Abstract
Background
Despite aggressive multimodal therapy, >50% of children with high-risk neuroblastoma (HRNB) relapse. Survival after relapse is rare, and no consensus currently exists on the most effective therapy.
Objective
To conduct a systematic review of the literature on effectiveness of re-induction chemotherapy in children with relapsed HRNB.
Methods
Database searches were performed to identify studies looking at response to 1st line chemotherapy for children >12 months at diagnosis with first relapse of HRNB. Studies not reporting separate outcomes for HRNB patients or of refractory patients only were excluded. Two independent reviewers extracted the data and assessed study quality using a modified Newcastle–Ottawa tool.
Results
Nine studies were identified fitting the inclusion criteria. All except one were single arm cohorts, and two were retrospective database reviews from single centres. One was a multicentre randomised controlled trial. All used a version of the validated International Neuroblastoma Response Criteria with 8 recording best ever response and 1 at a specified time, and 5 had central review. The proportion of relapsed patients varied from 24 to 100% with 30–93% receiving upfront myeloablative therapy. The response rate varied from 6 to 64%; however, because of heterogeneity, studies were not directly comparable, and no single treatment emerged as the most effective re-induction therapy.
Conclusions
To date, there is no clear superior re-induction therapy for 1st relapse of HRNB. Randomised controlled trials with separate arms for relapsed versus refractory disease are needed to determine optimal re-induction chemotherapy to act as a backbone for testing newer targeted agents.
Keywords: Neuroblastoma, High-risk, Relapse, Re-induction chemotherapy, Response assessment
Abbreviations: CCLG, Children's cancer and leukaemia group; COG, Children's oncology group; CR, Complete remission; EFS, Event-free survival; HRNB, High-risk neuroblastoma; INRC, International neuroblastoma response criteria; INRG, International neuroblastoma risk group; INSS, International neuroblastoma staging system; MAT, Myeloablative therapy; MR, Mixed response; NANT, New approaches to neuroblastoma therapy; NB, Neuroblastoma; NR, Not reported; OS, Overall survival; PD, Progressive disease; PICO, Patients, intervention, comparison and outcome; PR, Partial response; RCT, Randomised controlled trial; SD, Stable disease
Highlights
-
•
Objective response (complete remission and partial response) is achievable in 6–64% of patients.
-
•
Multiple drugs and regimes have been tested and show effect.
-
•
Relapsed and refractory patients need to be studied separately.
-
•
Currently, no one regime emerges as superior for re-induction chemotherapy at first relapse.
1. Background
Neuroblastoma (NB) is an embryonal tumour arising from the sympathetic nervous system originating in the adrenal gland or along the paravertebral sympathetic chain. It is a heterogeneous tumour classified into three risk groups (low, intermediate and high) depending on age, extent of disease, histology and cytogenetic abnormalities. Around 50% are high-risk neuroblastoma (HRNB) defined as unresectable or metastatic tumours with amplification of the MYCN oncogene in any age group or those over 18 months with metastatic disease [1].
Despite aggressive multimodal therapy, overall survival (OS) for HRNB is <50% at 5 years with most relapses (80%) occurring within 2 years of diagnosis [2]. Historically, survival after relapse was very rare. A review of relapsed stage 4 patients in the International Neuroblastoma Risk Group (INRG) database from 1990 to 2002 revealed 5-year OS of 8% and 4% for MYCN amplified disease [3]. An Italian retrospective review (1979–2004) found a 10-year OS for relapsed stage 4 patients of 2% [4]. UK data from a pilot epidemiological study found 3% OS at 10 years for relapsed HRNB [2]. A recent Children's oncology group meta-analysis showed a 4-year progression free survival of 6% and OS of 15% for high-risk patients enrolled on early phase trials for relapsed/refractory disease [5]. MYCN status, time to relapse and age have all been shown to affect length of survival after relapse with MYCN amplified disease progressing more rapidly, later relapse having a longer survival, and older children having a more chronic, smouldering disease [2], [3], [4], [5], [6], [7].
