Abstract
Background
Atypical fibroxanthoma (AFX) is a tumor that commonly presents on the head or neck in older individuals. Making a definitive diagnosis of AFX is challenging, and frequently, it is hard to distinguish from pleomorphic dermal sarcoma (PDS). There are no clear recommendations regarding the treatment of AFX, but an extensive surgery is actually considered the best option. Electrochemotherapy (ECT) is a novel therapeutic modality of local treatment in which the application of electrical pulses, enhancing cell membrane permeability, allows greater intracellular accumulation of chemotherapy drugs in the skin or subcutaneous tumors.
Case Report
We report a case of a 78-year-old male affected by a red, ulcerative, dermal, scalp nodule, which was treated with ECT with a complete clinical response. We have also reported literature data on this topic.
Results
In this case, ECT showed to be an effective and safe treatment for recurrent neoplasms of the head and neck, considering the complete response obtained and the absence of disease relapse after two years.
Conclusion
To the best of our knowledge, this is the first case report that shows great clinical results using ECT after surgery in relapsed AFX/PDS. However, more studies are needed to confirm our results.
1. Introduction
Electrochemotherapy (ECT) is the most developed electroporation-based cancer treatment, and it demonstrated to be highly effective, with complete response rates between 60% and 70% and objective response (complete and partial response) rates of 80% in primary or relapsed cutaneous tumors [1, 2]. This technique is based on the principle of electroporation (EP), the temporary increasing of cell membrane permeability that occurs when short high-voltage electric pulses are applied that allows a better penetration in the cells of hydrophilic drugs, which otherwise would not pass the membrane. The chemotherapeutic agents most commonly used in association with ECT are bleomycin and cisplatin, both administrable systemically or intratumorally. ECT is primarily indicated in melanoma, Kaposi sarcoma, and cutaneous or subcutaneous metastases. Recent studies have focused on its role in the treatment of nonmelanoma skin tumors, especially basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) of the head and neck [3–7]. Moreover, a meta-analysis showed that ECT is safe and cost-efficient as well as suitable for repetitive treatment [8]. Therefore, investigating the role of ECT for the treatment of atypical, aggressive, cutaneous tumors has gained a great deal of attention in the scientific community. In this article, with approval of the patient and review board of the Department of Clinical and Molecular Medicine of the “Sapienza” University of Rome, we report a case of atypical fibroxanthoma/polymorphic dermal sarcoma of the scalp, treated with ECT, resulting in long-term complete response, thus suggesting a possible new treatment opportunity for this type of cutaneous cancers.
2. Case Report
In February 2015, a 78-year-old white male was referred to us for the occurrence of a solitary rapidly growing nodule on the scalp. The nodule measured 3 cm in diameter. Clinical examination revealed a subcutaneous, bright red, ulcerated, dome-shaped lesion with irregular margins. The patient underwent an intervention of surgical excision, covering the substance loss with a skin graft from the left inguinal region. Subsequently, accurate haemostasis and suture were performed, and a compressive medication was applied. The histopathology described an ulcerated undifferentiated malignant neoplasm consisting of cells of different dimension and high mitotic index. The immunohistochemistry showed a weak positivity for S-100 and HMB45, negativity for CK, and moderate positivity for CD68. The surgical margins were clear and the diagnosis was atypical fibroxanthoma.
2.1. Follow-Up and Pathology Revision
After a month from the surgery, in May 2015, a new nodule appeared in correspondence of the graft, suggesting a recurrence (Figure 1). Considering disease relapse and the rarity of atypical fibroxanthoma, a histopathological revision of the case was requested. The second pathology report described a subcutaneous malignant neoplasm with spindle cells and pleomorphic epithelioid cells, with necrosis and atypical mitosis. De novo immunohistochemistry showed positivity for S-100 and ML actine, focal for HMB45 and MART-1, negativity for P63. Immunohistochemistry revision revealed negativity for CD68 and focal positivity for pan-CK and HMB45. This analysis suggested a diagnosis of undifferentiated pleomorphic sarcoma, but it has not excluded an acromial melanoma with aberrant ML actine expression.
Figure 1.
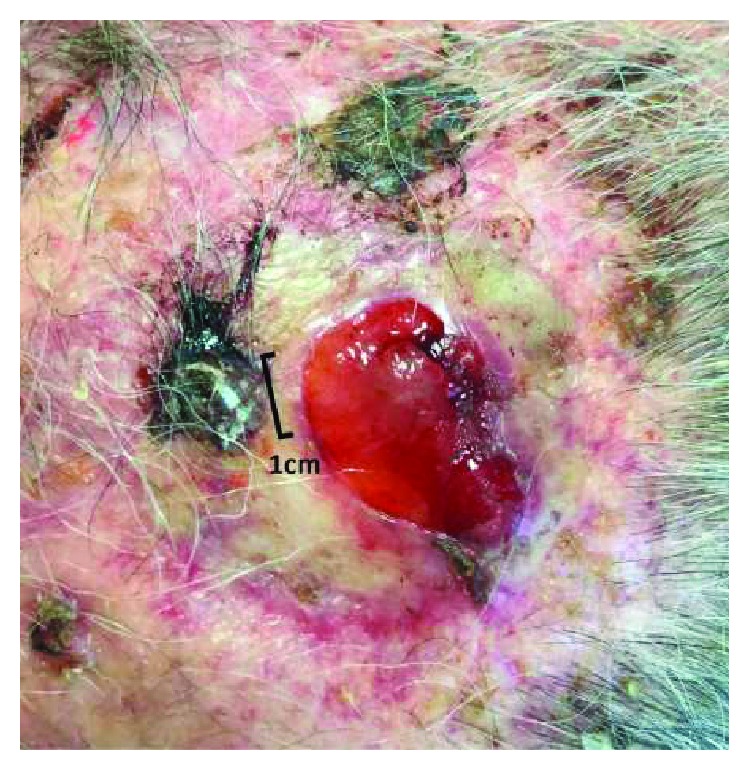
Recurrent lesion before ECT treatment.
2.2. ECT Treatment
A local treatment with ECT has been performed in the region of the cutaneous relapse using a Cliniporator® EPS-02 produced by IGEA®. The procedure is reported following the guidelines by Campana et al. [9]. Electroporation was performed after 8 minutes from the end of slow bleomycin intravenous infusion (Bleomycin TEVA, 28500 IU-15000 IU/m2 of body surface area, diluted in 100 cc of physiologic solution in 15 minutes). The patient underwent a general anesthesia (deep sedation with Propofol—Diprivan®) before an adjustable linear needle electrode by IGEA® was introduced into the tumor mass at a depth of 15 mm; also, a safety margin of 1 cm was treated around it. A series of 8 pulses of 1000 V/cm was delivered at a frequency of 5 kHz and duration of 100 microseconds, as recommended by ESOPE guidelines [10]. In order to guarantee that the tumor received a sufficient amount of bleomycin, the treatment was completed within 15 minutes after the end of the infusion. A sterile plate medication was finally applied on the treatment site. The patient did not report residual pain and did not have any kind of complication. Four weeks after ECT treatment, the mass diminished in diameter and appeared completely necrotized (Figure 2). Eight weeks after treatment, all that remained was an eschar that detached, leaving an erythematous zone of reepithelialization (Figure 3). This area disappeared within three months, with a complete restitutio ad integrum (Figure 4).
Figure 2.
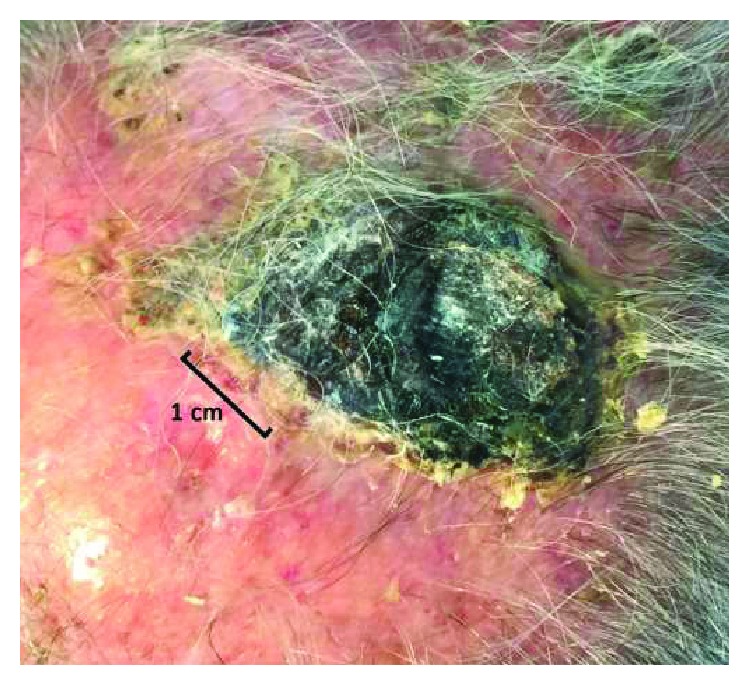
Eschar after ECT treatment.
