Abstract
Introduction
Thyroid hormones play a major role in the regulation of testicular maturation and growth and in the control of Sertoli and Leydig cell functions in adulthood. When naturally occurring, hypothyroidism causes male hypogonadotropic hypogonadism and Sertoli cell function disorders, but when iatrogenic and methimazole-induced its influence on the pituitary-testicular axis function with respect to Sertoli cells is poorly known.
Material and Methods
Male adult Wistar rats (n = 14) were divided into two groups: E – taking methimazole orally for 60 days, and C – control animals. After 60 d, the concentrations in serum of testosterone, follicle-stimulating and luteinising hormones, and inhibins A and B were measured. Testicles were examined morphologically: the apoptotic Sertoli cell percentage (ASC%) and number of these cells functional per tubular mm2 (FSCN/Tmm2) were calculated.
Results
In group E, inhibin A was higher while inhibin B was lower than in group C. ASC% was higher and FSCN/Tmm2 lower in group E than in group C.
Conclusion
A specific modulation of Sertoli cell function in the course of methimazole-induced hypothyroidism leads to a simultaneous concentration increase in inhibin A and decrease in B. Inhibin A might share responsibility for pituitary-testicular axis dysfunction and hypogonadotropic hypogonadism in this model of hypothyroidism.
Keywords: rats, hypogonadism, inhibins, sex hormones, thionamines, thyroid gland
Introduction
Pituitary-testicular axis dysfunctions are important causes of fertility and/or sexual function disorders. This is due in part to a decrease in thyroid hormone levels, as they play a key role in regulating maturation and growth of testicles as well as in controlling functions of Sertoli and Leydig cells both in adolescent and adult life, and restoring euthyroid state usually normalises impaired functions of the reproductive system (19, 27, 28). Naturally occurring hypothyroidism causes severe pituitary-testicular axis dysfunctions and hypogonadotropic hypogonadism in men, lowering gonadotropins, testosterone, and inhibin B concentrations (8).
Both in human and veterinary medicine, iatrogenic hypothyroidism may occur. It may attend cases of inappropriately conducted treatment of hyperthyroidism with methimazole (the treatment of choice) or with other antithyroid drugs (7, 14). This disease causes numerous multisystemic complications, not sparing the gonads; however, there are few studies that might explain its impact on testicular function, and none of them accurately defines the type of hormonal imbalance in the pituitary-testicular axis (1, 4, 6, 9-11, 20, 27). Ai et al. (1) found that methimazole-induced hypothyroidism produces impaired testicular function directly, without affecting pituitary function (1). They also found a statistically significant reduction in the number of Sertoli cells compared to the control group, but they did not include the significance of the inhibins produced by these cells in their study. Their results stand in opposition to those obtained by other authors who reported a reduction in gonadotropin-releasing hormone (GnRH) and pituitary gonadotropin levels in hypothyroid rats (24, 25).
Maintenance of correct relationships between hormones of the pituitary-testicular axis and steroid hormones is not only achieved by feedback concerning gonadotropins. Inhibins produced in the testicles are also crucial to forming such relationships (18). These multifunctional dimeric glycoproteins are integral members of the transforming growth factor beta (TGF-β) superfamily and negatively regulate follicle-stimulating hormone (FSH) in the pituitary gland and control spermatogenesis. This negative regulation is triggered by an increase in FSH level in the blood, while in healthy subjects this also affects the size of the Sertoli cell population and intensity of spermatogenesis (16). Luteinising hormone (LH) is unlikely to stimulate the release of inhibins, but it also inhibits; however, it appears that inhibins do not affect LH, as the administration of inhibin antiserum in rats resulted in an increase in FSH-b mRNA and protein levels in the pituitary gland but not in the transcription rate of LH subunits (3, 16). Inhibin consists of two subunits: α and β. The latter subunit occurs in two forms as inhibin A (βA) and inhibin B (βB), and this dimeric form is biologically active (5).
The aim of this study was to evaluate the pituitary-testicular axis function in male rats suffering from methimazole-induced hypothyroidism in relation to selected morphological features of Sertoli cells, while also explaining the role of inhibins.