There is no clear consensus on optimal therapy for relapse and a lack of randomised clinical trials. A recent review on relapse therapy for HRNB summarises the rationale and data for various chemotherapeutic approaches and suggests future therapies [8] However, this is an expert review, and there is no comment on study quality or comparison of efficacy. Guidelines exist on the Children's Cancer and Leukaemia Group website, [9] suggesting a number of different chemotherapeutic regimes; they are not a systematic comparison nor do they give a preference. To avoid unnecessary toxic treatment and to optimise cure, it is essential to identify the most effective treatment in relapsed HRNB, which will also provide a backbone for testing newer targeted agents. The aim of this study was to undertake a systematic review of work, published or available in abstract form, examining effectiveness of re-induction chemotherapy in children with newly relapsed HRNB.
2. Methods
2.1. Literature search
The systematic review followed guidelines contained in the NHS Centre for Reviews and Dissemination [10]. MEDLINE, EMBASE, Cochrane CENTRAL and SCOPUS bibliographic databases from inception to December 2017 were searched using NEUROBLASTOMA and a combination of terms and their alternatives: (i) RELAPS*, (ii) HIGH RISK/STAGE 4 and (iii) TREATMENT/THERAPY/CHEMOTHERAPY/RE-INDUCTION. The reference list of a previous review paper was cross-checked [8]. Websites including clinicaltrials.gov, American Society of Clinical Oncology and Advances in Neuroblastoma Research and Solving Kids’ Cancer were also reviewed for details of any relevant studies.
2.2. Study selection
Randomised controlled trials (RCTs), single arm observational studies and retrospective analyses where the population studied was children with relapsed HRNB (treated on a national high-risk protocol) were included. Studies combining relapsed and refractory patients were also included. The intervention assessed was first-line chemotherapy for relapsed disease and excluded patients with >2 lines of previous therapy. The outcome measure was response rate defined by a validated tool such as the International Neuroblastoma Response Criteria (INRC) [11], [12]. An objective response was defined as complete remission (CR) or partial remission (PR). Studies were excluded if they included patients at 2nd or subsequent relapse, studied refractory disease only, were not published in English, were studies of infant patients only or were phase I studies. Relapse was defined as recurrence or progression (any new lesion, soft tissue or bone) following an initial response (including partial) to any NB therapy [11].
Three authors of studies published in abstract format were contacted via email and asked to provide full data. All declined apart from one who had a follow-up paper accepted for publication [13]. However, this article did not meet the eligibility criteria because of inclusion of heavily pre-treated patients with a median number of prior relapses of two.
One reviewer (F.H.) assessed the papers for inclusion using PICO criteria (patients, intervention, comparison and outcome) from the record title and abstract. Full papers were assessed in detail for eligibility, and any controversies were reviewed by another independent adviser (D.A.T.).
2.3. Data extraction
Study characteristics and results were extracted by two independent reviewers (F.H. and N.O.B.) using a specially designed proforma (Supplementary Table 1). Trial methodology/quality was assessed subjectively and using a modified version of the Newcastle Ottawa Tool [14] for cohort studies after review of options [15]. A third independent adviser (D.A.T.) reviewed any discrepancies between the two reviewers. A cut-off of 60% was chosen for the proportion of relapsed patients and proportion of patients having initial high-dose myeloablative therapy (MAT) and autologous stem cell rescue for the study to be deemed a representative sample, since this research was focussed on first relapse of patients treated on a previous high-risk protocol.
3. Results
Electronic searching yielded 766 records, and an additional five other records were identified making a total of 771 records. Thirty-four full-text articles were assessed for study eligibility, and nine studies met the inclusion criteria. Most exclusions were because of all stages of disease being included without subgroup analysis or patients receiving more than two previous lines of chemotherapy (Fig. 1).
Fig. 1.
Flow diagram of included studies.
The characteristics of the nine included studies [16], [17], [18], [19], [20], [21], [22], [23], [24] are detailed in Table 1. The studies were undertaken between 1999 and 2015 and published between 2003 and 2017. Six were single arm, prospective studies with small cohorts of relapsed and refractory patients (25–40 patients) [17], [18], [19], [21], [22], [23]. One study had three different treatment arms depending on whether the patient had a central line in situ, and then a dose escalation was performed after the toxicity was deemed acceptable [22]. Two studies were single centre retrospective database reviews [16], [20]. Of the other studies, four were single arm, prospective multicentre studies within one country [17], [19], [22], [23], and two were European multicentre studies [18], [21]. Only one study [24] was a randomised controlled study.
Table 1.
