Abstract
Animal models play critical roles in exploring the pathogenesis of human diseases and designing novel therapeutic schemes. Acute experimental colitis (AEC) models have been reported to be established in mice principally by oral administration of dextran sulfate sodium (DSS). However, little knowledge is known about whether DSS can be used to induce the acute experimental enteritis (AEE). In this study, different concentrations of DSS (0%, 2%, 3%, and 5%) were used to induce AEC and AEE models in two cohorts. After the establishment of these two models, the symptoms of the mice induced by DSS were noted, the length and average weight of each colon and small intestine were measured, and hematoxylin and eosin (HE) staining was conducted for assessing the inflammatory infiltration in these models. Generally, the comparison of the inflammatory scoring between AEC and AEE models was analyzed. As a consequence, we found that, the mice with 2%–5% DSS administration in a week could develop into AEC models in two cohorts and AEE models in one cohort, followed by the signs of diarrhea, gross rectal bleeding, weight loss of the body, and shortened colon and intestine length, as compared with the control group. HE staining showed that the inflammatory scoring was dramatically increased by 3%–5% DSS in AEC models in two cohorts but slightly elevated in AEE models in one cohort. Meanwhile, as compared with the severe AEC models, the extent of inflammatory infiltration induced by 3%–5% DSS in AEE models was much milder. In conclusion, oral administration of 3%–5% DSS is a good inducer of AEC models, but not AEE models.
Keywords: acute experimental colitis, acute experimental enteritis, dextran sulfate sodium, inflammatory infiltration
Introduction
Intestines are always inflamed to some extent due to the persistent exposure to external stressors such as food, bacteria, and environmental chemicals.1 In response to external stimulants, intestinal epithelial and immune cells are collectively activated and the inflammatory compounds are produced to reinforce the intestinal barrier.1 Simultaneously, these secreted cytokines induce intestinal inflammation, leading to the epithelial tissue lesion and intestinal dysfunction. Acute colitis is a typical disease characterized by immoderate intestinal inflammation.2 Therefore, establishing an acute experimental colitis (AEC) or acute experimental enteritis (AEE) model is urgently needed to understand the pathogenesis of colitis.
Chemical-induced colitis models are widely used because of the fast onset of inflammation response and the relatively simple operation procedures. AEC induced by DSS is characterized by weight loss, bloody diarrhea, ulcer formation, loss of epithelial cells, and infiltrations with neutrophils.3 It mimics the key immunological and histopathological features of AEC. AEE is characterized by small intestinal mucosal erosion, edema, and bleeding. However, there is little knowledge about the models of AEE. Whether DSS can be used to induce AEE models remains unknown. In this study, different concentrations of DSS administration were used to develop into the AEC and AEE models in mice. We found that, oral administration of 3%–5% DSS is a good inducer of AEC models, but not AEE models.
Materials and methods
Animal models
Six to eight weeks C57BL/6 male mice (Shanghai West Pui Kai Experimental Animal Co. Ltd, China), fed in specific-pathogen-free (SPF) under standard laboratory conditions at animal laboratory center of our hospital, were used to establish the AEC and AEE models by oral administration of DSS. In cohort 1, mice were divided into four groups according to drinking distilled water containing 0%, 2%, 3%, and 5% DSS (Shanghai Aladdin Bio-Chem Technology Co., Ltd, China) ad libitum. In cohort 2, mice were raised by drinking distilled water containing 0% and 3.0% DSS (MP Bio, USA) ad libitum. Mice were fed normal chow, housed individually in a room at 22°C, and then sacrificed after oral administration of DSS for a week. Our experimental study was approved by the ethics committee of Shanghai Sixth People’s Hospital (no. 2018-0080).
Observation of DSS-induced fecal indications
Fecal indications were induced by DSS in mice. According to the criteria described by Wirtz et al.,4 mice in each group was observed daily in the morning, weight loss, stool consistency, and the degree of intestinal bleeding were recorded. Loose feces and blood in the feces were observed by naked eyes, and blood clot around the anus was considered as the gross blood per rectum.
Morphological analysis of DSS-induced animal models
After the mice were sacrificed, the gastrointestinal tissues were isolated and placed in 10% formalin solution (pH 7.2). The body weight and longitudinal length of each colon and small intestine were measured. Histological examinations were performed by H&E staining after paraffin sections of these colon and small intestine tissues.
