Abstract
The therapeutic armamentarium of inflammatory bowel disease is rapidly evolving with the development of novel treatment options including targeted monoclonal antibodies and small molecules. With these new therapies come additional safety and side effect concerns. Infections, malignancies, immunogenicity, and metabolic issues exist for each treatment. Management of these agents in the face of such complications is a challenge clinicians will encounter. In this article, we review the existing safety data behind the monoclonal antibodies and small molecules, suggest appropriate risk stratification and assessment considerations before and during therapy, and make expert recommendations on the management of potential complications or clinical scenarios.
Keywords: biologic, safety, risk, inflammatory bowel disease, Crohn’s, ulcerative colitis, complications, side effects
BACKGROUND
The last 2 decades have seen rapid growth in the inflammatory bowel disease (IBD) therapeutic armamentarium. The advent of the antitumor necrosis factor (TNF) agent infliximab in 1998 launched the “biologic era” of IBD therapeutics. The 2000s were the anti-TNF decade, with several agents following suit including adalimumab, certolizumab pegol, and golilumab. With broadened mechanistic understanding of IBD, additional monoclonal antibodies were designed including anti-integrin agents targeting leukocyte adhesion and trafficking (natalizumab and vedolizumab) and the anti-interleukin 12/23 antibody ustekinumab. Most recently, a unique orally administered small molecule targeting the Janus kinase pathway of inflammation (tofacitinib) was developed and recently received FDA approval for the treatment of moderate to severe ulcerative colitis. Other mechanisms of action are in the pipeline. While the number of available therapeutics with novel targets has increased, so have the immunologic, safety, and side effect considerations. In this article, we review some key treatment aspects of these newer agents including efforts to prevent adverse events, minimize risks, and address side effects or complications, should they arise. For this review, we will use the term “biologics” referring to all new agents, including tofacitinib, and will differentiate monoclonal antibodies as necessary. Due to curtailed clinical use of natalizumab related to JC virus reactivation, only vedolizumab will be discussed in this review.
Infection Risk
Infections, both common and opportunistic, remain one of the major safety considerations with biologics. Opportunistic infections are those that occur with increased frequency and severity in individuals with impaired immune function. For anti-TNFs, studies have demonstrated conflicting findings on potential common infection risk. Some have demonstrated an increased risk,1 while others reported contradictory findings.2–4 These contrasting results may be due in part to study design, disease type and severity, comorbid steroid and narcotic use, and patient age. Extensive reviews on the risk of infections with anti-TNFs have been previously published.5–8
Opportunistic infections are definitively associated with anti-TNFs and include both bacterial (tuberculosis, atypical mycobacterial, listeriosis) and fungal (histoplasmosis, coccidioidomycosis, candidiasis, aspergillosis, pneumocystosis) etiologies.9–11 While assessed before therapy initiation (see Safety—Before Starting section later on), tuberculosis (TB) reactivation still occurs in 0.05% (5 of 10,000) patients receiving anti-TNFs.12,13 The risk of opportunistic infection increases with age (odds ratio [OR] 1.1 per 5 years; 95% CI, 1.1–1.2) with patients over 50 years carrying 3 times (OR 3.0; 95% CI, 1.2–7.2) increased risk of opportunistic infection.14
Mechanistically, TNF is key for the containment of chronic viral infections. Thus, a major concern with anti-TNFs therapy is their potential impact on viral infections. Anti-TNF therapy has been associated with hepatitis B virus (HBV) reactivation and can result in fulminant hepatitis.15,16 This has occurred not only in patients with HBV surface antigen positivity but also in those with surface antigen negative/core antibody positive serology.17 There may be a protective effect of HBV surface antibody against reactivation in the latter group, though this is still debated. Conversely, anti-TNFs have not been shown to increase the risk of deterioration related to chronic hepatitis C virus (HCV).18
One of the appealing features of the gut-selective anti-integrin vedolizumab is its safety profile pertaining to infections. In a pooled post hoc analysis of the clinical trials, overall there was no increased risk of infection or serious infection compared with placebo.19 Rates of gastrointestinal infections, clostridial infections, and TB were higher in vedolizumab-treated patients compared with placebo, though the TB infections were largely felt to be primary infection in hyperendemic areas. While a concern limiting the use of its predecessor, natalizumab, there have been no reported cases of progressive multifocal leukoencephalopathy related to the JC virus with vedolizumab. In the clinical trial pooled analysis, risk factors for serious infection with vedolizumab were younger age, concurrent corticosteroids, and opiate use.
Similarly, in randomized clinical trials, ustekinumab demonstrated no increased infection frequency compared with placebo (2.3% vs 2.3%)20 and the Psoriasis Longitudinal Assessment and Registry (PSOLAR), which monitors ustekinumab for psoriasis, demonstrated an infection rate of 1.3 per 100 person years (PY) with ustekinumab compared with 5.75 per 100 with infliximab.21 Furthermore, there is a lower rate of TB reactivation with ustekinumab than anti-TNF (0.02 per 100 person years; infliximab 0.39; golilumab 0.24). In the UNITI trials of ustekinumab for Crohn’s disease, there was one subject that developed listeria meningitis and one subject that contracted an ophthalmic herpes infection. Consequently, longer-term monitoring is needed.
Conversely, in ulcerative colitis clinical trials, tofacitinib-treated participants had higher prevalence of all-cause infections compared with placebo (39.8% 10 mg BID and 35.9% 5 mg BID vs 24.2% placebo in OCTAVE Sustain), though the majority were mild or moderate, and the most common infection was nasopharyngitis.22 Furthermore, participants receiving tofacitinib demonstrated increased rates of herpes zoster virus (HZV) reactivation compared with placebo (7.6 per 100 person years; adjusted hazard ratio [AHR] 1.4; 95% CI, 1.09–1.81),23 with nearly 5% of participants in the higher dose maintenance arms experiencing HZV reactivation. However, all HZV reactivations were in 1 or 2 dermatomes (nonserious) and did not require toficitinib discontinuation.
Noncutaneous Malignancy Risk
Anti-TNFs were recently shown to be associated with increased risk of lymphoma in both monotherapy (AHR 2.41; 95% CI, 1.60–3.64) and combination therapy with thiopurines (AHR 6.11; 95% CI, 3.46–10.8) in a large French population cohort.24 There was historical concern of hepatosplenic T cell lymphoma risk in patients receiving anti-TNFs, particularly young males; however, a systematic review demonstrated that all patients who developed hepatosplenic T cell lymphoma received thiopurines either in combination with anti-TNFs or as monotherapy.25 There were no reported cases of hepatosplenic T cell lymphoma in patients receiving anti-TNF monotherapy. Anti-TNFs have not been associated with the development of other primary noncutaneous malignancies. Similarly, data from the vedolizumab dose-finding trials and GEMINI studies did not demonstrate an increased malignancy risk compared with expected population rates;26 however, in the pooled post hoc analysis, all noncutaneous malignancies occurred in vedolizumab-treated participants, including several gastrointestinal cancers (colorectal, appendiceal carcinoid, and hepatic neoplasm). These findings necessitate longer follow-up data.