Material and Methods
Animals. Male Wistar rats (220–260 g b.w., 10 weeks of age) were randomly divided into two groups: C (the control group) of seven healthy males and E (the experimental group) of seven males receiving methimazole. Rats were kept in an air-conditioned room with average humidity of 45%–47%, temperature of 22–23°C, and a 12/12 h light cycle. The rats underwent 14 days’ adaptation prior to the experiment. After this period, the rats were fed a commercial diet for laboratory animals (Agropol, Poland). Rats from the control group had access to tap water ad libitum, and the rats from experimental groups were given a 0.05% methimazole (Sigma-Aldrich, USA) water solution, administered ad libitum and known to be a dose that was very effective at inducing hypothyroidism (10). A fresh solution was prepared daily. In rats from the experimental group, hypothyroidism was confirmed with measurement of total thyroxine (TT4) concentration in serum with an ELISA (Cloud-Clone Corp., USA), before the end of the experiment. On the 58th day of the experiment, blood samples (0.5 mL) were collected from the lateral tail vein and allowed to clot. Next, these samples were centrifuged at 4°C for 30 min at 4,000 rpm to obtain serum. In group E, the concentration of TT4 was statistically significantly lower (mean ± SD: 6.8 nmol/L ± 0.9) than in group C (mean ± SD: 72.7 nmol/L ± 6.4).
Hormonal analysis. On the 60th day of the experiment, after a 12 h fast, animals aging 21 weeks were anesthetised using ketamine administered intramuscularly in a dose of 80 mg/kg b.w. (Vetaketam, Vet-Agro, Poland). Under general anaesthesia, blood was drawn by puncturing the right heart ventricle using a 0.8 mm needle. Blood samples were put into test tubes for coagulation in order to obtain serum, which was obtained by centrifuging at 4°C for 30 min at 4,000 rpm. The animals were then euthanised intracardially with pentobarbital sodium (Morbital, Biowet Puławy, Poland) in a dose of 0.6 mL/kg b.w. Testosterone (T) was measured with a CEA458Ge ELISA Kit for Testosterone (Testo) (Cloud-Clone Corp., USA) with a minimum detection limit of 53.7 pg/mL, intra-assay coefficient of variation <10%, inter-assay coefficient of variation <12%, and detection range of 123.5–10,000 pg/mL, according to the kit manufacturer’s information. FSH was assayed with a 201-11-0183 Rat (FSH) ELISA Kit (Sunred Shanghai Biological Technology Co., China) with a minimum detection limit of 0.202 IU/L, intra-assay coefficient of variation <9%, inter-assay coefficient of variation <11%, and detection range of 0.25–60 IU/L, according to the information accompanying the kit. LH was quantified with a 201-11-0180 Rat (LH) ELISA Kit (Sunred Shanghai Biological Technology Co.) with manufacturer specifications for minimum detection limit of 0.206 IU/L, intra-assay coefficient of variation <9%, inter-assay coefficient of variation <11%, and detection range of 0.3–60 IU/L. Inhibin A (INHA) was immunoabsorbed for measurement with an SEA395Ra ELISA Kit for Inhibin A (INHA) (Cloud-Clone Corp.) with a manufacturer-documented minimum detection limit of 6.0 pg/mL, intra-assay coefficient of variation <10%, inter-assay coefficient of variation <12%, and detection range of 15.6–1,000 pg/mL. Inhibin B (INHB) was gauged with the counterpart SEA760Ra kit (Cloud-Clone Corp.) with the only specification difference from the inhibin A kit being a minimum detection limit of 6.1 pg/mL.
Morphological analysis of testicles. Immediately after euthanasia, right testicles without epididymis were taken and fixed in a 10% buffered formalin solution and submitted for standard histological processing.
Apoptotic Sertoli cell percentage (ASC%). Sections 4 μ thick were stained with Hoechst 33342 and examined in fluorescence with the use of a BX63 microscope (Olympus, Japan) at 1,000 × magnification, differentiating apoptotic cells by their nuclear condensation and DNA fragmentation. The parameter was calculated as the number of apoptotic Sertoli cells relative to the total number of 100 Sertoli cells analysed.
