Abstract
Background
Adherent and invasive Escherichia coli (AIEC) is preferentially associated with ileal Crohn’s disease (CD). The role of AIEC in the development of inflammation and its regional tropism is unresolved. The presence of long polar fimbriae (LPF) in 71% of ileal CD AIEC suggests a role for LPF in the tropism and virulence of AIEC. The aim of our study is to determine if AIEC, with or without LpfA, induces intestinal inflammation in monoassociated IL-10-/- mice.
Methods
We compared murine AIEC strains NC101 (phylogroup B2, LpfA-) and CUMT8 (phylogroup B1, LpfA+), and isogenic mutant CUMT8 lacking lpfA154, with a non-AIEC (E. coli K12), evaluating histologic inflammation, bacterial colonization, mucosal adherence and invasion, and immune activation.
Results
IL-10-/- mice monoassociated with AIEC (either CUMT8, CUMT8:ΔlpfA, or NC101) but not K12 developed diffuse small intestinal and colonic inflammation. There was no difference in the magnitude and distribution of inflammation in mice colonized with CUMT8:ΔlpfA compared with wild-type CUMT8. Bacterial colonization was similar for all E. coli strains. Fluorescence in situ hybridization revealed mucosal adherence and tissue invasion by AIEC but not K12. Production of the cytokines IL-12/23 p40 by the intestinal tissue and IFN-γ and IL-17 by CD4 T cells correlated with inflammation.
Conclusions
IL-10-/- mice monoassociated with murine AIEC irrespective of LpfA expression developed chronic inflammation accompanied by IL-12/23 p40 production in the small and large intestines and IFN-γ/IL-17 production by CD4 T cells that model the interplay between enteric pathosymbionts, host susceptibility, and enhanced immune responses in people with IBD.
Keywords: ileitis, colitis, cytokines, T cells, adherent invasive E. coli
INTRODUCTION
Crohn’s disease (CD) affects an estimated 500,000–750,000 people in the United States and is considered a consequence of dysregulated immune responses to environmental and microbial components in genetically susceptible individuals.1–5 Histologically, CD is characterized by chronic mononuclear cell infiltration with aggregated macrophages that can form noncaseating granulomas.6 Although CD can affect the gastrointestinal tract anywhere from the mouth to the rectum, the terminal ileum and right colon are most often involved.6, 7
The intestinal immune system is activated in patients with inflammatory bowel disease (IBD), and the molecular basis of the activation has been the focus of intense study.8 The initial but overly simplified description of ulcerative colitis (UC) as a disease driven by Th2-associated cytokines (IL-5 and IL-13) and CD involving Th1 cytokines (IL-12 and IFN-γ) has been substantially revised since the discovery of IL-17, with numerous reports identifying IL-17-producing cells in mucosal samples obtained from patients with either CD or UC.9–13 Among the IL-17-producing cells identified in inflamed tissue, some concomitantly produce IFN-γ.12 Therefore, immune responses that occur in CD and UC patients should not be considered narrowly as the response of one type of helper T cell or another but instead are associated with production of a broad array of immune system components.
No single bacterial pathogen has been identified as the causative agent of IBD.1 Instead, investigations have focused on components of the normal, resident intestinal bacteria in IBD patients, specifically Escherichia coli strains. These E. coli are designated “adherent and invasive” because they bind to and penetrate the intestinal epithelium. AIEC are present in intestinal biopsy samples from IBD patients.14–16 However, to date, no clear cause and effect relationship between the presence of AIEC and disease development has been established.
We reported previously that IL-10-/- mice, born in germ-free conditions and subsequently monoassociated with a murine AIEC strain isolated from the cecal contents of a healthy mouse, designated E. coli NC101, rapidly develop histologically detectable colitis.17 The identification of genes encoding long polar fimbriae (lpfA141/154) in up to 71% of ileal CD AIEC that promote their uptake and translocation across intestinal epithelial microfold (M) cells into Peyer’s patches suggests that long polar fimbriae are involved in ileal tropism and virulence.15, 18 However, a mechanistic understanding of the role of AIEC in the development of intestinal inflammation and the molecular basis of their regional tropism is lacking. The goal of the present study was to determine if AIEC strains isolated from the ileal mucosa of mice with Toxoplasma gondii–induced ileitis (CUMT8),15, 19 or from the cecal contents of healthy mice (NC101), or E. coli K12 isolated from human stool, differ in their ability to colonize and induce inflammation and immune responses in the ileum, colon, and lymph nodes of IL-10-/- mice. A subsidiary aim was to evaluate the role of long polar fimbriae, associated with translocation of AIEC into Peyer’s patches,15, 18 through monoassociation of IL-10-/- mice with CUMT8 (lpfA+) and an isogenic mutant lacking lpfA.
Our results show that murine AIEC strains derived from inflamed ileum (CUMT8) or from normal, uninflamed cecum (NC101) are both able to colonize and induce small intestinal and colonic inflammation in IL10-/- mice that is accompanied by intestinal IL-12/23 p40 production and bacterial-induced CD4 T-cell activation and production of IFN-γ and IL-17, considered hallmarks of the effector immune response in patients with CD. Inflammation that develops in the IL-10-deficient mouse model is independent of the CD-AIEC virulence factor lpfA154. K12-monoassociated mice remain healthy.
METHODS
Bacteria
The murine AIEC E. coli strains NC101 (isolated from the cecal contents of a healthy 129S6/SvEv wild-type mouse housed in specific pathogen-free [SPF] conditions)17, 20 phylogroup B2, lacking lpfA, and CUMT8 (isolated from the ileal mucosa of mice with T. gondii and indomethacin induced ileitis)19 phylogroup B1, lpfA+, were used in these studies. An isogenic mutant derivative of AIEC CUMT8 that lacks lpfA154 (CUMT8:ΔlpfA) and has impaired ability to translocate across M cells was used to examine the effect of lpfA on tropism and virulence.15 A comparison between these 2 AIEC is shown in Table 1. E. coli K12 ATCC 29425, obtained from the American Type Culture Collection, Manassas, Virginia, does not contain any of the virulence genes, as shown in Table 1.
Table 1.
Bacterial Characteristics
| CUMT8 | NC101 | K12 | |
|---|---|---|---|
| Serotype | O8:H21 | O2:H (6 or 41) | OR:H48 |
| Phylogroup | B1 | B2 | A |
| Virulence genes | |||
| ratA | - | + | - |
| pmtI | - | - | - |
| colV | - | + | - |
| hcp | + | - | - |
| lpfA141 | - | - | - |
| lpfA154 | + | - | - |
| kpsMII | - | + | - |
| fyuA | - | + | - |
| iss | - | - | - |
| malX | - | + | - |
| gsp | + | + | - |
| afaBC | - | - | - |
| focG | - | - | - |
| ibeA | - | + | - |
| sfaDE | - | + | - |
| pduC | + | + | - |
| LT | - | - | - |
| Sta | - | - | - |
| STb | - | - | - |
| stx1 | - | - | - |
| stx2 | - | - | - |
| eae | - | - | - |
| cnf1 | - | - | - |
| cnf2 | - | - | - |
| pks | - | + | - |
Mice
Germ-free IL-10-/- mice on the 129S6/SvEv background and wild-type control mice (129S6/SvEv) were bred and maintained at the National Gnotobiotic Rodent Resource Center (NGRRC; University of North Carolina, Chapel Hill, NC) or the Center for Gastrointestinal Biology and Disease (CGIBD) Gnotobiotic Core (North Carolina State University, Raleigh, NC, USA). These germ-free mouse colonies were originally derived by hysterectomy at the Gnotobiotic Laboratory, University of Wisconsin (Madison, WI, USA). Mice were monoassociated at 8–10 weeks of age with 1 of 4 E. coli strains—CUMT8, CUMT8:ΔlpfA, NC101, or K12—by oral and rectal swabbing with viable cultured bacteria. Monoassociated mice were housed in separate isolators according to bacterial strain. Mice were evaluated between 3 and 12 weeks after colonization. Animal use protocols were approved by the Institutional Animal Care and Use Committees of North Carolina State University and the University of North Carolina at Chapel Hill.
Evaluation of Bacterial Colonization
At necropsy (12 weeks after monoassociation), the numbers of CUMT8, NC101, or K12 were determined by quantitative plating of serial dilutions of intestinal luminal contents on brain heart infusion agar. Plates were incubated for 24 hours under aerobic conditions at 37°C, and colonies were enumerated.
Histological Scoring
Swiss rolls of duodenum, jejunum, ileum, and colon were fixed with 10% neutral buffered formalin. The fixed tissue was embedded in paraffin and stained with hematoxylin and eosin (H&E). Sections were scored (0 = no inflammation to 4 = severe inflammation) by a single individual, blinded to experimental groups, using the criteria previously described.21, 22 Severe inflammation is characterized by extensive mononuclear cellular infiltration of the lamina propria and submucosa, crypt hyperplasia, goblet cell depletion, and disruption of tissue architecture.21
Intestinal Tissue Fragment Cultures
Intestinal tissue fragments were prepared from the duodenum, jejunum, ileum, and colon as previously described for the colon.17, 22, 23 Briefly, the sections of intestinal tissue were cut open lengthwise and then split in half longitudinally. One-half was used for histological assessment. Intestinal contents were removed from the other half, and the tissue was shaken at room temperature in RPMI containing 50 µg/mL gentamicin for 30 minutes at 280 rpm, then cut into 1-cm fragments. The fragments were gently blotted to remove excess media, then weighed, and 0.05 g were placed into wells of 24-well plates and incubated overnight at 37°C in 1 mL of RPMI 1640 medium supplemented with 5% fetal bovine serum (FBS), 50 µg/mL gentamicin, and 1% antibiotic/antimycotic (penicillin/streptomycin/amphotericin B; Invitrogen, Grand Island, NY, USA). Supernatants were collected after 18 hours and stored at –20°C. IL-12/23 p40 levels were quantified by enzyme-linked immunosorbent assay (ELISA) using commercially available monoclonal antibodies as capture and detection reagents (BD Biosciences), as previously described.23
Bacterial Lysate Preparation and Induction of CD4+ T-Cell Responses
Bacterial lysates were prepared from CUMT8, NC101, K12, or Enterococcus faecalis as previously described.17 Spleen cells from SPF 129S6/SvEv wild-type mice were T-cell-depleted as previously described,17 and antigen-presenting cells (APCs) were prepared by pulsing overnight with 10 µg/mL of bacterial lysate protein (CUMT8, NC101, K12, or E. faecalis) or keyhole limpet hemocyanin (KLH; Pierce, Rockford, IL, USA) as an unrelated antigen control.
Mesenteric lymph nodes (MLNs) were harvested from monoassociated IL-10-/- and wild-type mice, and CD4+ T cells were enriched by depleting B cells and CD8-positive cells using antibody-coated magnetic beads (Miltenyi Biotec, Auburn, CA, USA) as previously described.17, 24 More than 95% of the enriched MLN cells expressed CD4. MLN CD4+ T cells (2 × 105/well) were co-cultured for 72 hours with APCs (bacterial lysate–pulsed, T-cell-depleted wild-type spleen cells, 3 × 105/well) in flat-bottom 96-well cell culture plates, 0.2 mL per culture. Supernatants were collected after 72 hours and stored at –20°C. IFN-γ and IL-17 in culture supernatants were measured by ELISA using commercially available monoclonal antibodies as capture and detection reagents (BD Biosciences), as previously described.23
Fluorescence in Situ Hybridization
Formalin-fixed paraffin-embedded histological sections (4 µm) were mounted on glass slides (Fisher Scientific, Pittsburgh, PA, USA) and evaluated by fluorescence in situ hybridization (FISH) directed against eubacteria or E. coli, with probe specificity controlled by concurrent hybridization with the unrelated probe non-EUB-338 and inclusion of control slides spotted with E. coli, Streptococcus spp., and Proteus, as previously described.25 Hybridized samples were washed in phosphate-buffered saline (PBS), allowed to air dry, and mounted with a ProLong antifade kit (Molecular Probes Inc., Eugene, OR, USA). Sections were examined on a BX51 (Olympus America, Melville, NY, USA) epifluorescence microscope, and images were captured with an Olympus DP-7 camera.
Statistical Analysis
Histological scores were analyzed using the Kruskal-Wallis test with Dunn’s multiple comparison post test to compare groups of IL-10-/- mice monoassociated with the different E. coli strains. A separate pair-wise analysis using the Mann-Whitney test was used to compare histological scores between the 2 groups of mice (IL-10-/- compared with wild-type mice) for each E. coli strain. For cytokine measurements, values were compared using 1-way ANOVA with Tukey’s multiple comparison test (Graph Pad, San Diego, CA, USA).
RESULTS
AIEC CUMT8 and NC101 Induce Inflammation in the Duodenum, Jejunum, Ileum, and Colon of Monoassociated IL-10-/- Mice
IL-10-/- mice monoassociated with AIEC CUMT8 or NC101, but not with E. coli K12, developed histological evidence of inflammation throughout the small and large intestines (Figs. 1 and 2). The jejunum and ileum of AIEC-monocolonized IL-10-/- mice had mild to moderate cellular infiltration of the lamina propria that was accompanied by broad-based and blunted villi (Fig. 1E, F, I, and J) and crypt hyperplasia. Inflammation in the colon was characterized by cellular infiltration, crypt hyperplasia, and loss of goblet cells and was generally more severe than in the small intestine of CUMT8- or NC101-monoassociated IL-10-/- mice (Figs. 1 and 2).
FIGURE 1.
Histological representation of small intestine and colon of IL-10-/- and 129S6/SvEv wild-type mice monoassociated with different strains of E. coli. Photomicrographs of H&E-stained swiss-rolled sections of the duodenum, jejunum, ileum, and colon from mice monoassociated with E. coli CUMT8, NC101, or K12 for 12 weeks. Note the marked crypt hyperplasia, goblet cell depletion, and lamina propria cellular infiltration in sections of CUMT8- and NC101-monoassociated IL-10-/- mice shown in (A, B, E, F, I, J, M, and N). Sections from wild-type mice monoassociated with CUMT8 are shown in (D, H, L, and P). Sections from NC101- and K12-monoassociated wild-type mice (not pictured) were very similar histologically to sections from CUMT8-monoassociated wild-type mice.
FIGURE 2.
Histological scores of the small intestine and colon of IL-10-/- and 129S6/SvEv wild-type mice monoassociated with different strains of E. coli. Results show histological scores for sections of the duodenum (A), jejunum (B), ileum (C), and colon (D) from IL-10-/- and wild-type mice monoassociated for 12 weeks with CUMT8, NC101, or K12. Each point represents the score for an individual mouse (n = 8–16). Statistically significant differences in the histological inflammatory scores between IL-10-/- mice monoassociated with either CUMT8 or NC101 vs IL-10-/- mice monoassociated with K12 are indicated above each group. Statistically significant differences in the histological inflammatory scores between IL-10-/- and wild-type mice monoassociated with the same E. coli strains are indicated by asterisks as follows: *P < 0.05, **P < 0.01, and ***P < 0.001. Abbreviation: NS, not significant.
Inflammation was absent in wild-type mice monoassociated with the AIEC strains (Figs. 1 and 2, in agreement with previous reports on NC101 monoassociation17), which is consistent with the emerging view that AIEC are resident pathosymbionts or opportunistic pathogens that can induce inflammation in susceptible hosts.
Lack of Influence of Long Polar Fimbriae
To evaluate the role of long polar fimbriae in the development of intestinal inflammation, we monoassociated IL-10-/- mice with CUMT8 or CUMT8ΔlpfA. Inflammatory scores for the duodenum, jejunum, ileum, and colon obtained at 3, 7, and 12 weeks after colonization were not significantly different for mice monoassociated with CUMT8 compared with CUMT8:ΔlpfA154 (Table 2). Moreover, CUMT8 (lpfA154 positive)- and NC101 (lpfA154 negative)-colonized mice (Table 1) had comparable histologic scores in all observed regions of the small intestine and colon (Fig. 2).