In the phase 2 and UNITI studies of ustekinumab, there were reports of prostate, colorectal, and breast cancer in patients receiving ustekinumab, but the rates were not different from the general US population’s expected types and rates.20 Data from the clinical trials of ustekinumab in psoriasis and PSOLAR have not suggested any increased malignancy risk in patients receiving ustekinumab compared with controls.27–29
In the clinical trials of tofacitinib for ulcerative colitis, no tofactinib-treated patients developed noncutaneous malignancies.22 Pooling data from the rheumatoid arthritis (RA) clinical trials demonstrated no increase in age- or sex-adjusted standardized incidence rate (SIR) of malignancies (excluding nonmelanoma skin cancer) compared with surveillance, epidemiology, and end results (SEER) expected rates (SIR 1.0; 95% CI, 0.8–1.1).30,31 It should be noted that there is interest in JAK inhibition for the treatment of various malignancies, including EBV-associated lymphoma.32,33
In relation to recurrence of prior noncutaneous malignancy, studies to date have demonstrated no significant increased risk of recurrence with anti-TNFs.34 Long-term data on vedolizumab, ustekinumab, and tofacitinib is pending.
Cutaneous Malignancy Risk
In IBD patients, anti-TNF therapy has been associated with an increased risk of melanoma (OR 1.88; 95% CI, 1.08–3.29);35 however, other studies, including those focused on rheumatoid arthritis, have not confirmed this association.36 There is no significant increased risk of nonmelanoma skin cancer (NMSC) with anti-TNFs (OR 1.14; 95% CI, 0.95–1.36).35
In the pooled post hoc study of vedolizumab safety, 5 vedolizumab-treated patients developed cutaneous malignancies (2 melanoma, 3 NMSC). Those who developed melanoma previously received anti-TNF therapies, and those with NMSC were previously or concurrently treated with thiopurines. All dermatologic malignancies were reported as resolved. Longer-term follow-up is underway.
Clinical trials of ustekinumab in IBD did not demonstrate any significant signal for increased cutaneous malignancies compared with placebo.20 In the long-term follow-up studies of ustekinumab in treatment of psoriasis, no increased risk of malignancy has been reported.27
In the OCTAVE induction and maintenance trials of tofacitinib for ulcerative colitis, 6 patients developed NMSC compared with 1 placebo. There were no reports of melanoma in either group. The follow-up studies for tofacitinib in rheumatoid arthritis demonstrated no increased risk of NMSC compared with expected rates in RA. Interestingly, 1 study observed an increase in the NMSC incidence rate in higher dosing groups (10 mg BID vs 5 mg BID),31 but other reports dispute this finding.30 There was no increase in risk over tofacitinib-exposed time. Melanoma was not specifically evaluated in this study.
Immunologic Issues
Anti-TNFs have been associated with a wide array of immunologic entities including humoral immunogenicity, psoriatic and lupus-like reactions, and demyelinating processes.
An individual’s immune system can recognize a monoclonal antibody as a foreign epitope and generate an antidrug humoral response to the monocloncal antibody, a characteristic termed “immunogenicity.” These antidrug antibodies increase drug clearance, increase the risk of infusion reactions, and thereby influence clinical outcomes. With the wealth of existing literature, we will not belabor the humoral immunogenicity aspects of anti-TNFs. The development of immunogenicity can be reduced with concomitant immunomodulator use.37,38 Vedolizumab studies to date estimate the immunogenicity at 4% after 52 weeks of treatment without a significant rate increase over exposed time, but this increases to 10% 16 weeks after last dose.19 Concomitant immunomodulator reduced antidrug antibody formation from 4% to 3%. Rates of antidrug antibody formation with ustekinumab in the IM-UNITI trial were 2.3% at week 44.20 Due to its small molecule nature, immunogenicity with tofacitinib has not been described.
Psoriatic lesions with anti-TNFs have been well-described, with an estimated incidence of 0.6%–5.3% with a slight predilection for CD.39 The most commonly affected areas are hands and feet (palmoplantar), scalp, and ears. Timing of onset is variable and can occur at any point during therapy. Early suggestions that psoriatic lesions were secondary to high-drug trough concentrations have since been refuted. The complication appears to be a class-effect, with frequent recurrence reported when another anti-TNF is attempted.40 No psoriatic reactions to vedolizumab, ustekinumab, or tofacitinib have been reported. In fact, ustekinumab is FDA-approved for use in plaque psoriasis and psoriatic arthritis while tofacitinib carries an FDA indication for psoriatic arthritis.
Rates of drug-induced lupus reactions with anti-TNFs are estimated at <1% and have been reported for all anti-TNF agents.41,42 The 2 characteristic forms of anti-TNF-induced lupus include the cutaneous type (malar rash, photosensitive rash, purpura, and alopecia, with positive antinuceal antibody and antidouble-stranded DNA antibody) and the complete lupus type (autoantibodies, cutaneous manifestations, plus other extra-cutaneous involvement such as serositis, arthritis, renal abnormalities, etc.). The exact etiology of anti-TNF lupus reaction is unknown, but proposed hypotheses include anti-TNF-induced cellular apoptosis releasing DNA and lupus auto-antigens, increased B cell activity due to susceptibility to infections, and promotions of T-helper 2 immune responses in anti-TNF patients. Recurrence with anti-TNF rechallenge has been reported; however, 1 case of successful reexposure has also been described with adalilumab.43 One vedolizumab-treated participant developed cutaneous lupus in the GEMINI trials. Similarly, a single case of ustekinuamb-induced cutaneous lupus with recurrence upon rechallenge has been reported.44 Drug-induced lupus has not been reported with tofacitinib to date.
The exact incidence and cause of demyelinating processes with anti-TNFs is unknown. These demyelinating reactions predominantly affect the central nervous system and typically resolve after drug discontinuation; however, progressive clinical courses have also been described.45,46 A single case of ustekinumab-induced central nervous system demyelination has been reported in CD patients previously treated with 3 anti-TNFs.47 No cases of demyelinating conditions have been reported with vedolizumab or tofacitinib.
Metabolic and Hematologic Complications
Most biologic medications have been associated with at least 1 metabolic or hematologic side effect or derangement. Liver enzyme abnormalities with anti-TNFs have been described. These are typically asymptomatic and discovered incidentally, though anti-TNFs have also been associated with autoimmune hepatitis.48,49 Excluding autoimmune hepatitis, liver enzyme abnormalities with anti-TNFs are usually self-limited.50 Neutropenia is the most commonly reported anti-TNF hematologic complication, with 0.6%–5.7% of patients treated with anti-TNF ever developing neutropenia and more commonly reported in rheumatologic diseases than IBD.51 Classically mild and transient, anti-TNF-induced neutropenia rarely requires discontinuation.51,52
Hepatobiliary events were observed more frequently in vedolizumab-treated participants (0.3 per 100 PY; 95% CI, 0.2–0.5) compared with placebo (0.0 per 100 PY; 95% CI, 0.0–1.4) in the clinical trials, with hepatic steatosis the most common hepatobiliary event (0.2 per 100 PY; 95% CI, 0.1–0.3).19 There was no difference in isolated abnormal liver enzymes in vedolizumab (2.1 per 100 PY; 95% CI, 1.6–2.5) compared with placebo (2.8 per 100 PY; 95% CI, 0.6–5.1), and isolated liver enzyme abnormalities did not lead to vedolizumab discontinuation. No hematologic abnormalities were observed in the clinical trials of vedolizumab.53,54
No significant liver enzyme abnormalities have been observed in ustekinumab- or tofacitinib-treated patients. In the OCTAVE trials of tofacitinib, 2 tofacitinib-treated subjects with baseline low neutrophil counts developed absolute lymphopenia.22
More participants receiving tofacitinib had abnormal lipid profiles with higher total cholesterol, low-density lipoprotein (LDL), and high-density lipoprotein (HDL) compared with placebo.22 This same effect was seen in the rheumatoid arthritis tofacitinib clinical trials, where they observed a dose-dependent mean increase in LDL and HDL by approximately 10%–20%, with lipid increases correlating to reduction in inflammation.55 These increases were generally seen in the first 4 weeks of therapy, stabilized after 3 months of therapy, and have not been associated with cardiovascular events. Several mechanisms including lower baseline levels of LDL and HDL in autoimmune patients compared with healthy controls and tofacitinib-induced altered cholesterol ester metabolism have been suggested.56 There were also higher rates of creatine kinase elevation in tofacitinib participants, but no patients experienced concurrent myopathy or rhabdomyolysis.
