Abstract
A better understanding of the immune profile of non-small cell lung cancer (NSCLC) and the immunomodulatory impact of chemotherapy is essential to develop current therapeutic approaches. Herein, we collected peripheral blood from 20 healthy donors and 50 patients with advanced NSCLC, before and after chemotherapy, followed by phenotypic analysis of lymphocyte subsets and assessment of the correlation between their post-chemotherapy levels and progression-free survival (PFS). Results showed that, before chemotherapy, the levels of CD8+ lymphocytes, PD-1+CD4+, Th2, and Th17 cells were elevated in patients’ peripheral blood, in contrast to natural killer (NK) cells and Th1 cells. Besides, there was no remarkable difference in the frequency of PD-1+CD8+ cells between patients and healthy controls. After chemotherapy, the levels of CD8+ lymphocytes, NK, Th2, Th17, and Treg were declined, in contrast to the level of Th1 cells which was markedly increased. Importantly, chemotherapy had no impact on the frequencies of PD-1+CD8+ and PD-1+CD4+ cells. PFS was significantly better in patients with low percentage of PD-1+CD4+ T cells than those with high percentage. Patients with high content of Th1 cells showed longer PFS than those with low content. The low percentages of Th17 and Treg cells were correlated with longer PFS, even though the difference did not reach statistical significance. In conclusion, the imbalance of lymphocyte subsets is a hallmark of NSCLC. Furthermore, the high level of PD-1+CD4+ cells plays a crucial role in the progression of NSCLC and could be used as a prognostic marker; and the high level of Th1 could predict better clinical outcomes of chemotherapy.
Keywords: chemotherapy, immunomodulatory, immunophenotyping, lymphocytes, NSCLC, PFS
Introduction
Among several types of tumor, lung cancer is considered one of the most fatal and still the main cause of cancer-related deaths. In the last few years, there has been a growing interest in lung cancer immunotherapy due to its promising results in achieving significance and durable treatment responses with minimal manageable toxicity.1 Immune cells play dual roles in suppressing or promoting tumor development, metastasis, and progression. So that more understanding of tumor microenvironment is important to explore novel immune-based biomarkers and improve the efficacy of tumor immunotherapy.2
Tumors develop several methods to avoid detection and elimination by the immune system and inhibit immune cell function. For example, lung cancer cells undergo a slow process of immunoediting,3 secrete soluble proteins which disturb the routine processing by antigen-presenting cells (APCs),4 create a dense fibrotic stroma,5 downregulate major histocompatibility (MHC) class I expression,6 induce the expansion of CD4+ FoxP3+ regulatory T cells,7 and upregulate myeloid-derived suppressor cells (MDSCs).8 Previous studies indicated that PDL-1 is overexpressed in tumor cells or in non-transformed cells in the tumor microenvironment by which PD-1/PD-L1 interaction inhibits the proliferation, survival, and effector function of cytotoxic lymphocytes (CTL), and thus induces apoptosis of tumor infiltrated lymphocytes (TILs).9
Some studies reported that chemotherapeutic agents do not only kill tumor cells, but they also damage normal cells, especially immune cells, and thus disturb the antitumor immune response.10 The goal of this study was to provide an exhaustive investigation of various lymphocyte subsets in peripheral blood of patients with advanced NSCLC and evaluate the immunomodulatory effect of chemotherapy. We also sought to elucidate the correlation between post-chemotherapy level of each lymphocyte subset, after two cycles of chemotherapy, and the progression-free survival (PFS) of patients with NSCLC.
Materials and methods
Samples, reagents, and cell line
Peripheral blood samples were collected from 50 patients with NSCLC before and after 1 week of the second cycle of chemotherapy and from 20 age-matched healthy donors (Table 1). Blood sample was obtained using BD Vacutainer tubes containing acid–citrate–dextrose anticoagulant, solution A (ACD-A; BD Biosciences), from which peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Paque gradient centrifugation. Samples were collected after approval by the “Institutional Health Research Ethics Authority” and signing a written informed consent by patients and healthy donors. All antibodies used for staining were purchased from BD Biosciences (San Jose, CA).
Table 1.
