Abstract
Aims
Secondary mitral regurgitation (MR) drives adverse remodelling towards late heart failure stages. Little is known about the evolution of MR under guideline-directed therapy (GDT) and its relation to cardiac remodelling and outcome. We therefore aimed to assess incidence, impact, and predictors of progressive secondary MR in patients under GDT.
Methods and results
We prospectively enrolled 249 patients with chronic heart failure and reduced ejection fraction receiving GDT in this long-term observational study. Of patients with non-severe MR at baseline 81% remained stable whereas 19% had progressive MR. Those patients were more symptomatic (P < 0.001), had higher neurohumoral activation (encompassing various neurohumoral pathways in heart failure, all P < 0.05), larger left atrial size (P = 0.004) and more tricuspid regurgitation (TR, P = 0.02). During a median follow-up of 61 months (IQR 50–72), 61 patients died. Progression of MR conveyed an increased risk of mortality—univariately (HR 2.33; 95% CI 1.34–4.08; P = 0.003), that persisted after multivariate adjustment using a bootstrap-selected confounder model (adjusted HR 2.48; 95% CI 1.40–4.39; P = 0.002). In contrast, regression of MR was not associated with a beneficiary effect on outcome (crude HR 0.84; 95% CI 0.30–2.30; P = 0.73).
Conclusions
Every fifth patient with chronic heart failure suffers from MR progression. This entity is associated with a more than two-fold increased risk of death even after careful multivariable adjustment. Symptomatic status, left atrial size, TR, and neurohumoral pathways help to identify patients at risk for progressive secondary MR in an early disease process and open the possibility for closer follow-up and timely intervention.
Keywords: mitral regurgitation, secondary mitral regurgitation, functional mitral regurgitation, heart failure, heart failure with reduced ejection fraction, HFrEF, guideline-directed therapy, moderate mitral regurgitation, severe mitral regurgitation, progression of mitral regurgitation, secondary MR, functional MR, mitral valve, mitral valve insufficiency
Introduction
Secondary mitral regurgitation (MR) is a common finding in patients with congestive heart failure with significant impact on mortality despite guideline directed therapy GDT.1 Patients with severe MR have a profoundly increased risk of death—but only convey a minority of the spectrum of roughly 20% of the patients.2–7 A significant proportion of those patients already developed a full-grown failure phenotype with severe symptoms, markedly elevated neurohormones, profoundly dilated left ventricular (LV) cavities and severely reduced LV function indicating an exhausted compensatory reserve and the window for timely intervention might already been closed.1,8,9 In contrast, non-severe MR affects the majority of patients with systolic heart failure—roughly 80%—still independently increasing mortality.2,3,7 Furthermore, MR is well known to have a strong dynamic component, not only during the cardiac cycle at rest10 and with exercise11 but also over time progressing in severity and contributing to a transition towards late heart failure stages.12 Therefore, progressive MR has been highlighted by the recent guidelines as a distinct entity.13,14 However, in contrast to primary MR prior research regarding the evolution of MR is limited and restricted to post-infarct ischaemic MR.4–6
Whether chronic MR progression occurs despite GDT and whether those patients are at increased risk is currently unknown. We therefore sought to assess the independent prognostic impact of progressive MR on long-term mortality in patients with chronic heart failure under guideline-directed heart failure therapy. Furthermore, we aimed to describe morphological and functional changes related to progressive secondary MR and identify neurohumoral, morphological, and functional factors associated with progressive secondary MR.
Methods
Study population
From February 2001 to November 2006, patients with chronic heart failure with reduced ejection fraction (HFrEF) at the heart failure clinic of the Vienna General Hospital, a university-affiliated tertiary centre, were included in this observational, non-interventional study. HFrEF was defined as a history of left ventricular ejection fraction below 40% as well as history of heart failure signs in line with the heart failure guidelines.15 All patients underwent a comprehensive echocardiographic exam at our institution at baseline and yearly thereafter within 3 years after study inclusion. In an attempt to define a clinically relevant increase of non-severe MR, we defined MR progression as advance of at least one grade in severity with transition to at least moderate MR during 3 years of follow-up. Analogously, regression of severe secondary MR was defined as decrease of at least one grade. Patients with more than mild aortic or mitral stenosis or ≥moderate primary MR were excluded. The study was approved by the Ethics Committee of the Medical University of Vienna. Please see Supplementary material online for clinical measures and follow-up as well as laboratory measurements.
Echocardiographic assessment
Baseline and follow-up echocardiograms were performed using commercially available equipment (Vivid5 and Vivid7, GE Healthcare, and Acuson Sequoia, Siemens). Cardiac morphology was assessed using diameters in standard four- and two chamber views. Ejection fraction was calculated using the biplane Simpson method and semi-quantitative assessment of right heart function was performed by experienced readers using multiple acoustic windows and graded as normal, mild, mild-to-moderate, moderate, moderate-to-severe, and severe. Mitral regurgitation was quantified by an integrated approach comprising mitral valve morphology, width of the proximal regurgitant jet, proximal flow convergence, and pulmonary venous flow pattern.16 Valvular stenosis and regurgitation were quantified using an integrated approach and graded as none, mild, mild-to-moderate, moderate, moderate-to-severe, and severe according to the respective guidelines.16–18 Systolic pulmonary artery pressures (sPAP) were calculated by adding the peak tricuspid regurgitation (TR) systolic gradient to the estimated central venous pressure.