Figure 3.
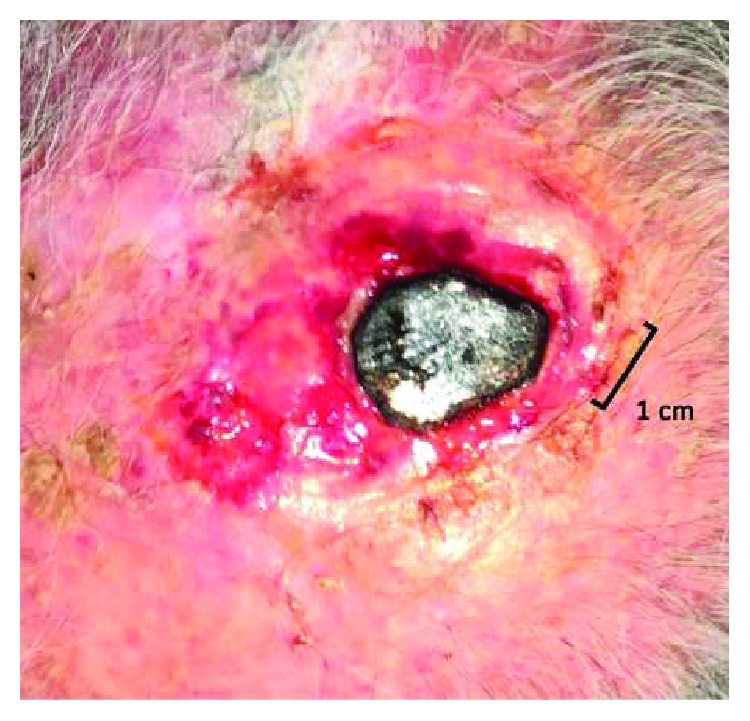
Eschar detached.
Figure 4.
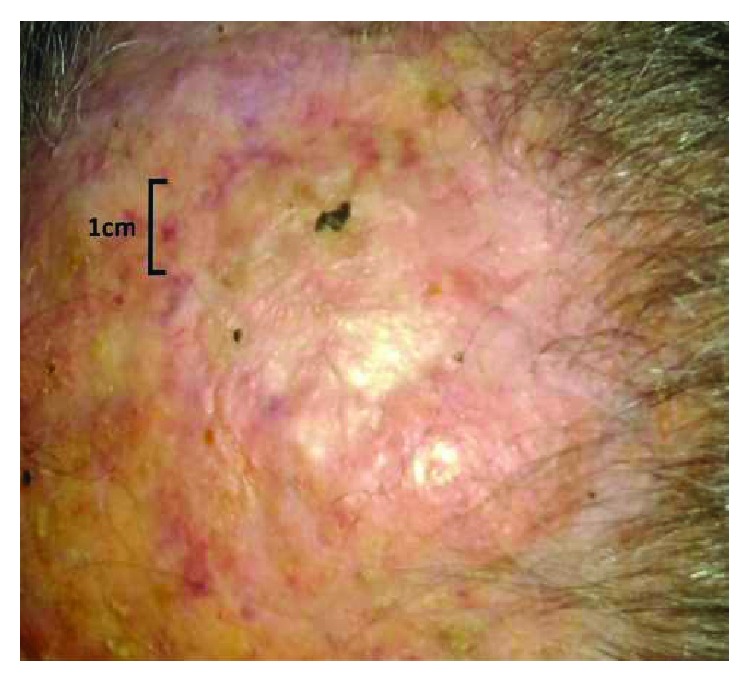
Restitutio ad integrum.