Functional Sertoli cell number per tubular mm2 (FSCN/Tmm2). This parameter was developed to determine the number of active (non-apoptotic) Sertoli cells in 1 mm2 cross-sectional area of the seminiferous tubule and was calculated from the following formula: FSCN/Tmm2 = SC/STA × (100–ASC%)/100. Sections 4 μ thick were stained with haematoxylin and eosin (HE) and examined under a BX62 light upright microscope (Olympus) with cellSens software (Olympus) for Microsoft Windows 10 Pro. The average number of Sertoli cells (SC), calculated in 10 seminiferous tubules in each individual separately, was referenced to these seminiferous tubules’ area (STA) expressed in mm2. For STA calculation, the average diameter was calculated from two perpendicular measurements of the tubule, and the difference between these two measurements had an acceptance criterion that it did not exceed 30 μm, in order to reject oval tubules. Only such tubules were examined. All morphological studies were made using a blank test method – slides were decoded when the results were obtained.
Statistical analysis. All values are presented as median and range. Statistical analysis was performed with Statistica software version 10.0 (StatSoft, now Tibco, USA) and a Mann–Whitney U test. The differences between groups were considered statistically significant at P ≤ 0.05. Correlations were calculated with the Spearman rank method.
Results
The results of hormone concentrations and testicular morphological parameters are presented in Table 1 and Fig. 1. Testosterone (T) concentration in group E was statistically significantly lower than in group C (P = 0.009). Similarly, FSH and LH concentrations in group E were lower than in group C, but no statistical significance was reported. Concentration of inhibin A (INHA) in group E was statistically significantly higher than in group C (P = 0.008), whereas inhibin B (INHB) in the group of hypothyroid rats was lower than in healthy animals, without statistical significance. ASC% in group E was higher than in group C, but was also without statistical significance. FSCN/Tmm2 in the experimental group was statistically significantly lower (P = 0.002) than in the control group.
Table 1.
Results of hormonal analysis and morphometric parameters in rat testicles
| Hormone | Group | |||
|---|---|---|---|---|
| C (n = 7) | E (n = 7) | |||
| Median | Range | Median | Range | |
| T (ng/dL) | 369.18 | 246.77–464.55 | 165.53* | 116.81–264.3 |
| FSH (IU/L) | 1.49 | 1.2–1.91 | 1.0 | 0.86–1.75 |
| LH (IU/L) | 0.57 | 0.3–0.74 | 0.54 | 0.41–0.74 |
| INHA (pg/mL) | 27.24 | 19.49–41.47 | 65.04* | 52.89–76.26 |
| INHB (pg/mL) | 285.47 | 253.99–510.35 | 169.58 | 152.64–380.48 |
| ASC% | 14 | 10–18 | 16 | 14–17 |
| FSCN/Tmm2 | 427.21 | 401.06–446.3 | 300.35* | 249.14–349.7 |
T – testosterone, FSH – follicle-stimulating hormone, LH – luteinising hormone, INHA – inhibin A, INHB – inhibin B, ASC% – apoptotic Sertoli cell percentage, FSCN/Tmm2 – functional Sertoli cell number per tubular mm2, * – statistically significant differences in comparison with control group for P ≤ 0.05
Fig. 1.
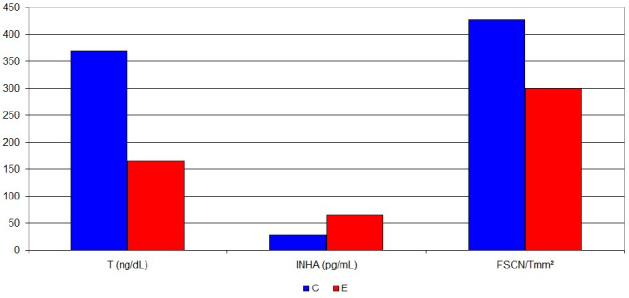
Statistically significant differences between experimental and control groups (median) in testosterone and inhibin A concentrations and functional Sertoli cell number per tubular mm2
In the group of hypothyroid rats, a positive correlation was observed between T and FSH (rho = 0.2), T and FSCN/Tmm2 (rho = 0.6), FSH and INHA (rho = 0.4), FSH and ASC% (rho = 0.35), FSH and FSCN/Tmm2 (rho = 0.5), INHA and FSCN/Tmm2 (rho = 0.6), and INHB and ASC% (rho = 0.45).
Negative correlations were found in group E between T and INHB (rho = −0.6), T and ASC% (rho = −0.67), T and FSCN/Tmm2 (rho = 0.6), FSH and INHB (rho = −0.63), INHA and INHB (rho = −0.4), INHA and ASC% (rho = −0.7), and INHB and FSCN/Tmm2 (rho = −0.5).
Discussion
In light of the results obtained, the conclusion of Herath et al. (12) that inhibin A is produced in adult male rats exclusively by Leydig cells in the case of testicular Leydig cell tumour and that it may be a specific marker of this type of neoplasm is debatable. Many authors stress that inhibin A is produced in small amounts and is undetectable in healthy men and male rats after puberty, but the detectability of this hormone in serum most likely depends on the method used and on its sensitivity (16, 30). It should be emphasised that the concentration of inhibin A obtained by us in all control subjects exceeded the detection threshold given by the manufacturer of the homologous diagnostic kit used in the study. Similar results of inhibin B concentrations to ours in male rats with hypothyroidism were obtained by Donnelly et al. (8) in hypothyroid men. We obtained an inhibin B ratio hypothyroid to normal males (0.59) similar to the ratio found by these authors (0.55). They also observed that decreased concentrations of inhibin B and normal FSH levels in patients with naturally occurring hypothyroidism suggest hypogonadotropic hypogonadism. The reduction in gonadotropin concentration reported by us, which was responsible for the lowering of testosterone levels in males with hypothyroidism, was not statistically significant, and the results were consistent with those obtained by Ai et al. (1), who used a similar model to our experimental model but with a higher dose of methimazole. It being the case that our results for gonadotropin and testosterone concentrations are also similar to those obtained by Donnelly et al. (8), we observed hypogonadotropic hypogonadism in males with methamizole-induced hypothyroidism, hence the decreased concentration of gonadotropins despite the zero increase in and concentration insufficient for inhibition of inhibin B. What mars the credibility of such an evident statement and requires further explanation is the increased concentration of inhibin A, which may be responsible for the dysfunctioning pituitary-testicular axis.
Probably inhibin A is the factor that inhibits the synthesis of gonadotropins; however, one must not exclude the paracrine activity of this or other transient factors as well as numerous endocrine substances that reduce the synthesis of steroid hormones in the testicles (13). Wagner et al. (27), basing on studies showing normal FSH and LH levels in hypothyroidism, reported that damage to the hypothalamic-pituitary-testicular axis in this disease does not occur in Leydig cells, but at the pituitary gland level, where lower hypothalamic stimulation and abnormal response to GnRH is shown. However, the same mechanisms that cause hypogonadism may be due to an elevated level of inhibin A, not necessarily directly due to deficiency in thyroid hormones. It was confirmed by Riwier et al. (22) who administered recombinant human inhibin A to rats and observed the inhibition of FSH release. Accordingly, inhibin A cannot be regarded as a “specific marker” but only “a marker for a group including” Leydig cell tumour, or more generally for hypogonadotropic hypogonadism in males, especially since its concentration in the study group was correlated with FSH concentration and with the number of active Sertoli cells.
