Abstract
The dysregulation of microRNAs (miRNAs) is associated with the pathogenesis of non-small cell lung cancer (NSCLC). However, the mechanisms by which miR-516a-5p contributes to NSCLC remain unclear. The association between miR-516a-5p expression and the clinicopathological characteristics and prognosis in patients with NSCLC was analyzed by The Cancer Genome Atlas (TCGA) data set. The targets of miR-516a-5p were identified by bioinformatic analysis and luciferase report assay. MTT and soft agar assays were conducted to investigate the function of miR-516a-5p in NSCLC cells. We found that the expression of miR-516a-5p was decreased in NSCLC tissues and associated with the age, pathological stage, and tumor size, acting as an independent prognostic factor of tumor recurrence in patients with NSCLC. Restoration of miR-516a-5p inhibited the cell viability and anchorage-independent growth of NSCLC cells, but its inhibitor had the opposite effects. Histone cluster 3 H2A (HIST3H2A) was further identified as a direct target of miR-516a-5p and displayed a negative correlation with miR-516a-5p expression in NSCLC tissues. Overexpression of HIST3H2A reversed the anti-proliferation effects induced by miR-516a-5p and acted as an independent prognostic factor of poor survival in patients with NSCLC. Altogether, our findings demonstrate that miR-516a-5p may function as a tumor suppressive factor in NSCLC cells by targeting HIST3H2A and might represent a potential indicator of tumor recurrence in patients with NSCLC.
Keywords: HIST3H2A, miR-516a-5p, NSCLC, proliferation
Introduction
Lung cancer is one of the most common malignancies with the highest morbidity and mortality worldwide.1 Non-small cell lung cancer (NSCLC) accounts for 80% of lung cancer cases, including adenocarcinoma, squamous cell carcinoma, and large cell carcinoma. The incipient symptom of NSCLC is unobvious, and most of them are diagnosed in advanced stage duo to their unlimited growth and distant metastasis.2 Thus, identification of the molecular biomarkers related to the progression of NSCLC is critical for improving their outcome.
MicroRNAs (miRNAs), a subtype of small non-coding RNAs, can repress the expression of their target mRNAs by binding to their 3‘-untranslated region (3′UTR). The dysregulation of miRNAs is associated with the growth and metastasis of NSCLC.3 They act as oncogenic factors4 or tumor suppressors5 involved in the pathogenesis of NSCLC. On one hand, miR-19b promotes the growth and apoptosis escape,6 and miR-661 facilitates the invasion and metastasis of NSCLC by inhibiting the RB transcriptional co-repressor 1.3 On the other hand, miR-183/-200c/-1258 suppresses the proliferation and invasion of NSCLC cells.7
There is little knowledge about the role of miR-516a-5p in cancers. Decreased expression of miR-516a-5p predicts a poor survival in neuroblastoma.8 But, miR-516a-5p expression is increased in abdominal aortic aneurysm and promotes the proteolytic degradation.9 In this study, we found that the decreased expression of miR-516a-5p was associated with the age, pathological stage, and tumor size, acting as an independent prognostic factor of tumor recurrence in patients with NSCLC. Re-expression of miR-516a-5p inhibited the proliferation of NSCLC cells by targeting Histone cluster 3 H2A (HIST3H2A) and might represent a potential marker for tumor recurrence in patients with NSCLC.
Materials and methods
Clinical data
The data including 262 NSCLC tissues, 23 pair-matched normal tissues, overall survival (OS) time and status, recurrence time and status as well as miR-516a-5p, and HIST3H2A expression levels were downloaded from The Cancer Genome Atlas (TCGA) database (https://genome-cancer.ucsc.edu). The protocols used in our study were approved by the Ethics Committee of Shanghai Chest Hospital. The pathological diagnosis for these tissues was conducted by two independent pathologists.
Identification of the target genes of miR-516a-5p
The target genes of miR-516a-5p were identified using the prediction tool TargetScanHuman7.1 (http://www.targetscan.org/vert_71/), and according to the cumulative weighted text score, five target genes of miR-516a-5p were selected for further investigation.
