to the autonomic nerve fibres that innervate the heart and the vessels, which in turn may lead to dysfunctional heart rate (HR) control. A disorder of the automatic nervous system (ANS) reflects an imbalance between the sympathetic and the parasympathetic activity that can be assessed by measuring heart rate variability (HRV), that is the variation between two consecutive heartbeats; the higher the variation, the higher is the parasympathetic activity. Higher parasympathetic activity protects against CV events, whereas low HRV predicts CV events.3
It was demonstrated in the LEADER (Liraglutide Effect and Action in Diabetes) trial that the time‐to‐event analysis for the composite end‐point, that is the rate of the first occurrence of CV death, nonfatal myocardial infarction or nonfatal stroke, was significantly lower among T2D patients treated with liraglutide compared with a placebo.4 This effect was mainly driven by a significantly lower rate of CV death, while the mechanisms behind this remain elusive. In clinical trials, treatment with glucagon‐like peptide‐1 receptor agonist (GLP‐1 RA) showed a small, however significant, increase in HR Preclinical and clinical experimental studies have demonstrated beneficial actions from GLP‐1 RA activation on the vessels, heart and brain, independently of glycaemic control.5
GLP‐1 RA may act on and change the activity of the ANS. This study aimed to investigate if liraglutide treatment, compared with glimepiride treatment, affects ANS in patients with T2D with subclinical heart failure.
2. TRIAL DESIGN
This post hoc study was an open, assessor‐blinded, randomized, controlled, parallel‐group trial and a part of a larger study identified as NCT01425580 (www.clinicaltrials.gov). The main results of this study have been published elsewhere.6
Briefly, T2D patients who had glycated haemoglobin A1c (HbA1c) of 6.3% ‐ 11% (45‐97 mmol/mol) were eligible if they had not been previously treated with GLP‐1 RA, dipeptidyl peptidase‐4 inhibitors or glimepiride. Patients who met these criteria were invited for echocardiographic screening, in which one of the following criteria had to be fulfilled: left ventricle ejection fraction ≤50% or evidence of diastolic dysfunction. The major exclusion criteria were as follows: type 1 diabetes, treatment with glitazones during the previous 6 months, treatment with sulphonylureas in the previous 3 months, insulin treatment within the previous month, heart failure according to the New York Heart Association classification 3‐4, past history of atrial fibrillation or flutter, presence of acute myocarditis or significant valvulopathies, uncontrolled hypertension, severe heart conduction disturbances or ventricular tachyarrhythmia within the previous 3 months, unstable angina or myocardial infarction in the previous 8 weeks, estimated glomerular filtration rate <30 mL/min, haemoglobin <90 g/L, BMI >40 kg/m2, severe gastrointestinal disease, history of acute or chronic pancreatitis, malign neoplasia within the last 5 years, current drug or alcohol abuse and pregnancy.
Patients were block‐randomized using sealed envelopes to receive either liraglutide or glimepiride during an 18‐week treatment period. The initial dose of liraglutide was 0.6 mg (sc), with an up‐titration of 0.6 mg every week to a final dose of 1.8 mg per day. The initial dose of the comparator was 2 mg glimepiride with an up‐titration of 1 mg every week, reaching a final dose of 4 mg per day. The regional ethics committees at both participating centres reviewed and approved the trial protocol, and the study followed the International Conference on Harmonization–Good Clinical Practice guidelines. All subjects provided written informed consent before enrolment.
2.1. Twenty‐four‐hour Holter monitoring (heart rate, heart rate variability and spectrum analysis)
Twenty‐four‐hour Holter monitoring (ambulatory electrocardiogram [ECG] recordings) was performed using Schiller Medilog AR12 Plus recorders (Schiller Medilog, Schiller AG, Baar, Switzerland). Three bipolar ECG leads (modified chest lead V1, V5 and aVF) were used. ECG data were extracted at baseline and after 18 weeks of treatment (liraglutide vs glimepiride).
The following parameters were calculated: for HR—mean, min/max, daytime (07:00 to 23:00) and midnight (24:00 to 6:00); for the time domain—mean beat‐to‐beat (NN) intervals, standard deviation of NN (SDNN), SD of the averages of NN intervals (SDANN) and SDNN of all 5‐minutes segment recordings (SDNN index), square root of the mean squared difference (RMSSD) and percentage of adjacent NN intervals differing by more than 50 ms (pNN50%). The RMSSD and pNN50% are associated with parasympathetic activity, whereas SDNN is associated with sympathetic activity.
Fast Fourier spectrum analyses were performed to measure cardiac nervous system autonomic balance. The derived spectrum included bands of very low frequency (VLF; <0.04 Hz) and low frequency (LH; 0.04‐0.15 Hz), that is an index of both sympathetic and parasympathetic activity, and high frequency (FH; 0.15‐0.4 Hz), indicating the most efferent (parasympathetic) activity. Finally, index bands of the total power were calculated.
