Abstract
OBJECTIVE:
This study intended to assess the occurrence of early signs of middle cerebral artery (MCA) on multidetector computed tomography (MDCT) in correlation with duration of the clinical features of stroke.
PATIENTS AND METHODS:
This retrospective study analyzed the electronic records of 20 patients with MCA infarction. The detected signs studied according to the onset of the clinical features of stroke to the time of CT imaging.
RESULTS:
Out of 20 patients with MCA infarction included in this study, the results revealed a significant relationship between the presence of insular ribbon sign and/or subtle hypodensity and hyperacute infarction (P < 0.001 and 0.003, respectively). Results revealed significant relationship between the occurrence of hypodense area, effacement of the cortical sulci, and compression of the ipsilateral lateral ventricle with acute infarction (6–72 h), (P = 0.006, 0.007, and 0.002) (odds ratio = 0.047, 0.050 and 0.028) and (95% confidence interval = 0.004–0.552, 0.004–0.597 and 0.002–0.367) respectively.
CONCLUSION:
MDCT can detect nearly half of MCA infarctions in the first 6 h. Insular ribbon sign and subtle hypodensity were the most significant findings in the first 6 h of stroke. Hypodense area was a significant sign after 6 h. Diabetes mellitus and ischemic heart disease were the most common risk factors. Hemiparesis was the most common clinical finding in MCA infarction.
Keywords: Early signs, middle cerebral artery infarction, multidetector computed tomography
Introduction
Brain infarction is irreversible damage to the brain tissue due to inadequate blood supply to the brain tissue because of occlusion of main cerebral artery either by thrombus or embolism. Stroke is a significant cause of death worldwide and a significant cause of long-term disability even in developed countries. Ischemic stroke (Infarction) is the majority (approximately 85%) of cerebral stroke.[1]
Cerebral infarction affects left cerebral hemisphere more than the right and most commonly affects the middle cerebral artery (MCA).[2] The incidence was reported to be higher in males than females before 85 years old.[3] Subclinical brain infarction affects nearly fifth of the stroke-free elderly population and significantly increases the risk of future stroke.[4]
Brain imaging has a significant role in early diagnosis, confirmation of brain infarction, and exclusion of hemorrhagic stroke. Computed tomography (CT) can usually detect signs of hyperacute infarction (<6 h), but it appears healthy in nearly 30% of cases.[5]
Early signs of brain infarction are hyperdense MCA sign, loss of insular ribbon, loss of the gray-white matter junction, effacement of the cortical sulci, subtle hypodensity in the cerebral tissue, parenchymal hypodensity, cerebral edema with compression of the ipsilateral lateral ventricle, and midline shift.[6]
Noncontrast CT remains the primary imaging modality for evaluation of patients with clinical features of acute stroke.[7]
This study aims to assess the detection rate of the early signs of MCA infarction on multidetector CT (MDCT) imaging in the first 3 days and to correlate between the time of occurrence of each sign on CT scan and the phase of infarction. This study is significant to highlight the role of MDCT imaging modality in the diagnosis of infarction in the first 6 h that is a critical time to avoid irreversible damage to the brain.
Patients and Methods
This was a cross-sectional retrospective study that involves 20 cases of hyperacute and acute brain infarction. The data were collected from the preserved electronic files in the hospital. All the patients underwent imaging of the brain by CT in the period from April 2017 to April 2018. Inclusion criteria were as follows: (1) patients with available time between first complaining and imaging, (2) patients with hyperacute and acute infarction, and (3) patients with a confirmed diagnosis. Exclusion criteria were as follows: (1) patients with infarction but no available full history, (2) patients with subacute infarction (older than three days), (3) patients with chronic infarction (older than two weeks), (4) patients with hemorrhagic stroke, (4) patients with brain neoplasms or other pathologies, and (5) patients with normal brain CT. We compared the occurrence of the early signs of MCA in acute and subacute phases.