3. Discussion
Atypical fibroxanthoma (AFX) is a tumor correlated to sun exposure that most commonly presents as a solitary red or pink papule or dermal ulcerative nodule of the head or neck of elderly people [11]. AFX affects males more frequently (76%) and it is associated with different risk factors, in particular xeroderma pigmentosum, Li-Fraumeni syndrome, and immunosuppression [12]. In particular, it has been demonstrated that AFX occurs more frequently in transplanted patients. It rarely metastasizes and infrequently recurs, with a rate of 7-10% [11, 13]. However, making a definitive diagnosis of AFX is challenging, and frequently, it is hard to distinguish from pleomorphic dermal sarcoma (PDS), previously known as undifferentiated pleomorphic sarcoma, squamous cell carcinoma (SCC), or malignant melanoma [12, 14, 15]. Head and neck localization represents 90% of the total, while the other 10% appears on the limbs or on the trunk [12]. Histological characteristics and immunohistochemical markers are essential for the right diagnosis; for example, the absence of immunostaining for cytocheratins, S-100, and HMB45 is useful for excluding both SCC and malignant melanoma [14]. In fact, AFX is a dermal-arising lesion of fibrohistiocytic origin and it is considered to be similar to PDS, more precisely a superficial less aggressive variant, sharing genetic alterations, such as chromosome 9p and 13q deletions [11, 12]. Moreover, a study demonstrated that UV-induced p53 mutations and CCND1/CDK4 were essential in tumorigenesis of both AFX and PDS, supporting the theory that AFX is a superficial variant of PDS [16]. Furthermore, activating mutations in HRAS and PIK3CA were also observed in both tumor types, even if with a greater frequency of HRAS mutations in PDS than AFX [16, 17]. Interestingly, in all AFX and PDS tumors, overexpression of p53, CCND1, and CDK4 was found. Despite these findings, it is still not fully understood whether AFX and PDS tumors are related neoplasms representing the extremes of a “spectrum” or whether they are two different malignant entities [14]. Taking into account all these considerations, a second consultation was requested for our case, confirming the difficulty to have an established diagnosis. In fact, if SCC could be excluded because of CK negativity, the weak positivity for S-100 and HMB45 did not allow ruling out a melanocytic origin. Anyway, we believe it was most likely a case of fibrohistiocyte-arising neoplasm, probably PDS, given the presence of necrosis and the subcutaneous extension. There are no clear recommendations for the treatment of these tumors, but a surgical exeresis is considered the best option, and it was thus undertaken. After relapse, given the superficial localization and the positive results of previous studies, the patient was considered for an electrochemotherapy treatment.
ECT is a local antitumor therapy that started being developed in the 80s [18]. Starting from the first clinical study in 1990, ECT demonstrated to be highly effective, with complete response rates of 73% and objective response (complete and partial response) rates of 85%. Another prospective study on heterogeneous cutaneous neoplasm showed tumor response rate at 60 days of 88% (complete, 50%) [19]. More recently, two studies considering head and neck cancers were published by EURECA (European Research on Electrochemotherapy in Head and Neck Cancer). One of them focused on head and neck skin cancer and showed OR rates ranging from 59% to 100%. In addition, 1-year overall survival and local DFS rates (76% and 89%, respectively) were favorable [3]. From the other, focusing on mucosal recurrent head and neck cancers, it resulted that electrochemotherapy is practicable and effective in recurrent mucosal head and neck cancer with an overall objective response of 56% in intention-to-treat analysis [4]. In 7% of the patients, a long-term complete remission with no evidence of disease was observed [4]. The standardization of ECT was obtained during a multicentric study (European Standard Operating Procedures of Electrochemotherapy (ESOPE)) in 2006 [20] and updated in July 2018 [10]. As previously written, the chemotherapeutic agents most commonly used in association with ECT are bleomycin and cisplatin. In our case, we utilized intravenous bleomycin as more frequently executed for cutaneous tumors. In fact, it has been demonstrated that cell electroporation increases the amount of bleomycin entering the cells up to several thousand times, while the effect