The scoring system of DSS-induced AEC models
The severities of DSS-induced AEC models were graded according to previous studies,4,5 and the scoring was conducted as follows: 0, normal morphology; 1, loss of goblet cells; 2, loss of goblet cells in large areas; 3, loss of crypts; and 4, loss of crypts in large areas. The scoring of inflammatory infiltration was shown as follows: 0, no infiltrate; 1, infiltrate around crypt basis; 2, infiltrate reaching to lamina muscularis mucosae; 3, extensive infiltration reaching the lamina muscularis mucosae and thickening of the mucosa with abundant edema; and 4, infiltration of the lamina submucosa. For neutrophile granulocyte count, colon tissue was observed at high magnification and scoring was as follows: 0, the number of neutrophils was 0; 1, there were neutrophils with a cell number ⩽ 10; 2, the number of neutrophils was 10–50; and 3, the number of neutrophils was ⩾ 50.
The scoring system of DSS-induced AEE models
Previous histologic score system for assessing colitis was modified for analyzing the small intestinal inflammation.5,6 The scoring of inflammatory infiltration was listed as follows: 0, no inflammation; 1, mild focal cellular infiltration; 2, mild lamina propria cellular infiltration; 3, more pronounced cellular infiltration; and 4, extensive cellular infiltration throughout the section. Score of small intestinal tissue structure was as follows: 0, normal villus architecture; 1, normal villous architecture; 2, early crypt epithelial hyperplasia with normal villus architecture; 3, thickened mucosa, marked epithelial hyperplasia and moderate distortion of villus architecture; and 4, severe architectural distortion. Score of neutrophile granulocyte count: 0, the number of neutrophils was 0; 1, there were neutrophils with a cell number ⩽ 10; 2, the number of neutrophils was 10–50; and 3, the number of neutrophils was ⩾ 50.
Statistical analysis
Statistical analyses were conducted by SPSS 17.0 (IBM, SPSS, Chicago, IL, USA) and GraphPad Prism. All data are conducted as the mean ± standard error of the mean (SEM). Analysis of variance (ANOVA) was used to analyze the differences between groups, and independent t-test was used to analyze the significance of two groups. P < 0.05 was considered statistically significant.
Results
Observations of the symptoms of DSS-induced animal models
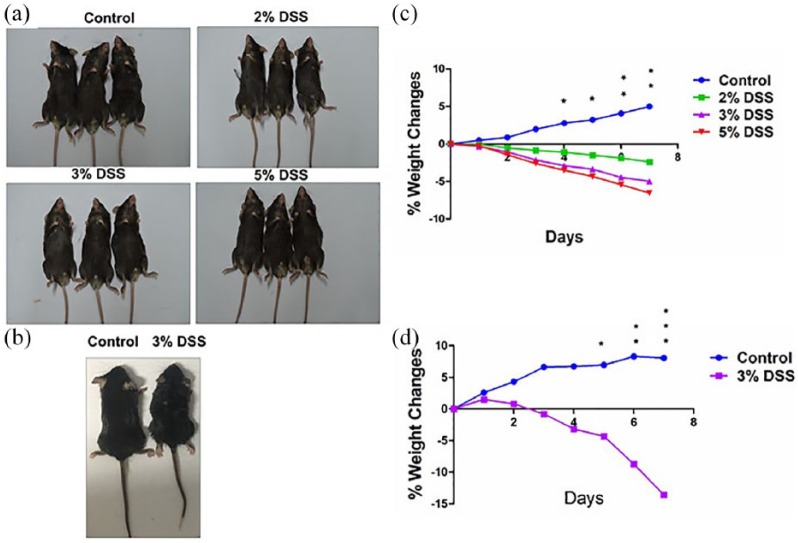
Mice gradually manifested loose stool, occult blood, and weight loss after oral administration of DSS for a week in both cohort 1 (Figure 1(a)) and cohort 2 (Figure 1(b)). In severe cases, gross blood adhered to the anus in addition to the above-mentioned symptoms. Weight loss was observed in mice with DSS administration as compared with control group (Figure 1(c) and (d); Table 1). There is a statistical difference between control and model groups from day 4 in cohort 1 and day 5 in cohort 2.
Figure 1.
Observation of DSS administration in mice. (a) Representative schematic of the mice treated by different concentrations of DSS in cohort 1. (b) Representative schematic of the mice treated by 3% DSS in cohort 2. (c) Comparison of the weight changes in mice treated by different concentrations of in cohort 1. (d) Comparison of the weight changes in mice treated by 3% DSS in cohort 2. Data were presented as mean ± SEM of two experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
Table 1.