Patients’ characteristics.
| Age (median) | 61 |
| Gender | Male: 41 |
| Female: 9 | |
| Smoking | Smoker: 31 |
| Non-smoker: 19 | |
| Pathology | Adenocarcinoma |
| Stages | VI |
| Chemotherapy regimen | 1. Cisplatin/pemetrexed 2. Nedaplatin/pemetrexed |
| Chemotherapy line | First line: 45 patients |
| Second line: 4 patients | |
| Third line: 1 patient |
Isolation of PBMC by Ficoll-Paque density gradient centrifugation
PBMCs were isolated from peripheral blood using Ficoll-Paque as previously described.11 Briefly, blood was diluted with an equal volume of phosphate-buffered saline (PBS), pH 7.4, and layered over equal volume of Ficoll-Paque PLUS (GE Healthcare Bio-Sciences AB, Uppsala, Sweden). Gradients were centrifuged at 400g for 30 min at room temperature in a swinging-bucket rotor without the brake applied. PBMC interface was carefully removed by pipetting and washed for three times with PBS containing 2% fetal bovine serum (FBS) by centrifugation at 250g for 10 min. Pellets were suspended in red blood cells (RBCs) (Invitrogen, Carlsbad, CA) and incubated for 10 min at room temperature with gentle mixing to lyse contaminating RBC. This was followed by washing with PBS containing 2% FBS. The cell viability was assessed by trypan blue exclusion assay with more than 95% viability in the collected samples. Non-viable cells were identified by staining with trypan blue, and cell viability was calculated using the total cell count and the count of non-viable cells. PBMCs were cryopreserved in liquid nitrogen in FBS (Invitrogen, Carlsbad, CA) containing 10% dimethyl sulfoxide (DMSO; Thermo Fisher Scientific, Rockford IL) and stored until required for downstream analyses.
Flow cytometry
One million of isolated PBMCs were washed with cold PBS followed by 30 min of incubation at 4°C in the dark with fluorochrome-labeled antibodies. To detect CD8+ T lymphocytes expressing PD-1 molecule, 1 × 106 of isolated PBMCs were stained with PE-conjugated anti-human CD3, FITC-conjugated anti-human CD8, and APC-conjugated anti-human PD-1. For NK cells, 1 × 106 of PBMCs were stained with FITC-conjugated anti-human CD3, PE-Cy5-conjugated anti-human CD16, and APC-conjugated anti-human CD56. Treg cells were detected by staining 1 × 106 of PBMC with FITC-conjugated anti-human CD4, PE-conjugated anti-human CD25, and ALEXA FLUOR 647-CD127. Incubations with matched immunoglobulin isotypes were done in parallel as controls. After incubation with antibodies, cells were washed twice with 1 mL of PBS and analyzed with a BD FACSCalibur benchtop flow cytometry. The data were analyzed using FlowJo 7.6 software (Flowjo LLC, Ashland, OR, USA).
For Th1, Th2, and Th17 cells, 1 × 106 of PBMCs were cultured in a 48-well plate in the presence of leukocyte activation cocktail (BD Biosciences, cat# 550583) for 5 h at 37°C in 5% CO2. Then, cells were washed in PBS supplemented with 3% FBS and blocked for nonspecific binding in 30% FBS for 30 min. Surface staining was performed using FITC-conjugated anti-human CD4 and Alexa Fluor 647-conjugated anti-human CD3, followed by intracellular staining with Cytofix/Cytoperm Kit (eBioscience, San Jose, CA) in accordance with the manufacturer’s instructions. Briefly, cells were fixed and permeabilized with Cytofix/Cytoperm solution for 20 min on ice followed by washing in Perm/Wash solution. Next, cells were stained for 30 min on ice with Percp-cy 5.5-conjugated anti-human interferon gamma (IFN-γ), APC-conjugated anti-human interleukin-4 (IL-4), or PE-conjugated anti-human IL-17. Finally, cells were resuspended in PBS buffer and analyzed by a BD FACSCalibur benchtop flow cytometry. The data were analyzed using FlowJo 7.6 software (Flowjo, LLC).
Statistical analysis
GraphPad Prism 5.0 (GraphPad software, San Diego, CA, USA) was used for all statistical analysis. All data are reported as means ± SD (standard deviation) and compared using analysis of variance (ANOVA). P values < 0.05 were considered to be statistically significant. The Kaplan–Meier survival curves were plotted to evaluate PFS; difference between high and low for each variable was analyzed by log-rank (Mantel–Cox) test.