Despite this fact, the site of inhibin synthesis raises a lot of doubts. The statistically significant increase in inhibin A and decrease inhibin B concentrations in serum in male rats with hypothyroidism may be indicative of specific modulation of Sertoli cell function (2, 12, 16, 21). Something having been found to indicate this location of inhibin synthesis is a high concentration of inhibins in the lumen of the seminiferous tubule, although a small pool of these hormones may be produced by the reproductive cells present there: spermatogonia, primary spermatocytes, and round spermatids (17, 18). The synthesis pathways of the two described inhibins are different. The coding genes are on different chromosomes: for example, the human gene encoding the βA subunit lies on chromosome 7 (7p15-p13) and the βB subunit on chromosome 2 (2cen-q13). Also the regulation of inhibin transcription is complex and dependent on various transcription factors, cofactors, and coactivators (18). Moreover, inhibins are synthesised in the form of prohormones, similarly to other proteins which belong to the TGF-β superfamily and must be activated to function in a dimerisation process, which further complicates the complexity and diversity of the processes of the formation of these hormones (29). Although the major positive regulator of inhibin secretion is protein kinase A, the signal protein kinase C signal transduction pathway seems to be more relevant with regard to inhibin B secretion (26). In the course of hypothyroidism, significant disturbances in protein kinase C expression may be responsible for the decrease in secretion of this hormone (23). Thyroid hormones use different signalling pathways to regulate critical biochemical steps in the Sertoli cell metabolism by genomic and extracellular pathways, but these mechanisms have not been fully understood. One of the most important mechanisms is the effect on glucose metabolism by modulating glucose transporter gene (GLUT1) expression as well as the effect on protein synthesis and independent accumulation of amino acids by these cells due to changes in membrane potential (27). The effect of hypothyroidism on the turnover of protein has already been the subject of our research, but it is worth mentioning that thyroid hormones are important regulators of development, differentiation, repair, and metabolism, and their main function is regulation of basic metabolism (11). All of these factors might affect the dysfunction of Sertoli cells and modulation of inhibin secretion in the course of hypothyroidism, but the knowledge in this area is limited and requires further investigation.
As mentioned earlier, the size of the Sertoli cell population is one of the factors affecting the concentration of inhibins in blood. Therefore, for a better understanding of pituitary-testicular axis function in hypothyroidism, we calculated a new index (FSCN/Tmm2), which observes the number of potentially active Sertoli cells per mm2 of the seminiferous tubule. In the experimental group, this easily calculated parameter was positively correlated with testosterone, FSH, and inhibin A concentrations, while it was negatively correlated with inhibin B. Therefore, it can be assumed that it evaluates the functional potential of Sertoli cells in a reliable way, as it takes into account their loss in the process of apoptosis. The phenomenon of programmed cell death may occur as a result of methimazole administration, with the severity of apoptosis being strongly correlated with the dose of this drug (15). This potential was statistically significantly lower in the experimental group, which could explain the decrease in inhibin B concentration. This value and additionally an increase in inhibin A concentration in the experimental group indicate that inhibin concentrations were not affected by the number of Sertoli cells, but by their dysfunction referred to above.
A specific modulation of Sertoli cell function in the course of methimazole-induced hypothyroidism leads to a simultaneous increase in concentration of inhibin A and a decrease in concentration of inhibin B. Among other factors, inhibin A might be responsible for the pituitary-testicular axis dysfunction and for hypogonadotropic hypogonadism in the course of our model of hypothyroidism.
Acknowledgements
The authors appreciate the contribution of Wiesław Sitkowski in helping with statistical analysis.
Footnotes
Conflict of Interest
Conflict of Interests Statement: The authors declare that there is no conflict of interests regarding the publication of this article.
Financial Disclosure Statement: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Animal Rights Statement: The experiments on animals were in accordance with the local Ethical Committee laws and regulations as regards care and use of experimental animals.