Induction of acute enteritis by dextran sulfate sodium administration in mice.
| Experiment | Groupa (% DSS) | No. of mice | Period (days) | Weight gain (g) | Grossb blood |
|---|---|---|---|---|---|
| 1 | 5 | 3 | 7 | –1.8 ± 0.1 | 3/3 |
| 3 | 3 | 7 | –1.3 ± 0.2 | 3/3 | |
| 2 | 3 | 7 | –0.6 ± 0.2 | 3/3 | |
| 0 | 3 | 7 | 1.3 ± 0.2 | 0/3 | |
| 2 | 3 | 4 | 7 | –3.0 ± 1.0 | 4/4 |
| 0 | 4 | 7 | 1.6 ± 0.9 | 0/4 |
Mice were given distilled water containing 0%, 2%, 3%, and 5% DSS ad libitum.
Number positive/total.
Assessment of DSS-induced AEC models
Mice were sacrificed after oral administration of DSS for a week, and the colon length and weight were measured. In cohort 1, the mean colon length (cm) for 2%, 3%, 5% DSS, and control group was 5.3 ± 0.4, 5.2 ± 0.1, 4.7 ± 0.3, and 5.6 ± 0.2, respectively (Supplementary Figure 1A). Statistical assessment indicated a significant difference between the model group and the control group (Supplementary Figure 1B). Generally, the mean colon length was markedly shortened by 5% DSS administration, as compared with the control group (P < 0.001), and the similar result was confirmed in cohort 2 (P < 0.001, Supplementary Figure 1C and D). We also measured the weight of colons in DSS-induced AEC, which showed that, the mean colon weight (g) for 2%, 3%, 5% DSS, and control group was 0.33 ± 0.03, 0.32 ± 0.04, 0.30 ± 0.02, and 0.29 ± 0.00, respectively, but had no difference between the groups (each P > 0.05, Supplementary Figure 1E).
Hematoxylin and eosin (HE) staining was then conducted for assessing tissue morphology and inflammatory infiltration of colons. Histological observation showed the inflammatory cell infiltration, including polymorphonuclear leukocytes and multiple erosive lesions in both cohort 1 (Supplementary Figure 2A) and cohort 2 (Supplementary Figure 2B). The mean pathological scoring for 2%, 3%, 5% DSS, and control group in cohort 1 was 2.0 ± 1.7, 4.3 ± 1.2, 8.3 ± 0.6, and 0.0 ± 0.0, respectively (Supplementary Figure 2C). We found that, the pathological scoring was increased followed by the increase in DSS concentrations, of which 5% DSS group had the highest scoring as compared with the control and 2% DSS groups (P < 0.001). This result was consistent with what was indicated in cohort 2 (P < 0.05, Supplementary Figure 2D).
Assessment of DSS-induced AEE models
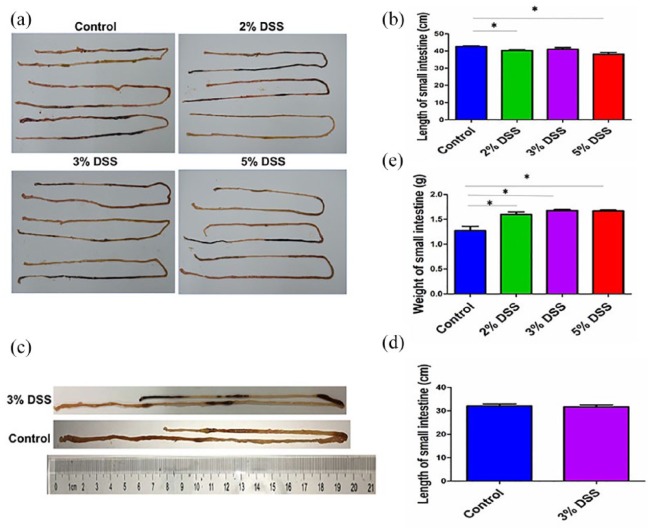
Whether DSS can be used to induce the AEE models was further estimated. In cohort 1, the observations revealed that, the length of small intestines in DSS-induced models was much shorter than those in control group (P < 0.05, Figure 2(a) and (b)). The mean length (cm) of the small intestine for 2%, 3%, 5% DSS, and control group was 40.3 ± 0.9, 41.0 ± 1.7, 38.1 ± 1.9, and 42.6 ± 0.8, respectively. Inconsistent with the results in cohort 1, the mean length of small intestine in 3% DSS group had no difference as compared with the control group in cohort 2 (P > 0.05, Figure 2(c) and (d)). In addition, the mean weight (g) of small intestine for 2%, 3%, 5% DSS, and control group were 1.60 ± 0.09, 1.68 ± 0.04, 1.67 ± 0.04, and 1.27 ± 0.15, respectively, and the weight of small intestine in DSS groups was increased as compared with that in control group (Figure 2(e)).