Results
CD3+CD8+ T cells, but not PD-1 expressing CD4+ and CD8+ T cells, were markedly decreased in the peripheral blood from patients with NSCLC after chemotherapy
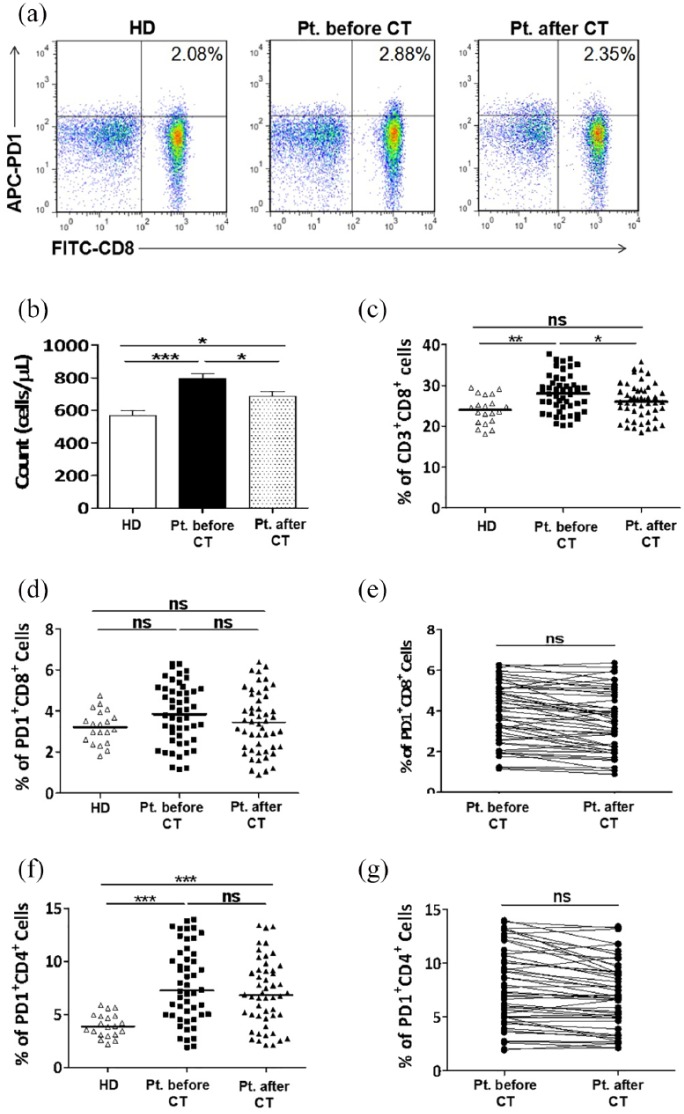
The absolute number and frequency of CD3+CD8+ T cells and PD-1 expression on CD8+ and CD4+ T cells were assessed in peripheral blood from healthy donors and patients with NSCLC before and after chemotherapy. Figure 1(a) is a representative flow cytometry showing the percentage of CD3+CD8+PD-1+ cells within PBMC from one healthy donor and from one patient before and after two cycles of chemotherapy.
Figure 1.
CD8+ T cells, but not PD-1 expressing CD4+ and CD8+ T cells, were significantly decreased in the peripheral blood from patients with NSCLC after two cycles of chemotherapy. PBMCs were collected from peripheral blood samples of 20 healthy donors and from 50 patients with NSCLC before and after chemotherapy: (a) Representative staining patterns of PD-1+ on CD8+ T cells (CD3+ gated) of one healthy donor and one patient before and after two cycles of chemotherapy. (b, c) The absolute number (b) and frequency (c) of CD3+CD8+ T cells in the peripheral blood of healthy donors and patients with NSCLC before and after chemotherapy. (d, f) Frequencies of PD-1-expressing CD3+CD8+ (d) and CD3+CD4+ (f) T cells in peripheral blood of healthy donors and patients with NSCLC before and after chemotherapy. (e, g) Representing the impact of chemotherapy on the frequencies of PD-1+CD8+ (e) and PD-1+CD4+ (g) T cells in peripheral blood of patients with NSCLC. HD: healthy donor; CT: chemotherapy.
As shown in Figure 1(b), the mean count of CD8+ T cells was significantly increased in peripheral blood from NSCLC patients compared to healthy donors (796.4 ± 31.06 versus 567.5 ± 31.36/µL). Similarly, their frequency was also elevated in patients with NSCLC compared to healthy subjects (28.05 ± 0.710% versus 23.98 ± 0.764%) (Figure 1(c)). After two cycles of chemotherapy, the mean absolute number of CD8+ T cells was declined to 687.1 ± 27.63/µL (Figure 1(b)), whereas their percentage was declined to 25.97 ± 0.664 without a significant difference before chemotherapy level and healthy subjects (Figure 1(c)).