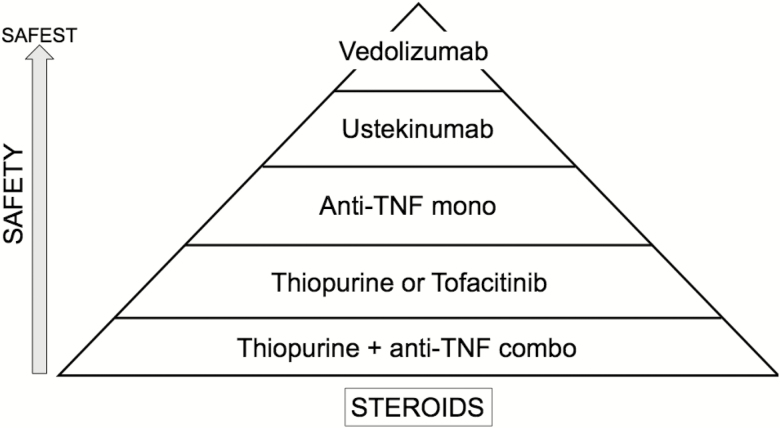
SAFETY PYRAMID
Based on the available risk data, the authors constructed a safety pyramid of the biologic agents and therapeutic regimens reflecting their own opinions and interpretation of available literature (Fig. 1).
FIGURE 1.
Proposed “safety pyramid” of inflammatory bowel disease medications. These pyramid positions reflect the authors’ views, interpretation of available data, and clinical practice.
SAFETY—BEFORE STARTING
Infection Risk Assessment
The first step to improving safety with these new agents is to assess an individual patient’s risk of therapeutic complications. Preparing a patient to start a biologic therapy is the ideal time to conduct such a risk assessment and implement interventions to reduce complications. To risk stratify for infections, clinicians should carefully review a patient’s medical history for comorbidities that would further increase susceptibility to infections (eg,, diabetes, renal disease, respiratory disorders).57 Age over 50 years is also a risk factor for increased infection risk with immunosuppression and should be included in therapeutic decision-making.14
Tuberculosis status should be evaluated before therapy. We recommend utilizing interferon-gamma release assays (Quantiferon-TB Gold and T-SPOT) over a purified protein derivative (PPD) for this purpose. If positive, a chest x-ray should be obtained to evaluate for active TB with referral to an infectious disease specialist for latent TB treatment. False negatives can occur with concomitant corticosteroids. With the ongoing risk of reactivation despite negative initial testing, periodic TB testing with interferon-gamma release assays during biologic therapy is recommended. As a practical point during therapy, we encourage routine TB testing every 2–3 years in a World Health Organization TB low-burden country and annually in high-burden locations.
Hepatitis B virus serology including HBV surface antibody (HBsAb), surface antigen (HBsAg), and core antibody (HBcAb) should be obtained before therapy.16 The 2015 American Gastroenterological Association guidelines recommend risk stratification based on HBV serology and the proposed therapy.58 In general, HBsAg-positive patients should receive suppressive antiviral therapy alongside monoclonal antibody therapy, whereas HBsAg-negative/HBcAb-positive patients should receive prophylactic HBV antiviral treatment if being placed on a monoclonal antibody or >4 weeks corticosteroid therapy. However, 2018 American Association for the Study of Liver Diseases (AASLD) guidelines offer the option of careful observation in the select HBsAg-negative/HBcAb-positive population.59 Patients receiving thiopurines, methotrexate, or short-course corticosteroids do not require HBV treatment. Currently, there are no recommendations for HBV treatment in tofacitinib therapy because most clinical trials excluded patients with evidence of HBV infection. A recent retrospective study from Taiwan with tofacitinib for rheumatoid arthritis found 50% HBV reactivation in patients with HBsAg positivity and no antiviral treatment and no reactivation in HBsAg-negative/HBcAb-positive patients without antivirals.60 No patients receiving antiviral therapy developed HBV reactivation. These findings support a similar approach to tofacitinib as monoclonal antibodies in the AASLD guidelines.
Clinicians should also consider baseline varicella zoster virus (VZV) and Epstein-Barr virus (EBV) testing, given the increased risk of reactivation or EBV-associated lymphoma development with acute EBV infections, especially in males. The latter is typically associated with thiopurines, but given the frequency of combination therapy (thiopurine plus biologic), it is reasonable to include with the remainder of the infection risk assessment in this discussion.
Vaccinations
Vaccinations are a key mitigating strategy of the infectious risk of biologics. Much has been previously written on the vaccination status to consider and evaluate before starting immunosuppressive treatment, and we will not review this data and recommendations in detail.61–64 A recommended list of items to review and vaccination status to assess derived from American College of Gastroenterology (ACG) guidelines62 are provided in Table 1. As gastroenterologists can often be the only physician IBD patients routinely see, many of the preventative health considerations with immunosuppressive therapy should be managed by the treating gastroenterologist.65–68
Table 1.
