Abstract
Background
Left atrial appendage closure is a non-pharmacological alternative for stroke prevention in high-risk non-valvular atrial fibrillation patients, but has not been widely studied in Asian patients. The prospective WASP registry assessed real-world outcomes for patients undergoing WATCHMAN implant in the Asia-Pacific region.
Methods
Data were collected from consecutive patients across 9 centres. Major endpoints included procedural success, safety and long-term outcomes including occurrence of bleeding, stroke/transient ischaemic attack/systemic embolism and all-cause mortality.
Results
Subjects (n = 201) had a mean age of 70.8 ± 9.4 years, high stroke risk (CHA2DS2-VASc: 3.9 ± 1.7), elevated bleeding risk (HAS-BLED: 2.1 ± 1.2) with 53% patients from Asian countries. Successful implantation occurred in 98.5% of patients; 7-day device/procedure-related SAE rate was 3.0%. After 2 years of follow-up, the rates of ischaemic stroke/TIA/SE and major bleeding were 1.9 and 2.2 per 100-PY, respectively, representing relative reductions of 77% and 49% versus expected rates per risk scores. The relative risk reductions versus expected rates were more pronounced in Asians vs. Non-Asians (89% vs 62%; 77% vs 14%). Other significant findings included larger mean LAA ostium diameter for Asians vs. Non-Asians (23.4 ± 4.1 mm vs. 21.2 ± 3.2 mm, p < 0.001) and hence requirement for larger median device size (27 mm for Asians, 24 mm for non-Asians [p < 0.0001]).
Conclusion
Real-world experience of left atrial appendage closure with WATCHMAN has demonstrated low peri-procedural risk, and long-term efficacy for stroke and bleeding prevention in a primarily Asian cohort.
Abbreviations: BMI, body mass index; CI, confidence interval; LAA, left atrial appendage; NOAC, Novel oral anticoagulant; NVAF, non-valvular atrial fibrillation; OAC, oral anticoagulation; SAE, serious adverse events; SE, systemic embolism; TOE, transoesophageal echocardiography; TIA, transient ischaemic attack; WASP, The Asia-Pacific Registry on WATCHMAN Outcomes in Real-Life Utilization
Keywords: Atrial fibrillation, Ischaemic stroke, Left atrial appendage, LAA device closure, Anticoagulant therapy
Graphical abstract
1. Introduction
Epidemiological studies have demonstrated significant differences in ischaemic stroke risk and risk of bleeding in Asian populations with non-valvular atrial fibrillation (NVAF) as compared with other ethnicities [[1], [2], [3]]. Increased stroke risk commencing from a younger age, as well as remarkably higher rates of intracranial bleeding, are some of the considerations when tailoring stroke prevention treatment for patients of Asian ethnicity [[1], [2], [3], [4]]. Left atrial appendage (LAA) closure with the WATCHMAN device (Boston Scientific, Marlboro, MA, USA) has emerged as a non-pharmacologic alternative that provides comparable stroke protection while significantly lowering bleeding rates when compared to warfarin [5]. The outcomes for LAA closure with this device have not been broadly assessed in an Asian population; thus, our prospective, multicentre registry aimed to collect real-world outcomes for patients in the Asia-Pacific region.
2. Methods
2.1. Study population
The Registry on WATCHMAN Outcomes in Real-Life Utilization (WASP) prospectively collected data on consecutive patients in the Asia-Pacific region undergoing implantation of a WATCHMAN device (NCT01972295). Certified implanting centres across Asia, Australia and the Middle East were approached to participate. The registry used identical design and study protocol to EWOLUTION [6]. Subjects were recruited at clinical sites by physician discretion with appropriate local and international guidelines to determine device eligibility. A contract research organization provided independent monitoring of the data, The study was approved by the respective institutional review boards for human research, complies with the Declaration of Helsinki, and informed consent was obtained from all participants.
2.2. Procedure
Procedures were performed by trained implanters and according to the device's Directions for Use. Peri-procedural oral anticoagulation (OAC) and intravenous (IV) heparin were managed at the Physician's discretion. Implant of the LAA closure device was performed under fluoroscopic and transoesophageal echocardiographic (TOE) guidance as previously described [5]. Post-procedural OAC was at the physician's discretion and was individualised according to patient history and physician preferences.
2.3. Follow-up
Follow-up for subjects was based on local standard practice; generally a clinical visit at 1 to 3 months post-procedure and annual follow-up visits. Additional telephone contact was also undertaken. TOE imaging was recommended at 6 weeks to reassess device position and any residual flow around the device. If satisfactory, OAC discontinuation and initiation of dual-antiplatelet therapy (aspirin 81–325 mg, clopidogrel 75 mg) was recommended for 6 months post-implant or at physician discretion. LAA occlusion was defined as satisfactory positioning of the device at the ostium covering all trabeculated portions of the LAA with no or minimal peri-device flow (<5 mm). Complete occlusion was defined as no peri-device flow.