2.2. Statistical analysis
Continuous data are presented as mean ± standard deviation (SD) or median with corresponding 1st quartile (Q1) and 3rd quartile (Q3), and categorical data are presented as percentages. Baseline characteristics of patients were compared using Student's t test or the Mann‐Whitney U test for continuous variables and Fisher's exact test for categorical variables. For repeated measures, a generalized linear mixed model with random effects in patients and fixed effects of the time‐period and treatment was used to compare the diurnal variation in hourly mean HR between groups. A generalized linear model was used to compare treatment changes in the 24‐hour Holter monitoring parameters between groups. An intention‐to‐treat (ITT) analysis was performed with five imputations by using the iterative Markov chain Monte Carlo method for missing values in continuous variables.7 A two‐sided P‐value of <0.05 was considered statistically significant. Data were analysed using IBM SPSS Statistics software, version 23.0 (IBM Corporation, Armonk, NY, USA) and Stata 15.1 (StataCorp, College Station, TX, USA).
3. RESULTS
The CONSORT flow chart of the study is provided in Figure 1. One hundred and five patients were screened, while 43 of these were excluded owing to screening criteria (n = 35), declining to participate (n = 3) or other reasons (n = 5). Subsequently, 62 patients were randomized to liraglutide (n = 33) vs glimepiride (n = 29). There were none lost to follow‐up; however, three patients withdrew their consent (Figure 1). For technical reasons (eg undetectable QRS segments in all three leads, high frequencies of ectopic complexes and distortions), another six patients were excluded from the per‐protocol analysis. Finally, 29 patients in the liraglutide group and 24 in the glimepiride group made up the final analysis (per‐protocol). Sensitivity analysis (ITT analysis) was also carried out for our end‐points.
Figure 1.
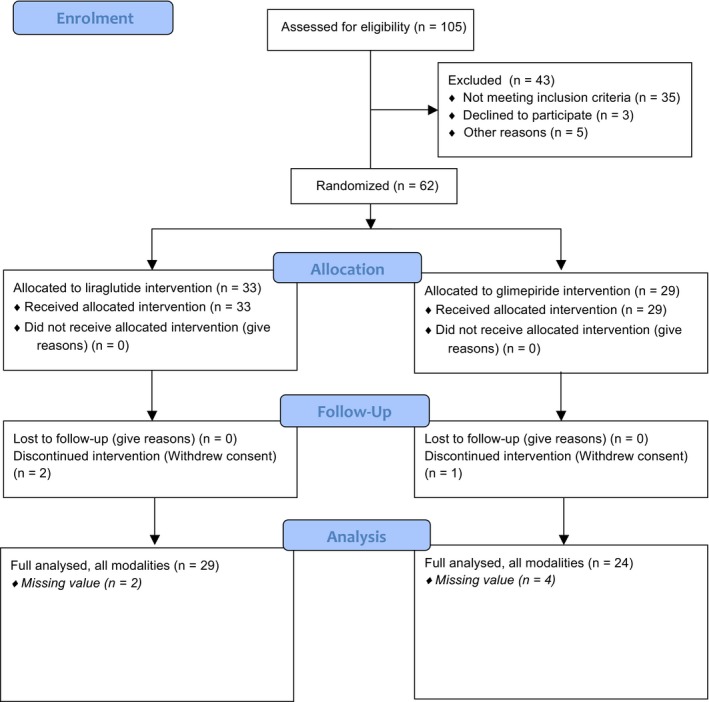
Flow chart for the study groups. 105 patients were screened, whereas 62 patients were eligible for the study, of these 33 vs 29 were randomized to liraglutide vs glimepiride, respectively. Due to technical hitches and dropouts, there were 29 in the liraglutide vs 24 in the glimepiride group, who were analysed per‐protocol, that is full data set
There was a statistically significant but small difference in HbA1c and triglycerides observed between groups (Table 1). In the liraglutide‐treated group, compared with the glimepiride‐treated group, there was a statistically significant treatment change for reduction in weight −3.7 kg, 95% confidence interval (CI): −5.0 to −2.4 kg vs −0.2 kg, 95% CI: −1.9 to −1.5 kg; P = 0.001 and waist circumference −3.1 cm, 95% CI: −4.1 to −2.1 cm vs −0.8 cm, 95% CI: −2.5 to −0.9 cm; P = 0.019, respectively. No such treatment change was observed for HbA1c −1.2%, 95% CI: −1.4 to −0.8% (−11.2 mmol/mol, 95% CI: −14.1 to −8.4 mmol/mol) vs −0.9%, 95% CI: −1.3 to −0.5% (−9.0 mmol/mol, 95% CI: −13.2 to −4.9 mmol/mol; P = 0.37) between groups.6
Table 1.