on intracellular concentration of cisplatin is less pronounced [21]. The reason of that finding must be searched in the different capability of the two drugs to permeate cells in normal condition. In fact, bleomycin is a completely nonpermeant drug that needs active carriers to be delivered throughout the cell membrane; on the contrary, cisplatin, a smaller and less hydrophilic molecule, is able to cross passively through the membrane so the increased permeability obtained by electroporation is extremely effective primarily with bleomycin. Bleomycin is a chemotherapeutic drug, whose main action is represented by DNA damaging and breaking, resulting in cell apoptosis. This mechanism mainly affects cycling cells, resulting in a selective killing of the neoplastic ones, preserving the healthy cells. The normal tissues located in proximity to the tumor are often infiltrated by neoplastic cells that can lead to disease relapse after an unpredictable period, and the classic tumor treatments for their aggressiveness do not treat margins extensively. Vice versa, considering the selectivity for tumor cells, with ECT, it is possible to treat safely large margins around the nodules with good results. In addition to the raised toxicity, ECT effectiveness also relies on other mechanisms. It determines transient local ischemia and vascular damage, reducing the blood flow by 80% and causing a drug entrapment in the tumor at the very time when the cell is permeable, by the so-called “vascular lock” [21, 22]. A study in 2012 performed with intravital microscopy demonstrated that after EP a consistent increase of permeability occurs in the blood vessels, while the flow is reduced because of a constriction of afferent arterioles [23]. Another recent study based on intravital microscopy demonstrated gap junction alterations as a result of EP application, stressing the key role of these structures in determining the increase of vessel permeability [24]. In the case of ECT, this can be used favorably. In this perspective, it is a fair assumption that this phenomenon would decrease drug washout and it must be considered for the drug administration timing. Furthermore, it has been demonstrated that vascular lock lasts longer in tumors than in the normal tissue and that the recovery of the initial blood flow takes hours [21, 22]. In fact, a long-lasting hypoxia in the area contributes to the high effectiveness of this treatment and is probably responsible for the hemorrhagic tumor bleeding stoppage [25]. Subsequently, the treatment of hemorrhagic and painful nodule, as occurred in our patient, is a very interesting indication for ECT. Moreover, it appears that ECT is capable to evoke an autoimmune response that is selective for neoplastic cells. In fact, the exposition of tumoral cells to EP alone determines the externalization of calreticulin, while the association with bleomycin provokes the exposition also of other damage-associated molecular profiles (DAMPs) as HMGB1 [26].
ECT use is approved for skin and subcutaneous tumors, independently of their histological origin [27]. However, it seems to be more efficient in the case of small, mildly aggressive, cutaneous primary or metastatic lesion. As previously described, cutaneous and subcutaneous cancers of different histological derivation have been successfully treated with ECT. For example, Macri et al. reported the case of a neck skin metastasis of oral SCC with a complete response and no signs of disease recurrence after two years [28]. Interestingly, similar results have been obtained also in two cases of dermatofibrosarcoma protuberans, confirming the versatility of this approach [29, 30]. On the contrary, there are only few studies considering PDS. Campana et al. published a phase II trial on soft tissue sarcomas with 8 cases of undifferentiated sarcoma and only one complete response, but there are no case reports or specific works on this kind of tumor [31].
4. Conclusions
To the best of our knowledge, no data concerning ECT as a locoregional treatment for AFX/PDS are available. Therefore, we present the first case in literature and hence the importance of this report in order to expand the application field of this new promising treatment also for AFX and PDS, considering its effectiveness and safety in elderly patients. However, further studies are needed to confirm our results.
Acknowledgments
We thank Pierandrea Conti, PhD, for language editing.
Conflicts of Interest
All the authors declare that they have no conflict of interest.