All the selected cases were reported by a high-qualified radiologist with 4 years' postdoctorate experience in CT imaging. Another high-qualified radiologist independently reported all the selected cases again to decrease the interobserver variance.
Ethics
Institutional ethical approval was obtained for this study. Data were collected from the preserved CT reports of the patients. Confidentiality of the patient's personal information was taken in mind.
Procedure
A 64-slice multidetector siemens CT machine was used in imaging all of the patients. The patients scanned according to the acute stroke CT protocol of brain imaging.[8]
Statistical analysis
Data were analyzed by the Statistical Package for the Social Sciences (SPSS), IBM, SPSS, version 23, USA. Descriptive statistics was reported as frequencies and percentages. Chi-square test and multivariate logistic regression were performed to describe the relationship between the variables.
Results
A total of 20 patients underwent CT imaging of the brain; age ranges from 10 years to 95 years (the mean age = 57.75 years). Males were affected more than females (13 [65%] vs. 7 [35%]). There was no significant difference between the affected sides of the brain (55% right vs. 45% left) as shown in Table 1.
Table 1.
Gender of the involved patients and the affected side of the brain
| Variables | Parameters | Frequency (%) | P |
|---|---|---|---|
| Gender | Male | 13 (65) | 0.18 |
| Female | 7 (35) | ||
| Affected side | Right | 11 (55) | 0.655 |
| Left | 9 (45) |
Exactly 45% of cases detected in the first 6 h (hyperacute stage) and 55% detected from 6 h to 3 days after the attack of stroke as demonstrated in Table 2.
Table 2.
Duration of clinical features of infarction before computed tomography imaging of the brain
| Period from beginning of the stroke signs | Frequency (%) |
|---|---|
| Hyperacute (<6 h) | 9 (45) |
| Acute (6-72 h) | 11 (55) |
| Total | 20 (100.0) |
Correlation between the stage of infarction and signs on CT scan revealed a significant relationship between the phase of infarction and insular ribbon sign, subtle hypodensity, hypodense area, effacement of the cortical sulci, and compression of the ipsilateral lateral ventricle (P = 0.001, 0.003, 0.006, 0.007, and 0.002, respectively)– [Table 3].
Table 3.
Correlation between the occurrence of signs of MCA infarction on MDCT and the stage of infarction
| Sign of infarction | Stage of infarction | Positive, n (%) | Negative, n (%) | Nonapplicable, n (%) | Total, n (%) | Significance | P |
|---|---|---|---|---|---|---|---|
| Hyperdense MCA sign | Hyperacute | 5 (55.6) | 4 (44.4) | 0 (0.0) | 9 (100) | No | 0.423 |
| Acute | 8 (72.7) | 3 (27.3) | 0 (0.0) | 11 (100) | |||
| Insular ribbon sign | Hyperacute | 3 (33.3) | 5 (55.6) | 1 (11.1) | 9 (100) | Positive | 0.001 |
| Acute | 1 (9.1) | 0 (0.0) | 10 (90.9) | 11 (100) | |||