Infection Risk Assessment, Vaccination Status, and Recommended Vaccination Doses for Inflammatory Bowel Disease Patients. Adapted from “ACG Clinical Guideline: Preventive Care in Inflammatory Bowel Disease.”62
| Inactivated Vaccine Available | Patient Population | Titer Check Pre-immunization | Dosing Regimen | Cornerstones Health Recommended? |
|---|---|---|---|---|
| Corynebacterium diphtheria, Clostridium tetani, Bordetella pertussis (Tdap) | All Patients If not given in last 10 yrs or Td ≥2 yrs |
No | Single dose of Tdap recommended at age 11 through 64 years; Td booster every 10 years | Yes |
| Hepatitis A | All Patients | Yes HAV Ab |
2 doses at 0 and 6 months | Yes |
| Hepatitis B | All Patients | Yes HBVsAb, HBVsAg, HBVcAb |
3 doses at 1, 1–2 and 4–6 months; check titers 1 month after the last dose; if no response, 3 options: revaccinate, double dose HBV vaccination or combined HAV/HBV vaccine | Yes |
| Human Papilloma Virus | Male and Female, aged 11–26 | No | 3 Doses at 0, 2, and 6 months | Yes, but recommend ages 9–26 |
| Influenza | All Patients | No | Annual immunization with trivalent inactivated influenza vaccine; “high dose” vaccine for patients 65 and older; live attenuated intranasal influenza vaccine is contraindicated in immunosuppressed patients | Yes |
| Neisseria meningitidis | All patients aged 11–19 High Risk (military, college, splenectomy, endemic area, HIV) |
No | Two or three doses depending on vaccine | Yes |
| Streptococcus pneumonia | All Patients | No | If no previous vaccination, PCV13 followed by a dose of PPSV23 after 2–12 months; if received 1 or more doses#8232;of PPSV23 should receive PCV13 one or more years after PPSV23; another dose of PPSV23 should be administered 5 years after the initial PPSV23 dose and at age 65 years or older if at least 5 years have elapsed since their previous PPSV23 dose | Yes |
| Herpes Zoster | Age >50 yrs Authors recommend in those starting tofacitinib, even if previously received Zostavax |
No | Shingrix—2 doses (2–6 mo apart). Shingrix now preferred herpes zoster vaccine. | Yes, for those receiving low dose immunosuppression and those anticipating immunosuppression. |
| Live Vaccine Available | ||||
| Measles Mumps Rubella | If unknown vaccination history; Do not give to immunosuppressed patients |
Yes | Two doses (>28 days apart) at least 4 weeks before starting immunosuppressive therapy | Contraindicated in patients planning to start immunosuppression within 3 mo |
| Varicella | If unknown vaccination history or prior infection OK to administer on “low dose” immunosuppressiona |
Yes VZV IgG |
2 doses (4–6 weeks apart) at least 1 month before starting immunosuppressive therapy | Yes |
| Herpes Zoster | Age >50 yrs Authors no longer recommend this vaccine. |
No | Zostavax—1 dose. No longer preferred herpes zoster vaccine. | No. Shingrix now the preferred vaccine. |
aLow dose immunosuppression defined by Infectious Disease Society of America as prednisone ≤ 20 mg/day, azathioprine ≤ 3.0 mg/kg/day, mercaptopurine ≤ 1.5 mg/kg/day, methotrexate ≤ 0.4 mg/kg/week.69
It is important to balance the rush to initiate immunosuppressive therapy and the optimization of vaccination immunogenicity; we recommend evaluating vaccination history with appropriate serologic testing ideally at the first clinical encounter. This allows the practitioner to provide necessary vaccinations when considering initiation of a biologic agent and time to develop an immune response while awaiting insurance approval (typically weeks in our experience). This approach will provide the optimal vaccination immunogenic window. Nonlive vaccinations are safe for administration, even in immunosuppressed individuals. Table 1 displays the recommended vaccinations for IBD patient populations along with dosing regimens. While live vaccinations are contraindicated in patients already on higher-dose immunosuppressive therapy or anti-TNF agents, Infectious Disease Society of America guidelines allow for live vaccines in those on low-dose immunosuppression (prednisone ≤20 mg/day, azathioprine ≤3.0 mg/kg/day, mercaptopurine ≤1.5 mg/kg/day, methotrexate ≤0.4 mg/kg/week).69 Similarly, the vedolizumab package insert states that patients may receive live vaccines if the benefits outweigh the risks (accessdata.fda.gov/drugsatfda_docs/label/2014/125476s000lbl.pdf), and some early data confirms this approach.70
New developments since the publication of American College of Gastroenterology (ACG) guidelines include the FDA approval of an inactivated recombinant herpes zoster virus (HZV) vaccination (Shingrix).71 With increased efficacy in immunocompetent participants compared with Zostavax, including in the elderly (>70 years old), and no reports of immune-mediated disease exacerbation, Shingrix is now the Centers for Disease Control preferred HZV vaccination (over Zostavax). We anticipate increased utilization of this new vaccine.72 Shingrix has not yet been tested in immunosuppressed individuals, so efficacy and safety in this cohort are currently unknown. In our experience, however, insurance providers largely do not yet reimburse for this vaccine, limiting the current practicality. Additional developments include a new 2-dose HBV vaccine, HEPLISAV-B, that was recently recommended by the Advisory Committee on Immunization Practices, but the clinical utility in IBD remains unknown. High-dose influenza vaccination has also recently received attention, and studies of organ transplant populations suggest the high-dose influenza vaccination (FluZoneHD) may impart improved serologic efficacy compared with standard-dose vaccination, but this has not been tested in IBD.73
Studies have demonstrated suboptimal vaccination rates in IBD patients; thus it is critical to maintain vaccination vigilance as part of routine care.74 Important ongoing vaccination health maintenance includes annual inactivated influenza and periodic pneumococcal vaccinations (Table 1). Along with our recommendation that gastroenterologists should take ownership of vaccinations in IBD patients, we also recommend that gastroenterologists treating IBD patients should regularly store both the influenza and pneumococcal vaccinations in their clinics to facilitate administration as this has been shown to improve vaccination rates.75 If unfeasible, we recommend providing patients directly with a prescription for the vaccination(s) needed to take to a local pharmacy for direct administration. For other approaches, direct communication and coordination with primary care physicians are vital to ensure completion. We would also emphasize the recommendation for HPV vaccination in both women and men ages 12–26, as this has been a neglected vaccination in our experience that has been shown to reduce cervical dysplasia rates.76 Lastly, we recommend evaluating for vaccination with the inactivated HZV vaccine, Shingrix, before initiating therapy with tofacitinib, given the increased risk of HZV reactivation with this small molecule.
Malignancy
Because of the malignancy concerns with biologic agents, a meticulous malignancy history including the malignancy type, timing, treatment, and last follow-up should be sought. Specific attention should be paid to skin (melanoma and nonmelanoma), hematologic (lymphoma), and cervical cancer histories, given the increased risk of these cancers in various biologic (and thiopurine) regimens.
Immunologic
A history of prior biologic treatment and antibody formation should be elicited. Comorbid immunologic diseases such as psoriasis, lupus, or multiple sclerosis should also be noted.
Metabolic and Hematologic
Prior hematologic abnormalities should be discussed. Baseline labs before therapy should include a complete blood count, renal, and liver functions in all patients. A baseline lipid panel in patients starting tofacitinib should be obtained. We do not routinely check creatine kinase levels.
Checklists
Given the complexity of considerations when initiating immunosuppressive therapy in IBD patients, we suggest the institution of checklists to facilitate completion of all necessary recommendations. Two such checklists can be found at cornerstoneshealth.org/wp-content/uploads/2018/08/Monitoring-and-Prevention-8.31.18.pdf and crohnscolitisfoundation.org/science-and-professionals/programs-materials/health-maintenance-checklist.pdf.
MANAGING COMPLICATIONS DURING TREATMENT
Once patients are initiated on immunosuppression with either monoclonal antibodies or tofacitinib, there are several safety issues that should be actively followed and assessed during the course of therapy. In this section, the authors readily acknowledge the lack of evidence-based guidance and make expert opinion recommendations for the management of complications that arise. The authors also recognize that efficacy and safety are not mutually exclusive and that all treatment considerations should be individualized, accounting for the benefits of therapy along with the risks. Active IBD can be considered an adverse event and thus deserves consideration when balancing these risks.
INFECTION MANAGEMENT
For all infections, we recommend stratification by infection severity. Severe infections include those requiring intensive care, those with multiple organs affected, or those meeting systemic inflammatory response criteria. In patients experiencing either a severe primary infection or reactivation of viral illness (eg, EBV, VZV, HSV), we recommend holding anti-TNF agents, ustekinumab, and tofacitinib (Table 2). With mild infections, we would continue these medications if biologic dosing is due. Given the gut selective nature of vedolizumab, with the exception of severe CMV colitis, we continue this agent. Once the virus is identified, treated, and infection has resolved, we restart anti-TNF therapy. If HZV is identified in a patient receiving tofacitinib, we would consider switching to alternative therapy, given the significantly increased risk of reactivation until if or when proper HZV vaccination can occur. Interruptions of tofacitinib therapy do not carry the immunogenic danger as monoclonal antibodies. It should be noted that while we recommend holding certain monoclonal antibodies with the theory of restoring the blocked mechanism amidst an infectious complication, the half-life of the monoclonal antibodies ranges from 9.5 days (infliximab) to 25.5 days (vedolizumab) and the immunologic effect may be even longer. Thus, for complete drug clearance and functional restoration, a 6- to 8-week period of drug abstinence would be required. Complete treatment and resolution of infectious complications generally necessitate shorter time periods. Therefore, clinical interpretation of this recommendation is necessary and must weigh the risk of IBD exacerbation upon withholding biologics with the rare risk of progression of infection by continued immunosuppression.