2.4. Serious adverse event reporting (SAE)
The trial required SAE reporting per ISO 14155 and the MEDDEV 2.7/3 12/2010. Oversight was provided by sponsor medical safety review of the source documents for all patient deaths. Events included procedure-related complications (e.g., serious pericardial effusion, device embolization, procedure-related stroke), all deaths and events related to excessive bleeding (e.g., intracranial or gastrointestinal bleeding) scored according to the BARC criteria [7]. Safety events were further classified as procedure-, device- or OAC-related efficacy endpoints included any occurrence of stroke (including ischaemic or haemorrhagic), transient ischaemic attack (TIA), death, or systemic embolism (SE).
2.5. Primary endpoints
The primary endpoints of the study were procedural success and safety, bleeding, all-cause mortality and efficacy of the device to prevent stroke/TIA/SE. Procedural success was defined as deployment and release of the device into the LAA with peri-device leak < 5 mm. Impact on bleeding was evaluated by comparing the observed bleeding event rates with the expected rate predicted by HAS-BLED score [8]. Efficacy was tested by comparing the actual stroke event rate with the CHA2DS2-VASc score predicted event rate [9].
2.6. Statistical methods
Country of implant was used as a surrogate for ethnicity with Asian subjects defined as patients from Indonesia, Malaysia, Hong Kong, Republic of Korea and Thailand; non-Asian subjects were defined as patients from Australia and Kingdom of Saudi Arabia. Australian subjects were manually audited to identify any Asian patients. Continuous variables are summarized using the mean, standard deviation, range and categorical variables with counts and percentages. Predicted risk of annual stroke (in the absence of therapy) and bleeding (during anticoagulant therapy) was extrapolated for each individual subject based on CHA2DS2-VASc and HAS-BLED scores using published literature [8,9] and then the average risk for the study population was used to determine the expected rates for comparison to observed rates and risk reduction calculations. Rates of stroke, TIA, SE, death and bleeding events are calculated as number of events per 100 patient-years (100-PY) and estimates of rates and 95% confidence intervals (CI) for each subgroup are derived from a Poisson Model. The Kaplan-Meier method was used to describe SAE rates at specific time points in follow-up. Fisher's exact test was used to compare binomial proportions, and the t-test to compare continuous variables.
3. Results
3.1. Patient demographics
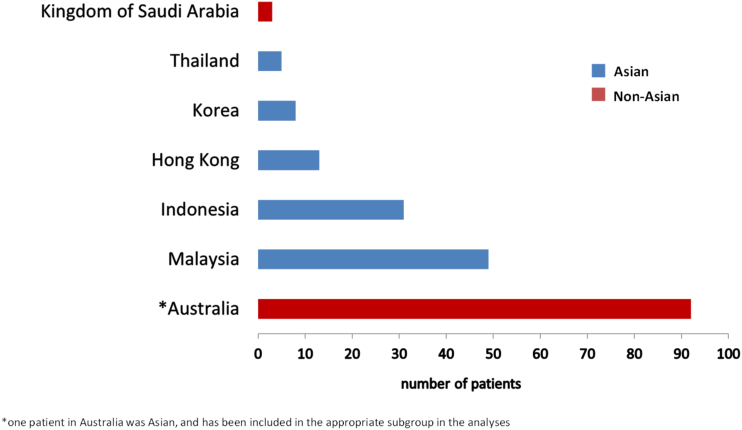
Enrolment in WASP commenced in January 2014 and concluded in October 2015, resulting in 201 patients across 7 countries including Australia, Asia and the Middle East. Australian and Saudi Arabian centres enrolled 95 patients, while more than half of patients (106/201) were from Asian countries (Indonesia, Malaysia, Hong Kong, Republic of Korea, Thailand) (Fig. 1 e-supplement).
Fig. 1 e-supplement.
Breakdown of study cohort by enrolling country with Asian patients in blue and Non-Asian patients in red. Note: *One Australian patient was Asian.
The mean age at consent was 70.8 ± 9 years (range 30–98) with 15.4% of patients aged ≥80 years and 67.2% male. Stroke risk scores for CHADS2 and CHA2DS2-VASc were 2.5 ± 1.4 and 3.9 ± 1.7, respectively, while the HAS-BLED score was 2.1 ± 1.2. Primary AF pattern was paroxysmal (51.0%) and contraindications to long-term OAC were recorded in 75.6% of patients. Baseline demographics and risk factors are summarized in Table 1.
Table 1.
Demographics and baseline characteristics.
| Characteristic | Asian N = 107 |
Non-Asian N = 94 |
p-Value |
|---|---|---|---|
| Age at time of consent (years) | 70.7 ± 9.4 (30.90) |
70.8 ± 9.4 (45.98) |
0.95 |
| Age ≥ 80 (%) | 14.0 | 17.0 | 0.56 |
| Gender (Male, %) | 62.6 | 72.3 | 0.18 |
| CHADS2 Score | 2.5 ± 1.3 | 2.4 ± 1.4 | 0.49 |
| CHA2DS2-VASc Score | 4.1 ± 1.7 | 3.7 ± 1.6 | 0.08 |
| CHA2Ds2-VASc Score (%) | 0.25 | ||
| ≤1 | 8.4 | 7.4 | |
| 2–3 | 29.9 | 41.5 | |
| ≥4 | 61.7 | 51.1 | |
| HAS-BLED Score | 2.2 ± 1.3 | 2.1 ± 0.9 | 0.66 |
| HAS-BLED Score (%) | 0.28 | ||
| <3 (%) | 65.4 | 73.4 | |
| ≥3 (%) | 34.6 | 26.6 | |
| CHF (%) | 20.6 | 10.6 | 0.08 |
| Hypertension (%) | 84.1 | 83.0 | 0.85 |
| Age ≥ 75(%) | 38.3 | 34.0 | 0.56 |
| Age 65–74 (%) | 43.0 | 44.7 | 0.89 |
| Diabetes (%) | 46.7 | 19.1 | <0.0001 |
| History of TIA/stroke (%) | 30.8 | 45.7 | 0.04 |
| Vascular disease (%) | 39.3 | 23.4 | 0.02 |
| Abnormal renal function (%) | 14.0 | 7.4 | 0.18 |
| Abnormal liver function (%) | 2.8 | 1.1 | 0.62 |
| History of ischaemic/haemorrhagic stroke (%) | 28.0 | 35.1 | 0.29 |
| Prior major bleeding or predisposition to bleeding (%) | 19.6 | 18.1 | 0.86 |
| Labile INRs (%) | 20.6 | 4.3 | 0.0006 |
| Concomitant use of drugs (%) | 33.6 | 50.0 | 0.02 |
| Alcohol abuse (%) | 2.8 | 13.8 | 0.007 |
| LV dysfunction (i.e. LVEF ≤ 40%) | 7.5 | 5.4 | 0.58 |
| AF pattern | |||
| % Paroxysmal AF | 54.2 | 47.3 | 0.40 |
Values are mean ± SD or %. CHF = congestive heart failure; LVEF = left ventricular ejection fraction.
3.2. Asian vs. non-Asian subgroup analysis
Post-hoc subgroup analysis was performed according to implanting geography as defined in the Statistical Analysis. One Australian patient was identified as Asian ethnicity and was included in the appropriate subgroup, yielding 107 Asian subjects and 94 non-Asian subjects. Significant differences emerged between Asian and non-Asian subjects, respectively, for underlying comorbidities with higher rates of diabetes (46.7% vs. 19.1%, p < 0.0001), vascular disease (39.3% vs. 23.4%, p = 0.02), and labile INRs (20.6% vs. 4.3%, p = 0.0006), while higher rates of prior TIA/ischaemic stroke were reported for non-Asian subjects (30.8% vs. 45.7%, p = 0.04), concomitant drug use (33.6% vs. 50.0%, p = 0.02) and alcohol abuse (2.8% vs. 13.8%, p = 0.01) (Table 1).
3.3. Procedural success
A total of 201 patients underwent implant attempts, with a successful implant achieved in 198 subjects (98.5%); 2 failures due to unfavourable anatomy (non-Asian) and 1 due to mismatch between device and LAA size (Asian). Closure (complete seal or leak < 5 mm) was achieved in all successful cases, with no difference between subgroups (Table 2).
Table 2.
Procedural results.
| Characteristic | Asian | Non-Asian | p-Value |
|---|---|---|---|
| Successful implant | 99.1% (105/106) |
97.9% (93/95) |
0.60 |
| LAA seal | |||
| Complete seal or Jet size < 5 mm | 100.0% (105/105) | 100.0% (93/93) | NS |
| Jet size ≥ 5 mm | 0.0% (0/105) |
0.0% (0/93) |
|
| LAA diameter | |||
| N | 106 | 92 | |
| Mean ± SD | 23.4 ± 4.1 | 21.2 ± 3.2 | <0.0001 |
| Median | 23.00 | 21.50 | |
| Last device size used (mm) | |||
| Mean ± SD | 27.4 ± 3.4 | 25.3 ± 3.2 | <0.0001⁎ |
| Median | 27 | 24 | |
| Compression of last device size used (mm) | |||
| Mean ± SD | 17 ± 7% | 17 ± 6% | 0.93 |
| Median | 16% | 17% |
p-Value = sample t-test across regions. LAA = left atrial appendage, mm = millimetres, NS = not significant.
Wilcoxon Rank-Sum test used to compare medians.
The majority of devices were successfully implanted without need for recapture in Asian patients compared to non-Asian subjects (59.8% vs. 43.0%, p = 0.06). Mean LAA diameter was significantly larger in Asians (23.4 ± 4.1 mm vs. 21.2 ± 3.2 mm, p < 0.001) and median device size used was 27 mm for Asians and 24 mm for non-Asians (p < 0.0001). There were significant differences in oversizing of the device size to maximum measured LAA diameter in Asian centres, but mean device compression was similar for both groups(17 ± 7% vs. 17 ± 6%, p = 0.93).