| Hypodense area | Hyperacute | 1 (11.1) | 8 (88.9) | 0 (0.0) | 9 (100) | Positive | 0.006 |
| Acute | 8 (72.7) | 3 (27.3) | 0 (0.0) | 11 (100) | |||
| Subtle hypodensity | Hyperacute | 5 (55.6) | 3 (33.3) | 1 (11.1) | 9 (100) | Positive | 0.003 |
| Acute | 0 (0.0) | 2 (18.2) | 9 (81.8) | 11 (100) | |||
| Loss of GM/WM interface | Hyperacute | 1 (5%) | 8 (40) | 0 (0.0) | 9 (40) | No | 0.275 |
| Acute | 0 (0.0) | 11 (55) | 0 (0.0) | 11 (55) | |||
| Effacement sulci | Hyperacute | 3 (33.3) | 6 (66.7) | 0 (0.0) | 9 (100) | Positive | 0.007 |
| Acute | 10 (90.9) | 1 (11.1) | 0 (0.0) | 11 (100) | |||
| Compression of ventricle | Hyperacute | 1 (11.1) | 8 (88.9) | 0 (0.0) | 9 (100) | Positive | 0.002 |
| Acute | 9 (81.8) | 2 (18.2) | 0 (0.0) | 11 (100) | |||
| Shifting midline | Hyperacute | 1 (11.1) | 8 (88.9) | 0 (0.0) | 9 (100) | No | 0.194 |
| Acute | 4 (36.4) | 7 (63.6) | 0 (0.0) | 11 (100) |
The table revealed a significant relationship between the stage of infarction and insular ribbon sign, hypodense area, and subtle hypodensity, effacement of the cortical sulci and compression of the ipsilateral lateral ventricle. No significant relationship between the stage of infarction and hyperdense MCA sign, loss of GM/WM interface or shifting of the midline. MCA: Middle cerebral artery, GM: Grey matter, WM: White matter
Table 4 revealed that diabetes mellitus (DM), ischemic heart disease (IHD), and hypertension (HTN) were risk factors in 10%, 10%, and 5% of the patients, respectively. Most cases (70%) had no obvious risk factors. All cases of MCA infarction presented with hemiparesis, 15% with facial palsy, 15% with aphasia or dysarthria, and only 5% with sudden blindness as shown in Figure 1.
Table 4.
Risk factors in patients with early middle cerebral artery infarctions
| Risk factor | Number and percentage of patients, n (%) | Notes |
|---|---|---|
| No obvious risk factors | 14 (70) | Most cases |
| DM, IHD, HTN | 2 (10) | Multiple risk factors |
| DM, HTN | 1 (5) | Multiple risk factors |
| DM, IHD | 1 (5) | Multiple risk factors |
| IHD | 1 (5) | Single risk |
| RHD (MS and AR) | 1 (5) | Single risk |
DM: Diabetes mellitus, IHD: Ischemic heart disease, HTN: Hypertension, RHD: Rheumatic heart disease, MS: Mitral stenosis, AR: Aortic regurgitation
Figure 1.
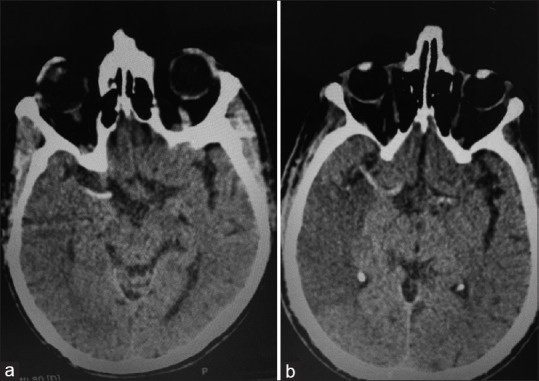
Noncontrast computed tomography images of different patients show (a) hyperdense middle cerebral artery signs in a patient with right middle cerebral artery hyperacute infarction. (b) hyperdense middle cerebral artery sign with obvious hypodense area in the territory of the right middle cerebral artery in an acute right middle cerebral artery infarction
Discussion
Early diagnosis of MCA infarction is a critical point for management to avoid severe complications and persistent handicap. This study focused on the role of MDCT scan in the detection of the early signs of MCA infarction in hyperacute and acute stages.