Baseline characteristics of the intention‐to‐treat population (ITT). Quantitative data are mean (±SD) or median (1st quartile, 3rd quartile), and categorical data are n (%)
| Liraglutide, n = 33 | Glimepiride, n = 29 | P‐value | |
|---|---|---|---|
| Age, year | 60.8 ± 7.6 | 63.3 ± 6.8 | 0.240a |
| Male | 24 (72.7%) | 21 (72.4%) | 1.000b |
| Diabetes duration, year | 5 (1, 10) | 3 (1, 7) | 0.368c |
| Current smoker | 3 (9.1%) | 4 (13.8%) | 0.852b |
| BMI kg/m2 | 30.5 ± 4.4 | 29.0 ± 3.2 | 0.152a |
| Body weight, kg | 92.8 ± 15.9 | 89.0 ± 9.9 | 0.411a |
| Waist circumference, cm | 109.0 ± 13.0 | 106.3 ± 9.7 | 0.366a |
| Mean systolic BP, mm Hg | 132 ± 14.0 | 129 ± 10.9 | 0.414a |
| Mean diastolic BP, mm Hg | 77 ± 7.9 | 77 ± 7.9 | 0.838a |
| eGFR, mL/min/1.72m2 | 88.3 ± 15.0 | 87.4 ± 13.1 | 0.799a |
| Complications | |||
| Hypertension | 29 (87.9%) | 21 (72.4%) | 0.224b |
| Hyperlipidaemia | 25 (75.8%) | 23 (79.31%) | 0.980b |
| Coronary artery disease | 10 (30.3%) | 11 (37.9%) | 0.714b |
| Stroke | 1 (3.0%) | 2 (6.9%) | 0.902b |
| Proliferative retinopathy | 1 (3.0%) | 1 (3.45%) | 1.000b |
| Treatment | |||
| Antiplatelet therapy | 11 (33.3%) | 12 (41.4%) | 0.696b |
| Anticoagulant therapy | 3 (9.1%) | 1 (3.5%) | 0.714b |
| ACE inhibitors/ARB blockers | 25 (75.8%) | 20 (69.0%) | 0.754b |
| Beta‐blockers | 14 (42.4%) | 13 (44.8%) | 1.000b |
| Calcium inhibitors | 13 (39.4%0) | 10 (34.5%) | 0.894b |
| Diuretics | 11 (33.3%) | 6 (20.7%) | 0.408b |
| Statins | 22 (66.7%) | 24 (82.8%) | 0.248b |
| Biochemical parameters | |||
| HbA1c, mmol/mol (IFCC) | 54 (50, 60) | 50 (49, 54) | 0.036c |
| HbA1c, % (NGSP) | 7.1 (6.7, 7.6) | 6.7 (6.6, 7.1) | 0.036c |
| Triglycerides, mmol/L | 2.0 (1.4, 2.6) | 1.5 (1.0, 2.2) | 0.029c |
| Total cholesterol, mmol/L | 4.4 (4.0, 6.0) | 4.6 (3.7, 4.9) | 0.530c |
| LDL‐cholesterol, mmol/L | 2.8 ± 1.2 | 2.5 ± 1.0 | 0.440a |
| HDL‐cholesterol, mmol/L | 1.1 ± 0.3 | 1.2 ± 0.3 | 0.417a |
ARB, angiotensin receptor blockers; BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin A1c; HDL, high‐density lipoprotein; IFCC, International Federation of Clinical Chemistry; LDL, low‐density lipoprotein; NGSP, The National Glycohaemoglobin Standardization Program.
Quantitative data are mean ± standard deviation or median (first quartile, third quartile), and categorical data are n (%).
Student's t test was used.
Doubled one‐sided P‐value from Fisher's exact test.
Mann‐Whitney U test was used.
3.1. Heart rate, heart rate variability and spectrum analysis (Fourier)
All data on HR, HRV and spectrum analysis are presented in Table 2. Depending on modalities and missing data from pre‐ and post‐treatment 24‐hour Holter recordings and owing to technical reasons (see above), there were 53 individuals (Table 2 and Figure 1) included in the final analysis (per‐protocol).
Table 2.