Table 2.
Kinetic Analysis of Intestinal Inflammation in IL-10-/- Mice Monoassociated With E. coli CUMT8 or the Mutant Lacking Long Polar Fimbriae (ΔlpfA)
| CUMT8 3 wk (n = 8) |
ΔlpfA 3 wk (n = 5) |
CUMT8 7 wk (n = 5) |
ΔlpfA 7 wk (n = 6) |
CUMT8 12 wk (n = 16) |
ΔlpfA 12 wk (n = 5) |
|
|---|---|---|---|---|---|---|
| Duodenum | 1.5 ± 0.76 | 1.3 ± 0.57 | 2.2 ± 0.45 | 2.0 ± 0.0 | 1.9 ± 0.48 | 2.6 ± 0.82 |
| Jejunum | 1.3 ± 0.6 | 1.5 ± 0.35 | 1.4 ± 0.42 | 2.2 ± 0.41 | 1.5 ± 0.56 | 1.2 ± 0.57 |
| Ileum | 1.4 ± 0.88 | 0.9 ± 0.42 | 1.6 ± 0.65 | 2.5 ± 0.45 | 1.7 ± 0.6 | 1.9 ± 0.74 |
| Colon | 1.6 ± 0.42 | 1.4 ± 0.42 | 2.8 ± 0.57 | 2.1 ± 0.38 | 2.3 ± 0.63 | 3.0 ± 0.35 |
Values represent the mean ± SD of histologic scores from different sections of the intestinal tracts of IL-10-/- mice monoassociated with E. coli that express (CUMT8) or does not express (ΔlpfA) due to deletion of long polar fimbriae sequence lpfA154. Histologic inflammation was not significantly different in any of the sections of the intestine when comparing CUMT8- and ΔlpfA-colonized IL-10-/- mice evaluated at any of the different time points.
Colonization of Mice by CUMT8, NC101, or K12
The ability of E. coli CUMT8, NC101, or K12 to colonize was determined by quantitative plating of luminal contents of the ileum. Each E. coli strain was able to efficiently colonize IL-10-deficient and IL-10-replete mice (Table 3), suggesting that the bacterial colonization density does not account for the differences in inflammation that we observed. Although the median amount of K12 obtained from the ileum of IL-10-/- mice was lower than the others, the results show substantial variability in bacterial counts among the mice, and there were no statistically significant differences comparing CUMT8-, NC101-, or K12-colonized IL-10-/- mice.
Table 3.
Bacterial Counts in Luminal Contents
| E. coli Strain | |||
|---|---|---|---|
| Mouse Strain | CUMT8 | NC101 | K12 |
| IL-10-/- | 110 (1–701)a | 264 (4–1550) | 22 (1–603) |
| Wild-type | 110 (9–793) | 306 (75–830) | 130 (1–280) |
aValues represent median × 107 (range) of bacterial counts obtained by quantitative plating of contents of the ileum of mice monoassociated for 12 weeks with the indicated E. coli strain (n = 8–12 per group). There were no statistically significant differences in luminal bacterial contents comparing CUMT8-, NC101-, or K12-monoassociated IL-10-/- mice with each other or comparing CUMT8-, NC101-, or K12-monoassociated wild-type mice with each other.
Spatial Distribution of E. coli in Mucosal Tissues From Monoassociated Mice
We used FISH with an oligonucleotide probe specific to E. coli/Shigella (E. coli, 16SrRNA) to visualize the spatial distribution of E. coli within the ileum. In the inflamed ileum of IL-10-/- mice, E. coli CUMT8 and NC101 were observed adhering to the epithelium, within the mucosa, and in acellular luminal debris (Fig. 3A and B). In contrast, K12 (Fig. 3C) was confined to acellular luminal debris on top of the mucus layer overlying the epithelium.
FIGURE 3.
Fluorescence in situ hybridization for E. coli in the ileum of IL-10-/- mice. In the inflamed ileum of IL-10-/- mice, E. coli CUMT8 (A) and NC101 (B) were observed adhering to the epithelium, within the mucosa, and in luminal cellular debris. In contrast, K12 (C) was confined to acellular luminal debris on top of the mucus layer overlying the epithelium. Bacteria are red (Cy3), and nuclei/DNA are blue (DAPI).