References
- 1.Sersa G., Miklavcic D., Cemazar M., Rudolf Z., Pucihar G., Snoj M. Electrochemotherapy in treatment of tumours. European Journal of Surgical Oncology (EJSO) 2008;34(2):232–240. doi: 10.1016/j.ejso.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 2.Sersa G., Cufer T., Paulin S. M., Cemazar M., Snoj M. Electrochemotherapy of chest wall breast cancer recurrence. Cancer Treatment Reviews. 2012;38(5):379–386. doi: 10.1016/j.ctrv.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Bertino G., Sersa G., De Terlizzi F., et al. European Research on Electrochemotherapy in Head and Neck Cancer (EURECA) project: results of the treatment of skin cancer. European Journal of Cancer. 2016;63:41–52. doi: 10.1016/j.ejca.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Plaschke C. C., Bertino G., McCaul J. A., et al. European Research on Electrochemotherapy in Head and Neck Cancer (EURECA) project: results from the treatment of mucosal cancers. European Journal of Cancer. 2017;87:172–181. doi: 10.1016/j.ejca.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Gargiulo M., Serra Mestre J. M., Cortese A., Murphy D. C., Parascandolo S., Razzano S. Long term effectiveness of electrochemotherapy for the treatment of lower lip squamous cell carcinoma. Journal of Cranio-Maxillofacial Surgery. 2018;46(11):1968–1974. doi: 10.1016/j.jcms.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Groselj A., Bosnjak M., Strojan P., Krzan M., Cemazar M., Sersa G. Efficiency of electrochemotherapy with reduced bleomycin dose in the treatment of nonmelanoma head and neck skin cancer: Preliminary results. Head Neck. 2018;40(1):120–125. doi: 10.1002/hed.24991. [DOI] [PubMed] [Google Scholar]
- 7.Campana L. G., Marconato R., Valpione S., et al. Basal cell carcinoma: 10-year experience with electrochemotherapy. Journal of Translational Medicine. 2017;15(1):p. 122. doi: 10.1186/s12967-017-1225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mali B., Jarm T., Snoj M., Sersa G., Miklavcic D. Antitumor effectiveness of electrochemotherapy: a systematic review and meta-analysis. European Journal of Surgical Oncology. 2013;39(1):4–16. doi: 10.1016/j.ejso.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 9.Campana L. G., Clover A. J., Valpione S., et al. Recommendations for improving the quality of reporting clinical electrochemotherapy studies based on qualitative systematic review. Radiology and Oncology. 2016;50(1):1–13. doi: 10.1515/raon-2016-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gehl J., Sersa G., Matthiessen L. W., et al. Updated standard operating procedures for electrochemotherapy of cutaneous tumours and skin metastases. Acta Oncologica. 2018;57(7):874–882. doi: 10.1080/0284186X.2018.1454602. [DOI] [PubMed] [Google Scholar]
- 11.McCalmont T. H. AFX: what we now know. Journal of Cutaneous Pathology. 2011;38(11):853–856. doi: 10.1111/j.1600-0560.2011.01802.x. [DOI] [PubMed] [Google Scholar]
- 12.Sakamoto A. Atypical fibroxanthoma. Clinical Medicine: Oncology. 2008;2:117–127. doi: 10.4137/CMO.S506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper J. Z., Newman S. R., Scott G. A., Brown M. D. Metastasizing atypical fibroxanthoma (cutaneous malignant histiocytoma): report of five cases. Dermatologic Surgery. 2005;31(2):221–225. doi: 10.1097/00042728-200502000-00019. [DOI] [PubMed] [Google Scholar]
- 14.Soleymani T., Tyler Hollmig S. Conception and management of a poorly understood spectrum of dermatologic neoplasms: atypical fibroxanthoma, pleomorphic dermal sarcoma, and undifferentiated pleomorphic sarcoma. Current Treatment Options in Oncology. 2017;18(8):p. 50. doi: 10.1007/s11864-017-0489-6. [DOI] [PubMed] [Google Scholar]
- 15.Fletcher C. D. Pleomorphic malignant fibrous histiocytoma: fact or fiction? A critical reappraisal based on 159 tumors diagnosed as pleomorphic sarcoma. The American Journal of Surgical Pathology. 1992;16(3):213–228. doi: 10.1097/00000478-199203000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Helbig D., Ihle M. A., Pütz K., et al. Oncogene and therapeutic target analyses in atypical fibroxanthomas and pleomorphic dermal sarcomas. Oncotarget. 2016;7(16):21763–21774. doi: 10.18632/oncotarget.7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griewank K. G., Schilling B., Murali R., et al. TERT promoter mutations are frequent in atypical fibroxanthomas and pleomorphic dermal sarcomas. Modern Pathology. 2014;27(4):502–508. doi: 10.1038/modpathol.2013.168. [DOI] [PubMed] [Google Scholar]
- 18.Mir L. M., Belehradek M., Domenge C., et al. Electrochemotherapy, a new antitumor treatment: first clinical trial. Comptes Rendus de l’Académie des Sciences - Series III. 1991;313:613–618. [PubMed] [Google Scholar]
- 19.Campana L. G., Testori A., Curatolo P., et al. Treatment efficacy with electrochemotherapy: a multi-institutional prospective observational study on 376 patients with superficial tumors. European Journal of Surgical Oncology. 2016;42(12):1914–1923. doi: 10.1016/j.ejso.2016.06.399. [DOI] [PubMed] [Google Scholar]
- 20.Marty M., Sersa G., Garbay J. R., et al. Electrochemotherapy - an easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: Results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. European Journal of Cancer Supplements. 2006;4(11):3–13. doi: 10.1016/j.ejcsup.2006.08.002. [DOI] [Google Scholar]
- 21.Gehl J., Skovsgaard T., Mir L. M. Vascular reactions to in vivo electroporation: characterization and consequences for drug and gene delivery. Biochimica et Biophysica Acta (BBA) - General Subjects. 2002;1569(1-3):51–58. doi: 10.1016/S0304-4165(01)00233-1. [DOI] [PubMed] [Google Scholar]
- 22.Cemazar M., Parkins C. S., Holder A. L., Chaplin D. J., Tozer G. M., Sersa G. Electroporation of human microvascular endothelial cells: evidence for an anti-vascular mechanism of electrochemotherapy. British Journal of Cancer. 2001;84(4):565–570. doi: 10.1054/bjoc.2000.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellard E., Markelc B., Pelofy S., et al. Intravital microscopy at the single vessel level brings new insights of vascular modification mechanisms induced by electropermeabilization. Journal of Controlled Release. 2012;163(3):396–403. doi: 10.1016/j.jconrel.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Markelc B., Bellard E., Sersa G., et al. Increased permeability of blood vessels after reversible electroporation is facilitated by alterations in endothelial cell-to-cell junctions. Journal of Controlled Release. 2018;276:30–41. doi: 10.1016/j.jconrel.2018.02.032. [DOI] [PubMed] [Google Scholar]
- 25.Gehl J., Geertsen P. F. Efficient palliation of haemorrhaging malignant melanoma skin metastases by electrochemotherapy. Melanoma Research. 2000;10(6):585–589. doi: 10.1097/00008390-200012000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Calvet C. Y., Famin D., André F. M., Mir L. M. Electrochemotherapy with bleomycin induces hallmarks of immunogenic cell death in murine colon cancer cells. Oncoimmunology. 2014;3(4, article e28131) doi: 10.4161/onci.28131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reinhold U. Electrochemotherapy for primary skin cancer and skin metastasis related to other malignancies. Anti-Cancer Drugs. 2011;22(8):711–718. doi: 10.1097/CAD.0b013e32834618da. [DOI] [PubMed] [Google Scholar]
- 28.Macri G. F., Greco A., Gallo A., Fusconi M., Marinelli C., de Vincentiis M. Use of electrochemotherapy in a case of neck skin metastasis of oral squamous cell carcinoma: case report and considerations. Head Neck. 2014;36 doi: 10.1002/hed.23552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonadies A., Elia F., Solivetti F. M., Vidiri A., Muscardin L., Bucher S. Electrochemotherapy of a multirecurrent dermatofibrosarcoma protuberans of the orbital margin: a case report. Anticancer Research. 2015;35(11):6121–6126. [PubMed] [Google Scholar]
- 30.Wiater K., Zdzienicki M., Morysiński T., et al. Effective treatment of recurrent, advanced dermatofibrosarcoma protuberans by electrochemotherapy. European Journal of Dermatology. 2013;23(2):260–261. doi: 10.1684/ejd.2013.1992. [DOI] [PubMed] [Google Scholar]
- 31.Campana L. G., Bianchi G., Mocellin S., et al. Electrochemotherapy treatment of locally advanced and metastatic soft tissue sarcomas: results of a non-comparative phase II study. World Journal of Surgery. 2014;38(4):813–822. doi: 10.1007/s00268-013-2321-1. [DOI] [PubMed] [Google Scholar]