References
- 1.Ai J., Zarifkar A., Takhshid M.A., Alavi J., Moradzadeh M.. The effect of thyroid activity on adult rat spermatogenesis. IJVR. 2007;8:155–160. [Google Scholar]
- 2.Andersson A.M., Muller J., Skakkebaek N.E.. Different roles of prepubertal and postpubertal germ cells and Sertoli cells in the regulation of serum inhibin B levels. J Clin Endocrinol Metab. 1998;83:4451–4458. doi: 10.1210/jcem.83.12.5360. [DOI] [PubMed] [Google Scholar]
- 3.Attardi B., Vaughan J., Vale W.. Regulation of FSH beta messenger ribonucleic acid levels in the rat by endogenous inhibin. Endocrinology. 1992;130:557–559. doi: 10.1210/endo.130.1.1727723. [DOI] [PubMed] [Google Scholar]
- 4.Azizi F., Ataie L., Hedayati M., Mehrabi Y., Sheikholeslami F.. Effect of long-term continuous methimazole treatment of hyperthyroidism: comparison with radioiodine. Eur J Endocrinol. 2005;152:695–701. doi: 10.1530/eje.1.01904. [DOI] [PubMed] [Google Scholar]
- 5.Burger H.G.. Clinical review 46: clinical utility of inhibin measurements. J Clin Endocrinol Metab. 1993;76:1391–1396. doi: 10.1210/jcem.76.6.8501140. [DOI] [PubMed] [Google Scholar]
- 6.Choo Y.K., Yoo W.S., Kim D.W., Chung H.K.. Hypothyroidism during antithyroid drug treatment with methimazole is a favorable prognostic indicator in patients with Graves’ disease. Thyroid. 2010;20:949–954. doi: 10.1089/thy.2009.0126. [DOI] [PubMed] [Google Scholar]
- 7.Cooper D.S.. Antithyroid drugs. N Engl J Med. 2005;352:905–917. doi: 10.1056/NEJMra042972. [DOI] [PubMed] [Google Scholar]
- 8.Donnelly P., Tan K., Winch D.. Inhibin B levels in hypothyroid males. Thyroid. 2013;23:1379–1382. doi: 10.1089/thy.2012.0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gołyński M., Lutnicki K.. Influence of β-endorphin on oxygen activity of neutrophils and total antioxidant status in rats after chronic administration of methimazole. Bull Vet Inst Pulawy. 2013;57:97–101. [Google Scholar]
- 10.Gołyński M., Szczepanik M., Lutnicki K., Adamek Ł., Gołyńska M., Wilkołek P., Sitkowski W., Kurek Ł., Dębiak P.. Biophysical parameters of rats’ skin after the administration of methimazole. Bull Vet Inst Pulawy. 2014;58:315–319. [Google Scholar]
- 11.Gołyński M., Szpetnar M., Tatara M.R., Lutnicki K., Gołyńska M., Kurek Ł., Szczepanik M., Wilkołek P.. Content of selected amino acids in the gastrocnemius muscle during experimental hypothyroidism in rats. J Vet Res. 2016;60:489–493. [Google Scholar]
- 12.Herath C.B., Watanabe G., Wanzhu J., Noguchi J., Akiyama K., Kuramoto K., Groome N.P., Taya K.. Elevated levels of inhibin-A and immunoreactive inhibin in aged male Wistar rats with testicular Leydig cell tumor. J Androl. 2001;22:838–846. [PubMed] [Google Scholar]
- 13.Hsueh A.J., Dahl K.D., Vaughan J., Tucker E., Rivier J., Bardin C.W., Vale W.. Heterodimers and homodimers of inhibin subunits have different paracrine action in the modulation of luteinizing hormone-stimulated androgen biosynthesis. Proc Natl Acad Sci USA. 1987;84:5082–5086. doi: 10.1073/pnas.84.14.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kallner G., Vitols S., Ljunggren J.G.. Comparison of standardized initial doses of two antithyroid drugs in the treatment of Graves’ disease. J Intern Med. 1996;239:525–529. doi: 10.1046/j.1365-2796.1996.489827000.x. [DOI] [PubMed] [Google Scholar]
- 15.Klatka M., Grywalska E., Surdacka A., Tarach J., Klatka J., Roliński J.. Peripheral blood lymphocyte apoptosis and its relationship with thyroid function tests in adolescents with hyperthyroidism due to Graves’ disease. Arch Med Sci. 2012;8:865–873. doi: 10.5114/aoms.2012.31618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luisi S., Florio P., Reis F.M., Petraglia F.. Inhibins in female and male reproductive physiology: role in gametogenesis, conception, implantation, and early pregnancy. Hum Reprod Update. 2005;11:123–135. doi: 10.1093/humupd/dmh057. [DOI] [PubMed] [Google Scholar]