Statistical analysis
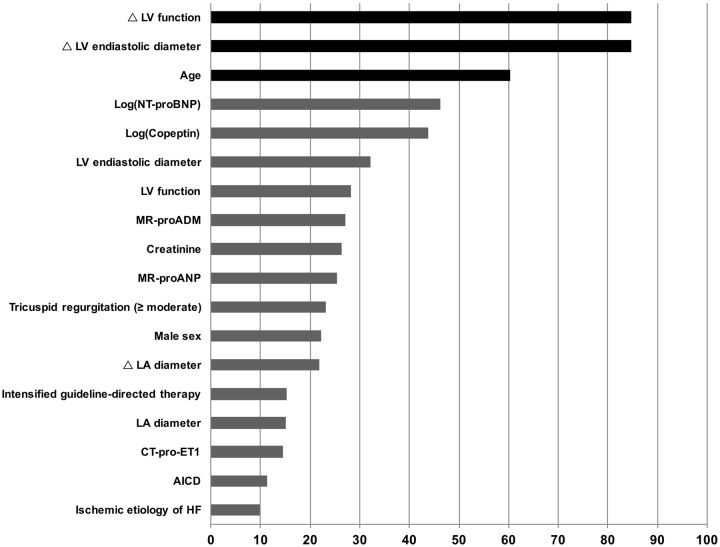
Continuous data were presented as median and interquartile range (IQR) and compared by using the Kruskal–Wallis test. Discrete data were presented as count and percentage and analysed by using a χ2 test. Univariable logistic regression analysis was used to estimate odds ratios associated with MR progression. Additionally, areas under the receiver-operating characteristic curve (ROC) were used to assess model discrimination of different baseline characteristics with respect to MR progression. Cox proportional hazard regression analysis was applied to assess the effect of MR progression on survival. In order to account for potential confounding effects, we first formed clinical clusters1 as follows: a clinical confounder cluster (encompassing: age, sex, ischaemic aetiology of heart failure, serum creatinine), an echocardiographic confounder cluster (encompassing: LV end-diastolic diameter, left atrial diameter, LV function, TR), an echocardiographic progression cluster (encompassing: change in LV end-diastolic diameter, change left atrial diameter and, change in LV function), a medical therapy cluster (encompassing: percent of maximal guideline recommended dose of RAS antagonist and beta-blocker, mineralocorticoid antagonist therapy, and implanted cardioverter defibrillator) and a neurohumoral cluster (encompassing: NT-proBNP, MR-proANP, MR-proADM, CT-proET-1, and Copeptin). All continuous variables in the aforementioned clusters were log-transformed and a stepwise regression analysis including the original and log-transformed variable was performed. The variable selected by this procedure was used for further analysis. Then a stepwise bootstrap resampling procedure including all variables from the aforementioned clinical clusters was used to identify best-fitting variables for the final multivariable Cox regression model. Five-hundred repeats with a P-value of 0.05 for selection were performed and variables selected in 50% of all repeats were included in the final confounder model (i.e. age, change in LV end-diastolic diameter, and change in LV function from baseline to follow-up; Figure 1). In order to test for interactions between MR progression and all variables in the final model, we used Cox proportional hazard regression models with MR progression, a variable in question and the interaction between both variables. We tested for collinearity in the multivariable model using the variance inflation factor. The proportional hazards assumption was tested and satisfied in all cases using Schoenfeld residuals. Interactions between MR progression and all variables included in the multivariable model were tested by entering interaction terms in the Cox proportional hazard regression models. The Kaplan–Meier analysis (log-rank test) was applied to assess the time-dependent discriminative power of MR progression. Two-sided P-values <0.05 were used to indicate statistical significance. The STATA11 software package (StataCorp, College Station, TX, USA) and SPSS 24.0 (IBM Corp, New York, NY, USA) were used for all analyses.
Figure 1.
Variable selection by a bootstrap resampling procedure based on stepwise Cox regression analysis. Variables selected in 50% of all repeats (black bars) were included in the final multivariable model.
Results
Baseline characteristics
We enrolled 249 patients with chronic heart failure with a history of reduced ejection fraction (HFrEF). Median age was 58 years (IQR 51–63) and 208 patients (84%) were male. The median Nt-proBNP was 2453 pg/mL (IQR 989–4981) and left ventricular function was still significantly reduced (≥moderate) in 86% of patients at index time. Forty-five percent of patients were in NYHA Class III and 20% in NYHA Class IV. Ninety-seven percent of patients (n = 242) received RAS antagonists up-titrated to a median dose of 100% of the maximal guideline recommended dosages, 189 patients (76%) received beta-blockers up-titrated to a median dose of 50% of the maximal guideline recommended dosages and 90 patients (36%) were treated with a mineralocorticoid receptor antagonist and 194 patients (78%) were under diuretic therapy. Among 249 patients, 58 had severe secondary MR at baseline. Forty-three patients (17%) underwent cardiac resynchronization therapy. Detailed baseline characteristics of the entire study population are displayed in Supplementary material online, Table S1.