The frequency of PD-1+ expression on CD8+ T cells had no statistically significant difference between patients with NSCLC and healthy controls (3.867 ± 0.214% versus 3.212 ± 0.179%) (Figure 1(d)), but it is important to mention that subgroup analysis showed that 41% of patients with NSCLC had high levels of PD-1 expression on their CD8+ T cells. Moreover, there was no obvious difference between the frequency of PD-1+CD8+ T cells before and after two cycles of chemotherapy (3.867 ± 0.214% versus 3.499 ± 0.223%) (Figure 1(d) and (e)).
The frequency of PD-1+ expression on CD4+ T cells was significantly higher in peripheral blood from patients with NSCLC than healthy donors (7.785 ± 0.542% versus 3.968 ± 0.244%) (Figure 1(f)), without a significant alteration after chemotherapy (6.968 ± 0.480) (Figure 1(f) and (g)).
These results indicate that the high level of CD8+ and PD-1+CD4+ T cells could be used as prognostic markers for NSCLC. More importantly, high expression of PD-1 molecule on CD4+ T cells could be responsible for the inhibition of antitumor immune activity in NSCLC; and the chemotherapy has no impact on the frequency of PD-1+CD8+ and PD-1+CD4+ T cells.
Chemotherapy significantly decreased the level of NK cells in the peripheral blood from patients with NSCLC
NK cells were characterized by harvesting PBMC from peripheral blood and staining for the following cell surface markers: CD3–, CD16+, and CD56+. Supplemental Figure 1(A) is a representative flow cytometry showing the percentage of CD3–CD16+CD-56+ cells within PBMC from one healthy donor and from one patient with NSCLC before and after two cycles of chemotherapy.
Results showed that the mean absolute number of NK cells in peripheral blood from patients with NSCLC was lower than healthy donors (199.0 ± 12.091 versus 261.1 ± 24.642/µL) (Supplemental Figure 1(B)). Besides, the frequency of NK cells in the peripheral blood from patients was also lower than that from healthy controls (22.18 ± 0.685% versus 26.33 ± 1.207%) (Supplemental Figure 1(C)). Results also showed that chemotherapy had a negative impact on the absolute count and the frequency of NK cells in patients with NSCLC, by which the mean absolute number was reduced to 165.3 ± 11.578/µL (Supplemental Figure 1(B)), and their frequency was reduced to 19.43 ± 0.582% (Supplemental Figure 1(C) and (D)).
These results may elucidate the mechanism of immune evasion by NSCLC through reducing the count and percentage of NK cells and referred to the cytotoxic impact of chemotherapeutic agents on NK cells.
Characterization of CD4+ T cell subsets (Th1, Th2, Th17, and Treg cells) in peripheral blood from patients with NSCLC
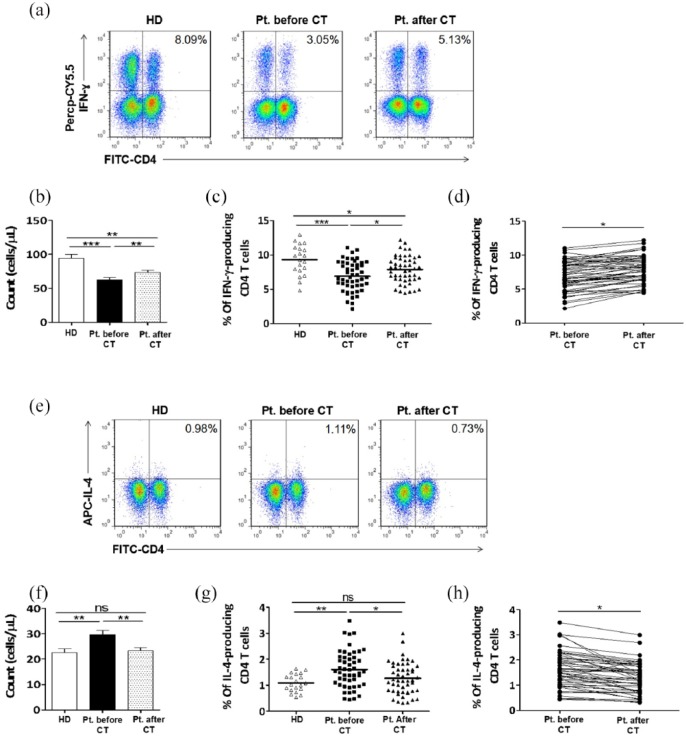
Th1 cells were characterized by IFN-γ production. Figure 2(a) is a representative flow cytometry showing the percentage of Th1 cells within peripheral blood from one healthy donor and from one patient with NSCLC before and after two cycles of chemotherapy. An elevated Th1 cell count has been observed in healthy donors compared to patients with NSCLC (94.05 ± 6.130 versus 62.91 ± 2.835/µL) (Figure 2(b)). Similarly, the frequency of Th1 cells in peripheral blood from healthy donors was also higher than that from patients with NSCLC (9.304 ± 0.489% versus 6.908 ± 0.320%) (Figure 2(c)). Interestingly, chemotherapy had a positive impact on the absolute count and the frequency of Th1 cells, by which the mean absolute number was increased to 73.70 ± 2.856/µL (Figure 2(b)), and their frequency was increased to 7.891 ± 0.289% (Figure 2(c) and (d)).