Table 2.
Suggested Management of Active Infections by IBD Medication Class
| Therapeutic Target | Viral, (eg, EBV, VZV, HSV) | Bacterial, (eg, Strep, Staph) |
Opportunistic, (eg, Fungal, Mycobacteria) |
C. difficile |
|---|---|---|---|---|
| TNF | Continue (hold if severe) |
Continue (hold if severe) |
Stop-Treat Severe: Do not restart Mild-Moderate: Consider restart vs. change medicationa |
Continue |
| Integrin | Continue | Continue | Continue | Consider holding dose Treat |
| IL12/23 | Continue (hold if severe) |
Continue (hold if severe) |
Stop-Treat Restart |
Continue |
| JAK | Stop-Treat Severe: Do not restart Mild-Moderate: Consider restart vs. change medicationa |
Continue (hold if severe) |
Stop-Treat Consider restart vs. change medicationa |
Continue |
IL: interleukin; JAK: Janus kinase
Severe infections include those requiring intensive care, those with multiple organs affected, or those meeting systemic inflammatory response criteria.
aDepends on the type and severity of infection and patient’s medication history and remaining medical options. Authors recommend restarting only if no other mechanism of action options exist and infection resolves rapidly with treatment.
Similarly, with bacterial infections, we recommend severity stratification, holding anti-TNFs, ustekinumab, and tofacitinib in severe infections and continuing vedolizumab if dosing is due (Table 2). Conversely, in the setting of Clostridium difficile (C. diff) infection, we recommend holding vedolizumab during C. diff treatment and restarting therapy after C. diff resolution. If a patient receiving an anti-TNF, ustekinumab, or tofacitinib is diagnosed with C. diff and dosing of the biologic is due, we initiate C. diff therapy, delay (or hold for tofacitinib) the biologic for 5–7 days, and ensure symptomatic improvement and clinical stability before dosing or restarting the biologic, along with completion of C. diff therapy. This approach helps balance the risk of an IBD relapse with concurrent infection treatment.
Given the well-documented risk of opportunistic infections with anti-TNF agents, we recommend stopping anti-TNF therapy once an opportunistic organism is suspected or identified (Table 2). Further dosing should be held until the infection is completely treated and resolved, and even then, consideration should be given to switching to alternative therapies. As an extension, given the relative paucity of Phase 4 data with other biologics, we recommend stopping ustekinumab and tofacitinib during evaluation and treatment, with potential to restart after infection is cleared. With the safety data to date and lack of increased opportunistic infectious risk in post hoc studies,19,26 we continue vedolizumab in this setting, unless the GI tract is the primary site of infection.
Noncutaneous Malignancy Management
For all cases of malignancy (cutaneous and noncutaneous) during therapy, we recommend a multidisciplinary approach involving the gastroenterologist and dermatologic or oncologic specialties with open and direct communication regarding the balance of IBD therapies with malignancy treatment. For noncutaneous solid tumors, we recommend continuation of the biologic agents unless concurrent cytotoxic chemotherapy is administered or there is metastatic involvement (Table 3). To avoid excessive immunosuppression with cytotoxic chemotherapy, we recommend holding anti-TNF, ustekinumab, and JAK inhibitor therapy with close clinical follow-up for rebound IBD activity after chemotherapy. Vedolizumab can be continued regardless of the chemotherapy.
Table 3.
Suggested Management of Biologics in the Setting of Active Malignancy
| Therapeutic Target | Non-Cutaneous | Cutaneous | ||
|---|---|---|---|---|
| Solid Tumor | Lymphoma | Non-Melanoma (Squamous Cell, Basal Cell) |
Melanoma | |
| TNF | Continue Stop if cytotoxic chemo or metastatica |
Stop-Treat, then Individualize: Restart vs Switch to non-anti-TNF |
Continue | Stop-Treat Switch to non-anti-TNF |
| Integrin | Continue | Continue | Continue | Continue |
| IL12/23 | Continue Stop if cytotoxic chemo or metastatica |
Continue Stop if cytotoxic chemoa |
Continue | Hold if chemoa |
| JAK | Continue Stop if cytotoxic chemo or metastatica |
Continue Stop if cytotoxic chemoa |
Continue, but monitor |
Hold if chemoa |
IL: interleukin; JAK: Janus kinase
aIf stopping biologic during chemotherapy, we recommend monitoring for rebound IBD flare once the chemotherapy is stopped.
For checkpoint inhibitors in patients without preexisting IBD, anti-TNFs and vedolizumab have been successfully used for treatment of checkpoint inhibitor-induced colitis. It is currently unknown how checkpoint inhibitors will influence underlying IBD, and thus, we recommend discussion with the treating oncologist and close clinical observation during therapy. In IBD patients not yet receiving biologics who develop worsening inflammation on checkpoint inhibitors, we recommend anti-TNF or vedolizumab therapy.
Similarly, if an individual receiving ustekinumab or tofacitinib is diagnosed with lymphoma, we recommend withholding these biologics if concurrent cytotoxic chemotherapy is administered, but if it is not, the individual should continue therapy. Given the associated lymphoma risk with anti-TNFs, we advocate for cessation of therapy during treatment and consideration of transitioning to an alternative mechanism of action upon diagnosis.
In patients with a history of prior malignancy in remission, we do not withhold any particular biologic therapy except in the case of metastatic melanoma, given this malignancy’s propensity for delayed recurrence. In this situation, we avoid anti-TNF therapy extrapolating the increased risk of melanoma with this antibody class.
Cutaneous Malignancy Management
If a patient develops NMSC, we recommend continuing all biologics. Given the possible signal with tofacitinib, we continue therapy but recommend close monitoring of clinical outcomes and development of additional lesions with a low threshold to alter therapy. In the setting of melanoma, we discontinue anti-TNFs during treatment and switch mechanism of action after completion of melanoma therapy. Similarly, we recommend holding ustekinumab and tofacitinib if chemotherapy is being administered. We recommend continuing vedolizumab throughout diagnosis and treatment.
Immunologic Issues Management
If a patient develops antidrug antibodies to a monoclonal antibody, we recommend stratifying by the concentration of antibody into high and low concentrations (Table 4). This segregation has not been standardized and varies depending on the type of antidrug antibody assay utilized (ELISA vs radioimmune vs mobility shift) and the laboratory performing the testing. A cutoff of <8 µg/mL for low concentration and ≥8 µg/mL for high concentration using an ELISA antidrug antibody assay for infliximab has been proposed.77 We recommend that providers utilize a single laboratory when feasible for drug and antibody testing and become familiar with results and interpretation. In the setting of low antibody concentration, we add concomitant immunomodulator if not previously prescribed and either increase the biologic dose or decrease the dosing interval if already receiving an immunomodulator in an attempt to overcome the antidrug antibodies.
Table 4.