AIEC Induces Endogenous IL12/23 p40 Secretion by Small Intestinal and Colonic Explants
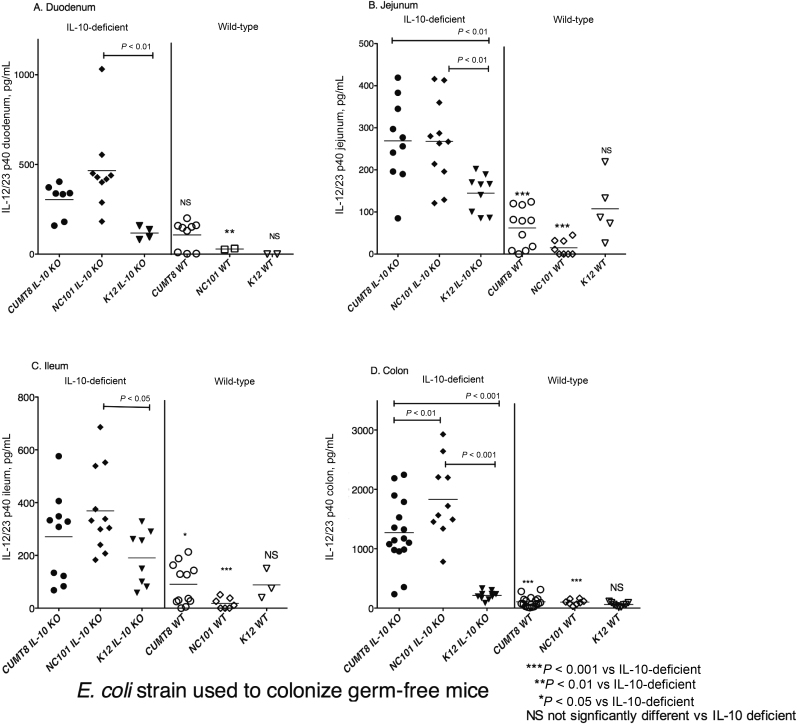
Endogenous IL-12/23 p40 detected in supernatants of small intestinal and colonic explant cultures is shown in Figure 4. IL-12/23 p40 concentrations were significantly higher in colonic fragment culture supernatants from IL-10-/- mice monoassociated with AIEC CUMT8 or NC101 compared with those colonized by K12 and compared with wild-type (WT) mice colonized by AIEC or K12 (Fig. 4D). Small intestinal cultures from the jejunum of AIEC CUMT8– or NC101–monoassociated IL-10-/- mice also contained significantly more IL-12/23 p40 than cultures from K-12-colonized IL10-/- mice or from AIEC- or K12-colonized wild-type mice (Fig. 4B). The amounts of IL-12/23 p40 in culture supernatants from various small intestinal segments of AIEC-colonized mice were about one-third to one-fourth of the amounts in colonic explant cultures (Fig. 4). Thus, colonization of IL-10-/- mice by AIEC is associated with enhanced spontaneous secretion of IL-12/23 p40 from intestinal segments relative to K12 and to AIEC-monoassociated wild-type mice.
FIGURE 4.
IL-12/23p40 in supernatants of small intestine and colon fragment cultures. Weighed fragments of the duodenum (A), jejunum (B), ileum (C), and colon (D) were cultured overnight, and the amount of IL-12/23p40 in supernatants was determined by ELISA. Each point represents the amount of IL-12/23 p40 in the supernatant of an individual culture. Statistically significant differences in the amounts of IL-12/23 p40 between IL-10-/- mice monoassociated with either CUMT8 or NC101 vs IL-10-/- mice monoassociated with K12 are indicated above each group. Statistically significant differences in the amounts of IL-12/23 p40 comparing IL-10-/- mice and wild-type mice monoassociated with the same E. coli strains are indicated by asterisks as follows: *P < 0.05, **P < 0.01, and ***P < 0.001. Abbreviation: NS, not significant.
AIEC Induces IFN-γ and IL-17 Secretion by CD4+ Cells From Monoassociated IL-10-/- Mice
Next, we evaluated activation of the acquired immune response by components of AIEC. The production of IFN-γ and IL-17 by CD4 cells isolated from the MLN of monoassociated mice is shown in Figure 5. Cytokine levels were measured in supernatants of CD4 cells activated by APCs pulsed with E. coli lysate from the same strain that was used to colonize the mice. Concentrations of IFN-γ were significantly higher in supernatants of in vitro–stimulated CD4+ T cells from CUMT8- and NC101-monoassociated IL-10-/- mice than K12-colonized IL-10-/- or WT mice colonized by AIEC or K12 (Fig. 5A). Likewise, CD4+ T cells from CUMT8- and NC101-colonized IL-10-/- mice produce significantly more IL-17 compared with CD4+ T cells from wild-type mice monoassociated with the same bacterial strains (Fig. 5B), although the amounts of IL-17 are 5- to 10-fold lower than the levels of IFN-γ that we detected in the same culture supernatants. We also used 2 negative controls, E. faecalis lysate and the large antigenic protein KLH. The amounts of IFN-γ and IL-17 in supernatants of control cultures containing MLN CD4 cells from each group of monoassociated mice plus APCs pulsed with either E. faecalis lysate or KLH were between 50- and 200-fold lower than levels of these cytokines detected in supernatants of E. coli lysate–pulsed APCs (data not shown). Overall, these results show that AIEC induces CD4+ cells from inflammation-prone IL-10-/- mice to produce IFN-γ and IL-17.
FIGURE 5.
IFN-γ and IL-17 in supernatants of CD4+ MLN cells stimulated with bacterial lysate–pulsed APC. CD4+ MLN cells were co-cultured with bacterial lysate–pulsed APCs for 72 hours. The bacteria used to pulse APCs were the same as the bacteria used to colonize the germ-free mice. The amounts of IFN-γ (A) and IL-17 (B) secreted into culture supernatants were measured by ELISA. Each point represents the amount of IFN-γ or IL-17 in the mean of the triplicate supernatants of CD4+ MLN cells from an individual mouse. Statistically significant differences in the amounts of IFN-γ and IL-17 between IL-10-/- mice monoassociated with either CUMT8 or NC101 vs IL-10-/- mice monoassociated with K12 are indicated above each group of values. Statistically significant differences in the amounts of IFN-γ and IL-17 between IL-10-/- mice and wild-type mice monoassociated with the same E. coli strain are indicated by asterisks ***P < 0.001. Abbreviation: NS, not significant.