3.4. Oral anticoagulant regimen
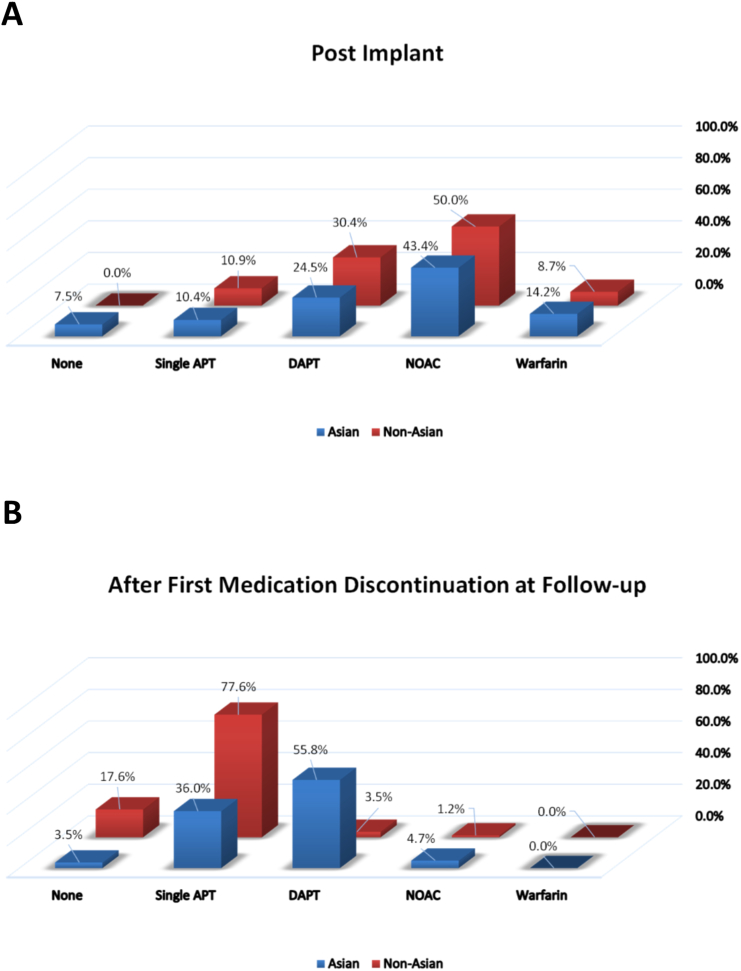
Baseline rates of anticoagulant or antiplatelet usage respectively for Asian and non-Asians was as follows — warfarin 13.1% and 4.3%; Novel oral anticoagulant (NOAC) 41.1% and 40.4%; dual-antiplatelet 15.0% and 11.7%; single-antiplatelet 18.7% and 19.1%; no therapy 12.1% and 24.5%. Post-implant and follow-up medications regimens are shown in Fig. 2 e-supplement. Anticoagulation with warfarin or NOAC was discontinued in 95% of patients by 90 days post-implant (96% Asian vs. 94% non-Asians) (Fig. 2B e-supplement). At last follow-up visit, the rates of anticoagulant and antiplatelet therapy respectively for Asian and non-Asians was as follows — warfarin 0% and 0%; NOAC 4.7% and 1.2%; dual-antiplatelet 55.8% and 3.5%; single-antiplatelet 36% and 77.6%; no therapy 3.5% and 17.6%.
Fig. 2 e-supplement.
Medications used by subjects (A) post-implant, and (B) at first medication discontinuation follow-up visit. Single APT = single-antiplatelet therapy, DAPT = dual-antiplatelet therapy, NOAC = novel oral anticoagulant.
3.5. Implant procedure safety
Procedure- or device-related SAEs within the first 7 and 30 days occurred at a rate of 3.0% and 3.5%, respectively. Six patients experienced SAEs on the day of the procedure: vascular damage to the groin (n = 1, non-Asian), pericardial effusion requiring pericardiocentesis (n = 2, Asian), oesophageal tear due to TEE (n = 1, Asian), hypotension (n = 1, non-Asian), and pulmonary oedema (n = 1, non-Asian). All patients experienced full recovery except for the oesophageal tear (fatal outcome on day 32). A femoral arteriovenous fistula was diagnosed and treated on day 28 (n = 1, Asian). The procedure- or device-related SAEs for Asian and non-Asian patients, respectively, at 7 and 30 days were 2.9% and 3.2% (p = 0.9) and 3.8% and 3.2% (p = 0.8).
3.6. Bleeding adverse events
There were 10 major bleeding events over 2 years of follow-up, with 8 events in 6 non-Asians and 2 in Asian patients (Table 3 e-supplement). The overall major bleeding SAE rate at 30-days was 1.0% versus 3.2% and at 12 months was 1.9% versus 5.4% for Asian and non-Asian patients, respectively.
The expected rate of major bleeding for the cohort predicted by HAS-BLED score [9] is 4.4 per 100-PY if taking warfarin, however the observed rate for the overall cohort was 2.2 per 100-PY [1.0 for Asian subjects, 3.7 per 100-PY for non-Asian subjects (p = 0.12)]. This represents a 49% relative risk reduction for bleeding events but a more pronounced relative risk reduction in Asian subjects as compared with non-Asians (77% versus 14%) (Fig. 3).
Fig. 3.