Figure 2.
Observations of DSS-induced AEE. (a) Representative schematic of small intestine in mice treated by different concentrations of DSS in cohort 1. (b) Comparison of the small intestine length in mice treated by different concentrations of DSS in cohort 1. (c) Representative schematic of small intestine in mice treated by 3% DSS in cohort 2. (d) Comparison of the small intestine length in mice treated by 3% DSS in cohort 2. (e) Comparison of the small intestine weight in mice treated by different concentrations of DSS in cohort 1. Data were presented as mean ± SEM of two experiments. *P < 0.05.
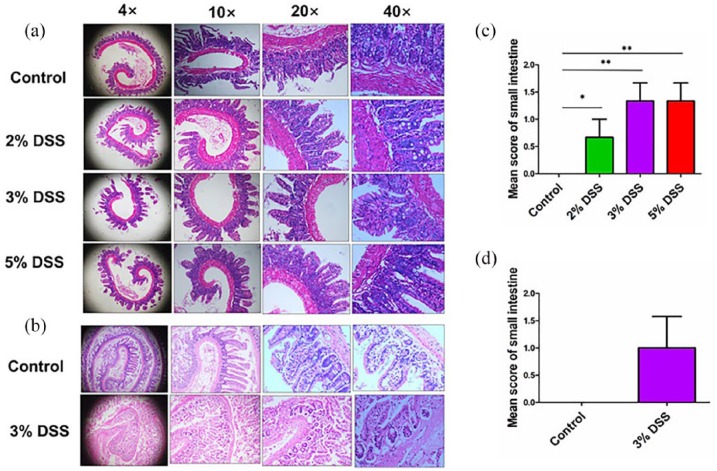
HE staining further indicated the slight inflammatory infiltration in both cohort 1 (Figure 3(a)) and cohort 2 (Figure 3(b)). In cohort 1, the pathological scoring for 2%, 3%, 5% DSS, and control group was 1.0 ± 0.0, 1.3 ± 0.6, 1.3 ± 0.6, and 0.0 ± 0.0, respectively (Figure 3(c)). Even for the 3% and 5% DSS groups, they displayed a mild focal inflammatory infiltration as compared with the control group in cohort 1. The pathological scoring in 3% DSS group had no difference as compared with the control group in cohort 2 (P > 0.05, Figure 3(d)).
Figure 3.
Histological observation of DSS-induced AEE. (a) Representative schematic of the H&E staining in small intestine tissues in mice treated by different concentrations of DSS in cohort 1. (b) Representative schematic of the H&E staining in small intestine tissue in mice treated by 3% DSS in cohort 2. (c) Comparison of histopathological scores of small intestine in mice treated by different concentrations of DSS in cohort 1. (d) Comparison of histopathological scores of small intestine in mice treated by 3% DSS in cohort 2. *P < 0.05; **P < 0.01.
Comparison of the inflammation infiltration between AEC and AEE models
We compared the inflammation infiltration between AEC and AEE models. The pathological scoring ⩽ 2 was defined as a mild inflammation infiltration, 2–4 was as moderate inflammation infiltration, and ⩾ 4 was regarded as severe inflammation infiltration. HE staining showed that, the pathological scoring of colons induced by DSS was significantly higher than those of small intestine tissues in both cohort 1 (Supplementary Figure 3A) and cohort 2 (Supplementary Figure 3B), which indicated that DSS could be used to induce the severe AEC, but mild AEE models.
Discussion
In this study, after oral administration of DSS, the mice showed the severe colitis characterized by the weight loss, bloody diarrhea, ulcer formation, loss of epithelial cells, and infiltrations with neutrophils, resembling the features of AEC. Numerous studies showed that, DSS-induced AEC models have been widely used to study the pathogenesis of colitis.5,7,8 Herein, we found that, the pathological scoring was dramatically elevated in DSS groups as compared with the control group, indicating that DSS might act as a good inducer to establish the severe AEC models. However, little knowledge is known about whether DSS can be used to induce the small intestine inflammation.