We also designed experiments to determine whether E. coli K12 produces bacterial antigens and other components capable of activating the immune response. For these studies, we evaluated cytokine production by CD4+ MLN cells from AIEC (E. coli NC101)-monoassociated IL-10-/- mice stimulated with K12 lysate–pulsed APC, and the reverse, that is, CD4+ MLN cells from E. coli K12–monoassociated IL-10-/- mice stimulated with NC101 lysate–pulsed APCs. As shown in Figure 6, K12 lysate–pulsed APCs activate CD4+ MLN cells from E. coli NC101–monoassociated IL-10-/- mice to produce IFN-γ and IL-17 (Fig. 6A and B). Although supernatants of cells co-cultured with K12 lysate–pulsed APCs generally contain lower amounts of the 2 cytokines compared with supernatants of cells co-cultured with NC101 lysate–pulsed APCs, E. coli K12 is clearly effective for in vitro restimulation of in vivo–activated CD4+ MLN cells. Moreover, supernatants of CD4+ MLN cells from E. coli K12–monoassociated IL-10-/- mice stimulated with K12 lysate–pulsed APCs contain equivalent or higher amounts of IFN-γ compared with supernatants of the same CD4+ cells stimulated with NC101-pulsed APCs (Fig. 6C). MLN cells from 5 of the 6 E. coli K12–monoassociated IL-10-/- mice also produced higher amounts of IL-17 in co-culture with K12 compared with NC101 lysate–pulsed APCs (Fig. 6D). Negative controls, as noted above, were on average 50–200-fold lower compared with the amount of each cytokine detected in supernatants of MLN CD4 cells stimulated with bacterial lysate–pulsed APC. These results demonstrate the capacity of K12 to effectively activate CD4+ cells and suggest that characteristics of AIEC aside from and/or in addition to bacterial products that activate innate and antigen-specific immune responses are necessary for induction and perpetuation of chronic intestinal inflammation in the IL-10-deficient mouse model.
FIGURE 6.
Comparison between E. coli NC101 and K12 for in vitro activation of CD4+ MLN cells. CD4+ MLN cells from either E. coli NC101 (A and B)– or K12 (C and D)–monoassociated IL-10-/- mice were co-cultured with bacterial lysate–pulsed APCs for 72 hours. The E. coli lysate used to pulse APCs is identified below the x-axis. The amounts of IFN-γ (A and C) and IL-17 (B and D) secreted into culture supernatants were measured by ELISA. Each point represents the amount of IFN-γ or IL-17 in the mean of the triplicate supernatants of CD4+ MLN cells from an individual mouse. Points connected by lines indicate results for an individual mouse.
DISCUSSION
Although no single pathogen has been implicated in the development of IBD, numerous investigations have demonstrated alterations (termed “dysbiosis”) in the composition of the resident intestinal bacteria that are associated with disease development.1 A near universal feature of the dysbiosis of IBD is the expansion of Enterobacteriaceae, including E. coli.1, 26, 27 After the isolation and identification of functionally altered E. coli with adherent and invasive properties obtained from the small intestine of a Crohn’s disease patient,28, 29 numerous reports showing the prevalence and speculations regarding the potential role of AIEC in the initiation and/or perpetuation of chronic intestinal inflammation have been published, as reviewed recently by Palmela et al.16 Here we describe the results of our studies using IL-10-/- mice that were born in germ-free conditions and subsequently colonized with 1 of following 3 E. coli strains: AIEC CUMT8 obtained from the ileum of a mouse infected with T. gondii; AIEC NC101, harvested from the cecum of an unmanipulated normal mouse housed under SPF conditions; or E. coli K12, a commonly used laboratory strain. Our results show that the 2 phylogenetically distinct, mouse-derived AIEC isolated from the ileum or the cecum induce similar degrees of histologically detectable chronic inflammation in the colon and small intestine of monoassociated IL-10-/- mice. The inflammation in AIEC-monoassociated IL-10-/- mice is accompanied by production of IL-12/23 p40 throughout the intestinal tract and activation of IFN-γ- and IL-17-producing E. coli–specific CD4+ T cells harvested from mesenteric lymph nodes. In comparison, intestinal inflammation does not develop in E. coli K12–monoassociated IL-10-deficient mice, and the amounts of IL-12/23 p40 produced in the intestinal tract and cytokine (IFN-γ and IL-17) secretion by in vitro restimulated MLN CD4+ T cells are dramatically lower compared with AIEC-monoassociated IL-10-deficient mice despite similar capacities of AIEC compared with K12 lysates to induce IFN-γ and IL-17 secretion in the same MLN-CD4+ cells.
A major functional property of AIEC strains is their ability to adhere to and invade epithelial cells in vitro. We demonstrated that the 2 AIEC stains CUMT8 and NC101 exhibited greater mucosal epithelial adherence and invasion in vivo compared with K12. We postulate that the ability of these AIEC strains to induce intestinal inflammation in susceptible IL-10-deficient mice is related to their in vivo mucosal adherent/invasive properties rather than intrinsic components that preferentially activate innate and antigen-stimulated immune responses.