Cell culture
NSCLC cell lines (A549 and NCI-H460) were purchased from Chinese Academy of Sciences Cell Bank and were cultured in Dulbecco’s Modified Eagle medium supplemented with 10% heat-inactivated fetal bovine serum in a humidified atmosphere containing 5% CO2 at 37°C.
Quantitative real-time PCR
Total RNA of NSCLC cells was extracted using TRIzol. Reverse transcription was performed by M-MLV and cDNA amplification using the SYBR Green Master Mix kit (Takara, Otsu, Japan). Total RNA for miRNAs was isolated using a High Pure miRNA isolation kit (Roche, Indianapolis, USA) and RT-PCR using a TaqMan MicroRNA Reverse Transcription kit (Life Technologies, Carlsbad, USA). Data were analyzed using the comparative Ct method (2−∆∆Ct). Three experiments were performed for each clone.
Western blot analysis
NSCLC cell lines were harvested and extracted using lysis buffer. Cell extracts were boiled in loading buffer, and equal amount of cell extracts was separated on 15% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels. Separated protein bands were transferred into polyvinylidene fluoride membranes. The primary antibodies against HIST3H2A (AA 2-130; 4A Biotech, Beijing, China) and β-actin (ab16039, Rabbit polyclonal antibody; Abcam, Cambridge, MA, USA) were diluted at a ratio of 1:1000 according to the instructions and incubated overnight at 4°C.
Luciferase reporter assay
NSCLC cell lines were seeded into 96-well plates in combination with the co-transfection with a mixture of 60 ng of luciferase, 6 ng of pRL-CMV Renilla luciferase reporter, and miR-516a-5p mimic or inhibitor. After 48 h of incubation, the luciferase activities were examined by a dual-luciferase reporter assay (Promega, Madison, WI, USA).
Plasmid, miR-516a-5p mimic, and inhibitor
Plasmid-mediated pcDNA3.1-HIST3H2A (HIST3H2A), miR-516a-5p mimic, and inhibitor and the control vectors were purchased from GenePharma (Shanghai, China). NSCLC cell lines were planted in 6-well plates 24 h prior to HIST3H2A plasmid, miR-516a-5p mimic, or inhibitor transfection with 50%–70% confluence and then were transfected with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacture instructions.
MTT, agar assays, and statistical analysis
MTT, agar assays, and statistical analysis were performed as previously described.5,10
Results
Downregulation of miR-516a-5p expression was associated with tumor recurrence in patients with NSCLC
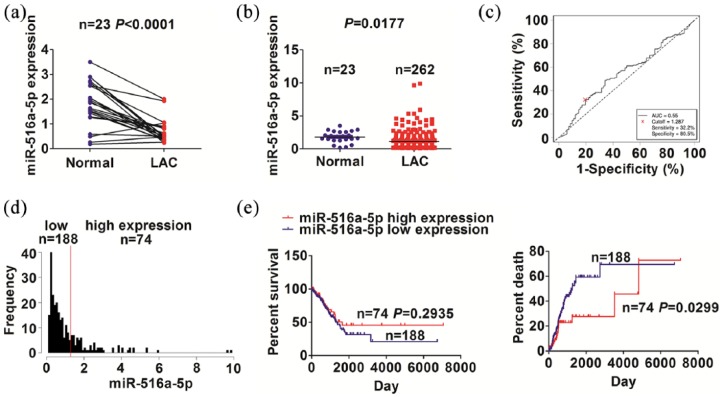
TCGA cohort indicated that miR-516a-5p expression levels were decreased in paired (n = 23, Figure 1(a)) and unpaired NSCLC tissues (n = 262, Figure 1(b)) as compared with the adjacent normal tissues (n = 23). In light of the OS time, survival status, and miR-516a-5p expression levels, a cut-off value of miR-516a-5p in NSCLC (Figure 1(c)) was acquired, and divided the patients into high-expression group and low-expression group (Figure 1(d)). We found that low expression of miR-516a-5p was associated with the age (P = 0.024), pathological stage (P = 0.025), and tumor size (P = 0.011), but had no association with other factors in patients with NSCLC (each P > 0.05, Supplementary Table S1). Kaplan–Meier showed that the patients with low miR-516a-5p expression had a higher tumor recurrence rate, but had no difference in OS, as compared with those with high miR-516a-5p expression (Figure 1(e)). Univariate and Multivariate Cox regression analyses uncovered miR-516a-5p expression as an independent prognostic factor of tumor recurrence in patients with NSCLC (P = 0.024, Table 1).
Figure 1.
The expression of miR-516a-5p was associated with tumor recurrence in NSCLC patients. (a, b) TCGA analysis of the expression levels of miR-516a-5p in paired and unpaired NSCLC tissues. (c) ROC curve was used to obtain a cut-off value of miR-516a-5p in NSCLC. (d) The grouping NSCLC patients was determined by the cut-off value of miR-516a-5p. (e) Kaplan–Meier analysis of the association of miR-516a-5p expression with OS and tumor recurrence in NSCLC patients.
Table 1.
Cox regression analysis of miR-516a-5p expression as a tumor recurrence predictor.
| Variables | Univariate Cox regression analysis |
Multivariate Cox regression analysis |
||
|---|---|---|---|---|
| RR (95% CI) | P-value | RR (95% CI) | P-value | |
| Age (years) | ||||
| ⩾60 versus <60 | 1.416 (0.857–2.341) | 0.174 | NA | NA |
| Gender | ||||
| Male versus Female | 0.771 (0.492–1.206) | 0.254 | NA | NA |
| Pathological stage | ||||
| III/ IV versus I/II | 1.555 (0.927–2.606) | 0.094 | NA | NA |
| T stage | ||||
| T3 + T4 versus T1 + T2 | 2.007 (1.081–3.728) | 0.027 | 2.032 (1.092–3.781) | 0.025 |
| N staging | ||||
| Positive versus Negative | 1.878 (1.213–2.906) | 0.005 | 1.909 (1.232–2.958) | 0.004 |
| M stage | ||||
| Positive versus Negative | 0.986 (0.632–1.538) | 0.951 | NA | NA |
| miR-516a-5p expression | ||||
| High versus Low | 0.546 (0.313–0.950) | 0.032 | 0.526 (0.301–0.919) | 0.024 |
RR: risk ratio; CI: confidence interval; NA: not analyzed.
MiR-516a-5p suppressed the proliferation and anchorage-independent growth of NSCLC cells
The transfection efficiency of miR-516a-5p mimic in A549 and NCI-H460 cell lines was confirmed by Quantitative real-time PCR (qRT-PCR) analysis (Figure 2(a)). We then found that miR-516a-5p reduced the cell viability (Figure 2(b)) and anchorage-independent growth (Figure 2(c)), as compared with the miRNA negative control (miR-NC) group. Proliferating cell nuclear antigen (PCNA) protein expression, indicated by Western blot, was decreased by miR-516a-5p, as compared with the miR-NC group in A549 and NCI-H460 cell lines (Figure 2(d)).
Figure 2.
miR-516a-5p inhibited the proliferation and anchorage-independent growth in NSCLC cells. (a) qRT-PCR analysis of the transfection efficiency of miR-516a-5p mimic in A549 and NCI-H460 cell lines. (b) MTT analysis of the effects of miR-516a-5p mimic on cell viability. (c) Soft agar analysis of the effects of miR-516a-5p mimic on anchorage-independent growth. (d) Western blot analysis of the effects of miR-516a-5p mimic on PCNA protein expression. (e) qRT-PCR analysis of the transfection efficiency of miR-516a-5p inhibitor in A549 and NCI-H460 cell lines. (f) MTT analysis of the effects of miR-516a-5p inhibitor on the cell viability. (g) Soft agar analysis of the effects of miR-516a-5p inhibitor on anchorage-independent growth. (h) Western blot analysis of the effects of miR-516a-5p inhibitor on PCNA protein expression. Data were the means ± SEM of three experiments.
*P < 0.05; **P < 0.01.
In addition, the transfection efficiency of miR-516a-5p inhibitor in A549 and NCI-H460 cell lines was verified by qRT-PCR analysis (Figure 2(e)). We found that miR-516a-5p inhibitor favored the cell viability (Figure 2(f)) and anchorage-independent growth (Figure 2(g)), as compared with the NC group. PCNA protein expression was increased by miR-516a-5p inhibitor as compared with the NC group in A549 and NCI-H460 cell lines (Figure 2(h)).
HIST3H2A was identified as a direct target of miR-516a-5p in NSCLC cells
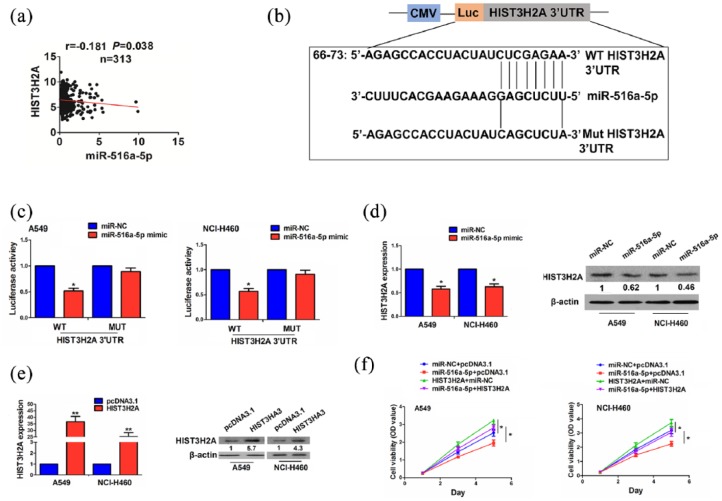
According to the cumulative weighted scores, five targets of miR-516a-5p were identified and their expression levels were estimated in paired and unpaired NSCLC tissues, indicating that only HIST3H2A possessed a significantly increased expression in paired and unpaired NSCLC tissues (Supplementary Figure S1) and exhibited a negative correlation with miR-516a-5p expression in NSCLC tissues (r = −0.181, P = 0.038; Figure 3(a)).
Figure 3.
Identification of HIST3H2A as a direct target of miR-516a-5p in NSCLC cells. (a) Pearson correlation analysis of the correlation of miR-516a-5p with HIST3H2A expression in NSCLC tissues. (b) The binding sites of miR-516a-5p with WT or Mut 3′UTR of HIST3H2A. (c) The luciferase activities of WT or Mut 3′UTR of HIST3H2A after the co-transfection with miR-516a-5p mimic and WT or Mut 3′UTR of HIST3H2A in NSCLC cells. (d) qRT-PCR and Western blot analysis of the effects of miR-516a-5p on HIST3H2A expression. (e) qRT-PCR and Western blot analysis of the transfection efficiency of HIST3H2A plasmid in A549 and NCI-H460 cell lines. (f) MTT analysis of the cell viability after co-transfection with the miR-516a-5p mimic and HIST3H2A plasmid in NSCLC cells. Data were the means ± SEM of three experiments.
*P < 0.05; **P < 0.01; ***P < 0.0001.
Luciferase reporter vector containing the wild type (WT) or mutant (Mut) 3′UTR of HIST3H2A (Figure 3(b)) was co-transfected with miR-516a-5p mimic into A549 and NCI-H460 cell lines, indicating that the luciferase activity of WT 3′UTR of HIST3H2A was reduced by miR-516a-5p, but that of Mut 3′UTR of HIST3H2A was unaffected as compared with the miR-NC group (Figure 3(c)). qRT-PCR and Western blot analysis showed that miR-516a-5p decreased the expression levels of HIST3H2A, as compared with the miR-NC group in A549 and NCI-H460 cells (Figure 3(d)).
The transfection efficiency of HIST3H2A plasmid in A549 and NCI-H460 cells was determined by qRT-PCR and Western blot analysis (Figure 3(e)). We found that overexpression of HIST3H2A promoted the cell viability and reversed the anti-proliferation effects induced by miR-516a-5p in NSCLC cells (Figure 3(f)).
Upregulation of HIST3H2A expression was associated with poor survival in patients
HIST3H2A expression levels were found dramatically increased in paired (n = 56) and unpaired NSCLC tissues (n = 398) (Supplementary Figure S2A). A cut-off value of HIST3H2A was acquired in NSCLC (Supplementary Figure S2B) and divided the patients into high-expression and low-expression groups (Supplementary Figure S2C). We found that high expression of HIST3H2A had no association with clinicopathological factors in NSCLC (each P > 0.05, Supplementary Table S2). Kaplan–Meier demonstrated that the patients with high HIST3H2A expression possessed a poorer survival, but had no difference in tumor recurrence, as compared with those with low HIST3H2A expression (Supplementary Figure S2D). Univariate and Multivariate Cox regression analyses revealed HIST3H2A expression as an independent prognostic factor of poor survival in patients with NSCLC (P = 0.027, Supplementary Table S3).
Discussion
Loss of miR-516a-5p expression is associated with a poor prognosis in patients with neuroblastoma.8 ERK inhibits the activity of miRNAs, but miR-516a-5p is unaffected by this signaling.11 Herein, we found that low expression of miR-516a-5p was associated with the age, pathological stage, and tumor size and acted as an independent prognostic factor of tumor recurrence in NSCLC patients, indicating miR-516a-5p as a potential marker for tumor recurrence in NSCLC.
MiR-516a contributes to the growth of ovarian cancer cells.12 But, we found that miR-516a-5p inhibited the proliferation of NSCLC cells, but miR-516a-5p inhibitor had the opposite effects, suggesting that miR-516a-5p might act as a tumor suppressor in NSCLC cells.
HIST3H2A was further identified as a direct target of miR-516a-5p and had a negative correlation with miR-516a-5p expression in NSCLC. High expression of HIST3H2A was associated with a poor survival in patients with NSCLC. We further confirmed that miR-516a-5p downregulated the expression of HIST3H2A, which reversed the anti-proliferative effects induced by miR-516a-5p in NSCLC cells, indicating that miR-516a-5p might inhibit the proliferation of NSCLC cells by targeting HIST3H2A.
Taken together, our findings demonstrated that low expression of miR-516a-5p acted as an independent prognostic factor of tumor recurrence in patients with NSCLC and miR-516a-5p repressed the proliferation of NSCLC cells by targeting HIST3H2A.
Supplemental Material
Supplemental material, Figure_S1 for MiR-516a-5p inhibits the proliferation of non-small cell lung cancer by targeting HIST3H2A by Xiang-Yun Ye, Ling Xu, Shun Lu and Zhi-Wei Chen in International Journal of Immunopathology and Pharmacology
Supplemental Material
Supplemental material, Figure_S2 for MiR-516a-5p inhibits the proliferation of non-small cell lung cancer by targeting HIST3H2A by Xiang-Yun Ye, Ling Xu, Shun Lu and Zhi-Wei Chen in International Journal of Immunopathology and Pharmacology
Supplemental Material
Supplemental material, Supplementary_data for MiR-516a-5p inhibits the proliferation of non-small cell lung cancer by targeting HIST3H2A by Xiang-Yun Ye, Ling Xu, Shun Lu and Zhi-Wei Chen in International Journal of Immunopathology and Pharmacology
Acknowledgments
X.-Y.Y. and L.X. contributed equally to this article.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval: This study was approved by the Hospital’s Protection of Human Subjects Committee.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Wu Jieping Medical Foundation (320.6750.12306), National Natural Science Foundation of China (81572250), State Administration of Traditional Chinese Medicine of the People’s Republic of China (JDZX2012121), and Science and Technology Commission of Shanghai (12401907000 and 11DZ1973203).
ORCID iD: Zhi-Wei Chen  https://orcid.org/0000-0002-8161-2388
https://orcid.org/0000-0002-8161-2388
Supplemental material: Supplemental material for this article is available online.
References
- 1. Siegel RL, Miller KD, Jemal A. (2018) Cancer statistics, 2018. CA: A Cancer Journal for Clinicians 68(1): 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Yang S, Liu Y, Li MY, et al. (2017) FOXP3 promotes tumor growth and metastasis by activating Wnt/β-catenin signaling pathway and EMT in non-small cell lung cancer. Molecular Cancer 16(1): 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu F, Cai Y, Rong X, et al. (2017) MiR-661 promotes tumor invasion and metastasis by directly inhibiting RB1 in non-small cell lung cancer. Molecular Cancer 16(1): 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang WC, Chin TM, Yang H, et al. (2016) Tumour-initiating cell-specific miR-1246 and miR-1290 expression converge to promote non-small cell lung cancer progression. Nature Communications 7: 11702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu M, Zhang Y, Zhang J, et al. (2018) MicroRNA-1253 suppresses cell proliferation and invasion of non-small-cell lung carcinoma by targeting WNT5A. Cell Death & Disease 9(2): 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baumgartner U, Berger F, HashemiGheinani A, et al. (2018) miR-19b enhances proliferation and apoptosis resistance via the EGFR signaling pathway by targeting PP2A and BIM in non-small cell lung cancer. Molecular Cancer 17(1): 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li J, Tan Q, Yan M, et al. (2014) miRNA-200c inhibits invasion and metastasis of human non-small cell lung cancer by directly targeting ubiquitin specific peptidase 25. Molecular Cancer 13: 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gattolliat CH, LeTeuff G, Combaret V, et al. (2014) Expression of two parental imprinted miRNAs improves the risk stratification of neuroblastoma patients. Cancer Medicine 3(4): 998–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chan CYT, Cheuk BLY, Cheng SWK. (2017) Abdominal aortic aneurysm-associated microrna-516a-5p regulates expressions of methylenetetrahydrofolate reductase, matrix metalloproteinase-2, and tissue inhibitor of matrix metalloproteinase-1 in human abdominal aortic vascular smooth muscle cells. Annals of Vascular Surgery 42: 263–273. [DOI] [PubMed] [Google Scholar]
- 10. Wang H, Chen W, Jin M, et al. (2018) CircSLC3A2 functions as an oncogenic factor in hepatocellular carcinoma by sponging miR-490-3p and regulating PPM1F expression. Molecular Cancer 17(1): 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sun HL, Cui R, Zhou J, et al. (2016) ERK activation globally downregulates miRNAs through phosphorylating exportin-5. Cancer Cell 30(5): 723–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. White NM, Chow TF, Mejia-Guerrero S, et al. (2010) Three dysregulated miRNAs control kallikrein 10 expression and cell proliferation in ovarian cancer. British Journal of Cancer 102: 1244–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Figure_S1 for MiR-516a-5p inhibits the proliferation of non-small cell lung cancer by targeting HIST3H2A by Xiang-Yun Ye, Ling Xu, Shun Lu and Zhi-Wei Chen in International Journal of Immunopathology and Pharmacology
Supplemental material, Figure_S2 for MiR-516a-5p inhibits the proliferation of non-small cell lung cancer by targeting HIST3H2A by Xiang-Yun Ye, Ling Xu, Shun Lu and Zhi-Wei Chen in International Journal of Immunopathology and Pharmacology
Supplemental material, Supplementary_data for MiR-516a-5p inhibits the proliferation of non-small cell lung cancer by targeting HIST3H2A by Xiang-Yun Ye, Ling Xu, Shun Lu and Zhi-Wei Chen in International Journal of Immunopathology and Pharmacology