- 17.Maddocks S., Sharpe R.M.. The route of secretion of inhibin from the rat testis. J Endocrinol. 1998;120:5–8. doi: 10.1677/joe.0.120r005. [DOI] [PubMed] [Google Scholar]
- 18.Makanji Y., Zhu J., Mishra R., Holmquist C., Wong W.P.S., Schwartz N.B., Mayo K.E., Woodruff T.K.. Inhibin at 90: from discovery to clinical application, a historical review. Endocr Rev. 2014;35:747–794. doi: 10.1210/er.2014-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maran R.R.. Thyroid hormones: their role in testicular steroidogenesis. Arch Androl. 2003;49:375–388. doi: 10.1080/01485010390204968. [DOI] [PubMed] [Google Scholar]
- 20.Okdach Y.A.. Methimazole affected spermatogenesis and enhanced proliferation of testicular macrophages in albino rats. World J Pharm Sci. 2013;1:55–60. [Google Scholar]
- 21.Risbridger G.P., Clements J., Robertson D.M., Drummond A.E., Muir J., Burger H.G., de Kretser D.M.. Immuno- and bioactive inhibin and inhibin alpha-subunit expression in rat Leydig cell cultures. Mol Cell Endocrinol. 1989;66:119–122. doi: 10.1016/0303-7207(89)90056-7. [DOI] [PubMed] [Google Scholar]
- 22.Rivier C., Schwall R., Mason A., Burton L., Vaughan J., Vale W.. Effect of recombinant inhibin on luteinizing hormone and follicle-stimulating hormone secretion in the rat. Endocrinology. 1991;128:1548–1554. doi: 10.1210/endo-128-3-1548. [DOI] [PubMed] [Google Scholar]
- 23.Rybin V., Steinberg S.F.. Thyroid hormone represses protein kinase C isoform expression and activity in rat cardiac myocytes. Circ Res. 1996;79:388–398. doi: 10.1161/01.res.79.3.388. [DOI] [PubMed] [Google Scholar]
- 24.Tohei A., Akai M., Tomabechi T., Mamada M., Taya K.. Adrenal and gonadal function in hypothyroid adult male rats. J Endocrinol. 1997;152:147–154. doi: 10.1677/joe.0.1520147. [DOI] [PubMed] [Google Scholar]
- 25.Toni R., Della Casa C., Castorina S., Cocchi D., Celotti F.. Effects of hypothyroidism and endocrine disruptor-dependent non-thyroidal illness syndrome on the GnRH-gonadotroph axis of the adult rat. J Endocrinol Investig. 2005;28:20–27. [PubMed] [Google Scholar]
- 26.Vänttinen T., Liu J., Hydén-Granskog C., Parviainen M., Penttilä I., Voutilainen R.. Regulation of immunoreactive inhibin A and B secretion in cultured human granulosa-luteal cells by gonadotropins, activin A, and insulin-like growth factor type-1 receptor. J Endocrinol. 2000;167:289–294. doi: 10.1677/joe.0.1670289. [DOI] [PubMed] [Google Scholar]
- 27.Wagner M.S., Wajner S.M., Maia A.L.. The role of thyroid hormone in testicular development and function. J Endocrinol. 2008;199:351–365. doi: 10.1677/JOE-08-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wajner S.M., Wagner M.S., Maia A.L.. Clinical implications of altered thyroid status in male testicular function. Arq Bras Endocrinol Metabol. 2009;53:976–982. doi: 10.1590/s0004-27302009000800011. [DOI] [PubMed] [Google Scholar]
- 29.Walton K.L., Kelly E.K., Chan K.L., Harrison C.A., Robertson D.M.. Inhibin biosynthesis and activity are limited by a prodomain-derived peptide. Endocrinology. 2015;156:3047–3057. doi: 10.1210/en.2014-2005. [DOI] [PubMed] [Google Scholar]
- 30.Woodruff T.K., Besecke L.M., Groome N., Draper L.B., Schwartz N.B., Weiss J.. Inhibin A and inhibin B are inversely correlated to follicle-stimulating hormone, yet are discordant during the follicular phase of the rat estrous cycle, and inhibin A is expressed in a sexually dimorphic manner. Endocrinology. 1996;137:5463–5467. doi: 10.1210/endo.137.12.8940372. [DOI] [PubMed] [Google Scholar]