Dysbiosis of intestinal microbial communities has been observed in IBD patients compared with healthy individuals, with higher proportions of Enterobacteriaceae, including adherent/invasive E. coli, especially in patients with ileal Crohn’s disease.1, 14, 26, 27, 30 Investigations are now focused on determining the bacterial constituents that distinguish AIEC associated with chronic intestinal inflammation in genetically susceptible individuals from organisms that do not play a role in disease. Our results show that CUMT8 with or without lpfA and NC101 that naturally lacks lpfA induced similar degrees of intestinal inflammation in IL-10-deficient mice, demonstrating that this attachment factor is not required to mediate inflammation in our murine model. However, in vitro studies using the prototypical AIEC LF82 or CUMT8 show that lpfA subunit 141 and 154 are involved in the ability of AIEC to traverse murine and human intestinal M cells of the follicle-associated epithelium, thus facilitating entry into the small intestinal lamina propria.15, 18 Highlighting the complex nature of the interplay between the intestinal microbiota and inflammation, Kim et al. reported that mice colonized with lpfA+ AIEC contain higher proportions of IL-10-producing intestinal CX3CR1+ macrophages and reduced intestinal inflammation after Salmonella Typhimurium infection compared with mice colonized with lpfA-deficient AIEC or K12.31 Therefore, epithelial adherence and invasion by AIEC may promote IL-10-mediated intestinal homeostasis.
AIEC expresses numerous components, in addition to lpfA, that may play a role in inducing or inhibiting intestinal inflammation. For example, propanediol dehydratase has been implicated in the pathogenicity of AIEC.15, 32 Both CUMT8 and NC101 contain the pduC gene that encodes a subunit of this enzyme. Wild-type CUMT8 (pduC+) induce higher intestinal IL-17 responses compared with pduC-deficient CUMT8.32 Identification of functionally relevant AIEC virulence factors provides insight into the mechanisms that these organisms employ to promote chronic intestinal inflammation and related carcinogenesis.33
In contrast to previous reports,17 we observed small intestinal inflammation in the duodenum, jejunum, and ileum of AIEC-monoassociated IL-10-/- mice, in addition to colitis, which has been widely reported. In the initial description of intestinal inflammation in this murine model,34 diffuse enterocolitis was observed in IL-10-/- mice raised in conventional housing (ie, the mice were not tested to determine specific pathogen-free status). Although small intestinal inflammation is unusual in murine models of intestinal inflammation, ileitis is a consistent phenotype of SAMP1/Yit Fc and TNFΔARE mice,35–37 and duodenitis was described as a feature of Enterococcus faecalis–monoassociated IL-10-/- mice and of Bacteroides vulgatus–monoassociated HLA-B27 transgenic rats.17, 21
The term “pathosymbiont” has been used to describe AIEC that have been implicated in IBD because the bacteria are found in normal, healthy individuals and, as our results corroborate, are not overtly pathogenic in wild-type mice. However, in the absence of IL-10, a crucial regulator of intestinal homeostasis,38, 39 AIEC causes disease. Thus, the AIEC-monoassociated IL-10-/- mouse represents a model that recapitulates many of the features of human IBD: induction of small intestinal and colonic disease by an opportunistic pathosymbiont, requirement for host genetic susceptibility, and bacterial component–mediated induction of the IL-12/23 p40, IFN-γ, and IL-17 cytokine responses.
Supported by: National Institutes of Health NIH R01 DK53347 (R.B.S.), UNC Center for Gastrointestinal Biology and Disease NIH P30 DK34987 (Histology Core and Gnotobiotic Core), National Gnotobiotic Rodent Resource Center NIH P40 OD010995, SPIRE fellowship K12GM000678 (J.M.S.), Gastroenterology Research Training NIH T32 DK007737 (K.J.W.), and The Crohn’s and Colitis Foundation.
Conflicts of interest: The authors have no conflicts of interest.
REFERENCES
- 1. Sartor RB, Wu GD.. Roles for intestinal bacteria, viruses, and fungi in pathogenesis of inflammatory bowel diseases and therapeutic approaches. Gastroenterology. 2017;152:327–339.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blander JM, Longman RS, Iliev ID, et al. Regulation of inflammation by microbiota interactions with the host. Nat Immunol. 2017;18:851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hall AB, Tolonen AC, Xavier RJ.. Human genetic variation and the gut microbiome in disease. Nat Rev Genet. 2017;18:690–699. [DOI] [PubMed] [Google Scholar]
- 4. Khor B, Gardet A, Xavier RJ.. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abraham C, Medzhitov R.. Interactions between the host innate immune system and microbes in inflammatory bowel disease. Gastroenterology. 2011;140:1729–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xavier RJ, Podolsky DK.. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. [DOI] [PubMed] [Google Scholar]
- 7. Morson BC. The early histological lesion of Crohn’s disease. Proc R Soc Med. 1972;65:71–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Park JH, Peyrin-Biroulet L, Eisenhut M, Shin JI.. IBD immunopathogenesis: a comprehensive review of inflammatory molecules. Autoimmun Rev. 2017;16:416–426. [DOI] [PubMed] [Google Scholar]
- 9. Nielsen OH, Kirman I, Rüdiger N, et al. Upregulation of interleukin-12 and -17 in active inflammatory bowel disease. Scand J Gastroenterol. 2003;38:180–185. [DOI] [PubMed] [Google Scholar]
- 10. Fujino S, Andoh A, Bamba S, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kobayashi T, Okamoto S, Hisamatsu T, et al. IL23 differentially regulates the Th1/Th17 balance in ulcerative colitis and Crohn’s disease. Gut. 2008;57:1682–1689. [DOI] [PubMed] [Google Scholar]