Immunologic Complications and Recommended Management Strategies
| Therapeutic Target | Drug Immunogenicity | Lupus-like | Demyelinating | Psoriasis |
|---|---|---|---|---|
| TNF | High Aba: Stop Low Ab: Add IMM and/or increase drug |
Stop Switch to a non-anti-TNF |
Stop Switch to a non-anti-TNF |
Mildb: Continue anti-TNF, Treat topically, +/-methotrexate Severe: Stop and Treat, +/- methotrexate, Switch to non-anti-TNF |
| Integrin | Continue | N/A | Continue | Continue |
| IL 12/23 | Continue | N/A | Continue | Effective Treatment for anti-TNF psoriasis |
| JAK | N/A | N/A | Continue | Continue |
IL: interleukin; JAK: Janus kinase; IMM: immunomodulator
aAntibody concentration interpretation depends on the assay utilized (ELISA vs radioimmune vs mobility shift) and no standard criteria have been defined. A cutoff of <8 µg/mL for low and ≥8 µg/mL for ELISA has been described for inflixiamb.77 The authors recommend providers utilize a single laboratory consistently and become familiar with the range and interpretation of results.
bMild dermatologic reactions defined as those encompassing <5% total body surface area, tolerable to patient, and not rapidly expanding. Severe reactions involve ≥5% body surface, are intolerable to the patient, or quickly enlarging.78
Lupus-like reactions and de novo demyelinating responses to anti-TNFs should precipitate withholding therapy during evaluation and treatment of the complication (Table 4). Discontinuation of the offending medication alone may result in improvement in a period of weeks to 6 months. However, involvement of appropriate specialty assistance (eg, rheumatology for lupus, neurology for demyelination) should be considered promptly as concurrent immunosuppression may need to be manipulated to treat the reaction and the potential severity of demyelinating processes. Both lupus-like and demyelinating reactions require a change in mechanism to non-anti-TNF therapy, given the class effect of these entities.
Treatment of psoriatic lesions in the setting of anti-TNFs include topical steroids depending on the extent and location, vitamin D analogues, keratolytics, and UV phototherapy (Table 4). Those with lesions involving <5% total body surface area, are tolerable to the patient, and not rapidly expanding can be treated topically in collaboration with dermatology.78 Unfortunately, topical therapy alone is effective in a minority of cases. The addition of methotrexate can also be beneficial in these cases. Severe (≥5% total body surface area, intolerable, or rapidly expanding) or refractory psoriasis may require discontinuation of anti-TNF therapy with a transition to alternative mechanism of action favoring ustekinumab because of its dermatologic use in psoriasis and addition of methotrexate.
Metabolic Conditions Management
During routine therapy, we recommend at least annual hematologic, renal, and liver function labs or more frequent as directed by specific therapies (eg, thiopurines) or patient symptoms.
If a patient receiving anti-TNF therapy develops isolated abnormal liver transaminases less than twice the upper limit of normal, we will continue therapy with ongoing observation (Table 5). If the transaminases are greater than this cutoff, we will evaluate for viral hepatitis and autoimmune hepatitis and withhold therapy. If autoimmune hepatitis is confirmed, we discontinue therapy with that particular agent. Otherwise, we recommend continuation of other biologics and small molecules with ongoing observation and consultation with a hepatologist if liver function tests worsen.
Table 5.
Metabolic Conditions Associated With New Agents and Suggested Management
| Therapeutic Target | Liver Enzyme Abnormality | Lipids/Creatine Kinase |
|---|---|---|
| TNF | Continue: if <2x ULN Stop: >2x ULN or (+) Hepatitis B or autoimmune hepatitis |
Continue |
| Integrin | Continue, but monitor | Continue |
| IL 12/23 | Continue | Continue |
| JAK | Continue | Continue, but monitor |
IL: interleukin; JAK: Janus kinase; ULN: upper limit of normal
For tofacitinib, we recommend monthly monitoring of lipid panel given the rate and rapidity of lipid abnormality onset after tofacitinib initiation delineated in clinical studies. Continued elevation after 12 weeks should prompt further evaluation and consideration of adjunctive statins or alternative therapies.
CONCLUSIONS
With the increasing IBD armamentarium now including monoclonal antibodies against a variety of targets along with novel small molecule therapies, the complexity of management has simultaneously intensified. Understanding the risks of side effects, reactions, and complications of the new agents is pivotal to an informed therapeutic decision and patient counseling. With appropriate risk stratification, vaccination strategies, and active monitoring, we propose several management strategies to optimize patient outcomes in the early period of these newer agents.
Conflicts of interests: Miguel Regueiro serves as a consultant and advisory boards for Abbvie, Janssen, UCB, Takeda, Miraca, Pfizer, Celgene, and Amgen. He also receives research support from Abbvie, Janssen, and Takeda. Benjamin Click serves as a consultant and advisory boards for Janssen.
Supported by: Benjamin Click reports support from National Institutes of Health (grant number 5T32DK063922-15).
REFERENCES
- 1. Lichtenstein GR, Feagan BG, Cohen RD, et al. . Serious infection and mortality in patients with Crohn’s disease: more than 5 years of follow-up in the TREAT™ registry. Am J Gastroenterol. 2012;107:1409–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fidder H, Schnitzler F, Ferrante M, et al. . Long-term safety of infliximab for the treatment of inflammatory bowel disease: a single-centre cohort study. Gut. 2009;58:501–508. [DOI] [PubMed] [Google Scholar]
- 3. Grijalva CG, Chen L, Delzell E, et al. . Initiation of tumor necrosis factor-α antagonists and the risk of hospitalization for infection in patients with autoimmune diseases. JAMA. 2011;306:2331–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lichtenstein GR, Rutgeerts P, Sandborn WJ, et al. . A pooled analysis of infections, malignancy, and mortality in infliximab- and immunomodulator-treated adult patients with inflammatory bowel disease. Am J Gastroenterol. 2012;107:1051–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dulai PS, Thompson KD, Blunt HB, et al. . Risks of serious infection or lymphoma with anti-tumor necrosis factor therapy for pediatric inflammatory bowel disease: a systematic review. Clin Gastroenterol Hepatol. 2014;12:1443–1451; quiz e88. [DOI] [PubMed] [Google Scholar]
- 6. Kopylov U, Afif W. Risk of infections with biological agents. Gastroenterol Clin North Am. 2014;43:509–524. [DOI] [PubMed] [Google Scholar]
- 7. Nanau RM, Cohen LE, Neuman MG. Risk of infections of biological therapies with accent on inflammatory bowel disease. J Pharm Pharm Sci. 2014;17:485–531. [DOI] [PubMed] [Google Scholar]
- 8. Targownik LE, Bernstein CN. Infectious and malignant complications of TNF inhibitor therapy in IBD. Am J Gastroenterol. 2013;108:1835–1842, quiz 1843. [DOI] [PubMed] [Google Scholar]
- 9. Bergstrom L, Yocum DE, Ampel NM, et al. . Increased risk of coccidioidomycosis in patients treated with tumor necrosis factor alpha antagonists. Arthritis Rheum. 2004;50:1959–1966. [DOI] [PubMed] [Google Scholar]