Figure 2.
Chemotherapy had positive and negative impacts on the number and frequency of Th1 cells and Th2 cells, respectively. PBMCs were collected from peripheral blood samples of 20 healthy donors and from 50 patients with NSCLC patients before and after chemotherapy. (a, e) Representative staining patterns of IFN-γ (a) and IL-4 (e) production by CD4+ T cells (CD3+ gated) of one healthy donor and one patient before and after two cycles of chemotherapy. (b, c) The absolute number (b) and frequency (c) of Th1 cells in the peripheral blood of healthy donors and patients with NSCLC before and after chemotherapy. (f, g) The absolute number (f) and frequency (g) of Th2 cells in the peripheral blood of healthy donors and patients with NSCLC before and after chemotherapy. (d, h) Representing the impact of chemotherapy on the frequencies of Th1 cells (d) and Th2 cells (h) in peripheral blood of patients with NSCLC. HD: healthy donor; CT: chemotherapy.
Th2 cells were characterized by IL-4 production. Figure 2(e) is a representative flow cytometry showing the percentage of Th2 cells within PBMC from one healthy donor and from one patient before and after two cycles of chemotherapy. The mean absolute number and percentage of Th2 cells were obviously higher in patients with NSCLC compared to healthy donors (29.72 ± 1.566 versus 22.60 ± 1.470/µL) (Figure 2(f)) and (1.606 ± 0.108% versus 1.086 ± 0.076%) (Figure 2(g)), respectively. In contrast to Th1 cells, the mean absolute number and frequency of Th2 cells were significantly reduced to 23.32 ± 1.186/µL (Figure 2(f)) and 1.236 ± 0.091 (Figure 2(g) and (h)), respectively, after two cycles of chemotherapy.
Th17 cells were characterized by IL-17. Supplemental Figure 2(A) is a representative flow cytometry showing the percentage of Th17 cells within PBMC from one healthy donor and from one patient before and after two cycles of chemotherapy. The mean absolute number and frequency of Th17 cells were significantly elevated in peripheral blood from patients with NSCLC compared to that from healthy donors (mean absolute number: 16.60 ± 1.158 versus 10.75 ± 0.897/µL) (Supplemental Figure 2(B)) and (frequency: 1.716 ± 0.097% versus 1.372 ± 0.063%) (Supplemental Figure 2(C)). After two cycles of chemotherapy, the mean absolute number of Th17 cells was declined to 12.79 ± 0.972/µL (Supplemental Figure 2(B)) and their frequency was decreased to 1.449 ± 0.086% (Supplemental Figure 2(C) and (D)).
The absolute number and frequency of Treg cells were also determined and assessed as CD4+CD25+CD127–. Supplemental Figure 2(E) is a representative flow cytometry showing the percentage of Treg cells within PBMC from one healthy donor and from one patient before and after two cycles of chemotherapy. The mean absolute number of Treg cells was significantly increased in patients with NSCLC (50.12 ± 2.620/µL) compared to healthy donors (32.10 ± 1.831/µL) (Supplemental Figure 2(F)). Similarly, the frequency of Treg cells was also higher in patients than that from healthy controls (6.349 ± 0.258% versus 4.226 ± 0.260%) (Supplemental Figure 2(G)). Herein, chemotherapy had a negative impact on Treg cells, by which their mean absolute number was decreased to 42.02 ± 2.251/µL (Supplemental Figure 2(F)) and their frequency was declined to 5.451 ± 0.226% (Supplemental Figure 2(G) and (H)).
Collectively, these results may indicate that the low level of Th1 cells and the high levels of Treg, Th2, and Th17 cells may play a decisive role in the antitumor immunity during the progression of NSCLC. Further studies could be needed to define the exact role of Th17 cells in NSCLC due to their dual function. Chemotherapy could have a potential to stimulate antitumor immunity via increasing the level of IFN-γ production by Th1 cells and reducing the levels of Treg, Th2, and Th17 cells.