Evolution of secondary mitral regurgitation
Of 191 patients with non-severe MR at baseline 157 (82%) remained stable, whereas 34 (18%) showed progressive MR within 3 years after study enrolment. Mean time from baseline echo to the echo showing progression of FMR was 2.2 ± 0.8 years. Detailed baseline characteristics according to MR progression are presented in the Supplementary material online, Table S1. Briefly, patients experiencing progression of MR were more symptomatic at baseline (NYHA Class IV 35% vs. 10%; P < 0.001). Furthermore, patients with subsequent progression of MR had higher levels of MR-proADM (P = 0.004), MR-proANP (P = 0.009), CT-proET1 (P = 0.01), and NT-proBNP (P = 0.03) compared to the non-progressing patients reflecting a more pronounced neurohumoral activation. Patients with progressive MR also had larger left atrial size at baseline (P = 0.004) and more often concomitant TR (P = 0.02). There were no differences in medical and device therapies between patients with subsequently progressive MR and those with stable MR.
Baseline parameters associated with progressive secondary mitral regurgitation
NYHA functional class at baseline was significantly associated with progression of MR (OR 2.22, 95% CI 1.32–3.71; P = 0.002) in the logistic regression analysis. Furthermore, higher levels of MR-proADM (OR 1.71, 95% CI 1.21–2.40; P = 0.002), MR-proANP (OR 1.48, 95% CI 1.07–2.04; P = 0.017), Copeptin (OR 1.41, 95% CI 1.03–1.93; P = 0.03) and CT-pro-ET1 (OR 1.52, 95% CI 1.08–2.14; P = 0.016) at baseline were significantly associated with MR progression. Regarding echocardiographic characteristics, the left atrial diameter (OR 1.70, 95% CI 1.16–2.50; P = 0.006) and the degree of concomitant TR (OR 2.24, 95% CI 1.46–3.42; P < 0.001) were significant risk factors for MR progression. In contrast, no significant effect of RAS antagonists (P = 0.21), beta-blocker therapy (P = 0.39), or mineralocorticoid antagonists (P = 0.32) could be observed. Detailed results of the logistic regression analysis are presented in Table 1.
Table 1.
Univariable logistic regression analysis assessing risk factors at baseline for MR progression
| SD | OR | 95% CI | P-value | ROC | |
|---|---|---|---|---|---|
| Baseline characteristics | |||||
| Age | 11 | 1.03 | 0.71–1.49 | 0.89 | 0.50 |
| Male sex | — | 0.70 | 0.27–1.78 | 0.45 | 0.53 |
| BMI | 4 | 0.75 | 0.50–1.10 | 0.14 | 0.58 |
| Ischaemic aetiology of HF | — | 0.75 | 0.34–1.65 | 0.48 | 0.53 |
| Hypertension | — | 0.77 | 0.37–1.62 | 0.50 | 0.53 |
| Diabetes | — | 0.46 | 0.17–1.26 | 0.13 | 0.56 |
| Atrial fibrillation | — | 0.87 | 0.31–2.45 | 0.79 | 0.51 |
| NYHA functional class | — | 2.22 | 1.32–3.71 | 0.002 | 0.65 |
| Estimated GFR, mL/min/1.73 m2 (IQR) | 28 | 0.74 | 0.49–1.10 | 0.13 | 0.61 |
| Neurohormones | |||||
| NT-proBNP | 281 | 1.12 | 0.80–1.55 | 0.52 | 0.62 |
| MR-proANP | 0.5 | 1.48 | 1.07–2.04 | 0.017 | 0.64 |
| MR-proADM | 17 | 1.71 | 1.21–2.40 | 0.002 | 0.66 |
| Copeptin | 17 | 1.41 | 1.03–1.93 | 0.034 | 0.55 |
| CT-pro-ET1 | 57 | 1.52 | 1.08–2.14 | 0.016 | 0.64 |
| Echocardiographic characteristics | |||||
| Left ventricular end-diastolic diameter | 9 | 1.44 | 0.98–2.11 | 0.07 | 0.59 |
| Left ventricular function | — | 1.38 | 0.84–2.26 | 0.20 | 0.55 |
| Left atrial diameter | 9 | 1.70 | 1.16–2.50 | 0.006 | 0.66 |
| Right atrial diameter | 9 | 1.26 | 0.87–1.82 | 0.22 | 0.56 |
| Right ventricular end-diastolic diameter | 7 | 1.37 | 0.95–1.99 | 0.10 | 0.60 |
| Systolic pulmonary artery pressure | 12 | 1.30 | 0.84–2.02 | 0.24 | 0.57 |
| Tricuspid regurgitation | — | 2.24 | 1.46–3.42 | <0.001 | 0.70 |
| Medication | |||||
| RAS antagonist | — | 0.31 | 0.05–1.94 | 0.21 | 0.52 |
| Beta-blockers | — | 1.63 | 0.53–4.99 | 0.39 | 0.53 |
| Mineralocorticoidantagonist | — | 1.46 | 0.69–3.12 | 0.32 | 0.55 |
Odds ratios (OR) refer to a 1-SD increase in continuous variables.
Bold values indicate statistical significance.
Progressive secondary mitral regurgitation: associated morphological and functional maladaptation
Detailed echocardiographic baseline and follow-up characteristics according to MR progression are presented in Table 2. Patients with subsequent MR progression and those with stable MR presented with similar morphology and function at baseline except for left atrial diameter and TR (Table 2). At baseline, the median left atrial diameter was 61 mm (55–69) in patients with stable MR vs. 65 mm (62–73) in progressive MR (P = 0.004). Fourteen (9%) patients with subsequently stable MR had ≥moderate TR vs. 8 (24%) patients with subsequent MR progression (P = 0.02). There was no significant difference in LV size [median left ventricular end-diastolic diameter 61 mm (IQR 55–68) in patients with stable MR vs. 65 mm (IQR 60–70) in patients with progressive MR; P = 0.09]. The number of patients with moderately reduced left ventricular function (34% vs. 26%, P = 0.37) and with severely reduced left ventricular function (45% vs. 62% P = 0.08) was similar at baseline among patients with subsequent MR progression and stable MR.
Table 2.
Echocardiographic characteristics at baseline and follow-up
| MR stable (n = 157) | MR progression (n = 34) | P-value | |
|---|---|---|---|
| Baseline exam | |||
| Left ventricular end-diastolic diameter, mm (IQR) | 61 (55–68) | 65 (60–70) | 0.09 |
| Left ventricular function | |||
| Moderately reduced (EF 30–40%), n (%) | 54 (34) | 9 (26) | 0.37 |
| Severely reduced (EF <30%), n (%) | 71 (45) | 21 (62) | 0.08 |
| Left atrial diameter, mm (IQR) | 61 (55–69) | 65 (62–73) | 0.004 |
| Right atrial diameter, mm (IQR) | 56 (51–64) | 58 (52–68) | 0.30 |
| Right ventricular end-diastolic diameter, mm (IQR) | 35 (31–38) | 37 (33–41) | 0.07 |
| Systolic pulmonary artery pressure, mmHg (IQR) | 43 (36–50) | 47 (37–57) | 0.31 |
| Tricuspid regurgitation (≥moderate), n (%) | 14 (9) | 8 (24) | 0.02 |
| Follow-up exam | |||
| Left ventricular end-diastolic diameter, mm (IQR) | 59 (54–66) | 64 (58–69) | 0.02 |
| Left ventricular function | |||
| Moderately reduced (EF 30–40%), n (%) | 45 (29) | 5 (15) | 0.15 |
| Severely reduced (EF <30%), n (%) | 85 (54) | 27 (79) | 0.001 |
| Left atrial diameter, mm (IQR) | 60 (55–68) | 67 (65–74) | <0.001 |
| Right atrial diameter, mm (IQR) | 57 (51–64) | 64 (59–68) | 0.001 |
| Right ventricular end-diastolic diameter, mm (IQR) | 35 (30–39) | 39 (36–43) | 0.002 |
| Systolic pulmonary artery pressure, mmHg (IQR) | 45 (38–56) | 56 (46–65) | 0.003 |
| Tricuspid regurgitation (≥moderate), n (%) | 22 (14) | 18 (53) | <0.001 |
Echocardiographic follow-up within three years after the baseline exam is displayed in Table 2. At follow-up, patients with MR progression had larger left- and right-ventricular size (left ventricular end-diastolic diameter of 59 mm vs. 64 mm, P = 0.02; right ventricular end-diastolic diameter of 35 mm vs. 39 mm; P = 0.002), more dilated atria (left atrial diameter of 60 mm vs. 67 mm, P < 0.001; right atrial diameter of 57 mm vs. 64 mm P = 0.001) and a higher estimated sPAP (45 mmHg vs. 56 mmHg, P = 0.003) at follow-up. There were more patients with severely reduced LV function at follow-up (54% vs. 79%, P = 0.001) and significantly more patients with more than mild-moderate secondary TR (14% vs. 53% P < 0.001, Table 2).
Evolution of secondary mitral regurgitation and outcome
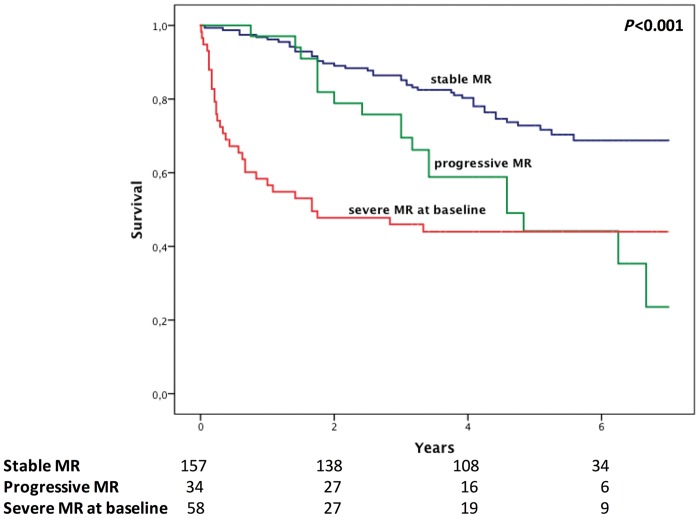
During a median follow up of 61 months (IQR 50–72 months), 61 patients with non-severe MR died. Progression of MR severity within 3 years of study enrolment was associated with significant long-term mortality in the crude Cox regression analysis with an HR of 2.33 (95% CI 1.34–4.08; P = 0.003). The results remained virtually unchanged after multivariate adjustment using a bootstrap-selected confounder model with an adjusted HR of 2.48 (95% CI 1.40–4.39; P = 0.002). Furthermore, we did not observe any significant interaction between MR progression and ischaemic or non-ischaemic MR (P-for-interaction = 0.88). Additionally, we did not observe any significant interactions between severe MR and any other variables included in the multivariable model, and we did not detect a significant collinearity in our multivariable models. The Kaplan–Meier analysis demonstrated a significant increase of long-term mortality in patients with progressive MR compared to patients with stable MR comparable to patients with severe MR at study enrolment (logrank P < 0.001, Figure 2). Furthermore, the Kaplan–Meier curves illustrate similar time to event-rates in the first 2 to 3 years in patients with subsequent progression and stable MR and a diverge of the curves thereafter, indicating a potential window to modify the disease course. Interestingly, among patients with severe MR at baseline, we observed a regression of MR in 13 patients (22%), which was not associated with a beneficiary effect on outcome (crude HR 0.84; 95% CI 0.30–2.30; P = 0.73).
Figure 2.
Kaplan Meier estimates of long-term mortality comparing patients with stable MR to patients with progressive MR and severe MR at baseline (overall: P < 0.001; stable vs. progressive MR P = 0.002; stable vs. severe MR at baseline: P < 0.001; progressive vs. severe MR at baseline: P = 0.81).
Discussion
The present data shows that every fifth patient with non-severe secondary MR experiences a progression of MR during the first 3 years of follow-up despite GDT. Patients that subsequently developed progressive MR had more symptoms, stronger neurohumoral activation, more dilated left atria, and more TR at baseline. Progressive MR conveys an adverse prognosis even after careful adjustment for clinical, echocardiographic, and neurohumoral confounders, as well as GDT. At follow-up, progressive MR was associated with more severe bi-ventricular and bi-atrial dilatation, higher estimated sPAP and more severely reduced LV function. Progressive MR remained a strong predictor of outcome even after adjustment for echocardiographic change in morphology and function. Factors associated with progressive MR were more severely impaired functional status, higher neurohumoral activation—except for NT-pro BNP, and more dilated left atrial size as well as more TR. Interestingly, regression of severe MR was not associated with improved long-term prognosis.
Current clinical practice in progressive MR
According to current heart valve guidelines, secondary MR Grade B corresponds to progressive MR but this entity has not yet been systematically defined. Motivated by recent findings from the CTSN trial19,20 the guidelines on valvular heart disease updated their recommendations regarding MV repair in patients with Grade B MR who are undergoing other cardiac surgery (Class IIb, Level B-R) due to the lack of benefit and increased risk of post-operative complications.13,15 The current data adds a new perspective being that this non-severe MR patient group includes individuals who will progress within 3 years to severe MR associated with poor outcome but also patients with subsequent stable disease under GDT. In agreement with the guidelines, it seems unnecessary to expose a patient to the risk of an additional procedure if the patient does not meet any features for progressive MR (e.g. only mild symptoms, merely mild neurohumoral activation, mildly dilated left atria and no significant secondary TR) as the present data shows that those patients do not show MR progression during an extended follow-up period and therefore might benefit most from GDT and close follow up. It has to be noted that this account to 80% of patients with non-severe MR hindering a general recommendation to intervene this population. In contrast, for those 20% of patients at risk for progressive MR which apparently can be defined by a bundle of clinical, neurohumoral, and echocardiographic variables, it might still be reasonable to add MV reconstruction during another cardiac procedure in an attempt to reduce associated adverse remodelling or perform trans-catheter MV repair, which needs to be elucidated by future studies.
Associated factors with progressive secondary mitral regurgitation
The present study, for the first time, defines factors associated with progressive secondary MR: more symptoms, higher neurohumoral activation—except for NT-pro BNP, larger left atrial size and more TR. These factors define a high-risk population among patients with heart failure, in need of closer follow-up. The combination of clinical, neurohumoral and morphological parameters sets the foundation for a complementary clinical workup to identify these patients before they develop a late heart failure stage. Importantly, NT-pro BNP—usually referred to as the gold standard biomarker in heart failure, is not useful to predict progressive MR in the present analysis. The exact mechanism behind this finding is not entirely clear, but might be related to the decreased afterload in the presence of MR—leading to a volume shift directed towards the left atrium. In fact 88% NT-pro BNP is being produced by LV myocardium and only a minority from the left atrium,21 which could lead to a paradoxically relative-low levels of the neurohormone that might not reflect the severity of the left ventricular failure. Our data indicates a possible advantage of MR-proANP, MR-proADM and CT-pro-ET1 to predict subsequent progressive MR. MR-proANP might well reflect the dynamic component with exercise as the differential excretion comes from the left atrium in response to stretch—and the fragments half-life is long enough to cover the dynamic range of the regurgitant lesion. Our data indicates that late outcome in patients with progressive MR is at least equivalent if not worse compared to patients with severe MR (Figure 2) with the clinically important disparity that they did not yet reach a late heart failure stage. Identifying those patients at significant risk early opens the intriguing possibility to alter the clinical course, either by an early switch to newer heart failure treatment regimens such as Sacubitril/Valsartan or allocation to new, low-risk, trans-catheter mitral valve repair techniques in an attempt, to not only reduce symptoms—but also to disrupt the vicious cycle of progressive MR.
Progressive secondary mitral regurgitation: associated morphological and secondary maladaptation
The present study lends evidence to the concept of secondary MR as a driving force for morphological and functional maladaptation: Interestingly, patients with subsequent MR progression had larger left atrial size, more symptoms and stronger neurohumoral activation at baseline. These observations indicate a possible role of a dynamic orifice as a predecessor of- or a regurgitant lesion in transition to progression. While the regurgitant jet at rest might be similar, patients with subsequent transition to progressive MR might already increase in regurgitant volume during exercise or even during daily life moderate activity and therefore display increased left atrial size at rest, and have more symptoms corresponding to the elevated pressures of the pulmonary circulation during exercise.11 These repetitively volume overloading states during exercise ultimately might be a driving force for eccentric left ventricular remodelling and heart failure, leading to the observed increased LV dilatation during the 3 years of follow-up with consecutively MR progression—then apparent at rest, with all the consequences: increased pulmonary artery pressures, increased TR, dilatation of the right heart, more systolic heart failure, and worse outcome.
Secondary MR in HFrEF occurs mainly due to left ventricular remodelling with associated papillary muscle displacement resulting in leaflet tethering that restricts valve closure. Atrial secondary MR might also be a contributing factor developing on top of the above mentioned mechanism due to the LV unloading backwards toward the left atrium with associated increase in LA pressure, complementary increase in LA size, and resulting pressure effects towards the pulmonary vasculature and the right heart. This chain of consecutive or concomitant events might add an additional driving force acting in concert with LV remodelling to induce mitral annular shape alterations augmenting progressive MR.
Progressive secondary mitral regurgitation and outcome
The present data indicates the significance of progressive MR—a 2.3- to 2.5-fold increased risk of death—even after careful adjustment for clinical and echocardiographic confounders, as well as adjustment for different pathways of neurohumoral activation and optimal medical therapy. The impact of long-term-progressive secondary MR has not been investigated before. Current data, comes exclusively from acute post-infarct studies on ischaemic MR and mainly from sub-studies of the VALIANT cohort. Amigoni et al. showed that there is a dynamic component after MI within 1 month associated with a three-fold increased risk of heart failure hospitalization and death.6 Meris et al.5 expanded this observation towards more chronic progression of IMR within the same cohort. They showed that 26% of patients have IMR progression within a timeframe of twenty month, also including the acute post-infarct phase, but did not relate this to outcome. Recently, Kwon et al.4 described progressive ischaemic MR within 7-month follow-up in 29% of patients, also acutely after myocardial infarction, with and without revascularization. Post-infarct progressive IMR was associated with a 1.2 times increased risk for death or need for heart transplantation. All three studies are distinctly different from the present one with regards to patient cohorts—the acute post-infarct phase vs. the chronic heart failure patient—and observation time, and therefore differ in the biological processes reflected: post-infarct IMR is likely a biphasic process with the acute event leading to changes in LV geometry and associated distortion of the MV apparatus resulting in restricted leaflet closure and acute ischaemic MR,22 followed by competing processes of LV remodelling23 and adaptive changes of the MV.24–26 During the chronic phase of ischaemic post-infarct remodelling as well as in chronic heart failure, the MV apparatus has more time to adapt and therefore the incidence of MR progression is likely lower—corresponding to the 19% observed in the present data. Another possible mechanism of less progressive MR within the present cohort may be the impact of GDT therapy on LV remodelling and consequently a tightened coaptational seal of the MV.