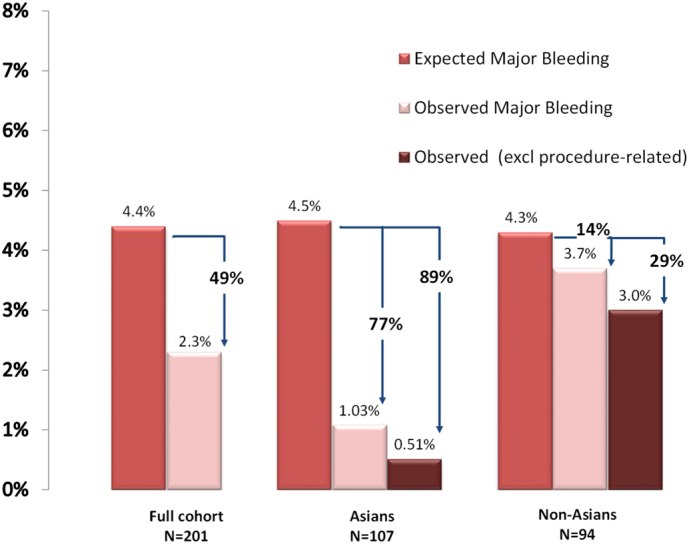
Calculated major bleeding risk based on HAS-BLED score, actual observed major bleeding rates and relative risk reduction for total cohort (left), Asian subgroup (centre) and Non-Asian subgroup (right) after 2 years follow-up.
3.7. TOE follow-up
Of the 198 successful implants, 170 (86%) patients had subsequent follow-up imaging (90/106 Asians, 81/92 non-Asian) at a mean follow-up time of 65.86 ± 30.18 days. Most imaging was performed with TOE, but 10 patients at 1 centre were studied post-implant with cardiac CT scans. No cases of device embolization were detected. Successful closure of the LAA with residual leak < 5 mm was documented in 100% of patients with a follow-up TOE.
Device-associated thrombus was detected in 5 patients (2 Asian, 3 non-Asian) (2.5%) at follow-up at 7, 45, 97, 216 and 386 days post-implant. Three patients were prescribed NOAC and 2 patients were taking antiplatelet therapy at the time of the observed thrombus. No neurological sequelae were detected and all thrombus were resolved at subsequent TOE follow-up after continuation or adjustment of OAC.
3.8. Long-term follow-up
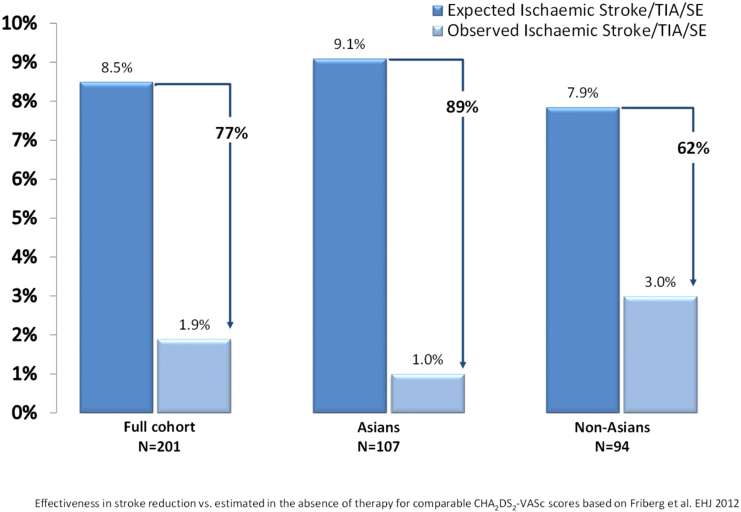
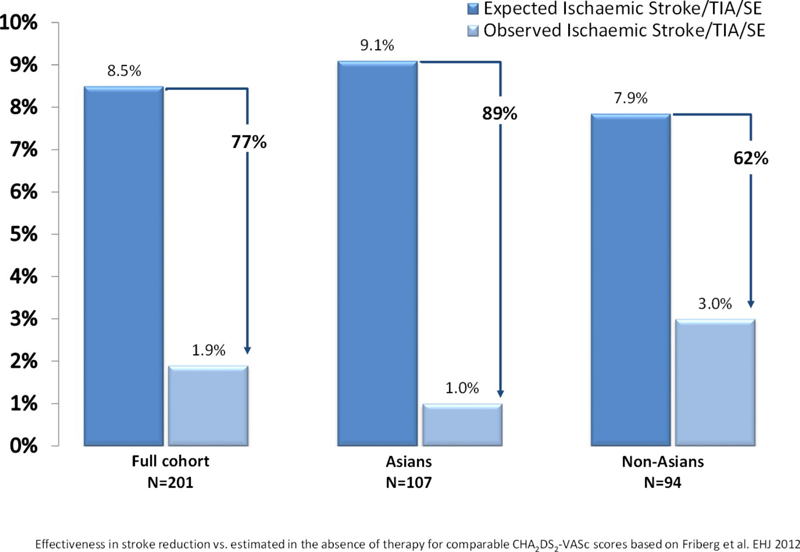
The mean follow-up time for the cohort was 684.1 ± 161.1 days (median 731). Six ischaemic strokes occurred during follow-up, 1 TIA, and no haemorrhagic stroke or intracranial bleeding events. Two strokes occurred in Asian patients — right hemiparesis (day 6) in patient with oesophageal tear from implant procedure, with eventual death (day 32) from sepsis and multi-organ failure; and one patient with left middle cerebral artery territory infarction (day 212) while on dual-antiplatelet therapy with eventual full neurological recovery. Four strokes occurred in non-Asian patients, all with full recovery — an unclassified small stroke (day 39) while on warfarin; a left frontal gyrus stroke (day 329) while on aspirin, a left sided frontal stroke (day 350) while on no therapy and a small middle cerebral artery territory stroke (day 414) while on dual-antiplatelet therapy. A non-Asian patient experienced a TIA during the procedure described as transient neurological deficit due to cerebral air embolism. The expected rate of stroke/TIA/SE predicted [8] assuming aspirin use is 8.5 per 100-PY, however the observed rate for the study was 1.93 per 100-PY [1.0 per 100-PY for Asian subjects, 3.0 per 100-PY for non-Asian subjects (p = 0.09)] (Table 4 e-supplement). This represents 77% relative risk reduction for the overall population, 89% reduction for Asian patients and 62% reduction for non-Asians (Fig. 4).