Effects of treatment change (baseline and at 18 weeks of treatment) for heart rate, heart variability and spectrum (frequency) analysis between groups treated with liraglutide vs glimepiride in combination with metformin
| Liraglutide | Glimepiride | Treatment change | Effect difference | P‐value | P‐valuea | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 18 weeks | Baseline | 18 weeks | Liraglutide | n | Glimepiride | n | ||||
| HR parameter | |||||||||||
| Mean HR (bpm) | 71.2 ± 9.9 | 78.0 ± 9.4 | 69.9 ± 12.2 | 72.7 ± 12.9 | 7.6 ± 11.5 | 31 | 2.5 ± 10.1 | 28 | 5.1 (−0.6, 10.8) | 0.079 | 0.096 |
| Maximum HR (bpm) | 126.8 ± 16.7 | 123.3 ± 17.2 | 129.5 ± 14.9 | 119.3 ± 18.5 | ‐1.9 ± 15.9 | 30 | ‐9.6 ± 19.8 | 28 | 7.6 (−1.8, 17.1) | 0.111 | 0.114 |
| Minimum HR (bpm) | 55.2 ± 8.1 | 55.6 ± 9.2 | 53.0 ± 7.2 | 53.3 ± 7.2 | 0.4 ± 9.0 | 30 | 0.4 ± 6.4 | 28 | ‐0.03 (−4.2, 4.1) | 0.990 | 0.869 |
| Daytime HR (bpm) | 77.7 ± 9.1 | 83.2 ± 11.2 | 77.6 ± 9.5 | 77.9 ± 10.8 | 6.1 ± 8.3 | 30 | 0.8 ± 7.2 | 28 | 5.4 (1.3, 9.5) | 0.011 | 0.023 |
| Night‐time HR (bpm) | 75.4 ± 6.6 | 75.9 ± 11.5 | 73.6 ± 6.3 | 68.5 ± 9.4 | 0.6 ± 13.2 | 30 | ‐5.2 ± 10.5 | 28 | 5.8 (−0.5, 12.1) | 0.072 | 0.067 |
| 24‐h time domain | |||||||||||
| Log Mean NN (ms) | 6.72 ± 0.16 | 6.73 ± 0.19 | 6.73 ± 0.16 | 6.75 ± 0.14 | 0.01 ± 0.14 | 32 | 0.01 ± 0.1 | 28 | 0 (−0.07, 0.06) | 0.935 | 0.878 |
| Log SDNN (ms) | 4.50 ± 0.38 | 4.54 ± 0.41 | 4.69 ± 0.3 | 4.60 ± 0.28 | 0.04 ± 0.46 | 32 | ‐0.08 ± 0.031 | 28 | 0.12 (−0.08, 0.33) | 0.237 | 0.236 |
| Log SDNNindex (ms) | 3.63 ± 0.33 | 3.67 ± 0.59 | 3.70 ± 0.41 | 3.72 ± 0.4 | 0.02 ± 0.64 | 29 | ‐0.01 ± 0.4 | 24 | 0.04 (−0.27, 0.34) | 0.808 | 0.513 |
| Log SDANN (ms) | 4.32 ± 0.43 | 4.34 ± 0.41 | 4.53 ± 0.31 | 4.47 ± 0.28 | 0.01 ± 0.45 | 29 | ‐0.04 ± 0.3 | 24 | 0.03 (−0.18, 0.25) | 0.746 | 0.526 |
| Log rMSSD (ms) | 3.14 ± 0.56 | 3.20 ± 0.88 | 3.27 ± 0.65 | 3.21 ± 0.63 | 0.07 ± 1.03 | 32 | ‐0.07 ± 0.61 | 27 | 0.14 (−0.31, 0.59) | 0.536 | 0.502 |
| Log pNN50 (%) | 0.92 ± 1.17 | 1.00 ± 1.58 | 1.05 ± 1.31 | 1.04 ± 1.22 | 0.11 ± 1.66 | 32 | ‐0.02 ± 1.13 | 28 | 0.12 (−0.62, 0.87) | 0.741 | 0.688 |
| Frequency domain | |||||||||||
| Log LF (ms2) | 6.08 ± 0.87 | 5.91 ± 1.08 | 5.95 ± 0.75 | 5.92 ± 0.76 | ‐0.20 ± 1.18 | 30 | ‐0.16 ± 0.74 | 24 | ‐0.04 (−0.59, 0.52) | 0.889 | 0.903 |
| Log HF (ms2) | 4.75 ± 1.15 | 4.65 ± 1.45 | 4.72 ± 0.81 | 4.78 ± 1 | ‐0.13 ± 1.6 | 30 | ‐0.09 ± 1.0 | 24 | ‐0.04 (−0.79, 0.71) | 0.916 | 0.967 |
| Log VLF (ms2) | 7.47 ± 1.15 | 7.18 ± 1.18 | 7.40 ± 0.94 | 7.34 ± 0.82 | ‐0.28 ± 1.11 | 30 | ‐0.12 ± 0.61 | 24 | ‐0.17 (−0.67, 0.34) | 0.516 | 0.902 |
| Log Total power (ms2) | 7.85 ± 1.08 | 7.56 ± 1.15 | 7.71 ± 0.86 | 7.69 ± 0.76 | ‐0.29 ± 1.05 | 30 | ‐0.10 ± 0.57 | 24 | ‐0.19 (−0.67, 0.29) | 0.427 | 0.781 |
General linear regression model was used for comparing effect difference.
P‐values of ITT analysis. Missing values were imputed using multiple imputation method and five imputed data sets were used for analyses.
At 18 weeks of treatment, there was a persistent increase in diurnal variation in hourly mean HR observed in the liraglutide‐treated group (Figure 2). There was an increased mean HR at daytime in the liraglutide‐treated group compared with the glimepiride‐treated group (Table 2). There was a trend for an increased mean HR at night‐time (P = 0.072) and for the 24‐hour period (P = 0.079) observed in the liraglutide‐treated group compared with the glimepiride‐treated group (Table 2). Sensitivity analysis (ITT analysis) did not change the results (Table 2).
Figure 2.
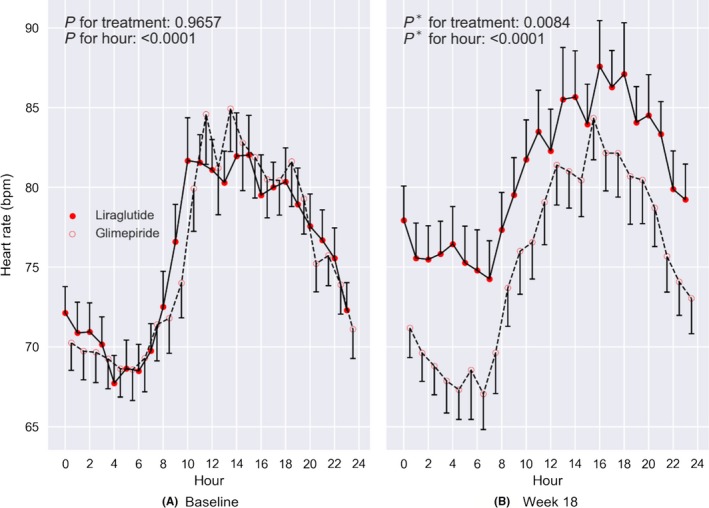
Diurnal variation in hourly mean HR between groups (liraglutide vs glimepiride). Error bars indicate mean ± standard error. Generalized linear mixed model for repeated measures was used to test the difference between the two groups. P * is P‐value adjusted for baseline heart rate. BPM; Beats per minute
In the 24‐hour time domain HRV, there were no statistically significant difference in treatment changes observed between groups for any of our variables, that is mean NN, SDDN, SDNN index, SDANN or pNN50% (Table 2). This non‐significant treatment changes were also demonstrated in the ITT analysis (Table 2).
In the spectrum analysis (Fourier), which can reveal changes between sympathetic and parasympathetic activity, no statistically significant treatment change for any of the frequency domain variables, that is VLF, LF, HF and total power were observed between groups (Table 2). ITT analysis did not change the results.
Since there were significant treatment changes in parameters that may have affected ANS balance, that is glycaemic control, weight and blood pressure, we further did a simple regression analysis between on the one hand HbA1c, systolic BP, diastolic BP and weight, and on the other hand mean HR and SDNN. Coefficient of regression among these parameters and variables of HR, HRV and spectrum analysis is presented in Table 3. In the liraglutide group, there were statistically significant association between treatment changes in mean NN (P = 0.006) and weight (P = 0.019) against SDNN; and a statistically significant association between treatment changes in LF (P < 0.001) and HF (P < 0.001) against RMSSD (Table 3). For the glimepiride group, there was only a significant correlation between treatment changes in LF (P = 0.015) and HF (P < 0.001) against RMSSD (Table 3).
Table 3.
Regression coefficients (β) R 2 values and P‐values from linear regression analysisa between treatment change (liraglutide vs glimepiride) for dependent and independent variables
| Dependent variable | Independent variable | Liraglutide | Glimepiride | ||||
|---|---|---|---|---|---|---|---|
| β | R 2 | P‐value | β | R 2 | P‐value | ||
| ΔHR | ΔHbA1c | −0.353 | 0.060 | 0.216 | 0.084 | 0.012 | 0.778 |
| ΔHR | ΔWeight | −0.190 | 0.016 | 0.780 | −0.130 | 0.007 | 0.810 |
| ΔHR | ΔsBP | −0.056 | 0.010 | 0.703 | −0.022 | 0.005 | 0.915 |
| ΔHR | ΔdBP | −0.135 | 0.011 | 0.591 | −0.046 | 0.022 | 0.839 |
| ΔSDNN | Δmean NN | 0.202 | 0.231 | 0.006 | −0.002 | 0.000 | 0.980 |
| ΔSDNN | ΔHbA1c | 0.454 | 0.006 | 0.724 | −0.604 | 0.017 | 0.450 |
| ΔSDNN | ΔWeight | 5.181 | 0.113 | 0.019 | −2.444 | 0.065 | 0.081 |
| ΔRMSSD | ΔHF | 0.070 | 0.747 | 0.000 | 0.063 | 0.365 | 0.000 |
| ΔRMSSD | ΔLF | 0.048 | 0.589 | 0.000 | 0.033 | 0.276 | 0.015 |
R 2 and P‐values of ITT analysis. Missing values were imputed using multiple imputation method and five imputed data sets were used for analyses.
4. DISCUSSION
In this open parallel study where T2D patients with subclinical heart failure were randomized to an 18‐week treatment period with liraglutide vs glimepiride in combination with metformin, we investigated treatment changes in mean HR and HRV by using 24‐hour Holter monitoring and spectrum analysis. At 18 weeks of liraglutide treatment, there was a persistent increased diurnal variation in hourly mean HR followed by an increase in mean HR daytime, compared with the glimepiride‐treated group. Between groups, there was no treatment change in the ANS suggesting no involvement from sympathetic or parasympathetic tonus.
In clinical trials, different GLP‐1 RA treatments, compared with placebo, have repeatedly demonstrated a small however significant increase in HR concomitant with a small reduction in blood pressure.4, 8, 9, 10 Mechanisms behind these observations remain elusive. Earlier studies in rodents have conclusively demonstrated that GLP‐1 R activation increases blood pressure and HR by different routes, that is peripheral and central nervous system.11 GLP‐1 R is expressed at a high level in the area postrema and nucleus tractus solitarius, areas in the brain known for the central regulation of blood pressure and HR12, 13 Findings in rodents support a dual role of GLP‐1 R activation on blood pressure and HR; on the one hand dependent on parasympathetic transmission from the central nervous system,13, 14 and on the other hand regulated by peripheral nervous structures by adrenergic and non‐adrenergic stimulation.11 This contrast clinical studies where a small reduction in blood pressure with a reciprocal small increase in HR frequently is reported4, 8, 9, 10 and suggested to be secondarily to vasodilatation, that is reflex tachycardia.15 However, recent reports have revealed GLP‐1 R expression in the heart only by the sinoatrial node (SAN) myocytes,16 whereas GLP‐1 RA treatment directly may stimulate SAN to increase HR17, 18
In the present study, we confirm previous observations, demonstrating an increase in HR due to liraglutide treatment. Patients treated with liraglutide had a persistent increased diurnal variation in hourly mean HR followed by a significant increase in HR for the daytime period although trended for increased mean HR night‐time and during the 24‐hour period. Since no significant treatment changes were observed at night‐time, it suggests a relative sympathetic enhancement, rather than inhibition of parasympathetic activity (more predominant night‐time). Notwithstanding this, there was no treatment change between groups in any of the HRV variables, nor in the high‐frequency analysis; therefore, it seems unlikely that changes in the sympathetic or the parasympathetic system can explain the increased HR demonstrated in the liraglutide‐treated group.
There was a treatment change for the reduction in weight in the liraglutide‐treated group and a robust reduction in HbA1c in both groups. This confirms the well‐characterized actions from GLP‐1 RA treatment.4, 8, 9, 10 Not only activation of SAN may give explanation for the increased HR evoked by GLP‐1 RA treatment. Also, expression of GLP‐1 R in the vasculature supports a physiological role of GLP‐1 R activation.19 Therefore, modulation of ANS balance and reflex tachycardia in response to vasodilation is another suggestion for the increased HR observed by GLP‐1 RA treatment. In the LEADER study, patients randomized to liraglutide had their blood pressure slightly decreased concomitant with a small increase in HR4 This may reflect a reciprocal situation where decreased blood pressure may increase HR In the present study, at 18 weeks treatment, no effect on blood pressure was observed. In contrast, there was a transient (after 2 weeks treatment) increased diastolic blood pressure in the liraglutide‐treated group (data published elsewhere).20 In simple regression analysis, no correlation was observed between treatment change in blood pressure and treatment change in HR This suggests other mechanisms than reflex tachycardia behind the increased HR evoked by liraglutide treatment.
There is an increased risk of premature CV death with more rapid HR21, 22 Also, low HRV, reflecting an imbalance in ANS, is a predictor of CV events.23 Despite a small, however significant, increase in HR in the large GLP‐1 RA CV outcome studies patients treated with GLP‐1 RA are not at higher risk of CV death compared with placebo.4, 8, 9, 10 In fact, in the LEADER study, patients treated with liraglutide were at lower risk for CV death compared with patients treated with placebo.4 Recently, it was demonstrated in newly diagnosed overweight T2D patients treated with liraglutide an increased HR concomitant with a reduction in HRV (increased nightly HR and decreased parasympathetic activity). This effect was independent of weight loss and any improvement in glycaemic control and suggested to be due to changes in sympathovagal balance.24 This contrasts to the present study results; although the increase in HR was much the same, we were not able to demonstrate any treatment change in the ANS balance (HRV or spectrum analysis). We cannot exclude that differences in diabetes duration, treatment duration, trial design or comorbidities may explain the difference between studies. One major difference between studies was that patients in the present study were diagnosed with subclinical heart failure (inclusion criteria) and therefore at high risk for CAN.25 Therefore, our findings cannot be generalized to other than patients with T2D with subclinical heart failure.
Previous studies suggest that GLP‐1 RA directly may activate SAN myocytes and increases HR26 Although receptors for GLP‐1 only are expressed by SAN myocytes, there is body of evidence demonstrating physiological action on the heart from GLP‐1 R activation.5 Some of these actions may be mediated through both GLP‐1 R‐dependent and GLP‐1 R‐independent pathways27; however, different actions by different GLP‐1 RA may also contribute to varying results.18 T2D patients treated with different GLP‐1 RA had an increase in HR (acute and after 12 weeks) that was not explained by changes in sympathetic activity, or a reflex to vasodilation, suggesting direct stimulation of SAN involved.18 Acute administration of exenatide into healthy overweight men17 and into T2D patients with heart failure28 increased HR that not was due to reflex tachycardia, however rather to the involvement of sympathetic action or stimulation of SAN. In another recent study where T2D patients received exenatide extended‐release, there was an increase in HR that did not appear to be related to sympathetic influence.29 In a current study, simple regression analysis revealed a significant correlation between treatment change in mean NN and weight against changes in SDNN in the liraglutide‐treated group, compared with the glimepiride‐treated group. Correlations were also demonstrated between changes in LF and HF against change in RMSSD in both groups. Despite this, there was no treatment change in any of the ANS parameters between groups, nor were there any treatment changes in the spectrum analysis parameters between groups. This supports (more than any plausible explanation of why liraglutide increases HR) the already known associations between changes in metabolic parameters and spectrum analysis parameters against changes in HRV parameters.
The limitations of this study include its open‐label design, which may have introduced some biases between groups. However, patients were randomized to one of the treatment groups and data were analysed blinded to minimize further biases. At baseline, there was a small, however significant, higher HbA1c and triglycerides concentrations observed in the group allocated to liraglutide. Although, no treatment changes (after 18 weeks) were observed in these parameters between groups, we cannot entirely role out that this difference might have affected our outcome. As the comparator group were treated with glimepiride, the possibility of hypoglycaemia‐induced tachycardia has to be considered. However, there was no severe hypoglycaemic event during the study (no groups) although a numerical (non‐significant) higher numbers of mild hypoglycaemia in the glimepiride group was observed.6 Despite this HR was not increased in patients treated with glimepiride compared to patients treated with liraglutide, making this consideration less important. Because of dropouts and technical hitches, full data were available for 85% of the study population (53 out of 62) and subsequently analysed per‐protocol. After multiple imputations and sensitivity analysis, the results were not changed, supporting the per‐protocol results.
Eighteen‐week treatment with liraglutide, compared with glimepiride, in combination with metformin significantly increased diurnal variation in hourly mean HR followed by an increase in mean daytime HR, with no effects on HRV. Increased HR induced by liraglutide treatment is suggested to be independent from sympathetic or parasympathetic involvement. Further studies are warranted to elucidate the mechanisms behind the increased HR observed with GLP‐1 RA treatment.
CONFLICT OF INTEREST
TN has received unrestricted grants from Astra Zeneca and consultancy fees from Boehringer Ingelheim, Eli Lilly, NovoNordisk, MSD, AMGEN and Sanofi‐Aventis. JJ has received consultancy fees from Astra Zeneca, Boehringer Ingelheim, Eli Lilly, NovoNordisk, Medtronic, MSD and Sanofi‐Aventis. No other disclosures are reported.
AUTHORS’ CONTRIBUTION
All authors contributed to the study conception and design. TN analysed data and wrote the first draft of the paper. All authors commented and took part of the revision of the paper.
AVAILABILITY OF THE DATA AND MATERIAL
Data sets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Nyström T, Santos‐Pardo I, Fang X, Cao Y, Hedberg F, Jendle J. Heart rate variability in type 2 diabetic subjects randomized to liraglutide or glimepiride treatment, both in combination with metformin: A randomized, open, parallel‐group study. Endocrinol Diab Metab. 2019;2:e00058 10.1002/edm2.58
Clinical Trial Registration: http://www.clinicaltrials.gov Unique identifier: NCT01425580.
Funding information
Financial support was provided through the Swedish Heart and Lung foundation, the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and the Karolinska Institutet. This was an investigator‐initiated and investigator‐designed clinical trial. The investigators received unrestricted grant from Novo Nordisk A/S, but the company was not involved in data collection, study management, analysis or interpretation of data. Nor was the company involved in the decisions regarding the submission of the manuscript.
REFERENCES
- 1. Rawshani A, Rawshani A, Franzen S, et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2018;379:633‐644. [DOI] [PubMed] [Google Scholar]
- 2. Gaede P, Lund‐Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580‐591. [DOI] [PubMed] [Google Scholar]
- 3. Maser RE, Mitchell BD, Vinik AI, Freeman R. The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta‐analysis. Diabetes Care. 2003;26:1895‐1901. [DOI] [PubMed] [Google Scholar]
- 4. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Drucker DJ. The ascending GLP‐1 road from clinical safety to reduction of cardiovascular complications. Diabetes. 2018;67:1710‐1719. [DOI] [PubMed] [Google Scholar]
- 6. Nyström T, Padro Santos I, Hedberg F, et al. Corrigendum: Effects on subclinical heart failure in type 2 diabetic subjects on liraglutide treatment vs. glimepiride both in combination with metformin. A randomized open parallel‐group study. Front Endocrinol (Lausanne). 2018;9:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30:377‐399. [DOI] [PubMed] [Google Scholar]
- 8. Holman RR, Bethel MA, Mentz RJ, et al. Effects of once‐weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377:1228‐1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834‐1844. [DOI] [PubMed] [Google Scholar]
- 10. Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247‐2257. [DOI] [PubMed] [Google Scholar]
- 11. Nyström T. The potential beneficial role of glucagon‐like peptide‐1 in endothelial dysfunction and heart failure associated with insulin resistance. Horm Metab Res. 2008;40:593‐606. [DOI] [PubMed] [Google Scholar]
- 12. Griffioen KJ, Wan R, Okun E, et al. GLP‐1 receptor stimulation depresses heart rate variability and inhibits neurotransmission to cardiac vagal neurons. Cardiovasc Res. 2011;89:72‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yamamoto H, Lee CE, Marcus JN, et al. Glucagon‐like peptide‐1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest. 2002;110:43‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barragan JM, Eng J, Rodriguez R, Blazquez E. Neural contribution to the effect of glucagon‐like peptide‐1‐(7–36) amide on arterial blood pressure in rats. Am J Physiol. 1999;277:E784‐791. [DOI] [PubMed] [Google Scholar]
- 15. Mendis B, Simpson E, MacDonald I, Mansell P. Investigation of the haemodynamic effects of exenatide in healthy male subjects. Br J Clin Pharmacol. 2012;74:437‐444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pyke C, Heller RS, Kirk RK, et al. GLP‐1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology. 2014;155:1280‐1290. [DOI] [PubMed] [Google Scholar]
- 17. Smits MM, Muskiet MH, Tonneijck L, et al. Exenatide acutely increases heart rate in parallel with augmented sympathetic nervous system activation in healthy overweight males. Br J Clin Pharmacol. 2016;81:613‐620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smits MM, Tonneijck L, Muskiet MH, et al. Heart rate acceleration with GLP‐1 receptor agonists in type 2 diabetes patients: an acute and 12‐week randomised, double‐blind, placebo‐controlled trial. Eur J Endocrinol. 2017;176:77‐86. [DOI] [PubMed] [Google Scholar]
- 19. Nyström T, Gutniak MK, Zhang Q, et al. Effects of glucagon‐like peptide‐1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab. 2004;287:E1209‐1215. [DOI] [PubMed] [Google Scholar]
- 20. Jendle J, Fang X, Cao Y, et al. Effects on repetitive 24‐hour ambulatory blood pressure in subjects with type II diabetes randomized to liraglutide or glimepiride treatment both in combination with metformin: a randomized open parallel‐group study. J Am Soc Hypertens. 2018;12:346‐355. [DOI] [PubMed] [Google Scholar]
- 21. Hozawa A, Ohkubo T, Kikuya M, et al. Prognostic value of home heart rate for cardiovascular mortality in the general population: the Ohasama study. Am J Hypertens. 2004;17:1005‐1010. [DOI] [PubMed] [Google Scholar]
- 22. Kannel WB, Kannel C, Paffenbarger RS Jr, Cupples LA. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J. 1987;113:1489‐1494. [DOI] [PubMed] [Google Scholar]
- 23. Stein PK, Barzilay JI, Chaves PH, et al. Novel measures of heart rate variability predict cardiovascular mortality in older adults independent of traditional cardiovascular risk factors: the Cardiovascular Health Study (CHS). J Cardiovasc Electrophysiol. 2008;19:1169‐1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kumarathurai P, Anholm C, Larsen BS, et al. Effects of liraglutide on heart rate and heart rate variability: a randomized, double‐blind. Placebo‐controlled crossover study. Diabetes Care. 2017;40:117‐124. [DOI] [PubMed] [Google Scholar]
- 25. Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care. 2003;26:1553‐1579. [DOI] [PubMed] [Google Scholar]
- 26. Nakatani Y, Kawabe A, Matsumura M, et al. Effects of GLP‐1 receptor agonists on heart rate and the autonomic nervous system using holter electrocardiography and power spectrum analysis of heart rate variability. Diabetes Care. 2016;39:e22‐23. [DOI] [PubMed] [Google Scholar]
- 27. Ban K, Noyan‐Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon‐like peptide 1 receptor are mediated through both glucagon‐like peptide 1 receptor‐dependent and ‐independent pathways. Circulation. 2008;117:2340‐2350. [DOI] [PubMed] [Google Scholar]
- 28. Nathanson D, Ullman B, Löfstrom U, et al. Effects of intravenous exenatide in type 2 diabetic patients with congestive heart failure: a double‐blind, randomised controlled clinical trial of efficacy and safety. Diabetologia. 2012;55:926‐935. [DOI] [PubMed] [Google Scholar]
- 29. Cacciatori V, Zoppini G, Bellavere F, et al. Long‐acting GLP‐1 receptor agonist exenatide influence on the autonomic cardiac sympatho‐vagal balance. J Endocr Soc. 2018;2:53‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]