The correlation between post-chemotherapy levels of lymphocyte subsets and prognosis
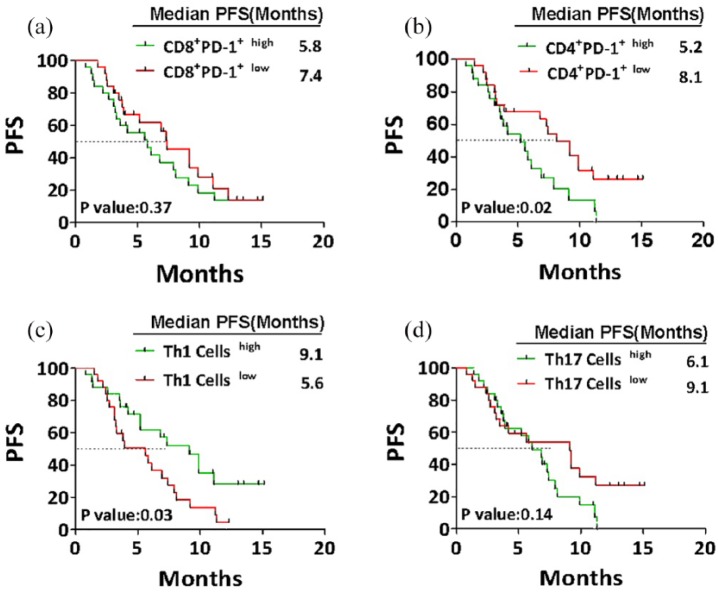
After two cycles of chemotherapy, 50 patients with NSCLC were divided into two groups, each with 25 patients according to the median relative contents of each immune cell in their peripheral blood. The median value was the cutoff to define the (high) and (low) contents of CD8+ T cells (median = 25.91%), PD-1+CD8+ T cells (median = 3.42%), PD-1+CD4+ T cells (median = 6.70%), NK cells (median = 19.40%), Th1 cells (median = 7.71%), Th2 cells (median = 1.16%), Th17 cells (median = 1.38), and Treg cells (median = 5.18%). Thereafter, the correlation between the relative contents of previous immune cells and patients’ PFS was analyzed by a Kaplan–Meier plot.
Results showed that the frequency of PD-1+CD8+ T cells had no remarkable correlation with PFS in NSCLC. Although the PFS in patients with high content of PD-1+CD8+ T cells was shorter than that in low content group, the difference was not statistically significant (median PFS = 5.8 versus 7.4 months; P = 0.377, HR = 1.341, 95% confidence interval (CI) = 0.699–2.657) (Figure 3(a)). In contrast, the low frequency of PD-1+CD4+ T cells significantly correlated with longer PFS compared with high frequency group (median PFS = 8.1 versus 5.2 months, P = 0.021, HR = 2.259, 95% CI = 1.127–4.525) (Figure 3(b)).
Figure 3.
The correlation between post-chemotherapy levels of lymphocyte subsets and patients’ PFS. After two cycles of chemotherapy, 50 patients with NSCLC were divided into two groups each with 25 patients according to the median frequency of CD8+PD-1+, CD4+PD-1+, Th1 cells, and Treg cells. The median value was assessed as cutoff point to define “high” and “low” contents. Kaplan–Meier curves representing the correlation between progression-free survival (PFS) of patients and the relative contents of CD8+PD-1+ (a), CD4+PD-1+ (b), Th1 cells (c), and Treg cells (d). Statistical significance was determined by a log-rank (Mantel–Cox) test.
Patients with high frequency of Th1 cells showed longer PFS than those with low frequency with a clear statistical difference (median PFS = 9.1 versus 5.6 months, P = 0.030, HR = 0.480, 95% CI = 0.247–0.931) (Figure 3(c)). Furthermore, low frequency of Th17 correlated with longer PFS compared to high frequency group, even though the difference did not reach statistical significance (median PFS = 9.1 versus 6.1 months, P = 0.142, HR = 1.647, 95% CI = 0.844–3.209) (Figure 3(d)). Similarly, the low level of Treg cells was correlated with longer PFS compared to high level group without a clear statistical difference (Table 2). There was no significant correlation between clinical outcomes and the frequency of CD8+ T cells, NK, and Th2 cells in different groups (Table 2). Finally, we analyzed the response to treatment in patients with high and low contents of CD4+PD-1+, Th1, and Treg cells (Supplemental Figure 3).