Fig. 4.
Calculated stroke/TIA/SE risk based on CHA2DS2-VASc score, actual observed stroke/TIA/SE rate and the relative risk reduction for total cohort (left), Asian subgroup (centre) and Non-Asian subgroup (right) after 2 years follow-up. TIA = transient ischaemic attack, SE = systemic embolism, RR = relative risk.
There were 12 deaths during the study [10 Asian, 2 non-Asian (p = 0.06)]. There was one procedural-related death in an Asian subject resulting from oesophageal tear as previously described. Other deaths were reported as unrelated to the procedure or device: liver malignancy, septic shock, thymus cancer, delirium, sudden cardiac arrest (day 153), acute pulmonary oedema (day 729), old age, sepsis, septic shock from pneumonia, head injury, and acute on chronic renal failure, resulting in an all-cause death rate of 3.23 per 100-PYs.
4. Discussion
This prospectively collected registry provides new information on the real-world use of LAA closure in an Asian population across multiple regions. The novelty of the findings is especially important due to increasing recognition of the challenges for stroke prevention in Asian patients with NVAF, as evidence suggests a lower age threshold at which increased risk is conferred for Asians (from age 50 years) compared with non-Asians [1,3]. This creates challenges from the potential increased duration of exposure to anticoagulation and long-term compliance. Rates of under-treatment with anticoagulation remain stubbornly high in Asian countries despite the global penetration of NOACs into clinical practice [10]. Compared to non-Asians, Asians are at significantly higher risk of warfarin-related intracranial bleeding (HR = 4.06) [2]. Although NOAC trial meta-analysis demonstrated greater proportional reduction compared with warfarin for rates of intracranial haemorrhage for Asian patients versus non-Asians, the absolute rates still remain higher [4,11]. Attention has also been focused on the possible link between macro and micro-angiopathies which are more prevalent in Asian populations and show an increased risk of intracranial haemorrhage [11]. Results from a Korean study suggested that among NVAF patients with a recent ischaemic stroke, 17.2% were classified as AF-unrelated, highlighting the complexity surrounding mixed aetiologies of stroke in Asians [12]. These data also highlight the significant numbers of Asian patients who may have contraindications to long-term anticoagulation; conversely LAA closure can achieve annual rates of ischaemic stroke and bleeding around 1% without an increased iatrogenic risk of intracranial bleeding and secured compliance [5]. The results in the current study are supported by a single-centre study from China which reported an annual stroke rate of 2.13% for a cohort of secondary prevention patients (mean CHA2DS2-VASc score of 4.09 ± 1.06) who underwent WATCHMAN LAA closure [13]. Mortality rates in Asian patients trended higher than non-Asian subjects in the current study although did not achieve statistical significance (p = 0.06) with most deaths non-cardiac related. A high 1-year mortality rate of 9.8% was noted in the EWOLUTION registry [6] with the probable explanation being OAC-contraindicated patients generally having higher comorbidity profiles and lower life expectancy.
4.1. Bleeding rates
The rate of major bleeding events in the study was 2.2 per 100-PY. There are several potential reasons for the small but significant ongoing bleeding rates in the study. Firstly, a proportion of events can be attributed to adverse effects of the post-implant medication regimen, likely impacting the short-term bleeding events. One study has examined the potential for using simplified or shortened oral anticoagulation or antiplatelet regimens in the post-implant period of LAA closure and healing [14]. Increased risk of intracranial haemorrhage has been noted for warfarin [2], NOACs [9] and aspirin [15] in Asian populations undergoing secondary stroke prevention, however no increased risk has been shown for clopidogrel [16]. This requires further consideration for post-implant strategies in Asian patients that may limit bleeding risk in this specific population. Secondly, the cohort includes significant numbers of patients with a documented history of major bleeding event. Unsurprisingly, over half of the bleeding events were attributable to a gastrointestinal cause, and most of these cases a history of gastrointestinal bleeding was the main indication for LAA closure. As rates of gastrointestinal bleeding are reported to be higher in non-Asian patients taking NOACs [4], the strategy of mechanical closure can provide an important stroke prevention alternative for these patients.
4.2. Procedural safety in Asian patients
Although the 7-day peri-procedural complication rate was low at 3.0%, there was a procedure-related death due to complications from a tear related to TOE performed to guide the implant. TOE technique during LAA device implant differs from typical diagnostic study due to the requirement for the patient to be supine, heparinised and may involve a more prolonged period of oesophageal manipulation. Oesophageal injury related to TOE was reported in one other small study on LAA device closure with the Amplatzer cardiac plug (Abbott, Abbott Park, IL) performed in 20 Asian patients [17]. Comparatively, rates of oesophageal injury in other WATCHMAN studies with predominantly non-Asian populations have been extremely low (1/542 in PROTECT AF, 0/1021 EWOLUTION) [5,6]. Some centres have suggested the use of micro-TOE probes to reduce oesophageal injury rates; further study of alternative imaging modality in this population deserves consideration.