- 12. Globig AM, Hennecke N, Martin B, et al. Comprehensive intestinal T helper cell profiling reveals specific accumulation of IFN-γ+IL-17+coproducing CD4+ T cells in active inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:2321–2329. [DOI] [PubMed] [Google Scholar]
- 13. Hegazy AN, West NR, Stubbington MJT, et al. Circulating and tissue-resident CD4+ T cells with reactivity to intestinal microbiota are abundant in healthy individuals and function is altered during inflammation. Gastroenterology. 2017;153:1320–1337.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Darfeuille-Michaud A, Boudeau J, Bulois P, et al. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn’s disease. Gastroenterology. 2004;127:412–421. [DOI] [PubMed] [Google Scholar]
- 15. Dogan B, Suzuki H, Herlekar D, et al. Inflammation-associated adherent-invasive Escherichia coli are enriched in pathways for use of propanediol and iron and M-cell translocation. Inflamm Bowel Dis. 2014;20:1919–1932. [DOI] [PubMed] [Google Scholar]
- 16. Palmela C, Chevarin C, Xu Z, et al. Adherent-invasive Escherichia coli in inflammatory bowel disease. Gut. 2018;67:574–587. [DOI] [PubMed] [Google Scholar]
- 17. Kim SC, Tonkonogy SL, Albright CA, et al. Variable phenotypes of enterocolitis in interleukin 10-deficient mice monoassociated with two different commensal bacteria. Gastroenterology. 2005;128:891–906. [DOI] [PubMed] [Google Scholar]
- 18. Chassaing B, Rolhion N, de Vallée A, et al. Crohn disease–associated adherent-invasive E. coli bacteria target mouse and human Peyer’s patches via long polar fimbriae. J Clin Invest. 2011;121:966–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Craven M, Egan CE, Dowd SE, et al. Inflammation drives dysbiosis and bacterial invasion in murine models of ileal Crohn’s disease. PLoS One. 2012;7:e41594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim SC, Tonkonogy SL, Karrasch T, et al. Dual-association of gnotobiotic IL-10-/- mice with 2 nonpathogenic commensal bacteria induces aggressive pancolitis. Inflamm Bowel Dis. 2007;13:1457–1466. [DOI] [PubMed] [Google Scholar]
- 21. Rath HC, Herfarth HH, Ikeda JS, et al. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. J Clin Invest. 1996;98:945–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sellon RK, Tonkonogy S, Schultz M, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moran JP, Walter J, Tannock GW, et al. Bifidobacterium animalis causes extensive duodenitis and mild colonic inflammation in monoassociated interleukin-10-deficient mice. Inflamm Bowel Dis. 2009;15:1022–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Veltkamp C, Tonkonogy SL, De Jong YP, et al. Continuous stimulation by normal luminal bacteria is essential for the development and perpetuation of colitis in Tg(epsilon26) mice. Gastroenterology. 2001;120:900–913. [DOI] [PubMed] [Google Scholar]
- 25. Simpson KW, Dogan B, Rishniw M, et al. Adherent and invasive Escherichia coli is associated with granulomatous colitis in boxer dogs. Infect Immun. 2006;74:4778–4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gevers D, Kugathasan S, Denson LA, et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe. 2014;15:382–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baumgart M, Dogan B, Rishniw M, et al. Culture independent analysis of ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of Clostridiales in Crohn’s disease involving the ileum. Isme J. 2007;1:403–418. [DOI] [PubMed] [Google Scholar]
- 28. Darfeuille-Michaud A, Neut C, Barnich N, et al. Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn’s disease. Gastroenterology. 1998;115:1405–1413. [DOI] [PubMed] [Google Scholar]
- 29. Boudeau J, Glasser AL, Masseret E, et al. Invasive ability of an Escherichia coli strain isolated from the ileal mucosa of a patient with Crohn’s disease. Infect Immun. 1999;67:4499–4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Halfvarson J, Brislawn CJ, Lamendella R, et al. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat Microbiol. 2017;2:17004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim M, Galan C, Hill AA, et al. Critical role for the microbiota in CX3CR1+ intestinal mononuclear phagocyte regulation of intestinal T cell responses. Immunity. 2018;49:151–163.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Viladomiu M, Kivolowitz C, Abdulhamid A, et al. IgA-coated E. coli enriched in Crohn’s disease spondyloarthritis promote TH-17-dependent inflammation. Sci Transl Med. 2017;9:eaaf9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Arthur JC, Perez-Chanona E, Mühlbauer M, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kühn R, Löhler J, Rennick D, et al. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. [DOI] [PubMed] [Google Scholar]
- 35. Bamias G, Okazawa A, Rivera-Nieves J, et al. Commensal bacteria exacerbate intestinal inflammation but are not essential for the development of murine ileitis. J Immunol. 2007;178:1809–1818. [DOI] [PubMed] [Google Scholar]
- 36. Pizarro TT, Pastorelli L, Bamias G, et al. SAMP1/YitFc mouse strain: a spontaneous model of Crohn’s disease-like ileitis. Inflamm Bowel Dis. 2011;17:2566–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kontoyiannis D, Pasparakis M, Pizarro TT, et al. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999;10:387–398. [DOI] [PubMed] [Google Scholar]
- 38. Shouval DS, Ouahed J, Biswas A, et al. Interleukin 10 receptor signaling: master regulator of intestinal mucosal homeostasis in mice and humans. Adv Immunol. 2014;122:177–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kole A, Maloy KJ.. Control of intestinal inflammation by interleukin-10. Curr Top Microbiol Immunol. 2014;380:19–38. [DOI] [PubMed] [Google Scholar]