In this study, hyperdense MCA sign was positive in 55.6% of cases in the first 6 h and 72.7% from 6 to 72 h. This was reported by Abd Elkhalek et al., as an accurate early sign for the diagnosis of MCA infarction with 100% sensitivity and 60% specificity.[9] However; this sign appeared in both hyperacute and acute infarction without significant difference. Chavhan and Shroff reported that hyperdense MCA sign occurs with the 2ndh from the onset of symptoms of stroke.[10]
The insular ribbon sign was observed as a reflection of edema in early infarction in 20% of cases of this study. It was significantly related to the hyperacute phase of infarction (within the first 6 h). This result was consistent with Sarikaya and Provenzale, who reported that insular ribbon sign occurred in 70% of cases at 2 h and occurred in 77% at 3 h after onset symptoms of stroke.[11]
Hypodense area on CT scan was occurred predominantly after 6 h (acute phase) in this study. It revealed a significant association with acute stroke rather than hyperacute. Consistently, Sarikaya and Provenzale reported this sign in 53% of cases at 6 h.[11] Another previous study by Aronovich et al. stated cerebral tissue hypodensity in the majority of patients in early ischemic stroke.[12]
In the current study, subtle hypodensity of cerebral tissue was detected in the first 6 h in 55.6% of cases and not discovered after 6 h. This sign is a significant predictor of hyperacute MCA infarction as reported by von Kummer et al.[13]
The effacement of cortical sulci and loss of gray-white matter interface were common CT findings in acute and hyperacute infarction. The current study observed that effacement of cortical sulci was more evident with increasing duration from the onset of stroke. Thus, it is more common in after 6 h than before. Similar to these findings, Nakano et al. reported that effacement of cortical sulci was an advanced sign of infarction and a predictor of hemorrhagic transformation.[14]
Compression of lateral ventricles and shifting of the midline were signs of early infarction because of cerebral edema. In this study, these signs were predominant after 6 h than before. In contrast to this study, Dostovic et al. reported cerebral edema only in 7.9% of acute cerebral infarction.[15]
In this study, four patients had more than one risk factor, and the most common risk factor was DM than IHD and HTN. These results are not compatible with Safeer et al., who reported that HTN was the most common risk factor.[16]
All patients in our study were presented with hemiparesis [Figure 2]. This result is consistent with Heiss, who reported that hemiparesis was the most clinical feature in malignant MCA infarction.[17]
Figure 2.
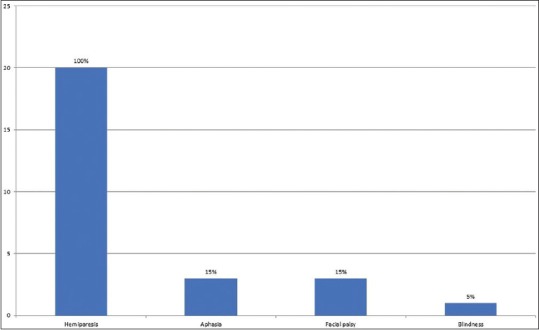
The clinical features of the patients with MCA
Regarding clinical features, all patients included in this study presented with sudden onset hemiparesis, only 15% of patients associated with either aphasia/dysarthria or facial palsy. These results are nearly similar to a previous study by Yew and Cheng (2009), who reported that 96% of ischemic stroke presented with sudden onset clinical features. They reported arm weakness in 63% and leg weakness in 54% of ischemic cases in their study.[18]
Limitations of this study
This study was limited by the following: (1) the sample size was not large enough and (2) the retrospective nature of the study that did not permit to follow up of the patients and record the physiopathological threshold and related pathological changes of each patient that is another parameter affecting the CT appearance of infarction.