4.3. LAA dimension in Asian patients
Another notable finding was a larger mean LAA ostial diameter and larger median device size used in Asian patients compared to non-Asians. This is important as LAA size was first recognized as an independent risk factor for stroke in the SPAF study [18]. Maximal LAA ostial diameter has previously been shown to correlate with left atrial volume and to be larger for patients with persistent as compared with paroxysmal forms of AF [19]. Furthermore, left atrial volume has also been shown to correlate with body mass index (BMI) [20]. However, no significant difference was apparent between the proportion of Asian vs. non-Asian patients with persistent forms of AF. Although BMI was not collected, Asian populations are generally smaller stature and lower BMI than comparable Western cohorts, so neither factor would appear to be an explanation for differences. The finding has practical implications for LAA device occlusion in Asian patients but warrants further research into ethnic differences in left atrial remodelling and stroke risk.
4.4. Clinical implications
While the effectiveness of OAC as a stroke reduction strategy in NVAF patients has been well established, the inherent bleeding risks still provide a conundrum for those at higher risk of bleeding complications, leading to variable physician and patient compliance especially in Asian regions [10]. LAA closure can provide effective stroke risk reduction without the bleeding risks associated with long-term pharmacological therapy, but until now has limited outcome data in Asian populations. This prospective real-world registry demonstrates that Asian patients appear to derive the same stroke benefit from LAA closure as non-Asians, while achieving the expected bleeding benefits associated with limited exposure to anticoagulation. Current Clinical Guidelines support the use of left atrial appendage device closure for patients with high stroke risk and contraindications to long-term oral anticoagulant treatment [21]. The current study provides additional evidence that the Guidelines are also directly applicable to patients of Asian ethnicity.
4.5. Study limitations
There are inherent limitations that arise from a real-world registry design including non-conformity of treatment (e.g. variation in post-discharge OAC) and non-conformity of follow-up (e.g. incomplete TOE follow-up). Additionally, all TOE measurements are subject to operator interpretation and imaging system variability since no independent image adjudication was employed. Country of origin was used as a surrogate for Asian ethnicity, which is generally considered valid due to low rates of ethnic diversity in Asian countries, however future studies should specifically define race. While this is the largest LAA closure registry in the region, the cohort size and infrequent event rates limits subgroup analyses. The predictive accuracy of estimating expected stroke and bleeding risks by mean CHA2DS2-VASc and HAS-BLED scores is unknown in Asian patients in the setting of NOAC rather than warfarin use. Thus the potential for under or over-estimation of risk reduction with LAAC cannot be excluded.
Because of the recognized differences in stroke epidemiology and responses to treatment across different racial groups, further large-scale studies are required. As new therapies for atrial fibrillation penetrate Asian countries, there will be better scope to study potential regional differences across Asia.
5. Conclusions
Real world experience of LAA closure with WATCHMAN has demonstrated a high successful implant rate and low peri-procedural risk in a predominantly Asian cohort. Consistent with other WATCHMAN studies, long-term outcomes show efficacious stroke prevention with low bleeding risks for patients with Asian ethnicity.
The following are the supplementary data related to this article.
Supplementary tables
Funding
Work was supported by Boston Scientific Inc., Marlboro, MA, USA. PS is supported by a Practitioner Fellowship from the National Health and Medical Research Council of Australia and by National Heart Foundation of Australia.
Conflict of interest statement
KP received honoraria for clinical proctorship and advisory board participation from Boston Scientific (BSC) and St Jude Medical/Abbott (SJM/ABT); PS has served on the advisory board for Biosense-Webster, SJM/ABT, BSC; University of Adelaide receives on behalf of PS lecture and/or consulting fees from Biosense-Webster, SJM/ABT, BSC and research funding from SJM/ABT, BSC; JA received honoraria from BSC; KMS and NG are employees of BSC; OR received honoraria from BSC; TS, JLKC, HP, MC have nothing to disclose.