Regression of severe secondary mitral regurgitation
The present data indicates that a proportion of patients with severe MR show regression of mitral valve regurgitation under guideline-directed therapy. However, the regression of mitral valve regurgitation fails to relieve the associated hazard of mortality. This lends evidence to the concept that once the diagnosis of severe MR has been established little can be achieved regarding prognosis in this patient cohort. Several reasons for this observation apply: backed up by basic scientific studies on the timing of volume overload relieve in ischaemic MR,8,9 recent findings in HFrEF suggest an intermediate failure phenotype as a possible window of opportunity for timely intervention. Long-standing severe MR, even if regressing to a moderate degree during GDT, might already fueled LV remodelling towards late heart failure with irreversibly impact on cardiac remodelling and molecular changes. Furthermore basic science23 as well as clinical studies1 emphasize the impact of MR even if moderate. Indeed, the decrease in MR did not have the momentum to reduce MR beyond moderate MR suggesting the need of treatment strategies on top of GDT such as trans-catheter mitral valve repair. This hypothesis is reinforced by recent data on residual MR after trans-catheter MV repair (TMVR).27,28 Procedural failure including residual moderate MR—the main challenge of TMVR techniques—is consistently associated with poor short- and long-term outcomes and the degree of MR reduction beyond moderate is consistently associated with reverse LV and left atrial remodelling.27 Further studies such as RESHAPE-HF and COAPT will likely elucidate the potential of TMVR on top of GDT to reduce the burden of severe MR.
Limitations
The study reflects the experience of a single tertiary care-center. However, this ensures the inclusion of a homogenous patient population and a consistent quality of imaging procedures as well as the adherence to a consistent clinical routine. Further, it has to be mentioned that our data are only hypothesis generating in regard to early intervention of progressive MR and this hypothesis specifically the bundle for prediction has to be confirmed by large randomized trials. The sample size for regression of MR under guideline directed therapy during follow-up is too small to draw conclusions for the general population and is rather hypothesis generating. Nevertheless, to the best of our knowledge, these data contain the most comprehensive information on MR and prognosis at present. We did not systematically perform bioimpedance measurements to determine fluid status at time of follow-up. However, all included patients were recruited at the heart failure outpatient clinic and therefore clinically significant decompensation at time of follow-up can be excluded. Moreover, we can exclude that any patient underwent interventional mitral valve repair during study enrolment or follow-up. However, data regarding surgical mitral valve interventions at other hospitals were not available.
Conclusion
The majority of chronic heart-failure patients is suffering from non-severe MR and a significant proportion of these develop progressive secondary MR—an important clinical entity with significant impact on adverse remodelling and outcome despite GDT. Symptomatic status, left atrial size, TR, and neurohumoral pathways help to identify patients at risk for progressive secondary MR in an early disease process and open the possibility for closer follow-up and timely intervention. Whether the dismal clinical course can be altered either as an add-on procedure during cardiac surgery or as a low-risk standalone catheter based procedure remains to be demonstrated.
Supplementary data
Supplementary data are available at European Heart Journal - Cardiovascular Imaging online.
Supplementary Material
Acknowlegements
The authors thank Sophia Gottsauner-Wolf from the Medical University of Vienna for valuable help with data collection.
Funding
P.E.B. received an Erwin-Schrödinger Stipend (FWF Austrian Science Fund).
Conflict of interest: None declared.
References
- 1. Goliasch G, Bartko PE, Pavo N, Neuhold S, Wurm R, Mascherbauer J. et al. Refining the prognostic impact of functional mitral regurgitation in chronic heart failure. Eur Heart J 2018;39:39–46. [DOI] [PubMed] [Google Scholar]
- 2. Rossi A, Dini FL, Faggiano P, Agricola E, Cicoira M, Frattini S. et al. Independent prognostic value of functional mitral regurgitation in patients with heart failure. A quantitative analysis of 1256 patients with ischaemic and non-ischaemic dilated cardiomyopathy. Heart 2011;97:1675–80. [DOI] [PubMed] [Google Scholar]
- 3. Grigioni F, Enriquez-Sarano M, Zehr KJ, Bailey KR, Tajik AJ.. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation 2001;10:33–64. [DOI] [PubMed] [Google Scholar]
- 4. Kwon DH, Kusunose K, Obuchowski NA, Cavalcante JL, Popovic ZB, Thomas JD. et al. Predictors and prognostic impact of progressive ischemic mitral regurgitation in patients with advanced ischemic cardiomyopathy: a multimodality study. Circ Cardiovasc Imaging 2016;9:e004577. [DOI] [PubMed] [Google Scholar]
- 5. Meris A, Amigoni M, Verma A, Thune JJ, Kober L, Velazquez E. et al. Mechanisms and predictors of mitral regurgitation after high-risk myocardial infarction. J Am Soc Echocardiogr 2012;25:535–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Amigoni M, Meris A, Thune JJ, Mangalat D, Skali H, Bourgoun M. et al. Mitral regurgitation in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both: prognostic significance and relation to ventricular size and function. Eur Heart J 2007;28:326–33. [DOI] [PubMed] [Google Scholar]
- 7. Hillis GS, Moller JE, Pellikka PA, Bell MR, Casaclang-Verzosa GC, Oh JK.. Prognostic significance of echocardiographically defined mitral regurgitation early after acute myocardial infarction. Am Heart J 2005;150:1268–75. [DOI] [PubMed] [Google Scholar]
- 8. Beaudoin J, Levine RA, Guerrero JL, Yosefy C, Sullivan S, Abedat S. et al. Late repair of ischemic mitral regurgitation does not prevent left ventricular remodeling: importance of timing for beneficial repair. Circulation 2013;128:S248–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beeri R, Yosefy C, Guerrero JL, Abedat S, Handschumacher MD, Stroud RE. et al. Early repair of moderate ischemic mitral regurgitation reverses left ventricular remodeling: a functional and molecular study. Circulation 2007;116:I-288–93. [DOI] [PubMed] [Google Scholar]
- 10. Hung J, Otsuji Y, Handschumacher MD, Schwammenthal E, Levine RA.. Mechanism of dynamic regurgitant orifice area variation in functional mitral regurgitation: physiologic insights from the proximal flow convergence technique. J Am Coll Cardiol 1999;33:538–45. [DOI] [PubMed] [Google Scholar]
- 11. Pierard LA, Lancellotti P.. The role of ischemic mitral regurgitation in the pathogenesis of acute pulmonary edema. N Engl J Med 2004;351:1627–34. [DOI] [PubMed] [Google Scholar]
- 12. Dal-Bianco JP, Bartko PE, Levine RA.. Anticipating the vicious circle of postinfarction mitral regurgitation. Circ Cardiovasc Imaging 2016;9:e005070.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Fleisher LA. et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation 2017;135:e1159–95. [DOI] [PubMed] [Google Scholar]
- 14. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ. et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease: the Task Force for the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2017;38:2739–91. [DOI] [PubMed] [Google Scholar]
- 15. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ. et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–200. [DOI] [PubMed] [Google Scholar]
- 16. Lancellotti P, Moura L, Pierard LA, Agricola E, Popescu BA, Tribouilloy C. et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr 2010;11:307–32. [DOI] [PubMed] [Google Scholar]
- 17. Baumgartner HC, Hung JC-C, Bermejo J, Chambers JB, Edvardsen T, Goldstein S. et al. Recommendations on the echocardiographic assessment of aortic valve stenosis: a focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging 2017;18:254–75. [DOI] [PubMed] [Google Scholar]
- 18. Lancellotti P, Tribouilloy C, Hagendorff A, Moura L, Popescu BA, Agricola E. et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 1: aortic and pulmonary regurgitation (native valve disease). Eur J Echocardiogr 2010;11:307–44. [DOI] [PubMed] [Google Scholar]
- 19. Michler RE, Smith PK, Parides MK, Ailawadi G, Thourani V, Moskowitz AJ. et al. Two-year outcomes of surgical treatment of moderate ischemic mitral regurgitation. N Engl J Med 2016;374:1932–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smith PK, Puskas JD, Ascheim DD, Voisine P, Gelijns AC, Moskowitz AJ. et al. Surgical treatment of moderate ischemic mitral regurgitation. N Engl J Med 2014;371:2178–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. LaPointe MC. Molecular regulation of the brain natriuretic peptide gene. Peptides 2005;26:944–56. [DOI] [PubMed] [Google Scholar]
- 22. Otsuji Y, Handschumacher MD, Schwammenthal E, Jiang L, Song JK, Guerrero JL. et al. Insights from three-dimensional echocardiography into the mechanism of functional mitral regurgitation: direct in vivo demonstration of leaflet tethering geometry. J Am Coll Cardiol 1997;29:39114. [DOI] [PubMed] [Google Scholar]
- 23. Beeri R, Yosefy C, Guerrero JL, Nesta F, Abedat S, Chaput M. et al. Mitral regurgitation augments post-myocardial infarction remodeling failure of hypertrophic compensation. J Am Coll Cardiol 2008;51:476–86. [DOI] [PubMed] [Google Scholar]
- 24. Dal-Bianco JP, Aikawa E, Bischoff J, Guerrero JL, Handschumacher MD, Sullivan S. et al. Active adaptation of the tethered mitral valve: insights into a compensatory mechanism for functional mitral regurgitation. Circulation 2009;120:334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dal-Bianco JP, Aikawa E, Bischoff J, Guerrero JL, Hjortnaes J, Beaudoin J. et al. Myocardial infarction alters adaptation of the tethered mitral valve. J Am Coll Cardiol 2016;67:275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bartko PE, Dal-Bianco JP, Guerrero JL, Beaudoin J, Szymanski C, Kim DH. et al. Effect of losartan on mitral valve changes after myocardial infarction. J Am Coll Cardiol 2017;70:1232–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grayburn PA, Foster E, Sangli C, Weissman NJ, Massaro J, Glower DG. et al. Relationship between the magnitude of reduction in mitral regurgitation severity and left ventricular and left atrial reverse remodeling after MitraClip therapy. Circulation 2013;128:1667–74. [DOI] [PubMed] [Google Scholar]
- 28. Kaneko H, Neuss M, Weissenborn J, Butter C.. Impact of residual mitral regurgitation after MitraClip implantation. Int J Cardiol 2017;227:813–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.