Summary of included studies and their characteristics.
| Study | Type | Patient no. | Aim/intervention | % Relapsed | % MAT |
|---|---|---|---|---|---|
| Ashraf 2013 [16] | Retrospective database review in single centre | 27 | Describe response, survival and toxicity of cyclophosphamide and topotecan in children with 1st relapse of NB | 96 | 93 |
| Bagatell 2011 [17] | Prospective single arm cohort in COG centres | 27a | Determine response rate of irinotecan and temozolomide in relapsed/refractory NB | 77 | NR |
| Di Giannatale 2014 [18] | Prospective single arm cohort in Europe | 38 | Assess objective response rate of 2 cycles of topotecan & temozolomide chemo | 66 | 61 |
| Garaventa 2003 [19] | Prospective single arm cohort in Italy | 25 | Evaluate anti-tumour activity and tolerability of topotecan/vincristine/doxorubicin) in children with advanced NB | 24 | 52 |
| Kushner 2010 [20] | Retrospective database review in single centre | 30a | Assess likelihood of response to high dose cyclophosphamide/topotecan/vincristine | 100 | 70 |
| Rubie 2006 [21] | Prospective single arm cohort in Europe | 25 | Determine response rate of NB to temozolomide | 60 | 64 |
| Simon 2007 [22] | Prospective single arm cohort in Germany | 40 | Trial of topotecan & etoposide in the treatment of patients with relapsed HRNB | 100 | 30 |
| Simon 2007 [23] | Prospective single arm cohort in Germany | 33a | Trial of topotecan/cyclophosphamide/etoposide in the treatment of patients with HRNB | 100 | 52 |
| Mody 2017 [24] | Randomised Control Trial in COG centres | 35 | Comparison of temozolomide & irinotecan chemotherapy with additional temsirolimus or dinutuximab in 1st relapse of HRNB | 56 & 53 | 50 & 59 |
MAT, myeloablative therapy with autologous stem cell rescue; NR, not reported; NB, neuroblastoma; HRNB; high-risk neuroblastoma; COG, Children's oncology group.
n = number of participants from the entire cohort in eligible sub group(s).
3.1. Evaluation of studies meeting inclusion criteria
All studies included patients with HRNB ranging from three studies [20], [22], [23] comprising all relapsed patients to just 24% in one study [19]. The percentage of patients who had received MAT with stem cell rescue as prior treatment varied from 30 to 93% but was not reported in one study [17]. Only one study documented prior use of immunotherapy [24]. In some studies, only certain subgroups of the total study cohort were suitable for inclusion: one study [17] split their cohort into two strata with 28 patients in stratum 1 who had measurable disease, but only 50% of these were stage 4 at diagnosis and others stage 1–3. Not all of these non-stage 4 patients had MYCN amplification, and therefore not all were defined as high-risk patients. Stratum 2 had 27 patients with disease evaluable by bone marrow or meta-iodo benzyl guanidine (mIBG) only, and all were high-risk at diagnosis so only this arm was included. Another [20] reported a total of 126 patients split into four groups—new recurrence, primary and secondary refractory and progressive disease. Only the subgroup of new recurrence (30 patients) was included. A further study [23] included a total of 44 patients split into two cohorts: 33 had new recurrences and were included, and 11 were newly diagnosed patients, so were excluded.
3.2. Response assessment
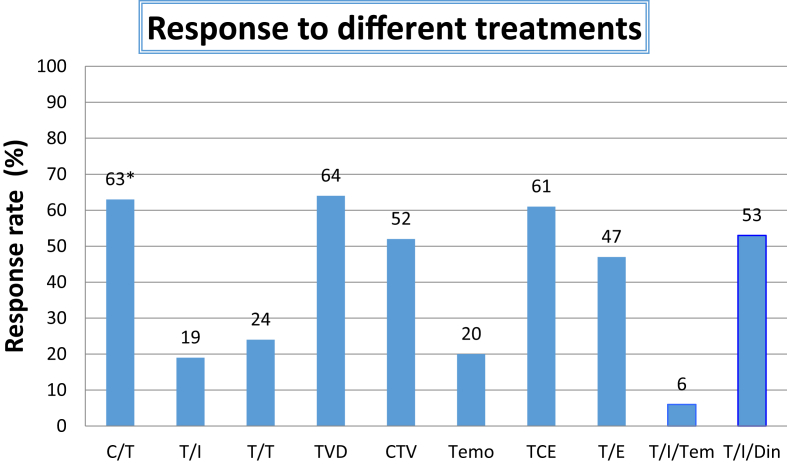
Table 2 provides a description of response assessments performed in each study and outcome. All studies described an objective response rate to treatment using validated criteria, although for two, this was a secondary outcome [22], [23]. All studies used the INRC [11], although one study [16] defined it as the New Approaches to Neuroblastoma Therapy criteria, which is a modified version of INRC. Most described best ever response, but one used response at a pre-defined time point [17]. Eight of the studies defined response as CR and PR, but one study included mixed response (MR) [16]. Response varied from 6 to 64% (Fig. 2).
Table 2.
Summary of response assessment in each study.
| Study | Drug | Timing of response assessment | Response rate (%) |
|---|---|---|---|
| Ashraf 2013 [16] | Topotecan/cyclophosphamide | Best | 63a |
| Bagatell 2011 [17] | Temozolomide/irinotecan | After 3 cycles | 19 |
| Di Giannatale 2014 [18] | Temozolomide/topotecan | Best | 24 |
| Garaventa 2003 [19] | Topotecan/vincristine/doxorubicin | Best | 64 |
| Kushner 2010 [20] | Cyclophosphamide/topotecan/vincristine | Best | 52 |
| Rubie 2006 [21] | Temozolomide | Best | 20 |
| Simon 2007 [22] | Topotecan/etoposide | Best | 47 |
| Simon 2007 [23] | Topotecan/cyclophosphamide/etoposide | Best | 61 |
| Mody 2017 [24] | Temozolomide/irinotecan + temsirolimus + dinutuximab |
Best | 6 53 |
Denotes that the response includes mixed response.
Fig. 2.
Bar chart displaying the objective response rate (complete remission & partial remission) to different chemotherapeutic strategies detailed in Table 1. C/T, Cyclophosphamide/Topotecan; T/I, Temozolomide/Irinotecan; T/T, Topotecan/Temozolomide; TVD, Topotecan/Vincristine/Doxorubicin; CTV, Cyclophosphamide/Topotecan/Vincristine; Temo, temozolomide TCE, Topotecan/Cyclophosphamide/Etoposide; T/E, Topotecan/Etoposide; T/I/T, temozolomide/Irinotecan/Temsirolimus; T/I/D, temozolomide/Irinotecan/Dinutuximab. *Mixed response is included in response
3.3. Quality assessment
Study quality is shown in Table 3. Two studies were not as representative of the desired patient group because of a low percentage of relapsed patients in the cohort and the low number who had received previous high-dose chemotherapy treatment, respectively [19], [23]. All studies described treatment exposure and adherence adequately. All studies used a validated tool for response assessment with five studies having central review of response, but little information was provided about blinding of reviewers [17], [18], [19], [21], [24]. Since the primary outcome was response, there was no requirement for long follow-up, and all patients were available for assessment. Two studies were retrospective single-centre studies, so were not representative of a wide cohort. Because only one study [24] was a randomised controlled study, a formal tool for quality assessment was not used, but appropriate methods of randomisation were used, and the two arms were relatively similar for important prognostic characteristics including MYCN status. The only difference was the percentage with bone marrow disease, which was 33% in the temsirolimus arm and 76% in the dinutuximab arm.
Table 3.
Quality assessmenta of studies.
| Study | Selection |
Outcome |
Other | Composite score | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representative |
Ascertainment of exposure | Outcome not present at start | Assessment of outcome |
Follow-up | |||||
| >60% relapse | >60% MAT | Validated tool | Central review | ||||||
| Ashraf 2013 [16] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Retrospective single centre | 6 | |
| Bagatell 2011 [17] | ✓ | NR | ✓ | ✓ | ✓ | ✓ | ✓ | Alternative definition of high risk | 6 |
| Di Giannatale 2014 [18] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 7 | |
| Garaventa 2003 [19] | ✓ | ✓ | ✓ | ✓ | ✓ | 5 | |||
| Kushner 2010 [20] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Retrospective single centre | 6 | |
| Rubie 2006 [21] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 7 | |
| Simon 2007 [22] | ✓ | ✓ | ✓ | ✓ | ✓ | Alternative definition of high risk; Variation in treatment delivered |
5 | ||
| Simon 2007 [23] | ✓ | ✓ | ✓ | ✓ | Alternative definition of high risk | 4 | |||
| Mody 2017 [24] | ✓ | ✓ | ✓ | ✓ | ✓ | Randomised trial | 5+ | ||
MAT, myeloablative therapy with autologous stem cell rescue.
✓ denotes study meets the criteria, NR, not reported.
Alternative definition of high risk refers to [1]: The US groups including children over 18 months with localised unresectable tumours & unfavourable histology group as high risk, whereas in the UK/rest of Europe, these groups are classed as intermediate risk [2]; The German studies include localised resectable tumours with MYCN amplification as high risk when others do not and exclude the 12–18 months with metastatic disease but no MYCN amplification from their high-risk group.
Quality assessed using modified Newcastle Ottawa Scale [13].
4. Discussion
Historically, trials undertaken in relapsed neuroblastoma describe a very heterogeneous patient group. Often, relapses of all stages of disease are included as well as inclusion of a combination of refractory and relapsed patients. This review focussed on relapsed HRNB. Relapsed patients respond differently compared with refractory patients [19], [20], with the latter less likely to show an objective response to chemotherapy but with a longer time to progression and better OS [25]. One study had just 24% relapsed patients (6 patients) [19]. However, the remaining studies comprised predominantly relapsed patients with three having an entirely relapsed cohort. MYCN amplification is associated with a poorer OS and a shorter survival time post relapse [2], [3], [26], [27]. Older patients without MYCN amplification often have a more chronic, smouldering disease with longer survival [6]. Therefore, age distribution and presence of MYCN amplification is important in interpretation of results. Seven studies reported the proportion of patients with MYCN amplification, and this ranged from 10 to 38%. The percentage of stage 4 patients with MYCN amplification is around 30% [28], and in high-risk disease, this is slightly higher because of localised MYCN amplified tumours being included. A pilot study of relapsed patients found the rate of MYCN amplification to be 42%, [2] so the proportions reported in these studies are slightly lower than expected. Relapses occurring earlier after diagnosis are associated with a shorter length of survival [3], [27], but the effect of other prognostic factors (age, MYCN amplification and time to relapse) were not reported in studies included in this review.
Neuroblastoma staging is standardised worldwide using the International neuroblastoma staging system and INRG criteria [1], [11]. However, the decision to treat patients on national high-risk protocols varies, e.g. in most of Europe, HRNB is defined as patients over 12 months with metastatic disease, MYCN amplified localised unresectable disease and infants with MYCN amplified metastatic disease [1]. However, German protocols include MYCN amplified resectable tumours on their high-risk protocols [29], [30] but exclude metastatic disease without MYCN amplification diagnosed between 12 and 18 months. Both German and North American protocols include children over 18 months with unresectable localised tumours showing International Neuroblastoma Pathology Classification unfavourable histology with or without unfavourable genetics. Thus, patient groups may be slightly different, potentially affecting outcome and response.
Not all centres use MAT and stem cell rescue in upfront treatment of HRNB. Refractory patients are less likely to have had previous MAT or immunotherapy. Response to relapse therapy may be different in those who have had prior MAT or immunotherapy. The included studies varied with respect to the proportion who had received prior MAT with four studies not having the desired 60% of patients having this treatment [19], [22], [23], [24]. Studies also lacked description of on-going therapy after the regimen reported in the papers. Some studies continued until disease progression, and others were for a prescribed number of cycles, with the aim of obtaining a response and then continuing to consolidation therapy (although this was not described). In most therapeutic phase III trials, OS and event-free survival (EFS) are the primary outcome measures and with the aim of ultimately improving OS of relapsed HRNB. However, because of the low survival and lack of standardised therapy post re-induction therapy and that many of the included trials did not report EFS or OS, comparison was impossible. Time to progression could not be used because of the varying treatment strategies given after the investigative treatment; therefore, response rate was the only suitable outcome measure but may not equate to survival.
The widespread use of standard definitions of response allows confidence that studies are comparing similar outcomes although one study included MR [16]. In 2012, revisions were made to the INRC to use Response Evaluation Criteria in Solid Tumours for response assessment of primary and metastatic soft tissues sites and to classify bone marrow involvement of ≤5% as minimal disease allowing these patients to be eligible for a PR depending on other site responses. Urinary catecholamine levels were removed from response assessment [12]. These changes arose with the advent of more modern imaging technology and a lack of sensitivity and specificity from the older techniques. Therefore, we recognise that even though validated INRC was used throughout the included studies, they have limitations and are not now the gold standard reassessment tool. The use of central review in five of the studies helped to reduce bias in response categorisation.
Whilst direct comparisons are limited, it was interesting that one study [21] reported a response rate (RR) of 20% to temozolomide alone, and two other studies [17], [18] assessing temozolomide with the addition of a second agent such as irinotecan or topotecan were very similar (19 & 24%), yet the RCT study [24] showed that the temozolomide/irinotecan and temsirolimus arm had a disappointing response rate of just 6%. It is not clear why it was so much lower in this group. There were no major differences in the patients included in these studies with a mix of relapsed and refractory patients mostly with metastatic disease. It may be worth noting that the three regimes with a >60% response rate contained topotecan [16], [19], [23]. In the United States, topotecan has been moved into frontline treatment because of its efficacy at relapse in the hope that it could reduce relapse rate [31].
A double-blind RCT is the gold standard method for comparison of therapeutic efficacy. A large phase II RCT, comparing cyclophosphamide and topotecan with topotecan alone demonstrated a slight, but non-significant improvement in response in the combination arm, which did not translate into improvement in survival [26]. This study was excluded because of inclusion of non–high-risk patients, without separate high-risk analysis [30]. A pilot study of temozolamide, irinotecan, rapamycin and dasatanib (RIST) in relapsed and refractory neuroblastoma patients published in abstract form showed an objective major response rate (CR and PR) of 71%, and a larger trial is now underway [32]. The on-going European BEACON trial will provide additional information on potentially suitable backbone chemotherapy [33]. A non-randomised cohort study found no benefit of adding bevacizumab to temozolomide and irinotecan in relapsed and refractory patients, and it will be important to see if this is confirmed by the BEACON study too [13]. This review is limited by the strict inclusion criteria, which were chosen at the outset in order not to replicate previous work and with the aim to identify the best treatment for patients at first relapse with the intention of cure rather than palliation. However, the authors recognise that this has led to exclusion of several other regimes [26], [34], [35].
The current review is subject to publication bias since all included studies were published in peer-reviewed journals despite searching for unpublished literature. Study heterogeneity with regard to risk group and previous treatments made formal quality assessment/scoring difficult and direct comparison of results and meta-analysis impossible.
5. Conclusion
Children with relapsed high-risk neuroblastoma show response to a variety of chemotherapy agents. However, patient and prior treatment heterogeneity in published studies precludes determination of the most effective re-induction strategy for children with relapsed high-risk neuroblastoma. International and perhaps worldwide RCTs in patients having similar upfront treatments powered to look at individual subgroups (relapsed versus refractory, MYCN amplification status) are required to determine the ideal backbone upon which to test novel targeted agents to try and cure more children with relapsed high-risk neuroblastoma.
Conflict of interest statement
None declared.
Acknowledgements
The authors thank Andy Bryant for provision of quality assessment tools; the North of England Children's Cancer Research (NECCR) Fund and the Great North Children's Hospital, Newcastle for funding FH for a Masters of Clinical Research, Newcastle Hospital Charity and Cancer Research UK (C8561/A12084), Action Medical Research & Great Ormond Street Children's Charity (GN2390) and NECCR for supporting epidemiological research.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejca.2018.12.032.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Cohn S.L., Pearson A.D., London W.B., Monclair T., Ambros P.F., Brodeur G.M. The international neuroblastoma risk group (INRG) classification system: an INRG task force report. J Clin Oncol. 2009;27(2):289–297. doi: 10.1200/JCO.2008.16.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basta N.O., Halliday G.C., Makin G., Birch J., Feltbower R., Bown N. Factors associated with recurrence and survival length following relapse in patients with neuroblastoma. Br J Canc. 2016;115(9):1048–1057. doi: 10.1038/bjc.2016.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.London W., Castel V., Monclair T., Ambros P., Pearson A., Cohn S. Clinical and biologic features predictive of survival after relapse of neuroblastoma: a report from the international neuroblastoma risk group project. J Clin Oncol. 2011;29(24):3286–3292. doi: 10.1200/JCO.2010.34.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garaventa A., Parodi S., De Bernardi B., Dau D., Manzitti C., Conte M. Outcome of children with neuroblastoma after progression or relapse. A retrospective study of the Italian neuroblastoma registry. Eur J Cancer. 2009;45(16):2835–2842. doi: 10.1016/j.ejca.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 5.London W.B., Bagatell R., Weigel B.J., Fox E., Guo D., Van Ryn C. Historical time to disease progression and progression-free survival in patients with recurrent/refractory neuroblastoma treated in the modern era on Children's Oncology Group early-phase trials. Cancer. 2017;123(24):4914–4923. doi: 10.1002/cncr.30934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mosse Y.P., Deyell R.J., Berthold F., Nagakawara A., Ambros P., Monclair T. Neuroblastoma in older children, adolescents and young adults: a report from the international neuroblastoma risk group project. Pediatr Blood Canc. 2014;61:627–635. doi: 10.1002/pbc.24777. [DOI] [PubMed] [Google Scholar]
- 7.Simon T., Berthold F., Klingebiel T., Kremens B., De Carolis B., Hero B. Do relapsed high risk neuroblastoma patients have a second chance? Pediatr Blood Canc. 2009;53(5):736. [Google Scholar]
- 8.Morgenstern D.A., Baruchel S., Irwin M.S. Current and future strategies for relapsed neuroblastoma: challenges on the road to precision therapy. Journal of pediatric hematology/oncology. 2013;35(5):337–347. doi: 10.1097/MPH.0b013e318299d637. [DOI] [PubMed] [Google Scholar]
- 9.Morgenstern D., Barone G., Moreno L., Anderson J., Brock P., Elliott M. 2015. Options for the treatment of patients with relapsed/progressive high-risk neuroblastoma.www.cclg.org.uk CCLG website. [Google Scholar]
- 10.NHS Centre for Reviews and Dissemination . University of York: NHS Centre for Reviews and Dissemination; 1996. Undertaking Systematic reviews of Research on Effectiveness: CRD guidelines for those carrying out or comissioning reviews. Report No.: CRD Report 4. [Google Scholar]
- 11.Brodeur G.M., Pritchard J., Berthold F., Carlsen N.L., Castel V., Castelberry R.P. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol : official journal of the American Society of Clinical Oncology. 1993;11(8):1466. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 12.Park J.R., Bagatell R., Cohn S.L., Pearson A.D., Villablanca J.G., Berthold F. Revisions to the international neuroblastoma response criteria: a consensus statement from the national cancer institute clinical trials planning meeting. J Clin Oncol. 2017;35(22):2580–2587. doi: 10.1200/JCO.2016.72.0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Modak S., Kushner B.H., Basu E., Roberts S.S., Cheung N.V. Combination of bevacizumab, irinotecan, and temozolomide for refractory or relapsed neuroblastoma: results of a phase II study. Pediatr Blood Canc. 2017;64(8) doi: 10.1002/pbc.26448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wells G, Shay B. Data extraction for non randomised systematic reviews [Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 15.Deeks J.J., Dinnes J., D'Amico R., Sowden A.J., Sakarovitch C., Song F. Evaluating non-randomised intervention studies. Health Technol Assess. 2003;7(27):1–173. doi: 10.3310/hta7270. iii-x. [DOI] [PubMed] [Google Scholar]
- 16.Ashraf K., Shaikh F., Gibson P., Baruchel S., Irwin M.S. Treatment with topotecan plus cyclophosphamide in children with first relapse of neuroblastoma. Pediatr Blood Canc. 2013;60(10):1636–1641. doi: 10.1002/pbc.24587. [DOI] [PubMed] [Google Scholar]
- 17.Bagatell R., London W.B., Wagner L.M., Voss S.D., Stewart C.F., Maris J.M. Phase II study of irinotecan and temozolomide in children with relapsed or refractory neuroblastoma: a Children's Oncology Group study. J Clin Oncol. 2011;29(2):208–213. doi: 10.1200/JCO.2010.31.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Giannatale A., Dias-Gastellier N., Devos A., Mc Hugh K., Boubaker A., Courbon F. Phase II study of temozolomide in combination with topotecan (TOTEM) in relapsed or refractory neuroblastoma: a European Innovative Therapies for Children with Cancer-SIOP-European Neuroblastoma study. Eur J Cancer. 2014;50(1):170–177. doi: 10.1016/j.ejca.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Garaventa A., Luksch R., Biasotti S., