Summary
Objective
To investigate early signs of cardiovascular arterial remodelling in paediatric patients with Cushing syndrome (CS) in comparison with normative values from healthy children.
Study Design
The metrics used to assess cardiac health were from thoracic aorta and carotid MRI. Scans were performed on 18 children with CS (mean: 12.5 ± 3.1 years, range: 6.0‐16.8 years, 10 female). Pulse wave velocity (PWV), aortic distensibility (AD) and carotid intima‐media thickness (cIMT), well‐validated measurements of cardiac compromise, were measured from the images and compared to normative age‐matched values where available.
Results
Patients with CS had significantly higher PWV compared to age‐adjusted normal median control values (4.0 ± 0.7 m/s vs. 3.4 ± 0.2 m/s, respectively, P = 0.0115). PWV was positively correlated with midnight plasma cortisol (r = 0.56, P = 0.02). Internal and common cIMT were negatively correlated with ascending AD (r = −0.75, P = 0.0022, r = −0.69, P = 0.0068, respectively).
Conclusion
Pulse wave velocity data indicate that paediatric patients with CS have early evidence of cardiovascular remodelling. The results suggest the opportunity for monitoring as these changes begin in childhood.
Keywords: cardiology, heart disease, hypercortisolemia, paediatrics, pulse wave velocity
1. INTRODUCTION
Cushing syndrome (CS) is a rare condition caused by a chronic excess of glucocorticoids that affects 2‐5 individuals per million people annually. Ten per cent of these cases occur in children.1 Endogenous CS is most commonly caused by an adrenocorticotropic hormone (ACTH) producing pituitary adenoma, which is called Cushing disease (CD).1, 2 CD affects about 75% of paediatric CS patients over the age of 7.1, 3 Endogenous CS can also be caused by an ACTH independent adrenal tumour or an ACTH or corticotropin‐releasing hormone (CRH)‐producing ectopic tumour.1, 2
Hypercortisolism leads to signs and symptoms including obesity, growth deceleration, striae, moon face, facial plethora, posterior cervical fat pad, acanthosis nigricans, menstrual irregularity, kidney stones, hypercoagulability, diminished bone health, diabetes and hypertension.1, 2 Even after cure, individuals with CS may face complications including cognitive challenges, cardiovascular disease and recurrence of CS.1, 6, 7
Cardiovascular complications are the leading cause of death in adult patients with CS.9 Adults with CS have been found to have a cardiovascular mortality rate that is 4‐5 times higher than the overall population.9, 10, 13, 14 Even after patients are in remission, risk factors including hypertension, high low‐density lipoprotein (LDL) and total cholesterol, adiposity, diabetes mellitus, insulin resistance and increased arterial rigidity may remain unchanged.6, 10, 15 Additionally, cardiovascular remodelling may be one of the permanent sequelae of long‐standing CS and underscores the importance of early detection and treatment in children.16 A recent meta‐analysis of 14 studies of atherosclerosis in adult patients with CS showed that CS is significantly associated with subclinical markers of atherosclerosis and cardiovascular risk.17
An excess of cortisol is also linked to vascular damage, which may lead to cardiovascular complications in CS patients.18 It has been shown that adult patients with CS have significantly increased carotid intima‐media thickness and a higher incidence of well‐defined plaque walls than control populations with the same cardiovascular risk factors.6, 17 Furthermore, adult patients with CS had increased left and right ventricular hypertrophy, and lower left atrial ejection fractions and increased end‐diastolic left ventricular segmental thickness compared to the normal population.11 In contrast, only one prior study specifically evaluating cardiovascular involvement in children with CS has been published to date.7, 8 In this prior study, a cohort of 23 female paediatric patients with CS were shown to have increased blood pressure and disadvantageous differences in epicardial fat thickness, intima‐media thickness and N‐terminal pro‐B‐type natriuretic peptide compared to a control population.7, 8 Cardiovascular remodelling may be one of the permanent sequelae of long‐standing CS.10 These findings underscore the importance of early detection and treatment in children.