Table 2.
Comparison of median PFS between patients based on the contents of CD8+ T cells, NK, Th2, and Th17 cells.
| High | Low | HR | 95% CI | P value | |
|---|---|---|---|---|---|
| CD8+ T cells | 7.3 months | 5.8 months | 1.091 | 0.564–2.171 | 0.818 |
| NK cells | 7.3 months | 5.6 months | 0.592 | 0.372–1.423 | 0.251 |
| Th2 cells | 6.1 months | 7.9 months | 1.429 | 0.729–2.798 | 0.298 |
| Treg | 6.8 months | 9.1 months | 1.587 | 0.817–3.082 | 0.172 |
PFS: progression-free survival; CI: confidence interval.
In conclusion, these results indicate that the expression of PD-1 on CD4+ T cells might play the main role in the progression of NSCLC. Besides, the low level of Th1 cells and high levels of Treg and Th17 cells could be correlated with worse clinical outcomes in patients with NSCLC.
Discussion
A better understanding of NSCLC immunology and the immunomodulatory impact of chemotherapy on immune cells could be a cornerstone to improve the current treatment choices and facilitates finding novel strategies.
CD8+ T cells are functionally impaired and are poorly responsive in lung cancer patients.12 Our results showed that there was no correlation between the high level of CD8+ T cells and clinical outcomes. Results also demonstrated that the high expression level of PD-1 on CD4+ T, but not CD8+, cells is correlated with poor clinical outcomes, suggesting that PD-1+CD4+ T cells play more decisive role than PD-1+CD8+ T cells in NSCLC.
We found that after chemotherapy, 70% of patients with progressed disease had high levels of Treg cells in their blood, indicating the NSCLC-induced immune suppression could be resulted from the effect of Treg cells. Interestingly, results revealed that the level of Th1 cells was increased after chemotherapy and correlated with better clinical outcomes, indicating the importance of blood content of Th1 cells and their IFN-γ.
Even though our findings indicated that chemotherapy had no impact on the frequency of PD-1 expression on CD8+ or CD4+ T cells of peripheral blood, we found that chemotherapeutic agents such as cisplatin and paclitaxel could upregulate the expression of PD-L1 on A549 human lung cancer cells in vitro in a dose-dependent manner (Supplemental Figure 4). We also found that chemotherapy could be beneficial in inducing antitumor immune response through decreasing the levels of Treg, Th2, and Th17 cells and increasing the level of Th1 cells.
This study demonstrated that NSCLC is strongly associated with the imbalance of lymphocyte subsets and proved that the immunomodulatory impact of chemotherapy could be beneficial for NSCLC treatment through downregulation of immunosuppressive lymphocytes. We believe that future multicenter studies with larger sample sizes are required to confirm these results.
Supplemental Material
Supplemental material, Supplementary_Figure_1 for Immunomodulatory effects of chemotherapy on blood lymphocytes and survival of patients with advanced non-small cell lung cancer by Mohanad Aldarouish, Xiangyu Su, Jianbing Qiao, Chanchan Gao, Yan Chen, Anwei Dai, Tianyu Zhang, Yongqian Shu and Cailian Wang in International Journal of Immunopathology and Pharmacology
Supplemental Material
Supplemental material, Supplementary_Figure_2 for Immunomodulatory effects of chemotherapy on blood lymphocytes and survival of patients with advanced non-small cell lung cancer by Mohanad Aldarouish, Xiangyu Su, Jianbing Qiao, Chanchan Gao, Yan Chen, Anwei Dai, Tianyu Zhang, Yongqian Shu and Cailian Wang in International Journal of Immunopathology and Pharmacology
Supplemental Material
Supplemental material, Supplementary_Figure_3 for Immunomodulatory effects of chemotherapy on blood lymphocytes and survival of patients with advanced non-small cell lung cancer by Mohanad Aldarouish, Xiangyu Su, Jianbing Qiao, Chanchan Gao, Yan Chen, Anwei Dai, Tianyu Zhang, Yongqian Shu and Cailian Wang in International Journal of Immunopathology and Pharmacology
Supplemental Material
Supplemental material, Supplementary_Figure_4 for Immunomodulatory effects of chemotherapy on blood lymphocytes and survival of patients with advanced non-small cell lung cancer by Mohanad Aldarouish, Xiangyu Su, Jianbing Qiao, Chanchan Gao, Yan Chen, Anwei Dai, Tianyu Zhang, Yongqian Shu and Cailian Wang in International Journal of Immunopathology and Pharmacology
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: This study was approved by the Medical Ethics Committee of Zhongda Hospital affiliated to Southeast University and carried out in accordance to the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (No. 81271699).