- 10. Lee JH, Slifman NR, Gershon SK, et al. . Life-threatening histoplasmosis complicating immunotherapy with tumor necrosis factor alpha antagonists infliximab and etanercept. Arthritis Rheum. 2002;46:2565–2570. [DOI] [PubMed] [Google Scholar]
- 11. Velayos FS, Sandborn WJ. Pneumocystis carinii pneumonia during maintenance anti-tumor necrosis factor-alpha therapy with infliximab for Crohn’s disease. Inflamm Bowel Dis. 2004;10:657–660. [DOI] [PubMed] [Google Scholar]
- 12. Hou JK, Kramer JR, Richardson P, et al. . Tuberculosis screening and reactivation among a national cohort of patients with inflammatory bowel disease treated with tumor necrosis factor alpha antagonists. Inflamm Bowel Dis. 2017;23:254–260. [DOI] [PubMed] [Google Scholar]
- 13. Lorenzetti R, Zullo A, Ridola L, et al. . Higher risk of tuberculosis reactivation when anti-TNF is combined with immunosuppressive agents: a systematic review of randomized controlled trials. Ann Med. 2014;46:547–554. [DOI] [PubMed] [Google Scholar]
- 14. Toruner M, Loftus EV Jr, Harmsen WS, et al. . Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134:929–936. [DOI] [PubMed] [Google Scholar]
- 15. Gisbert JP, Chaparro M, Esteve M. Review article: prevention and management of hepatitis B and C infection in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2011;33:619–633. [DOI] [PubMed] [Google Scholar]
- 16. Loras C, Gisbert JP, Mínguez M, et al. ; REPENTINA study; GETECCU (Grupo Español de Enfermedades de Crohn y Colitis Ulcerosa) Group Liver dysfunction related to hepatitis B and C in patients with inflammatory bowel disease treated with immunosuppressive therapy. Gut. 2010;59:1340–1346. [DOI] [PubMed] [Google Scholar]
- 17. Madonia S, Orlando A, Scimeca D, et al. . Occult hepatitis B and infliximab-induced HBV reactivation. Inflamm Bowel Dis. 2007;13:508–509. [DOI] [PubMed] [Google Scholar]
- 18. Lin MV, Blonski W, Buchner AM, et al. . The influence of anti-TNF therapy on the course of chronic hepatitis C virus infection in patients with inflammatory bowel disease. Dig Dis Sci. 2013;58:1149–1156. [DOI] [PubMed] [Google Scholar]
- 19. Colombel JF, Sands BE, Rutgeerts P, et al. . The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut. 2017;66:839–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Feagan BG, Sandborn WJ, Gasink C, et al. ; UNITI–IM-UNITI Study Group Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016;375:1946–1960. [DOI] [PubMed] [Google Scholar]
- 21. Loftus EV, Augustin M, Bissonnette R, et al. . Prevalence of inflammatory bowel disease among patients with psoriasis and incidence of serious infections in this subset: results from the PSOLAR registry. Gastroenterology. 2016;150:S805. [Google Scholar]
- 22. Sandborn WJ, Su C, Sands BE, et al. ; OCTAVE Induction 1, OCTAVE Induction 2, and OCTAVE Sustain Investigators Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376:1723–1736. [DOI] [PubMed] [Google Scholar]
- 23. Curtis JR, Xie F, Yun H, et al. . Real-world comparative risks of herpes virus infections in tofacitinib and biologic-treated patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1843–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lemaitre M, Kirchgesner J, Rudnichi A, et al. . Association between use of thiopurines or tumor necrosis factor antagonists alone or in combination and risk of lymphoma in patients with inflammatory bowel disease. JAMA. 2017;318:1679–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kotlyar DS, Osterman MT, Diamond RH, et al. . A systematic review of factors that contribute to hepatosplenic T-cell lymphoma in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2011;9:36–41.e1. [DOI] [PubMed] [Google Scholar]
- 26. Luthra P, Peyrin-Biroulet L, Ford AC. Systematic review and meta-analysis: opportunistic infections and malignancies during treatment with anti-integrin antibodies in inflammatory bowel disease. Aliment Pharmacol Ther. 2015;41:1227–1236. [DOI] [PubMed] [Google Scholar]
- 27. Fiorentino D, Ho V, Lebwohl MG, et al. . Risk of malignancy with systemic psoriasis treatment in the psoriasis longitudinal assessment registry. J Am Acad Dermatol. 2017;77:845–854.e5. [DOI] [PubMed] [Google Scholar]
- 28. Gordon KB, Papp KA, Langley RG, et al. . Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742–751. [DOI] [PubMed] [Google Scholar]
- 29. Papp K, Gottlieb AB, Naldi L, et al. . Safety surveillance for ustekinumab and other psoriasis treatments from the psoriasis longitudinal assessment and registry (PSOLAR). J Drugs Dermatol. 2015;14:706–714. [PubMed] [Google Scholar]
- 30. Cohen SB, Tanaka Y, Mariette X, et al. . Long-term safety of tofacitinib for the treatment of rheumatoid arthritis up to 8.5 years: integrated analysis of data from the global clinical trials. Ann Rheum Dis. 2017;76:1253–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Curtis JR, Lee EB, Kaplan IV, et al. . Tofacitinib, an oral janus kinase inhibitor: analysis of malignancies across the rheumatoid arthritis clinical development programme. Ann Rheum Dis. 2016;75:831–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ando S, Kawada JI, Watanabe T, et al. . Tofacitinib induces G1 cell-cycle arrest and inhibits tumor growth in epstein-barr virus-associated T and natural killer cell lymphoma cells. Oncotarget. 2016;7:76793–76805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bilori B, Thota S, Clemente MJ, et al. . Tofacitinib as a novel salvage therapy for refractory T-cell large granular lymphocytic leukemia. Leukemia. 2015;29:2427–2429. [DOI] [PubMed] [Google Scholar]
- 34. Shelton E, Laharie D, Scott FI, et al. . Cancer recurrence following immune-suppressive therapies in patients with immune-mediated diseases: a systematic review and meta-analysis. Gastroenterology. 2016;151:97–109.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Long MD, Martin CF, Pipkin CA, et al. . Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology. 2012;143:390–399.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kopylov U, Vutcovici M, Kezouh A, et al. . Risk of lymphoma, colorectal and skin cancer in patients with IBD treated with immunomodulators and biologics: a quebec claims database study. Inflamm Bowel Dis. 2015;21:1847–1853. [DOI] [PubMed] [Google Scholar]
- 37. Colombel JF, Sandborn WJ, Reinisch W, et al. ; SONIC Study Group Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–1395. [DOI] [PubMed] [Google Scholar]
- 38. Panaccione R, Ghosh S, Middleton S, et al. . Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology. 2014;146:392–400.e3. [DOI] [PubMed] [Google Scholar]
- 39. Ko JM, Gottlieb AB, Kerbleski JF. Induction and exacerbation of psoriasis with TNF-blockade therapy: a review and analysis of 127 cases. J Dermatolog Treat. 2009;20:100–108. [DOI] [PubMed] [Google Scholar]
- 40. Cullen G, Kroshinsky D, Cheifetz AS, et al. . Psoriasis associated with anti-tumour necrosis factor therapy in inflammatory bowel disease: a new series and a review of 120 cases from the literature. Aliment Pharmacol Ther. 2011;34:1318–1327. [DOI] [PubMed] [Google Scholar]
- 41. Guerin M, Haettich B, Bara C, et al. . Lupus attributable to anti-TNF therapy and revealed by interstitial granulomatous dermatitis. Rheumatol Int. 2012;32:2937–2940. [DOI] [PubMed] [Google Scholar]
- 42. Mocci G, Marzo M, Papa A, et al. . Dermatological adverse reactions during anti-TNF treatments: focus on inflammatory bowel disease. J Crohns Colitis. 2013;7:769–779. [DOI] [PubMed] [Google Scholar]