Increased carotid intima‐media thickness (IMT) reflects morphological changes associated with atherosclerosis and is a strong predictor for future clinical cardiovascular events. Previous studies have established normal values for carotid artery intima‐media thickness and distensibility in paediatric patients, using high‐resolution vascular ultrasound.19 A study analysing 43 patients with 16‐79% stenosis underwent both B‐mode ultrasound and high‐resolution MRI examinations of their carotid arteries found that there was a very high correlation between these two methods of examination.20
Aortic pulse wave velocity (PWV) is a well‐validated clinical measure of central arterial stiffness and is predictive of increased mortality, coronary heart disease and stroke.21, 22 PWV has been an accurate measure of cardiac complications in individuals with diabetes, obesity and end‐stage renal disease.22, 25, 26 PWV has been used to measure aortic stiffness in children in prior studies; however, it has not been studied in children with CS.22 Additionally, aortic distensibility (AD) has been shown to be a sensitive marker of arterial stiffening in children.21 While cardiovascular complications in adults with CS have been well documented, less is known about cardiovascular risk in paediatric patients. The aim of this study was to assess early signs of cardiovascular remodelling associated with CS in paediatric patients through measurements of AD, PWV and cIMT.
2. METHODS
2.1. Study participants
Patients who were diagnosed with CD and participated in the clinical protocol NCT00001595 at the National Institutes of Health Clinical Center between July 2014 and August 2017 and who underwent thoracic aorta and carotid MRI were included in the analysis. The protocol was approved by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Institutional Review Board. Informed consent and assent were obtained. Clinical information for all patients including midnight serum cortisol levels, plasma ACTH levels, systolic and diastolic z‐scores, body mass index (BMI) z‐scores, height z‐score, blood glucose levels, lipid profiles and family history was collected.
The diagnosis of CD was made according to standard clinical and biochemical criteria including elevated urinary free cortisol (UFC), loss of diurnal serum cortisol rhythm, CRH stimulation test consistent with CD and/or suppression of morning cortisol from an overnight administration of 8 mg dexamethasone, adjusted for body weight.2, 3, 11, 27 Patients with pituitary adenomas clearly seen on MRI underwent transsphenoidal surgery (TSS), while more radiographically ambiguous cases underwent inferior petrosal sinus sampling to localize the source of ACTH before undergoing TSS. All patients had pathological confirmation of an ACTH‐positive pituitary adenoma on immunohistochemistry staining.
2.2. Image acquisition
Thoracic aorta and carotid artery MRIs were offered to all paediatric CD patients who were able to tolerate the procedure without sedation and who did not have MRI contraindications (implanted non‐MRI safe devices or foreign metallic bodies) or claustrophobia. Imaging was done on a 3.0 Tesla scanner (Trio Tim, Siemens, Munich, Germany) using ECG gating and breath‐holding. A 6‐element body surface coil was used for radiofrequency signal detection. Sagittal oblique views of the aortic arch were obtained using a black‐blood spin echo sequence (slice thickness, 6 mm; matrix, 256 × 256) to visualize the aorta. At the level of the pulmonary artery bifurcation perpendicular to the aorta, a phase contrast gradient echo pulse sequence with through‐plane velocity encoding acquired velocity data in the ascending and descending aorta. The maximal encoding velocity encoding was 150 cm/s; the slice thickness was 6 mm, and the temporal resolution used was 20 ms; and the matrix was 256 × 192. An aortic cine series was acquired at the same slice location using a fast retrospectively gated gradient echo sequence (slice thickness, 6 mm; matrix, 256 × 256; temporal resolution, 20 ms). Carotid MRIs were acquired using dedicated carotid coils. In‐plane spatial resolution was 2 mm; cross‐sectional spatial resolution was 0.6 mm. Axial T2‐weighted and T1‐weighted slices were acquired from the level of the carotid bulb through to the bifurcation of the internal and external carotid arteries.
2.3. Image analysis
Images were analysed using Syngo Multimodality Workplace to measure Δx, the distance between the ascending and descending aorta measurement locations as measured along an intra‐luminal midline. Medis QMass Software (Leiden, The Netherlands) was used to analyse the phase contrast data and find Δt, the time delay of the distal flow curve relative to the proximal flow curve determined by the difference in time points at the mid‐points of the systolic upslope of the ascending and descending aorta. PWV was then calculated using the equation:
Figure 1.
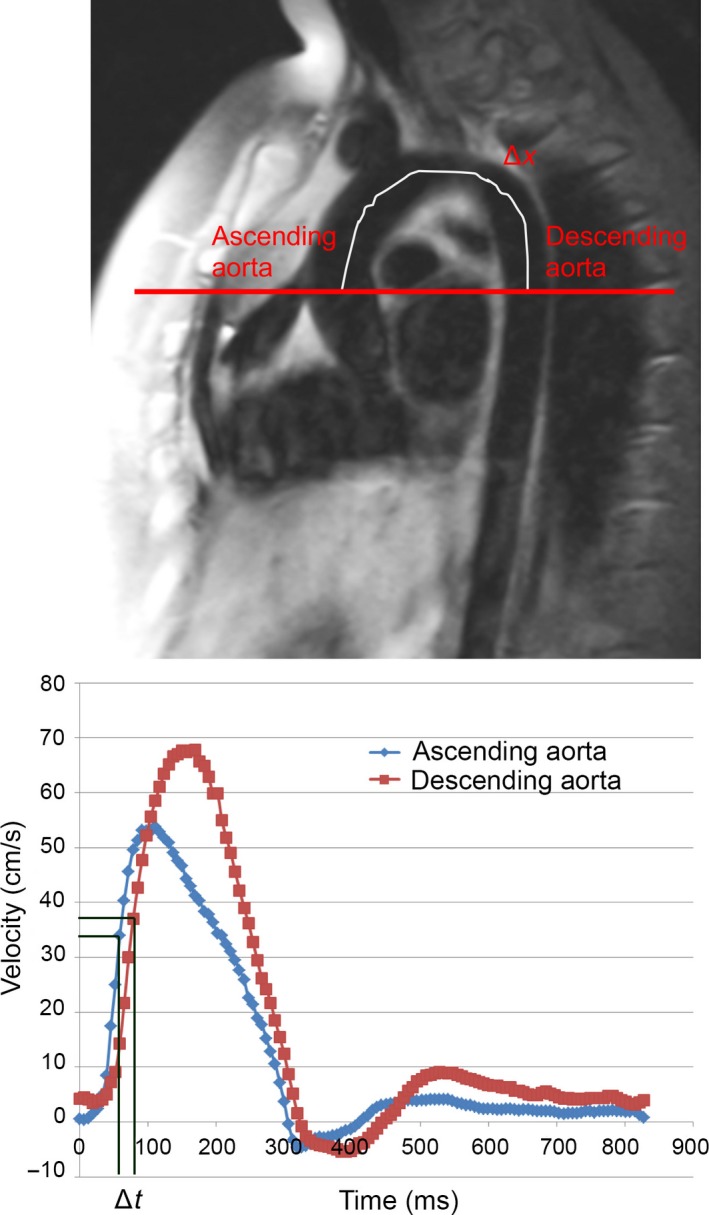
Aortic pulse wave velocity (PWV). The distance between the ascending and descending aorta, Δx, was measured along the midline through the aortic arch as shown. The transit delay, Δt, was determined by the difference in time points at the mid‐points of the systolic upslope of the ascending and descending aorta as shown. PWV was calculated as Δx/Δt. Cross‐sectional aortic areas were estimated at the ascending and descending aortas and used to calculate PWV and aortic distensibility (AD)
The cross‐sectional areas of the ascending and descending aorta were measured. Aortic distensibility was calculated from the maximal and minimal cross‐sectional areas and the systolic and diastolic blood pressures measured the closest to the time of acquisition of the carotid MRI using the equation:
A max and A min represent the maximum and minimum cross‐sectional areas of the aorta in mm2. P max and P min represent the systolic and diastolic blood pressures, respectively. Both ascending aortic distensibility and descending aortic distensibility were calculated using this equation.
The acquired data were then compared to control data for healthy age‐matched children in the literature using the same imaging modality.28 Medis QPlaque software was used for analysis of the carotid images. Automatic regions of interest and segmentation of the carotid internal lumen and external vessel wall borders were created with manually adjusted assistance. The vessel wall was segmented into 12 regions, and the vessel thickness value was averaged from these regions.
2.4. Statistical analysis
Data were described by frequency distributions and percentages or by mean ± standard deviation. A P‐value <0.05 was considered statistically significant. Data were assessed for distributional assumptions, and either t tests or nonparametric Wilcoxon rank‐sum tests compared continuous data between groups. Spearman's rank correlations tested the relation between various cardiovascular remodelling measures and clinical features. Data analysis was carried out using SAS v 9.4 (SAS Institute, Inc, Cary, NC).