Informed consent: Informed consent was obtained from all individual participants included in the study.
ORCID iD: Mohanad Aldarouish  https://orcid.org/0000-0001-9470-0224
https://orcid.org/0000-0001-9470-0224
Supplemental material: Supplemental material for this article is available online.
References
- 1. Aldarouish M, Wang C. (2016) Trends and advances in tumor immunology and lung cancer immunotherapy. Journal of Experimental and Clinical Cancer Research: CR 35(1): 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Markman JL, Shiao SL. (2015) Impact of the immune system and immunotherapy in colorectal cancer. Journal of Gastrointestinal Oncology 6(2): 208–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Matsushita H, Vesely MD, Koboldt DC, et al. (2012) Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature 482(7385): 400–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kida H, Ihara S, Kumanogoh A. (2013) Involvement of STAT3 in immune evasion during lung tumorigenesis. Oncoimmunology 2(1): e22653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Salmon H, Donnadieu E. (2012) Within tumors, interactions between T cells and tumor cells are impeded by the extracellular matrix. Oncoimmunology 1(6): 992–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ramnath N, Tan D, Li Q, et al. (2006) Is downregulation of MHC class I antigen expression in human non-small cell lung cancer associated with prolonged survival? Cancer Immunology, Immunotherapy: CII 55(8): 891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Woo EY, Yeh H, Chu CS, et al. (2002) Cutting edge: Regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. Journal of Immunology: Official Journal of the American Association of Immunologists 168(9): 4272–4276. [DOI] [PubMed] [Google Scholar]
- 8. Dieu-Nosjean MC, Antoine M, Danel C, et al. (2008) Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology 26(27): 4410–4417. [DOI] [PubMed] [Google Scholar]
- 9. Qin A, Coffey DG, Warren EH, et al. (2016) Mechanisms of immune evasion and current status of checkpoint inhibitors in non-small cell lung cancer. Cancer Medicine 5(9): 2567–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rebe C, Ghiringhelli F. (2015) Cytotoxic effects of chemotherapy on cancer and immune cells: How can it be modulated to generate novel therapeutic strategies? Future Oncology (London, England) 11(19): 2645–2654. [DOI] [PubMed] [Google Scholar]
- 11. Pham TNQ, King D, Macparland SA, et al. (2008) Hepatitis C virus replicates in the same immune cell subsets in chronic hepatitis C and occult infection. Gastroenterology 134(3): 812–822. [DOI] [PubMed] [Google Scholar]
- 12. Prado-Garcia H, Romero-Garcia S, Aguilar-Cazares D, et al. (2012) Tumor-induced CD8+ T-cell dysfunction in lung cancer patients. Clinical & Developmental Immunology 2012: 741741. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Figure_1 for Immunomodulatory effects of chemotherapy on blood lymphocytes and survival of patients with advanced non-small cell lung cancer by Mohanad Aldarouish, Xiangyu Su, Jianbing Qiao, Chanchan Gao, Yan Chen, Anwei Dai, Tianyu Zhang, Yongqian Shu and Cailian Wang in International Journal of Immunopathology and Pharmacology
Supplemental material, Supplementary_Figure_2 for Immunomodulatory effects of chemotherapy on blood lymphocytes and survival of patients with advanced non-small cell lung cancer by Mohanad Aldarouish, Xiangyu Su, Jianbing Qiao, Chanchan Gao, Yan Chen, Anwei Dai, Tianyu Zhang, Yongqian Shu and Cailian Wang in International Journal of Immunopathology and Pharmacology
Supplemental material, Supplementary_Figure_3 for Immunomodulatory effects of chemotherapy on blood lymphocytes and survival of patients with advanced non-small cell lung cancer by Mohanad Aldarouish, Xiangyu Su, Jianbing Qiao, Chanchan Gao, Yan Chen, Anwei Dai, Tianyu Zhang, Yongqian Shu and Cailian Wang in International Journal of Immunopathology and Pharmacology
Supplemental material, Supplementary_Figure_4 for Immunomodulatory effects of chemotherapy on blood lymphocytes and survival of patients with advanced non-small cell lung cancer by Mohanad Aldarouish, Xiangyu Su, Jianbing Qiao, Chanchan Gao, Yan Chen, Anwei Dai, Tianyu Zhang, Yongqian Shu and Cailian Wang in International Journal of Immunopathology and Pharmacology