- 43. Cush JJ. Unusual toxicities with TNF inhibition: heart failure and drug-induced lupus. Clin Exp Rheumatol. 2004;22:S141–S147. [PubMed] [Google Scholar]
- 44. Guarneri C, Lentini M, Polimeni G, et al. . Ustekinumab-induced drug eruption resembling lymphocytic infiltration (of jessner-kanof) and lupus erythematosus tumidus. Br J Clin Pharmacol. 2016;81:792–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Seror R, Richez C, Sordet C, et al. ; Club Rhumatismes et Inflammation Section of the SFR Pattern of demyelination occurring during anti-TNF-α therapy: a French National Survey. Rheumatology (Oxford). 2013;52:868–874. [DOI] [PubMed] [Google Scholar]
- 46. Lozeron P, Denier C, Lacroix C, et al. . Long-term course of demyelinating neuropathies occurring during tumor necrosis factor-alpha-blocker therapy. Arch Neurol. 2009;66:490–497. [DOI] [PubMed] [Google Scholar]
- 47. Badat Y, Meissner WG, Laharie D. Demyelination in a patient receiving ustekinumab for refractory Crohn’s disease. J Crohns Colitis. 2014;8:1138–1139. [DOI] [PubMed] [Google Scholar]
- 48. Ghabril M, Bonkovsky HL, Kum C, et al. ; US Drug-Induced Liver Injury Network Liver injury from tumor necrosis factor-α antagonists: analysis of thirty-four cases. Clin Gastroenterol Hepatol. 2013;11:558–564.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rodrigues S, Lopes S, Magro F, et al. . Autoimmune hepatitis and anti-tumor necrosis factor alpha therapy: a single center report of 8 cases. World J Gastroenterol. 2015;21:7584–7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shelton E, Chaudrey K, Sauk J, et al. . New onset idiosyncratic liver enzyme elevations with biological therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2015;41:972–979. [DOI] [PubMed] [Google Scholar]
- 51. Bessissow T, Renard M, Hoffman I, et al. . Review article: non-malignant haematological complications of anti-tumour necrosis factor alpha therapy. Aliment Pharmacol Ther. 2012;36:312–323. [DOI] [PubMed] [Google Scholar]
- 52. Hastings R, Ding T, Butt S, et al. . Neutropenia in patients receiving anti-tumor necrosis factor therapy. Arthritis Care Res (Hoboken). 2010;62:764–769. [DOI] [PubMed] [Google Scholar]
- 53. Feagan BG, Rutgeerts P, Sands BE, et al. ; GEMINI 1 Study Group Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699–710. [DOI] [PubMed] [Google Scholar]
- 54. Sandborn WJ, Feagan BG, Rutgeerts P, et al. ; GEMINI 2 Study Group Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369:711–721. [DOI] [PubMed] [Google Scholar]
- 55. Charles-Schoeman C, Gonzalez-Gay MA, Kaplan I, et al. . Effects of tofacitinib and other dmards on lipid profiles in rheumatoid arthritis: implications for the rheumatologist. Semin Arthritis Rheum. 2016;46:71–80. [DOI] [PubMed] [Google Scholar]
- 56. Charles-Schoeman C, Fleischmann R, Davignon J, et al. . Potential mechanisms leading to the abnormal lipid profile in patients with rheumatoid arthritis versus healthy volunteers and reversal by tofacitinib. Arthritis Rheumatol. 2015;67:616–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ananthakrishnan AN, Cagan A, Cai T, et al. . Diabetes and the risk of infections with immunomodulator therapy in inflammatory bowel diseases. Aliment Pharmacol Ther. 2015;41:1141–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Reddy KR, Beavers KL, Hammond SP, et al. ; American Gastroenterological Association Institute American gastroenterological association institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148:215–219; quiz e16. [DOI] [PubMed] [Google Scholar]
- 59. Terrault NA, Lok ASF, McMahon BJ, et al. . Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chen YM, Huang WN, Wu YD, et al. . Reactivation of hepatitis B virus infection in patients with rheumatoid arthritis receiving tofacitinib: a real-world study. Ann Rheum Dis. 2018;77:780–782. [DOI] [PubMed] [Google Scholar]
- 61. Abreu C, Sarmento A, Magro F. Screening, prophylaxis and counselling before the start of biological therapies: a practical approach focused on IBD patients. Dig Liver Dis. 2017;49:1289–1297. [DOI] [PubMed] [Google Scholar]
- 62. Farraye FA, Melmed GY, Lichtenstein GR, et al. . ACG clinical guideline: preventive care in inflammatory bowel disease. Am J Gastroenterol. 2017;112:241–258. [DOI] [PubMed] [Google Scholar]
- 63. Long MD, Gulati A, Wohl D, et al. . Immunizations in pediatric and adult patients with inflammatory bowel disease: a practical case-based approach. Inflamm Bowel Dis. 2015;21:1993–2003. [DOI] [PubMed] [Google Scholar]
- 64. Sands BE, Cuffari C, Katz J, et al. . Guidelines for immunizations in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:677–692. [DOI] [PubMed] [Google Scholar]
- 65. Melmed GY. Immunizations and IBD: whose responsibility is it? If I’m the prescribing doctor, shouldn’t it be mine?Inflamm Bowel Dis. 2012;18:41–42. [DOI] [PubMed] [Google Scholar]
- 66. Selby L, Hoellein A, Wilson JF. Are primary care providers uncomfortable providing routine preventive care for inflammatory bowel disease patients?Dig Dis Sci. 2011;56:819–824. [DOI] [PubMed] [Google Scholar]
- 67. Wasan SK, Coukos JA, Farraye FA. Vaccinating the inflammatory bowel disease patient: deficiencies in gastroenterologists knowledge. Inflamm Bowel Dis. 2011;17:2536–2540. [DOI] [PubMed] [Google Scholar]
- 68. Yeung JH, Goodman KJ, Fedorak RN. Inadequate knowledge of immunization guidelines: a missed opportunity for preventing infection in immunocompromised IBD patients. Inflamm Bowel Dis. 2012;18:34–40. [DOI] [PubMed] [Google Scholar]
- 69. Rubin LG, Levin MJ, Ljungman P, et al. ; Infectious Diseases Society of America 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58:e44–e100. [DOI] [PubMed] [Google Scholar]
- 70. Wichmann A, Krugliak Cleveland N, Rubin DT. Safety and efficacy of live measles vaccine administered to a Crohn’s disease patient receiving vedolizumab. Am J Gastroenterol. 2016;111:577. [DOI] [PubMed] [Google Scholar]
- 71. Lal H, Cunningham AL, Godeaux O, et al. ; ZOE-50 Study Group Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372:2087–2096. [DOI] [PubMed] [Google Scholar]
- 72. Cunningham AL, Lal H, Kovac M, et al. ; ZOE-70 Study Group Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375:1019–1032. [DOI] [PubMed] [Google Scholar]
- 73. Natori Y, Shiotsuka M, Slomovic J, et al. . A double blind randomized trial of high dose vs. standard dose influenza vaccine in adult solid organ transplant recipients. Clin Infect Dis. 2018;66:1698–1704. [DOI] [PubMed] [Google Scholar]
- 74. Selby L, Kane S, Wilson J, et al. . Receipt of preventive health services by IBD patients is significantly lower than by primary care patients. Inflamm Bowel Dis. 2008;14:253–258. [DOI] [PubMed] [Google Scholar]
- 75. Reich JS, Miller HL, Wasan SK, et al. . Influenza and pneumococcal vaccination rates in patients with inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2015;11:396–401. [PMC free article] [PubMed] [Google Scholar]
- 76. The FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–1927. [DOI] [PubMed] [Google Scholar]
- 77. Vermeire S, Noman M, Van Assche G, et al. . Autoimmunity associated with anti-tumor necrosis factor alpha treatment in Crohn’s disease: a prospective cohort study. Gastroenterology. 2003;125:32–39. [DOI] [PubMed] [Google Scholar]
- 78. Collamer AN, Guerrero KT, Henning JS, et al. . Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: a literature review and potential mechanisms of action. Arthritis Rheum. 2008;59:996–1001. [DOI] [PubMed] [Google Scholar]