3. RESULTS
Of the total patient cohort enrolled in the protocol 97‐CH0076, a subset of 18 patients had thoracic aorta MRIs (n = 17) or carotid MRI (n = 15) and were analysed. Patient clinical characteristics are summarized in Table 1. The mean age of the patients at evaluation was 12.5 ± 3.1 years old with a range of 6.0‐16.8 years, and there were 10 (56%) females and 7 males. The majority of the patients were Caucasian (15 patients, 83%), two patients were African American and one patient was Asian. The control population consisted of 71 children and young adults age 16.4 ± 7.6 years, range 2.3‐28.3 years. There were 41 females (58%) and 30 males. The BMI of the total control population was 19.4 ± 3.5 kg/m2.
Table 1.
Clinical characteristics of 18 paediatric patients with Cushing syndrome at the time of transsphenoidal surgery
| Mean ± SD | Normal range | |
|---|---|---|
| Midnight cortisol | 16.1 ± 6.8 mcg/dL | <4.4 µg/dL |
| Plasma ACTH | 53.0 ± 25.8 pg/mL | 5‐46 pg/mL |
| Glucose | 99.6 ± 14.9 mg/dL | 74‐106 mg/dL |
| Prevalence of hypertension, systolic | 38% | |
| Prevalence of hypertension, diastolic | 17% | |
| Height SDS | −0.78 ± 1.41 | |
| BMI/BMI z‐score | 25.8 ± 12.5/1.93 ± 0.78 | |
| Period of disease before diagnosis | 2.3 ± 1.7 y | |
| Total cholesterol | 169.2 ± 32.3 mg/dL |
Acceptable: <170 mg/dL Borderline high risk: 170‐199 mg/dL Elevated: ≥200 mg/dL |
| HDL | 52.2 ± 11.1 mg/dL | <35 mg/dL |
| LDL | 96.9 ± 29.2 mg/dL |
Acceptable: <110 mg/dL Borderline high risk: 110‐129 mg/dL Elevated: ≥130 mg/dL |
Children with CS had an average PWV of 4.0 ± 0.7 m/s, while the respective age‐ and sex‐adjusted normal value was 3.4 ± 0.2 m/s. The difference between PWV values for CS patient and normal populations was statistically significant (delta 0.59 ± 0.86 m/s, P = 0.0115). PWV in patients with CS did not correlate with BMI z‐score, ACTH, UFC, total cholesterol, LDL cholesterol or blood pressure; however, PWV positively correlated with midnight cortisol levels (r s = 0.56, P = 0.018; Figure 2).
Figure 2.
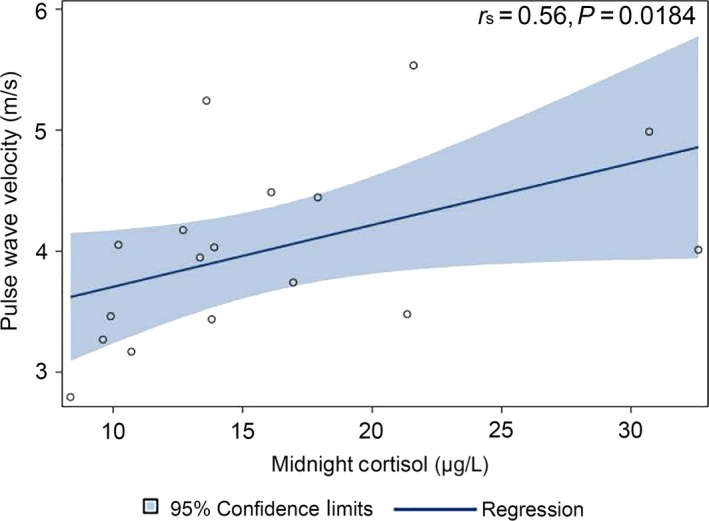
Pulse wave velocity (PWV) in 18 paediatric patients with Cushing syndrome (CS) was positively correlated with midnight cortisol levels
Additionally, internal and common carotid artery thickness (cIMT) was measured as an indicator of cardiovascular remodelling in CS patients. The mean internal cIMT of paediatric CS patients was 0.67 ± 0.24 mm (n = 15), and the mean common cIMT was 0.67 ± 0.15 mm (n = 15). The average distensibility for the descending and ascending aorta in the patients with CS was 13 ± 7×10−3 mm Hg and 15 ± 8×10−3 mm Hg, respectively. There was a statistically significant inverse correlation between ascending aortic distensibility and both the internal cIMT (r s = −0.74, P = 0.0022) and the common cIMT (r s = −0.69, P = 0.007) (Figure 3). Additionally, there was an inverse correlation between the descending aortic distensibility and the internal cIMT (r s = −0.55, P = 0.0433).
Figure 3.
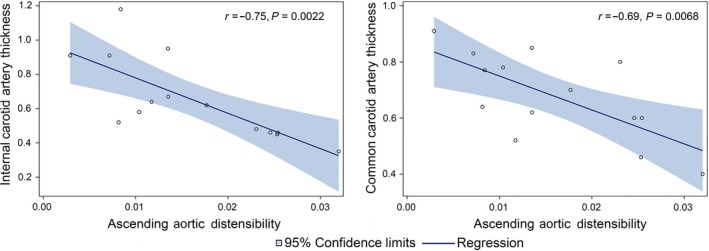
Ascending aortic distensibility (AD) in 18 paediatric patients with Cushing syndrome (CS) was inversely correlated with both internal and common carotid artery thickness (cIMT)
4. DISCUSSION
This study assessed the cardiovascular complications associated with CS in paediatric patients using PWV, carotid arterial intimal thickness and aortic distensibility. Our findings suggest that paediatric patients with CS have evidence of early cardiovascular remodelling. This supports the current literature which describes long‐term cardiovascular remodelling as a sequela of CS in adults. Our data indicate that cardiovascular remodelling occurs at a much younger age than previously shown, with differences from normative data seen as young as 6 years of age.
The pathophysiology underlying increased PWV in CS is thought to be due to an increase in connective tissue volume; this remodelling may not be reversible after cure.18 In our study, PWV was significantly higher in patients with CS than the normative age‐ and sex‐adjusted values. The increase in PWV may be an early indicator of worsened bioelastic function in the aorta, which is representative of cardiovascular dysfunction.28 Furthermore, PWV was correlated with midnight cortisol levels, suggesting that the incidence of cardiovascular complications may be associated with the severity of the primary disease. Our prior study has specifically included midnight cortisol as one factor that is linked to disease severity.29 Additionally, the ratio of midnight to morning cortisol levels has also been reported to indicate the extent of hypercortisolemia.30 Previous studies in CS patients have noted the difficulty in distinguishing between cardiovascular remodelling caused by increased cortisol and metabolic disease.14, 31 Patients with CS have been shown to have hypertension, high LDL and total cholesterol, adiposity, diabetes mellitus, insulin resistance and increased arterial rigidity even after cure.6, 10 These manifestations of CS are potential causes of cardiovascular complications. Thus, the link between CS and cardiovascular remodelling is unsurprising due to patient's symptoms that have been associated with both CS and cardiovascular complications.
This study was limited by the small size of the patient cohort due to the rare nature of CS and movement artefact for some of the patients. In addition, this study was limited in that we did not have control measurements for patients with the same height, weight and metabolic parameters. Previous studies have shown that obese children have increased arterial stiffening, especially in central arteries.32 Due to the small sample size, additional differences between hypertension and normotensive patients were unable to be analysed. Additionally, four patients were taking antihypertensive medication at the time of the scan. This could have affected the results, though it would be expected that this would make the difference between CS patients and the normative controls less significant. Thus, the differences seen between CS patients and the sex‐ and age‐adjusted children do not take into account any of these potential covariates.
In conclusion, paediatric patients with CS are at a higher risk of cardiovascular complications than the general population. These findings indicate the importance of early detection of CS in paediatric patients to avoid long‐term comorbidities. Furthermore, these results indicate that the cardiovascular health of patients with CS needs to be followed closely from a young age. Patients are continuing to be followed to assess whether cardiovascular remodelling is a long‐term consequence of CS as these children grow into adulthood.
ETHICAL APPROVAL
Consent has been obtained from each patient after full explanation of the purpose and nature of all procedures used.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
Hailey Blain acquired, analysed and interpreted the data, conceived and designed the study, drafted the manuscript, and statistically analysed the data. Ninet Sinaii analysed the data, drafted the manuscript and statistically analysed the data. Deena Zeltser, Charalampos Lyssikatos, Elena Belyavskaya and Margaret Keil, PhD acquired and interpreted the data, drafted and supervised the manuscript. David A. Bluemke acquired the data, drafted and supervised the manuscript, and provided technical support. Constantine Stratakis conceived and designed the study, critically revised the manuscript for important intellectual content and supervised the manuscript. W. Patricia Bandettini conceived and designed the study, acquired and interpreted the data, drafted and supervised the manuscript, and provided technical and material support. Maya Lodish conceived and designed the study, acquired and interpreted the data, drafted and supervised the manuscript, statistically analysed the data, and obtained funding.
ACKNOWLEDGEMENTS
Research reported in this publication was supported by the Division of Intramural Research of the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Blain H, Sinaii N, Zeltser D, et al. Aortic pulse wave velocity in children with Cushing syndrome: A window into a marker of early cardiovascular disease. Endocrinol Diab Metab. 2019;2:e00054 10.1002/edm2.54
Funding information
This work was supported by the Division of Intramural Research of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
DATA ACCESSIBILITY
Individual participant data will not be available.
REFERENCES
- 1. Lodish M. Cushing's syndrome in childhood: update on genetics, treatment, and outcomes. Curr Opin Endocrinol Diabetes Obes. 2015;22(1):48‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Newell‐Price J. Cushing's syndrome. Clin Med (Lond). 2008;8(2):204‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Libuit LG, Karageorgiadis AS, Sinaii N, et al. A gender‐dependent analysis of Cushing's disease in childhood: pre‐ and postoperative follow‐up. Clin Endocrinol. 2015;83(1):72‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Birdwell L, Lodish M, Tirosh A, et al. Coagulation profile dynamics in pediatric patients with cushing syndrome: a prospective, observational comparative study. J Pediatr. 2016;177:227‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rahman SH, Papadakis GZ, Keil MF, Faucz FR, Lodish MB, Stratakis CA. Kidney stones as an underrecognized clinical sign in pediatric cushing disease. J Pediatr. 2016;170:273‐277.e271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Albiger N, Testa RM, Almoto B, et al. Patients with Cushing's syndrome have increased intimal media thickness at different vascular levels: comparison with a population matched for similar cardiovascular risk factors. Horm Metab Res. 2006;38(6):405‐410. [DOI] [PubMed] [Google Scholar]
- 7. Bassareo PP, Fanos V, Zaffanello M, Mercuro G. Early markers of cardiovascular dysfunction in young girls affected by Cushing's syndrome before and after successful cure. J Pediatr Endocrinol Metab. 2010;23(6):627‐635. [DOI] [PubMed] [Google Scholar]
- 8. Bassareo PP, Marras AR, Pasqualucci D, Mercuro G. Increased arterial rigidity in children affected by Cushing’s syndrome after successful surgical cure. Cardiol Young. 2010;20(06):610‐614. [DOI] [PubMed] [Google Scholar]
- 9. Etxabe J, Vazquez JA. Morbidity and mortality in Cushing's disease: an epidemiological approach. Clin Endocrinol (Oxf). 1994;40(4):479‐484. [DOI] [PubMed] [Google Scholar]
- 10. Faggiano A, Pivonello R, Spiezia S, et al. Cardiovascular risk factors and common carotid artery caliber and stiffness in patients with Cushing's disease during active disease and 1 year after disease remission. J Clin Endocrinol Metab. 2003;88(6):2527‐2533. [DOI] [PubMed] [Google Scholar]
- 11. Kamenicky P, Redheuil A, Roux C, et al. Cardiac structure and function in Cushing's syndrome: a cardiac magnetic resonance imaging study. J Clin Endocrinol Metab. 2014;99(11):E2144‐2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roux C, Kachenoura N, Raissuni Z, et al. Effects of cortisol on the heart: characterization of myocardial involvement in cushing's disease by longitudinal cardiac MRI T1 mapping. J Magn Reson Imaging. 2017;45(1):147‐156. [DOI] [PubMed] [Google Scholar]
- 13. Dekkers OM, Horvath‐Puho E, Jorgensen JO, et al. Multisystem morbidity and mortality in Cushing's syndrome: a cohort study. J Clin Endocrinol Metab. 2013;98(6):2277‐2284. [DOI] [PubMed] [Google Scholar]
- 14. Bayram NA, Ersoy R, Sen DO, et al. The relationship between aortic stiffness and left ventricular function in patients with Cushing's disease: aortic stiffness in Cushing's disease. Endocrine. 2010;37(2):280‐285. [DOI] [PubMed] [Google Scholar]
- 15. Lodish MB, Sinaii N, Patronas N, et al. Blood pressure in pediatric patients with Cushing syndrome. J Clin Endocrinol Metab. 2009;94(6):2002‐2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Graversen D, Vestergaard P, Stochholm K, Gravholt CH, Jorgensen JO. Mortality in Cushing's syndrome: a systematic review and meta‐analysis. Eur J Intern Med. 2012;23(3):278‐282. [DOI] [PubMed] [Google Scholar]