Conclusion
MDCT can detect nearly half of MCA infarctions in the first 6 h. Insular ribbon sign and subtle hypodensity were significant findings in the first 6 h of the cerebral ischemic stroke. Hypodense area was a significant sign after 6 h. Hyperdense MCA sign is an essential sign in both hyperacute and acute phases of MCA infarction. DM and IHD were the most common risk factors. Hemiparesis was the most common clinical finding in MCA infarction.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Gibson CL. Cerebral ischemic stroke: Is gender important? J Cereb Blood Flow Metab. 2013;33:1355–61. doi: 10.1038/jcbfm.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hedna VS, Bodhit AN, Ansari S, Falchook AD, Stead L, Heilman KM, et al. Hemispheric differences in ischemic stroke: Is left-hemisphere stroke more common? J Clin Neurol. 2013;9:97–102. doi: 10.3988/jcn.2013.9.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turtzo LC, McCullough LD. Sex differences in stroke. Cerebrovasc Dis. 2008;26:462–74. doi: 10.1159/000155983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta A, Giambrone AE, Gialdini G, Finn C, Delgado D, Gutierrez J, et al. Silent brain infarction and risk of future stroke: A systematic review and meta-analysis. Stroke. 2016;47:719–25. doi: 10.1161/STROKEAHA.115.011889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Islam MN, Kuddus R, Chowdhury NS, Akhter MD, Salahuddin G, Parvin S, et al. Radiologic evaluation of hyperacute brain infarction: A review. Mymensingh Med J. 2014;23:621–35. [PubMed] [Google Scholar]
- 6.Hand P, Wardlaw J. CT for acute ischemic stroke. Pract Neurol. 2001;1:82–92. [Google Scholar]
- 7.Birenbaum D, Bancroft LW, Felsberg GJ. Imaging in acute stroke. West J Emerg Med. 2011;12:67–76. [PMC free article] [PubMed] [Google Scholar]
- 8.Leiva-Salinas C, Wintermark M, Kidwell CS. Neuroimaging of cerebral ischemia and infarction. Neurotherapeutics. 2011;8:19–27. doi: 10.1007/s13311-010-0004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abd Elkhalek YI, Elia RZ. Qualitative and quantitative value of hyperdense MCA sign as a prognostic marker for infarction. Egypt J Radiol Nucl Med. 2016;47:1043–8. [Google Scholar]
- 10.Chavhan GB, Shroff MM. Twenty classic signs in neuroradiology: A pictorial essay. Indian J Radiol Imaging. 2009;19:135–45. doi: 10.4103/0971-3026.50835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarikaya B, Provenzale J. Frequency of various brain parenchymal findings of early cerebral ischemia on unenhanced CT scans. Emerg Radiol. 2010;17:381–90. doi: 10.1007/s10140-010-0870-2. [DOI] [PubMed] [Google Scholar]
- 12.Aronovich BD, Reider-Groswasser II, Segev Y, Bornstein NM. Early CT changes and outcome of ischemic stroke. Eur J Neurol. 2004;11:63–5. doi: 10.1046/j.1351-5101.2003.00708.x. [DOI] [PubMed] [Google Scholar]
- 13.von Kummer R, Dzialowski I, Gerber J. Therapeutic efficacy of brain imaging in acute ischemic stroke patients. J Neuroradiol. 2015;42:47–54. doi: 10.1016/j.neurad.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Nakano S, Iseda T, Yoneyama T, Wakisaka S. Early CT signs in patients with acute middle cerebral artery occlusion: Incidence of contrast staining and haemorrhagic transformations after intra-arterial reperfusion therapy. Clin Radiol. 2006;61:156–62. doi: 10.1016/j.crad.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 15.Dostovic Z, Dostovic E, Smajlovic D, Ibrahimagic OC, Avdic L. Brain edema after ischaemic stroke. Med Arch. 2016;70:339–41. doi: 10.5455/medarh.2016.70.339-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Safeer M, Tariq M, ur Rahman U. Frequency of risk factors of cerebral infarction in stroke patients. A study of 100 cases in Nasser Teaching Hospital, Peshawar. Pak J Med Sci. 2008;24:109–13. [Google Scholar]
- 17.Heiss WD. Malignant MCA infarction: Pathophysiology and imaging for early diagnosis and management decisions. Cerebrovasc Dis. 2016;41:1–7. doi: 10.1159/000441627. [DOI] [PubMed] [Google Scholar]
- 18.Yew KS, Cheng E. Acute stroke diagnosis. Am Fam Physician. 2009;80:33–40. [PMC free article] [PubMed] [Google Scholar]