References
- 1.Chao T.F., Lip G.Y., Liu C.J. Validation of a modified CHA2DS2-VASc score for stroke risk stratification in Asian patients with atrial fibrillation: a nationwide cohort study. Stroke. 2016;47:2462–2469. doi: 10.1161/STROKEAHA.116.013880. [DOI] [PubMed] [Google Scholar]
- 2.Shen A.Y., Yao J.F., Brar S.S., Jorgensen M.B., Chen W. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J. Am. Coll. Cardiol. 2007;50:309–315. doi: 10.1016/j.jacc.2007.01.098. [DOI] [PubMed] [Google Scholar]
- 3.Bai Y., Wang Y.L., Shantsila A., Lip G.Y.H. The global burden of atrial fibrillation and stroke: a systematic review of the clinical epidemiology of atrial fibrillation in Asia. Chest. 2017;152:810–820. doi: 10.1016/j.chest.2017.03.048. [DOI] [PubMed] [Google Scholar]
- 4.Wang K.L., Lip G.Y., Lin S.J., Chiang C.E. Non-vitamin K antagonist oral anticoagulants for stroke prevention in Asian patients with nonvalvular atrial fibrillation: meta-analysis. Stroke. 2015;46:2555–2561. doi: 10.1161/STROKEAHA.115.009947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holmes D.R., Jr., Doshi S.K., Kar S. Left atrial appendage closure as an alternative to warfarin for stroke prevention in atrial fibrillation: a patient-level meta-analysis. J. Am. Coll. Cardiol. 2015;65:2614–2623. doi: 10.1016/j.jacc.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 6.Boersma L.V., Ince H., Kische S. Efficacy and safety of left atrial appendage closure with WATCHMAN in patients with or without contraindication to oral anticoagulation: 1-year follow-up outcome data of the EWOLUTION trial. Heart Rhythm. 2017;14:1302–1308. doi: 10.1016/j.hrthm.2017.05.038. [DOI] [PubMed] [Google Scholar]
- 7.Mehran R., Rao S.V., Bhatt D.L. Standardized bleeding definition for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–2747. doi: 10.1161/CIRCULATIONAHA.110.009449. [DOI] [PubMed] [Google Scholar]
- 8.Friberg L., Rosenqvist M., Lip G.Y. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182,678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur. Heart J. 2012;33:1500–1510. doi: 10.1093/eurheartj/ehr488. [DOI] [PubMed] [Google Scholar]
- 9.Lip G.Y.H., Frison L., Halperin J.L., Lane D.A. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED score. J. Am. Coll. Cardiol. 2011;57:173–180. doi: 10.1016/j.jacc.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 10.Huisman M.V., Rothman K.J., Paquette M. The changing landscape for stroke prevention in AF: findings from the GLORIA-AF registry phase 2. J. Am. Coll. Cardiol. 2017;69:777–785. doi: 10.1016/j.jacc.2016.11.061. [DOI] [PubMed] [Google Scholar]
- 11.Bang O.Y., Hong K.S., Heo J.H. Asian patients with stroke plus atrial fibrillation and the dose of non-vitamin K oral anticoagulants. J. Stroke. 2016;18:169–178. doi: 10.5853/jos.2016.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim S.J., Ryoo S., Kwon S. Is atrial fibrillation always a culprit of stroke in patients with atrial fibrillation plus stroke? Cerebrovasc. Dis. 2013;36:373–382. doi: 10.1159/000355571. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y., Zhang Y., Huang W., Huang K., Xu B., Su X.I. Primary and secondary stroke prevention using left atrial appendage closure with Watchman devices in atrial fibrillation patients: a single center experience from mainland China. Pacing Clin. Electrophysiol. 2017;40:607–614. doi: 10.1111/pace.13020. [DOI] [PubMed] [Google Scholar]
- 14.Tung M.K., Ramkumar S., Cameron J.D. Retrospective cohort study examining reduced intensity and duration of anticoagulant and antiplatelet therapy following left atrial appendage occlusion with the WATCHMAN device. Heart Lung Circ. 2017;26:477–485. doi: 10.1016/j.hlc.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Diener H.C., Bogousslavsky J., Brass L.M. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331–337. doi: 10.1016/S0140-6736(04)16721-4. [DOI] [PubMed] [Google Scholar]
- 16.Uchiyama S., Tanahashi N., Minematsu K., COMPASS (SFY6913) Study Group Clopidogrel two doses comparative 1-year assessment of safety and efficacy (COMPASS) study in Japanese patients with ischemic stroke. Cerebrovasc. Dis. 2012;34:229–239. doi: 10.1159/000342655. [DOI] [PubMed] [Google Scholar]
- 17.Lam Y.Y., Yip G.W., Yu C.M. Left atrial appendage closure with AMPLATZER cardiac plug for stroke prevention in atrial fibrillation: initial Asia-Pacific experience. Catheter. Cardiovasc. Interv. 2012;79:794–800. doi: 10.1002/ccd.23136. [DOI] [PubMed] [Google Scholar]
- 18.The Stroke Prevention in Atrial Fibrillation Investigators Predictors of thromboembolism in atrial fibrillation: II. Echocardiographic features of patients at risk. Ann. Intern. Med. 1992;16:6–12. doi: 10.7326/0003-4819-116-1-6. [DOI] [PubMed] [Google Scholar]
- 19.Walker D.T., Humphries J.A., Phillips K.P. Anatomical analysis of the left atrial appendage using segmented, three-dimensional cardiac CT: a comparison of patients with paroxysmal and persistent forms of atrial fibrillation. J. Interv. Card. Electrophysiol. 2012;34:173–179. doi: 10.1007/s10840-011-9638-1. [DOI] [PubMed] [Google Scholar]
- 20.Huang G., Parikh P.B., Malhotra A., Gruberg L., Kort S. Relation of body mass index and gender to left atrial size and atrial fibrillation. Am. J. Cardiol. 2017;120:218–222. doi: 10.1016/j.amjcard.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 21.Kirchhof P., Benussi S., Kotecha D. ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609–1678. doi: 10.1093/europace/euw295. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables