Abstract
Youths with attention-deficit/hyperactivity disorder symptomatology often exhibit residual inattention and/or hyperactivity in adulthood; however, this is not true for all individuals. We recently reported that dimensional, multi-informant ratings of hyperactive/inattentive symptoms are associated with ventromedial prefrontal cortex (vmPFC) structure. Herein, we investigate the degree to which vmPFC structure during adolescence predicts hyperactive/inattentive symptomatology at 5-year follow-up. Structural equation modeling was used to test the extent to which adolescent vmPFC volume predicts hyperactive/inattentive symptomatology 5 years later in early adulthood. 1104 participants (M = 14.52 years, standard deviation = 0.42; 583 females) possessed hyperactive/inattentive symptom data at 5-year follow-up, as well as quality controlled neuroimaging data and complete psychometric data at baseline. Self-reports of hyperactive/inattentive symptomatology were obtained during adolescence and at 5-year follow-up using the Strengths and Difficulties Questionnaire (SDQ). At baseline and 5-year follow-up, a hyperactive/inattentive latent variable was derived from items on the SDQ. Baseline vmPFC volume predicted adult hyperactive/inattentive symptomatology (standardized coefficient = −0.274, P < 0.001) while controlling for baseline hyperactive/inattentive symptomatology. These results are the first to reveal relations between adolescent brain structure and adult hyperactive/inattentive symptomatology, and suggest that early structural development of the vmPFC may be consequential for the subsequent expression of hyperactive/inattentive symptoms.
Keywords: attention-deficit/hyperactivity disorder, neuroimaging, ventromedial prefrontal cortex
Introduction
Attention-deficit/hyperactivity disorder (ADHD) symptomatology frequently persists across the span of development. Longitudinal research indicates that functionally impairing symptoms continue into adolescence and adulthood in approximately 60–80% of cases diagnosed during childhood (Barkley et al. 1990; McGough and Barkley 2004). Despite such findings, longitudinal relations between adolescent brain structure and adult ADHD symptomatology remain virtually unstudied. Prospective longitudinal neuroimaging studies offer an invaluable opportunity to identify early brain-based markers of future emotional and behavioral problems. Investigating links between adolescent brain structure and adult psychopathology may further elucidate the neural underpinnings of adult ADHD symptomatology, as well as help to characterize different disease trajectories. Ultimately, such efforts may inform early intervention and prevention strategies.
The ventromedial prefrontal cortex (vmPFC), comprised of medial portions of the orbitofrontal cortex as well as ventral portions of the medial prefrontal cortex, has long been implicated in ADHD symptomatology and impulse control (Bechara 2005; Faraone et al. 2015). Prior studies demonstrate that the vmPFC is involved in aspects of reward processing, including reward valuation, as well as receipt of reward (Knutson et al. 2003; Liu et al. 2011). Indeed, motivation-based dysfunction models of ADHD have been proposed, positing that altered reward processes underpin ADHD behaviors such as hypersensitivity to delay and discounting of future reward (Sonuga-Barke et al. 1994; Sagvolden et al. 1998; Sonuga-Barke 2005). Portions of the vmPFC also constitute a critical node in the brain’s default mode network (DMN)—a functional brain network that, more recently, has been implicated in ADHD pathophysiology. Specifically, the default-mode interference hypothesis of ADHD postulates that activity in the DMN, which is normally diminished during goal-directed tasks, persists into periods of task-related processing and, consequently, interferes with task-specific processing (Sonuga-Barke and Castellanos 2007).
In the largest voxel-based morphometry (VBM) study to date on adolescent ADHD symptomatology, Albaugh et al. (2017) reported that parent and youth ratings of ADHD symptoms were each negatively associated with gray matter volume (GMV) in an overlapping portion of the vmPFC (Albaugh et al. 2017). In particular, reduced GMV in the vmPFC was tied to aspects of inattentive symptomatology in adolescents. Further, Albaugh et al. (2017) found that reaction time variability—posited to reflect attentional lapses—was negatively associated with GMV in an overlapping region of the vmPFC. Similarly, in the largest VBM study to date on adult ADHD, a significant negative correlation was revealed between vmPFC GMV and dimensional measures of inattentive symptomatology (Maier et al. 2015). Taken together, vmPFC volume may be a critical marker for inattentive symptomatology. It is possible that vmPFC structure during adolescence is not only related to concomitant symptoms of inattention, but may also be tied to the subsequent trajectories of ADHD symptomatology.
When characterizing longitudinal relations between adolescent brain structure and subsequent ADHD psychopathology, it may be beneficial to assess symptomatology in a quantitative fashion. Indeed, empirically based assessment of psychopathology has provided strong support for dimensionality with regard to a number of psychiatric conditions, including ADHD (Hudziak et al. 2007). There have been reports of an association between subclinical symptoms of hyperactivity and impulsivity in typically developing youths and evidence of delayed cortical thickness maturation—interestingly, delayed thickness maturation was revealed in areas of the cortex that have been previously implicated in clinically significant ADHD symptoms (Shaw et al. 2011; Ducharme et al. 2012). Such evidence supports the use of dimensional measures of psychopathology, as emphasized by the National Institute of Mental Health’s Research Domain Criteria program (Morris and Cuthbert 2012). In addition to assessing psychopathology using dimensional measures, studying large population-based samples affords the opportunity to capture naturally occurring variance in behavioral phenotypes, including psychopathology. Unfortunately, few studies have examined the neural correlates of hyperactivity/inattention in population-based samples.
In the present study, we employ structural equation modeling (SEM) in order to examine the degree to which adolescent vmPFC volume predicts hyperactive/inattentive symptoms during early adulthood in a large, population-based sample of 1104 youths. In a subset of participants, we also test the degree to which hyperactive/inattentive symptoms and vmPFC structure are related at study follow-up, during early adulthood.
Materials and Methods
Sample
Neuroimaging and behavioral data were obtained from the IMAGEN study conducted across 8 European sites, which includes 2223 adolescents recruited from schools at age 14 years (standard deviation [SD] = 0.41 years; age range = 12.9–15.7 years). A description of recruitment and assessment procedures, as well as study exclusion and inclusion criteria, has been published elsewhere (Schumann et al. 2010). In the present study, a total of 1104 participants possessed ADHD symptom data at the 5-year follow-up, as well as quality controlled neuroimaging data and complete psychometric and demographic data at baseline. Of these 1104 participants, 976 (88.4%) possessed quality controlled neuroimaging data at the 5-year follow-up as well as complete psychometric and demographic data at baseline.
Assessment of Hyperactivity and Inattention
The Development and Well-Being Assessment (DAWBA) is a computer-based package of questionnaires, interviews, and rating techniques used to assess adolescent psychopathology (Goodman et al. 2000). In the present study, ADHD symptom counts were derived from the parent version of the DAWBA administered at baseline and were used solely in defining the vmPFC ROI (see below for further details) used in SEM analysis.
At baseline and 5-year follow-up, the self-report version of the Strengths and Difficulties Questionnaire (SDQ) was used to assess symptoms of hyperactivity and inattention (Goodman 1997). The SDQ is a reliable and valid measure of youth emotional and behavior symptoms, on which scores are predictive of increased probability of clinician-rated psychiatric disorders and have retest stability over 4–6 months (Goodman 2001). Importantly, concurrent validity has been established between the Child Behavior Checklist Attention Problems subscale—arguably the most widely accepted dimensional measure of hyperactive/inattentive symptomatology in youths—and the SDQ Hyperactive/Inattentive scale (r = 0.75) (Mieloo et al. 2012).
Demographic Measures
The puberty development scale (PDS) was used to assess the pubertal status of participants (Petersen et al. 1988). The socioeconomic status (SES) score was derived by summing the following variables: Mother’s Education Score, Father’s Education Score, Family Stress Unemployment Score, Financial Difficulties Score, Home Inadequacy Score, Neighborhood Score, Financial Crisis Score, Mother Employed Score, and Father Employed Score (Whelan et al. 2014).
MRI Acquisition
MRI scanning was conducted at the 8 IMAGEN assessment sites using 3T whole body MRI systems. Image acquisition utilized a set of parameters that were compatible with all scanners in order to ensure comparability of data across the different scanners. Details surrounding image acquisition protocols and quality checks have been described elsewhere, including extensive standardization across MRI scanners (Schumann et al. 2010).
Structural MRI
High-resolution anatomical MRIs were acquired with a 3D T1-weighted magnetization prepared gradient echo sequence (MPRAGE) based on the ADNI protocol (http://adni.loni.usc.edu/methods/documents/mri-protocols/).
MRI Data Preprocessing
Preprocessing of the structural T1-weighted data was performed with Statistical Parametric Mapping version 8 (Wellcome Department of Neuroimaging, London, UK, http://www.fil.ion.ucl.ac.uk/spm/software/spm8/), using standard automated pipelines (Schumann et al. 2010). T1-weighted MRI processing included image segmentation into gray matter, white matter and cerebrospinal fluid tissue classes, preceded by an iterative registration to the Montreal Neurological Institute template space, using SPM’s optimized normalization routine (Ashburner and Friston 2005). For VBM, gray matter images were smoothed with a Full Width at Half Maximum Gaussian kernel of 8 mm, warped to standard MNI space and modulated by multiplying the linear and nonlinear component of the Jacobian determinants generated during spatial normalization (Ashburner and Friston 2000).
ROI Definition
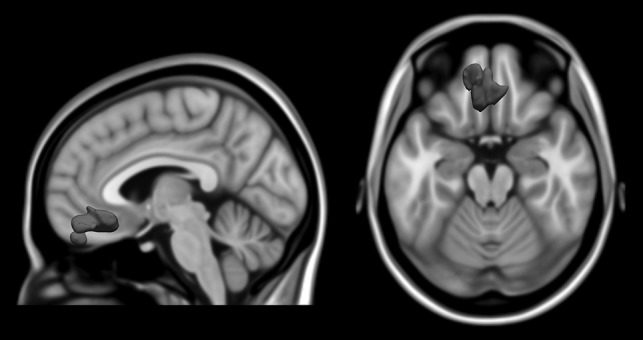
Parent reports of ADHD symptoms (obtained at baseline) were used to define the vmPFC ROI (shown in Supplementary Fig. S1). Specifically, baseline regional GMV was regressed against baseline total ADHD symptom count—using parent reports on the DAWBA—while controlling for age, sex, total GMV, site, pubertal development, Performance IQ, Verbal IQ, and SES. As outlined in Albaugh et al. (2017), this regression analysis included 1538 adolescents and revealed a negative association in bilateral vmPFC (3424 voxels, x = −4, y = 30, z = −20; peak Z score = 4.12).
Although the spatial resolution of MRI does not allow for reliable identification of cytoarchitectonic areas in humans, we have attempted to apply the cytoarchitectonic scheme of Ongur et al. (2003) using anatomical landmarks. Moving in the caudal to rostral direction along the gyrus rectus, the ROI likely includes areas 32pl, 14c, 14r, and 11 m, as well as areas 10 m and 10r along the medial wall (Ongur et al. 2003). The lateral extent of the ROI likely includes portions of area 13.
Statistical Analyses
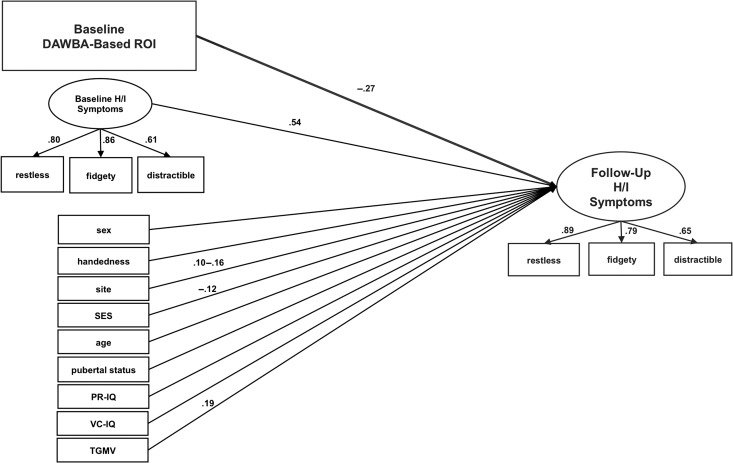
SEM was employed to test the extent to which adolescent vmPFC volume was associated with self-reported hyperactive/inattentive symptomatology at 5-year follow-up, while accounting for the effects of sex, age, pubertal status, IQ, handedness, site, SES, and total GMV, as well as baseline self-reports of hyperactive/inattentive symptomatology. By controlling for baseline symptoms, we tested the extent to which baseline vmPFC structure accounted for unique variance in follow-up H/I symptoms—independent of baseline symptomatology. At baseline and follow-up, a hyperactive/inattentive latent variable was derived from items on the youth version of the SDQ. Three SDQ items from the hyperactive/inattentive subscale were used to indicate the latent variable (“I am restless, I cannot stay still for long,” “I am constantly fidgeting or squirming,” “I am easily distracted, I find it difficult to concentrate”). This was due to the fact that the 2 positively coded items (“I think before I do things,” “I finish the work I’m doing. My attention is good”) did not covary with the other items, likely reflecting their positive scaling. The tendency for positively worded items on the SDQ to cluster together, irrespective of the subscale they belong to, has been previously reported by other groups (DiStefano and Motl 2006; Palmieri and Smith 2007; Van Roy et al. 2008). Analysis was carried out using the statistical package Mplus (http://www.statmodel.com). We utilized the Weighted Least Squares with Mean and Variance Adjusted Chi Square Test Statistic estimator (WLSMV), which is robust to violations of multivariate normality (Muthén LK and Muthén BO 2001–2016). We also repeated our analysis using standard multiple linear regression, utilizing the 5-item SDQ Hyperactive/Inattentive summary scores at baseline and follow-up (rather than indicating latent variables).
In order to assess if brain regions during adolescence—other than the vmPFC—might be associated with adult hyperactive/inattentive symptoms, we performed a subsequent exploratory whole-brain analysis. Specifically, a whole-brain voxel-wise analysis was conducted using the general linear model, performed with the VBM toolbox of SPM8. Regional GMV, measured at baseline, was regressed against self-reports of hyperactive/inattentive symptomatology obtained at 5-year follow-up. Age at baseline, sex, handedness, total GMV, site, pubertal development, performance IQ, verbal IQ, and SES were controlled for in the analysis. An initial height threshold of P ≤ 0.001 was implemented at the voxel level, with a corrected family-wise error (FWE; P ≤ 0.05) subsequently applied to identify significant clusters.
Results
Demographic and Behavioral Measures
Demographic and psychometric information for participants is provided in Table 1. For the 1104 participants included in the main SEM analysis, self-report ratings of hyperactive/inattentive symptomatology at follow-up were inversely correlated with SES (r = −0.114, P < 0.001) and Verbal IQ (r = −0.068, P = 0.023). In addition, self-reported SDQ H/I scores at follow-up were positively correlated with self-reported SDQ H/I scores at baseline (r = 0.434, P < 0.001). Baseline parent-reported DAWBA symptom counts were correlated with baseline self-reported SDQ H/I scores (r = 0.345, P < 0.001) as well as follow-up self-reported SDQ H/I scores (r = 0.235, P < 0.001) (Supplementary Table S1).
Table 1.
Summary statistics for demographic and psychometric variables
| N = 1104 | N = 976 (Available follow-up imaging) | |
|---|---|---|
| Age at baseline (in years) (mean ± SD) | 14.52 ± 0.42 | 14.52 ± 0.42 |
| Sex | 52.8% F (583), 47.2% M (521) | 53.0% F (517), 47.0% M (459) |
| SES (mean ± SD) | 18.28 ± 3.92 | 18.37 ± 3.88 |
| Verbal IQ (mean ± SD) | 112.75 ± 14.00 | 112.76 ± 13.99 |
| Performance IQ (mean ± SD) | 109.83 ± 14.61 | 109.88 ± 14.59 |
| Baseline H/I score on youth SDQ (mean ± SD) | 3.80 ± 2.11 | 3.82 ± 2.10 |
| Baseline DAWBA symptom count (mean ± SD) | 3.59 ± 5.32 | 3.54 ± 5.32 |
| Follow-up H/I score on youth SDQ (mean ± SD) | 3.41 ± 2.14 | 3.39 ± 2.13 |
| Participants scoring at, or above, youth SDQ H/I cut-off of 7 at follow-up | 93 | 82 |
H/I = hyperactive/inattentive scale.
Imaging Analyses
ROI-Based Analysis
Table 2 displays results from the ROI-based SEM analysis. The model (Figure 1) showed good fit (root mean square error of approximation = 0.030; comparative fit index = 0.941; Tucker–Lewis index = 0.925). Our analysis revealed that there was a significant direct effect of baseline vmPFC volume on hyperactive/inattentive symptoms at 5-year follow-up (standardized coefficient = −0.274, P < 0.001) where smaller volumes at baseline were associated with higher levels of hyperactive/inattentive symptoms at 5-year follow-up—independent of baseline self-reports of hyperactive/inattentive symptoms. Results were not meaningfully altered when age and pubertal stage at time of MRI scan were removed from the model, or while controlling for other SDQ subscales (including mood and anxiety symptoms captured on the Emotion subscale, as well as oppositional/rule-breaking behaviors captured on the Conduct subscale). These latter findings suggest that co-occurring psychopathology was not confounding our results.
Table 2.
Summary of ROI-based structural equation modeling analysis
| Direct effects on latent H/I variable at 5-year Follow-up | ||
|---|---|---|
| Std. beta | Sig. | |
| Baseline ROI GMV | −0.274 | <0.001 |
| Sex | 0.065 | 0.224 |
| Hand | 0.006 | 0.871 |
| Site1 | 0.104 | 0.036 |
| Site2 | 0.155 | 0.003 |
| Site3 | 0.159 | 0.001 |
| Site4 | −0.024 | 0.593 |
| Site5 | −0.049 | 0.328 |
| Site6 | −0.010 | 0.835 |
| Site7 | −0.025 | 0.630 |
| SES | −0.123 | 0.002 |
| Age | −0.002 | 0.959 |
| Puberty | −0.048 | 0.303 |
| IQ PR | 0.008 | 0.845 |
| IQ VC | 0.029 | 0.505 |
| Baseline total GMV | 0.188 | 0.014 |
| Baseline latent H/I variable | 0.535 | <0.001 |
SES = socioeconomic status; puberty = pubertal development scale; IQ PR = perceptual IQ; IQ VC = verbal IQ; H/I = hyperactive/inattentive; ROI = region of interest; GMV = gray matter volume (N = 1104). Bold indicates a significance threshold of p < 0.05.
Figure 1.
The model used to study the relationship between baseline vmPFC GMV and follow-up hyperactive/inattentive symptomatology (N = 1104). Only statistically significant parameters are reported. A range of parameters is reported for site because it was coded via 7 binary dummy-variables. All covariates were assessed at baseline.
It is noteworthy that very similar results were obtained when standard multiple linear regression analysis was performed in which SDQ hyperactive/inattentive summary scores (using all 5 items) were used rather than latent variables (Supplementary Table S2). More specifically, follow-up SDQ Hyperactive/Inattentive summary scores were regressed on sex, age, pubertal status, performance IQ, verbal IQ, handedness, site, SES, baseline total GMV, baseline SDQ Hyperactive/Inattentive summary score, and baseline vmPFC ROI volume.
Using only the 976 participants with available follow-up imaging data, we attempted to include vmPFC volume (assessed at 5-year follow-up) into the structural equation model—in particular, as a mediating variable in between baseline vmPFC and follow-up hyperactive/inattentive symptoms. This resulted in a lack of model convergence. Upon further investigation, this reflected the fact that follow-up vmPFC volume was not significantly correlated with hyperactive/inattentive symptoms at baseline or 5-year follow-up (see Supplementary Tables S3–S5). Baseline vmPFC volume, however, was significantly correlated with follow-up vmPFC volume (r = 0.846, P < 0.001). Post hoc partial correlation analysis revealed a significant association between baseline vmPFC volume and follow-up hyperactive/inattentive SDQ summary score while controlling for follow-up vmPFC volume, baseline hyperactive/inattentive SDQ summary score, as well as sex, handedness, site, SES, age at baseline, pubertal development at baseline, baseline total GMV, and follow-up total GMV (r = −0.084, P = 0.009).
Whole-Brain Analysis
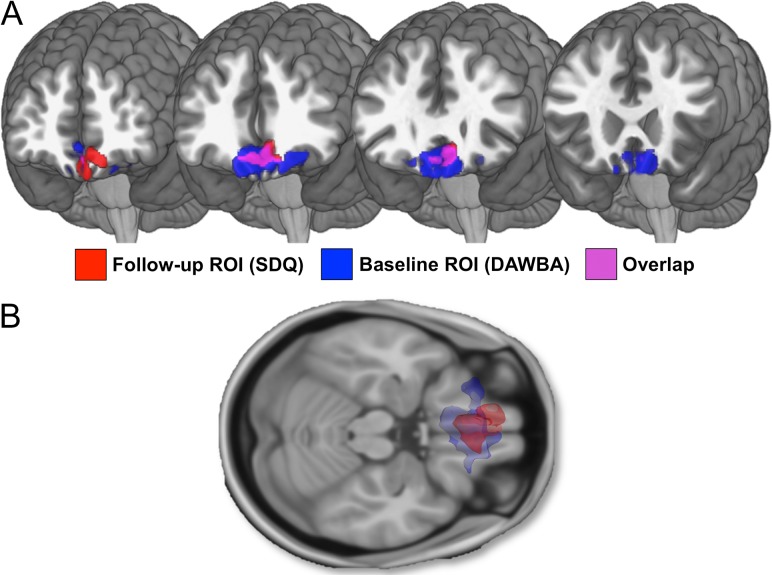
Regressing baseline regional GMV against follow-up hyperactive/inattentive SDQ summary scores revealed a negative association in the vmPFC (1351 voxels, x = −12, y = 46, z = −17; peak Z value = 5.04) (Fig. 2). No other associations survived correction for multiple comparisons. Figure 3 depicts the spatial overlap between the parent-defined ROI used for the a priori analyses above and the results from this whole-brain analysis.
Figure 2.
Results from whole brain voxel-wise analyses regressing baseline regional gray matter volume against SDQ HYPERACTIVE/INATTEntive score (assessed approximately 5 years later at follow-up). Age, sex, handedness, total gray matter volume (GMV), site, pubertal development, performance IQ, verbal IQ, and socioeconomic status were controlled for in the analysis. An initial height threshold of P ≤ 0.001 was implemented at the voxel level, with a corrected family-wise error (FWE; P ≤ 0.05) subsequently applied to identify significant clusters (N = 1104). In axial view, left is left.
Figure 3.
(A) Blue depicts baseline regional GMV related to parent-reported hyperactive/inattentive symptomatology (assessed at baseline) (see Albaugh et al., 2017, for further details; N = 1538). Red depicts baseline regional GMV related to self-reported hyperactive/inattentive summary score (assessed approximately 5 years later at follow-up) on the Strengths and Difficulties Questionnaire (N = 1104). Pink represents overlap in results. Age, sex, handedness, total gray matter volume (GMV), site, pubertal development, performance IQ, verbal IQ, and socioeconomic status were controlled for in the analysis. An initial height threshold of P ≤ 0.001 was implemented at the voxel level, with a corrected family-wise error (FWE; P ≤ 0.05) subsequently applied to identify significant clusters. (B) 3D reconstruction of results. Blue depicts baseline regional GMV related to parent-reported ADHD symptomatology (assessed at baseline) (see Albaugh et al., in press, for further details; N = 1538). Red depicts baseline regional GMV related to self-reported ADHD symptoms (assessed approximately 5 years later at follow-up) on the Strengths and Difficulties Questionnaire (N = 1104). Results shown in axial view.
When controlling for baseline H/I self-report scores in the above VBM analysis, findings hold when an initial height threshold of P ≤ 0.005 is implemented at the voxel level, with a corrected family-wise error (FWE; P ≤ 0.05) subsequently applied to identify significant clusters.
Discussion
To our knowledge, this is the first report of a longitudinal association between adolescent brain structure and hyperactive/inattentive symptomatology in early adulthood. Critically, vmPFC structure during adolescence was linked to hyperactive/inattentive symptomatology in early adulthood. In our SEM and standard multiple linear regression analyses, smaller ventromedial prefrontal volume at baseline predicted greater hyperactive/inattentive symptomatology at 5-year follow-up. It is important to note that, in these analyses, we controlled for baseline symptomatology. Further, covarying for mood and anxiety psychopathology, as well as conduct problems, did not meaningfully alter our results. Thus, our findings indicate that adolescent vmPFC volume accounts for unique variance in self-reported hyperactive/inattentive symptoms at 5-year follow-up—independent of self-reported baseline symptomatology. Taken together, vmPFC morphology during adolescence may possess predictive utility with regard to future symptoms of hyperactivity/inattention in early adulthood.
The vmPFC has been previously associated with concomitant ADHD symptomatology in adolescents and adults. In recent work by Albaugh et al. (2017), it was found that vmPFC GMV during adolescence was negatively associated with concomitant parent and youth reports of inattention. In this same study, it was also reported that reaction time variability was negatively associated with GMV in an overlapping region of the vmPFC. Similar results were obtained in the largest brain structural imaging study to date on adult ADHD, where a significant negative correlation was revealed between vmPFC GMV and a dimensional measure of inattentive symptomatology (Maier et al. 2015). Taken together, these previous studies further implicate the vmPFC in the pathophysiology of inattention. The present study extends findings from these previous reports, demonstrating that adolescent vmPFC structure is associated with hyperactive/inattentive symptomatology approximately 5 years later in early adulthood, independent of baseline symptomatology.
Interestingly, the vmPFC represents a central node in the brain’s DMN, a brain network that has been hypothesized to play a role in the pathophysiology of ADHD symptoms. Specifically, the default-mode interference hypothesis posits that activity in the DMN, which is typically attenuated during goal-directed tasks, can persist into periods of task-related processing and, as a result, compete with task-specific neural processing (Sonuga-Barke and Castellanos 2007). The vmPFC represents a primary hub in the brain’s DMN—a network believed to play a central role in mind-wandering and task-unrelated thought. Although speculative, it is possible that the volumetric reductions in the vmPFC may be linked to both concomitant and future DMN dysfunction. In a recent study by Salavert et al. (2015), ADHD participants exhibited reduced deactivation of the vmPFC during a working memory task. The authors suggest that failure to deactivate the medial prefrontal cortex is tied to lapses of attention, and that this may be a central feature of ADHD symptomatology (Salavert et al. 2015). In the context of the present study, reduced vmPFC volume during adolescence may serve as a marker for increased vulnerability to future DMN dysfunction—more specifically, an impaired ability to deactivate portions of the DMN. Future studies are needed to test this hypothesis.
In the context of the DMN, it is noteworthy that mind-wandering—or the drifting of attention away from external, task-related activities towards self-generated cognitions—has been previously tied to the vmPFC. Numerous functional imaging studies have implicated the vmPFC in mind-wandering (Andrews-Hanna, Reidler, Huang, et al. 2010; Fox et al. 2015). Bertossi and Ciaramelli (2016) recently found that patients with vmPFC damage reported significantly reduced off-task thoughts and less frequent daydreaming when compared with controls. The extent and overlap of patients’ brain lesions studied by Bertossi and Ciaramelli (2016) share a striking resemblance to the ROI used in the present study. As noted by others, the vmPFC belongs to the “medial temporal lobe (MTL)-subsystem” of the DMN (Andrews-Hanna, Reidler, Sepulcre, et al. 2010). As hypothesized by Bertossi and Ciaramelli, the vmPFC—and its shared connections with MTL structures—may be central to the mental construction of past events, or possible future scenarios (Bertossi and Ciaramelli 2016). According to their hypothesis, vmPFC patients may experience a relative dearth of internally generated thoughts about the past and future, and there is little competition from the internal milieu with regard to the allocation of attentional resources (Bertossi and Ciaramelli 2016). Although speculative, it is plausible that aberrant functioning and/or connectivity of the vmPFC could also lead to an abundance of internally generated stimuli that outcompete external stimuli for attentional resources. Interestingly, this aberrant functioning and/or connectivity of the vmPFC may underpin aspects of normative, as well as clinically significant, inattention. It is also worth mentioning that over-activation of the subcallosal cingulate area (Brodmann Area 25)—an area closely neighboring the caudal extent of the ROI used in the present study—has been tied to the shifting of attention away from external stimuli and towards negative, self-referential thoughts (Choi et al. 2015).
Findings from the present study may also reflect altered maturation of neural pathways involved in reward processing. A number of functional neuroimaging studies have found evidence of hyporesponsiveness during reward anticipation in adolescent and adult ADHD samples (Scheres et al. 2007; Strohle et al. 2008). It was recently reported that vmPFC-lesioned neurosurgical patients exhibited reduced ventral striatal activity during the anticipation of reward, as well as decreased nucleus accumbens volumes, relative to neurologically healthy controls (Pujara et al. 2016). Intriguingly, in the context of the present study, structural alterations in the vmPFC during adolescence may be related to enduring functional deficits in reward processing.
Few imaging studies have attempted to test longitudinal associations between brain metrics and ADHD-related outcomes. In a seminal longitudinal study by Shaw et al. (2006), 163 children with ADHD (mean age at study entry, 8.9 years) and 166 controls underwent MRI scanning, with the majority of participants undergoing MRI scanning 2 times or more. Clinical evaluations were conducted at follow-up (mean follow-up, 5.7 years). In brief, children with worse clinical outcome possessed thinner left medial prefrontal cortex at baseline relative to controls and ADHD participants with better outcomes. This finding appears in line with results from the present study indicating that reduced ventromedial prefrontal volume during adolescence is associated with greater ADHD symptomatology in early adulthood. Mattfeld et al. (2014) recently used resting state MRI to characterize patterns of functional connectivity within 3 groups: (I) patients with persistent ADHD diagnoses in both childhood and adulthood, (II) patients who had met criteria for ADHD diagnosis in childhood but not during adulthood, and (III) controls who did not meet criteria for ADHD diagnosis during childhood or adulthood (Mattfeld et al. 2014). Importantly, participants were scanned as adults. Positive functional correlation between 2 major midline nodes of the DMN—the vmPFC and posterior cingulate—was reduced in patients with a persistent ADHD diagnosis, but not in remitted patients or controls. Furthermore, whereas control participants exhibited significant negative correlations between resting state activity in medial prefrontal and bilateral dorsolateral prefrontal regions, these regions were not significantly anti-correlated in participants with persistent or remitted ADHD. These findings suggest that DMN dysfunction may indeed be related to trajectories of ADHD symptomatology.
It is noteworthy that baseline vmPFC volume was associated with hyperactive/inattentive symptoms at follow-up; however, follow-up vmPFC volume was not significantly associated with baseline or follow-up symptomatology. Although seemingly at odds with Maier et al. (2015), this finding appears in line with several morphometric studies of adult ADHD in which volumetric reductions were limited to the dorsal anterior cingulate and areas comprising the dorsal attention network (Seidman et al. 2006; Makris et al. 2007). Given the relatively protracted structural development of the vmPFC—particularly with regard to cortical surface expansion (Sowell et al. 2004)—it may be a region where delayed brain maturation could still be observed at time of baseline assessment. Interestingly, results from the present study appear to dovetail with findings of Ducharme et al. (2012). Studying a large population-based sample of typically developing youths, Ducharme et al. (2012) revealed negative associations between Child Behavior Checklist Attention Problems score and orbitofrontal (including portions of the vmPFC) cortical thickness early on in development; however, this relation was not observed in older youths. Thus, our results appear to support previous reports of clinical and subclinical ADHD symptoms being associated with reduced rates of brain structural change. Moreover, it is notable that self-reported hyperactive/inattentive symptoms at follow-up were related to vmPFC structure 5 years earlier even when partialling out the influence of this region’s volume at follow-up. This suggests that the earlier developmental trajectory of this region may prove to be consequential for the subsequent expression of hyperactive/inattentive symptoms.
We have demonstrated anatomical convergence with regard to the association between baseline brain structure and baseline parent reports of ADHD symptoms, and the longitudinal association between baseline brain structure and subsequent self-reported hyperactive/inattentive symptomatology in early adulthood (controlling for baseline self-reports of hyperactive/inattentive symptomatology). Given that this anatomical overlap was observed primarily in ventromedial prefrontal cortices, these results further implicate this brain region in the pathophysiology of ADHD symptomatology. Thus, vmPFC structure during adolescence is not only related to concomitant hyperactivity/inattention, but also future hyperactivity/inattention in adulthood—with smaller volumes during adolescence being associated, on average, with greater hyperactive/inattentive symptomatology in adulthood.
Intriguingly, findings from the present study suggest that aspects of prefrontal structure during adolescence may, ultimately, be of clinical significance in the context of adult ADHD. Although speculative, it is possible that more refined assessments of orbital and ventromedial prefrontal morphology during adolescence may help to identify youths at greatest risk for clinically significant symptom change. It is possible that aberrant vmPFC volume during adolescence, when coupled with particular genetic and/or environmental factors, may increase likelihood of clinically significant symptomatology in adulthood. Future studies may benefit from investigating the extent to which environmental and genetic factors may serve to moderate the relationship between adolescent prefrontal structure and adult hyperactive/inattentive symptomatology.
Finally, it should be noted that aspects of the vmPFC have been implicated in a number of different psychopathologies and behaviors, including anxiety, depression, impulse control, psychopathy, and reward valuation (Hiser and Koenigs 2017). This observation likely reflects several important points. First, the majority of previous neuroimaging studies have utilized relatively simple approaches to characterizing psychopathology. With the advent of more sophisticated statistical approaches, such as bifactor models of psychopathology (Lahey et al. 2017), it is possible that a more general psychopathology factor—a factor that cuts across different classes of psychopathology and accounts for observed correlations across different symptom domains—may help to elucidate why particular brain areas are implicated in numerous psychopathologies. Second, the vmPFC has been identified as a hub node in the brain’s “rich club” network—a constellation of brain regions that possess rich connections and are densely interconnected (van den Heuvel and Sporns 2013). Thus, the vmPFC is ideally situated to exert influence on numerous brain networks; its rich connectivity may account for the vmPFC’s putative role in numerous psychopathologies and behaviors.
The present study possesses a number of methodological strengths. We utilized a large longitudinal, population-based sample, capturing naturally occurring variation in ADHD symptomatology. We also assessed hyperactive/inattentive symptoms as a quantitative trait rather than following a strict categorical approach. These methodological approaches serve to greatly bolster statistical power. Nonetheless, given that we have focused on regional GMV in our analyses, we are unable to definitively comment on the neurophysiological underpinnings of the VBM findings. Similarly, we are unable to comment on possible ties to aberrant structural and/or functional connectivity. Future studies are needed to address these issues. We were limited by the fact that only self-reports of ADHD symptomatology were obtained at follow-up. Thus, our SEM analysis rested solely upon self-reports of hyperactive/inattentive symptoms using the SDQ. One potential weakness of the present study is that the vmPFC ROI was defined vis-à-vis phenotypic reports of ADHD, and phenotypic reports of ADHD are the predicted variable in the model—thus, our ROI predictor and dependent variable may not be entirely independent. As a result, there is a possibility that the association between baseline vmPFC and baseline symptoms was due to chance, and, as a result, we have simply carried forward a false positive effect. Several factors reduce the likelihood of this possibility. We defined the baseline ROI using parent-reported ADHD symptom counts obtained from the DAWBA interview; however, the predicted variable in our primary analysis was self-reported Hyperactive/Inattentive scores on the SDQ. Thus, we used different methods to obtain parent and youth reports of ADHD symptomatology (symptom count from interview versus standardized questionnaire, respectively). Most importantly, the correlation between parent-reported DAWBA symptom counts and youth-reported SDQ H/I was low. As a result, if the association between baseline vmPFC and baseline symptoms were truly due to chance, one would not expect to find a relationship between baseline vmPFC and the follow-up self-report measure. Unfortunately, we did not have information with regard to prescription stimulant usage, which may have qualified the relationship between brain structure and hyperactive/inattentive symptoms over the developmental window studied.
In conclusion, vmPFC structure, which has been previously linked to concomitant ADHD symptomatology, also informs ADHD symptom trajectories from adolescence into early adulthood. These findings suggest that vmPFC structure in adolescence may have clinical utility by informing ADHD symptom trajectories. More granular assessment of adolescent vmPFC morphology may increase predictive utility in future studies.
Supplementary Material
Footnotes
Conflicts of interest: Dr Banaschewski has served as an advisor or consultant to Bristol-Myers Squibb, Desitin Arzneimittel, Eli Lilly, Medice, Novartis, Pfizer, Shire, UCB, and Vifor Pharma; he has received conference attendance support, conference support, or speaking fees from Eli Lilly, Janssen McNeil, Medice, Novartis, Shire, and UCB; and he is involved in clinical trials conducted by Eli Lilly, Novartis, and Shire; the present work is unrelated to these relationships. Dr Gallinat has received research funding from the German Federal Ministry of Education and Research, AstraZeneca, Eli Lilly, Janssen-Cilag, and Bristol-Myers Squibb; he has received speaking fees from AstraZeneca, Janssen-Cilag, and Bristol-Myers Squibb. Dr Barker has received honoraria from General Electric for teaching on scanner programming courses. The other authors report no biomedical financial interests or potential conflicts of interest.
Funding
This work was supported by the European Union–funded FP6 Integrated Project IMAGEN “Reinforcement-related behaviour in normal brain function and psychopathology” (Grant no. LSHM-CT-2007-037286); FP7 projects IMAging GEnetics for MENtal Disorders (Grant no. 602450), AGGRESSOTYPE (Grant no. 602805), and MATRICS (Grant no. 603016); Innovative Medicine Initiative Project EU-AIMS (Grant No. 115300-2); Medical Research Council Grants “Developmental pathways into adolescent substance abuse” (Grant no. 93558) and Consortium on Vulnerability to Externalizing Disorders and Addictions (Grant no. MR/N000390/1); Swedish Funding Agencies VR, FORTE, and FORMAS; Medical Research Council and Wellcome Trust Behavioural and Clinical Neuroscience Institute, University of Cambridge, National Institute for Health Research Biomedical Research Centre at South London, and Maudsley National Health Service Foundation Trust and King’s College London; Bundesministeriumfür Bildung und Forschung (Grant nos 01GS08152 and 01EV0711), eMED SysAlc01ZX1311A, and Forschungsnetz AERIAL; Deutsche Forschungsgemeinschaft (Grant nos SM 80/7-1, SM 80/7-2, and SFB 940/1); National Institutes of Health “Axon, Testosterone and Mental Health During Adolescence” (Grant no. RO1 MH085772-01A1); National Institutes of Health Consortium (Grant no. U54 EB020403), supported by a National Institutes of Health cross-alliance that funds Big Data to Knowledge Centres of Excellence; Tobacco Centers of Regulatory Science (Grant no. P50DA036114 to H.G.); and Center of Biomedical Research Excellence (award P20GM103644-01A1) from the National Institute of General Medical Sciences (to A.P., H.G., R.A., B.C., and P.S.).
References
- Albaugh MD, Orr C, Chaarani B, Althoff RR, Allgaier N, D’Alberto N, Hudson K, Mackey S, Spechler PA, Banaschewski T, et al. 2017. Inattention and reaction time variability are linked to ventromedial prefrontal volume in adolescents. Biol Psychiatry. 82:660–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Huang C, Buckner RL. 2010. a. Evidence for the default network’s role in spontaneous cognition. J Neurophysiol. 104:322–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. 2010. b. Functional-anatomic fractionation of the brain’s default network. Neuron. 65:550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. 2000. Voxel-based morphometry—the methods. NeuroImage. 11:805–821. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. 2005. Unified segmentation. NeuroImage. 26:839–851. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Fischer M, Edelbrock CS, Smallish L. 1990. The adolescent outcome of hyperactive children diagnosed by research criteria: I. An 8-year prospective follow-up study. J Am Acad Child Adolesc Psychiatry. 29:546–557. [DOI] [PubMed] [Google Scholar]
- Bechara A. 2005. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 8:1458–1463. [DOI] [PubMed] [Google Scholar]
- Bertossi E, Ciaramelli E. 2016. Ventromedial prefrontal damage reduces mind-wandering and biases its temporal focus. Soc Cogn Affect Neurosci. 11:1783–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KS, Riva-Posse P, Gross RE, Mayberg HS. 2015. Mapping the “Depression Switch” during intraoperative testing of subcallosal cingulate deep brain stimulation. JAMA Neurol. 72:1252–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiStefano C, Motl RW. 2006. Further investigating method effects associated with negatively worded items on self-report surveys. Struct Equ Modeling. 13:440–464. [Google Scholar]
- Ducharme S, Hudziak JJ, Botteron KN, Albaugh MD, Nguyen TV, Karama S, Evans AC, Brain Development Cooperative G . 2012. Decreased regional cortical thickness and thinning rate are associated with inattention symptoms in healthy children. J Am Acad Child Adolesc Psychiatry. 51:18–27 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Asherson P, Banaschewski T, Biederman J, Buitelaar JK, Ramos-Quiroga JA, Rohde LA, Sonuga-Barke EJ, Tannock R, Franke B. 2015. Attention-deficit/hyperactivity disorder. Nat Rev Dis Primers. 1:15020. [DOI] [PubMed] [Google Scholar]
- Fox KC, Spreng RN, Ellamil M, Andrews-Hanna JR, Christoff K. 2015. The wandering brain: meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. NeuroImage. 111:611–621. [DOI] [PubMed] [Google Scholar]
- Goodman R. 1997. The Strengths and Difficulties Questionnaire: a research note. J Child Psychol Psychiatry. 38:581–586. [DOI] [PubMed] [Google Scholar]
- Goodman R. 2001. Psychometric properties of the strengths and difficulties questionnaire. J Am Acad Child Adolesc Psychiatry. 40:1337–1345. [DOI] [PubMed] [Google Scholar]
- Goodman R, Ford T, Richards H, Gatward R, Meltzer H. 2000. The Development and Well-Being Assessment: description and initial validation of an integrated assessment of child and adolescent psychopathology. J Child Psychol Psychiatry. 41:645–655. [PubMed] [Google Scholar]
- Hiser J, Koenigs M. 2017. The multifaceted role of the ventromedial prefrontal cortex in emotion, decision making, social cognition, and psychopathology. Biol Psychiatry. pii: S0006-3223(17)32203-5. 10.1016/j.biopsych.2017.10.030. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudziak JJ, Achenbach TM, Althoff RR, Pine DS. 2007. A dimensional approach to developmental psychopathology. Int J Methods Psychiatr Res. 16(Suppl 1):S16–S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. 2003. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. NeuroImage. 18:263–272. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Krueger RF, Rathouz PJ, Waldman ID, Zald DH. 2017. A hierarchical causal taxonomy of psychopathology across the life span. Psychol Bull. 143:142–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J. 2011. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 35:1219–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier S, Perlov E, Graf E, Dieter E, Sobanski E, Rump M, Warnke A, Ebert D, Berger M, Matthies S, et al. 2015. Discrete global but no focal gray matter volume reductions in unmedicated adult patients with attention-deficit/hyperactivity disorder. Biol Psychiatry. 80:905–915. [DOI] [PubMed] [Google Scholar]
- Makris N, Biederman J, Valera EM, Bush G, Kaiser J, Kennedy DN, Caviness VS, Faraone SV, Seidman LJ. 2007. Cortical thinning of the attention and executive function networks in adults with attention-deficit/hyperactivity disorder. Cereb Cortex. 17:1364–1375. [DOI] [PubMed] [Google Scholar]
- Mattfeld AT, Gabrieli JD, Biederman J, Spencer T, Brown A, Kotte A, Kagan E, Whitfield-Gabrieli S. 2014. Brain differences between persistent and remitted attention deficit hyperactivity disorder. Brain. 137:2423–2428. [DOI] [PubMed] [Google Scholar]
- McGough JJ, Barkley RA. 2004. Diagnostic controversies in adult attention deficit hyperactivity disorder. Am J Psychiatry. 161:1948–1956. [DOI] [PubMed] [Google Scholar]
- Mieloo C, Raat H, van Oort F, Bevaart F, Vogel I, Donker M, Jansen W. 2012. Validity and reliability of the strengths and difficulties questionnaire in 5–6 year olds: differences by gender or by parental education? PLoS One. 7:e36805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S, Cuthbert B. 2012. Research Domain Criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues Clin Neurosci. 14:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. 2001. –2016. Mplus: User’s guide. Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Ongur D, Ferry AT, Price JL. 2003. Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol. 460:425–449. [DOI] [PubMed] [Google Scholar]
- Palmieri PA, Smith GC. 2007. Examining the structural validity of the strengths and difficulties questionnaire (SDQ) in a US sample of custodial grandmothers. Psychol Assessment. 19:189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. 1988. A self-report measure of pubertal status: reliability, validity, and initial norms. J Youth Adolesc. 17:117–133. [DOI] [PubMed] [Google Scholar]
- Pujara MS, Philippi CL, Motzkin JC, Baskaya MK, Koenigs M. 2016. Ventromedial prefrontal cortex damage is associated with decreased ventral striatum volume and response to reward. J Neurosci. 36:5047–5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagvolden T, Aase H, Zeiner P, Berger D. 1998. Altered reinforcement mechanisms in attention-deficit/hyperactivity disorder. Behav Brain Res. 94:61–71. [PubMed] [Google Scholar]
- Salavert J, Ramos-Quiroga JA, Moreno-Alcazar A, Caseras X, Palomar G, Radua J, Bosch R, Salvador R, McKenna PJ, Casas M, et al. 2015. Functional imaging changes in the medial prefrontal cortex in adult ADHD. J Atten Disord. 10.1177/1087054715611492. [DOI] [PubMed] [Google Scholar]
- Scheres A, Milham MP, Knutson B, Castellanos FX. 2007. Ventral striatal hyporesponsiveness during reward anticipation in attention-deficit/hyperactivity disorder. Biol Psychiatry. 61:720–724. [DOI] [PubMed] [Google Scholar]
- Schumann G, Loth E, Banaschewski T, Barbot A, Barker G, Buchel C, Conrod PJ, Dalley JW, Flor H, Gallinat J, et al. , consortium I . 2010. The IMAGEN study: reinforcement-related behaviour in normal brain function and psychopathology. Mol Psychiatry. 15:1128–1139. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Valera EM, Makris N, Monuteaux MC, Boriel DL, Kelkar K, Kennedy DN, Caviness VS, Bush G, Aleardi M, et al. 2006. Dorsolateral prefrontal and anterior cingulate cortex volumetric abnormalities in adults with attention-deficit/hyperactivity disorder identified by magnetic resonance imaging. Biol Psychiatry. 60:1071–1080. [DOI] [PubMed] [Google Scholar]
- Shaw P, Gilliam M, Liverpool M, Weddle C, Malek M, Sharp W, Greenstein D, Evans A, Rapoport J, Giedd J. 2011. Cortical development in typically developing children with symptoms of hyperactivity and impulsivity: support for a dimensional view of attention deficit hyperactivity disorder. Am J Psychiatry. 168:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Lerch J, Greenstein D, Sharp W, Clasen L, Evans A, Giedd J, Castellanos FX, Rapoport J. 2006. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 63(5):540–549. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. 2005. Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biol Psychiatry. 57:1231–1238. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Castellanos FX. 2007. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neurosci Biobehav Rev. 31:977–986. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Houlberg K, Hall M. 1994. When is “impulsiveness” not impulsive? The case of hyperactive children’s cognitive style. J Child Psychol Psychiatry. 35:1247–1253. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. 2004. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 24:8223–8231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohle A, Stoy M, Wrase J, Schwarzer S, Schlagenhauf F, Huss M, Hein J, Nedderhut A, Neumann B, Gregor A, et al. 2008. Reward anticipation and outcomes in adult males with attention-deficit/hyperactivity disorder. NeuroImage. 39:966–972. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O. 2013. An anatomical substrate for integration among functional networks in human cortex. J Neurosci. 33:14489–14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Roy B, Veenstra M, Clench-Aas J. 2008. Construct validity of the five-factor Strengths and Difficulties Questionnaire (SDQ) in pre-, early, and late adolescence. J Child Psychol Psychiatry. 49:1304–1312. [DOI] [PubMed] [Google Scholar]
- Whelan R, Watts R, Orr CA, Althoff RR, Artiges E, Banaschewski T, Barker GJ, Bokde AL, Buchel C, Carvalho FM, et al. 2014. Neuropsychosocial profiles of current and future adolescent alcohol misusers. Nature. 512:185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.