- 17. Lupoli R, Ambrosino P, Tortora A, Barba L, Lupoli GA, Di Minno MN. Markers of atherosclerosis in patients with Cushing's syndrome: a meta‐analysis of literature studies. Ann Med. 2017;49(3):206‐216. [DOI] [PubMed] [Google Scholar]
- 18. Ferrau F, Korbonits M. Metabolic comorbidities in Cushing's syndrome. Eur J Endocrinol. 2015;173(4):M133‐M157. [DOI] [PubMed] [Google Scholar]
- 19. Doyon A, Kracht D, Bayazit AK, et al. Carotid artery intima‐media thickness and distensibility in children and adolescents: reference values and role of body dimensions. Hypertension. 2013;62(3):550‐556. [DOI] [PubMed] [Google Scholar]
- 20. Underhill HR, Kerwin WS, Hatsukami TS, Yuan C. Automated measurement of mean wall thickness in the common carotid artery by MRI: a comparison to intima‐media thickness by B‐mode ultrasound. J Magn Reson Imaging. 2006;24(2):379‐387. [DOI] [PubMed] [Google Scholar]
- 21. Redheuil A, Yu WC, Wu CO, et al. Reduced ascending aortic strain and distensibility: earliest manifestations of vascular aging in humans. Hypertension. 2010;55(2):319‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rider OJ, Banerjee R, Rayner JJ, et al. Investigating a liver fat: arterial stiffening pathway in adult and childhood obesity. Arterioscler Thromb Vasc Biol. 2016;36(1):198‐203. [DOI] [PubMed] [Google Scholar]
- 23. Ripley DP, Negrou K, Oliver JJ, et al. Aortic remodelling following the treatment and regression of hypertensive left ventricular hypertrophy: a cardiovascular magnetic resonance study. Clin Exp Hypertens. 2015;37(4):308‐316. [DOI] [PubMed] [Google Scholar]
- 24. Heesen WF, Beltman FW, Smit AJ, et al. Reversal of pathophysiologic changes with long‐term lisinopril treatment in isolated systolic hypertension. J Cardiovasc Pharmacol. 2001;37(5):512‐521. [DOI] [PubMed] [Google Scholar]
- 25. Van Bortel LM, Struijker‐Boudier HA, Safar ME. Pulse pressure, arterial stiffness, and drug treatment of hypertension. Hypertension. 2001;38(4):914‐921. [DOI] [PubMed] [Google Scholar]
- 26. McCulloch MA, Mauras N, Canas JA, et al. Magnetic resonance imaging measures of decreased aortic strain and distensibility are proportionate to insulin resistance in adolescents with type 1 diabetes mellitus. Pediatric diabetes. 2015;16(2):90‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Batista DL, Riar J, Keil M, Stratakis CA. Diagnostic tests for children who are referred for the investigation of Cushing syndrome. Pediatrics. 2007;120(3):e575‐e586. [DOI] [PubMed] [Google Scholar]
- 28. Voges I, Jerosch‐Herold M, Hedderich J, et al. Normal values of aortic dimensions, distensibility, and pulse wave velocity in children and young adults: a cross‐sectional study. J Cardiovasc Magn Reson. 2012;14:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gkourogianni A, Sinaii N, Jackson SH, et al. Pediatric Cushing disease: disparities in disease severity and outcomes in the Hispanic and African‐American populations. Pediatr Res. 2017;82(2):272‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tirosh A, Lodish MB, Lyssikatos C, Belyavskaya E, Papadakis GZ, Stratakis CA. Circadian plasma cortisol measurements reflect severity of hypercortisolemia in children with different etiologies of endogenous Cushing syndrome. Horm Res Paediatr. 2017;87(5):295‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pivonello R, Faggiano A, Lombardi G, Colao A. The metabolic syndrome and cardiovascular risk in Cushing's syndrome. Endocrinol Metab Clin North Am. 2005;34(2) ):327–339. [DOI] [PubMed] [Google Scholar]
- 32. Hudson LD, Rapala A, Khan T, Williams B, Viner RM. Evidence for contemporary arterial stiffening in obese children and adolescents using pulse wave velocity: a systematic review and meta‐analysis. Atherosclerosis. 2015;241(2):376‐386. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Individual participant data will not be available.