Previous studies showed that nonsteroidal anti-inflammatory drugs (NSAIDs) can cause severe adverse effects including ulcers, erosions, bleeding, perforation, and strictures in gastrointestinal tract including stomach and small intestine and be used to induce the small intestine injury in mice.9,10 Systemic side-effects and induction cycles of NSAIDs result in the limitation of their application in animal models. In this study, we attempted to induce the AEE models using DSS administration and found that the length of small intestine was shortened, and its weight was increased by DSS as compared with the control group. HE staining showed that, 5% DSS could induce the slight AEE models in comparison with the control group.
Altogether, these findings demonstrated that, DSS was more suitable for inducing severe AEC models, but had the slight effects on the establishment of AEE models. Nevertheless, our study might provide the insights into the establishment of AEE models.
Supplemental Material
Supplemental material, Supplemental_data for Is dextran sulfate sodium a good inducer of acute experimental enteritis? by Wei Chen, Jing Zhang, Chen Li, Quan Pan, Jingtong Wu, Lina Fan, Chunyan Chen, Xiaoqing Huang, Fei Teng and Jinshui Zhu in International Journal of Immunopathology and Pharmacology
Footnotes
Author note: Wei Chen, Jing Zhang, and Chen Li contributed equally to this article.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the grants from the National Nature Science Foundation of China (nos 81573747 and 81873143), Shanghai Science and Technology Commission Western Medicine Guide Project (no. 17411966500), and Shanghai Jiaotong University School of Medicine Transformation Medicine and Innovation Center Research Project (no. TM201723).
ORCID iDs: Jing Zhang  https://orcid.org/0000-0002-9412-3567
https://orcid.org/0000-0002-9412-3567
Jinshui Zhu  https://orcid.org/0000-0001-8537-8747
https://orcid.org/0000-0001-8537-8747
Supplemental material: Supplemental material for this article is available online.
References
- 1. Shimizu M. (2017) Multifunctions of dietary polyphenols in the regulation of intestinal inflammation. Journal of Food and Drug Analysis 25(1): 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Singh D, Srivastava S, Pradhan M, et al. (2015) Inflammatory bowel disease: Pathogenesis, causative factors, issues, drug treatment strategies, and delivery approaches. Critical Reviews in Therapeutic Drug Carrier Systems 32(3): 181–214. [DOI] [PubMed] [Google Scholar]
- 3. Okayasu I, Hatakeyama S, Yamada M, et al. (1990) A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 98(3): 694–702. [DOI] [PubMed] [Google Scholar]
- 4. Wirtz S, Popp V, Kindermann M, et al. (2017) Chemically induced mouse models of acute and chronic intestinal inflammation. Nature Protocols 12(7): 1295–1309. [DOI] [PubMed] [Google Scholar]
- 5. Takagawa T, Kitani A, Fuss I, et al. (2018) An increase in LRRK2 suppresses autophagy and enhances Dectin-1-induced immunity in a mouse model of colitis. Science Translational Medicine 10(444): eaan8162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moran JP, Walter J, Tannock GW, et al. (2010) Bifidobacterium animalis, causes extensive duodenitis and mild colonic inflammation in monoassociated interleukin-10-deficient mice. Inflammatory Bowel Diseases 15(7): 1022–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ruiz PA, Morón B, Becker HM, et al. (2016) Titanium dioxide nanoparticles exacerbate DSS-induced colitis: Role of the NLRP3 inflammasome. Gut 66(7): 1216–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Polytarchou C, Hommes DW, Palumbo T, et al. (2015) MicroRNA214 is associated with progression of ulcerative colitis, and inhibition reduces development of colitis and colitis-associated cancer in mice. Gastroenterology 149(4): 981–992.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sha S, Vong LB, Chonpathompikunlert P, et al. (2013) Suppression of NSAID-induced small intestinal inflammation by orally administered redox nanoparticles. Biomaterials 34(33): 8393–8400. [DOI] [PubMed] [Google Scholar]
- 10. Kim EK, Cho JH, Jeong AR, et al. (2017) Anti-inflammatory effects of simvastatin in nonsteroidal anti-inflammatory drugs-induced small bowel injury. Journal of Physiology and Pharmacology 68(1): 69–77. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_data for Is dextran sulfate sodium a good inducer of acute experimental enteritis? by Wei Chen, Jing Zhang, Chen Li, Quan Pan, Jingtong Wu, Lina Fan, Chunyan Chen, Xiaoqing Huang, Fei Teng and Jinshui Zhu in International Journal of Immunopathology and Pharmacology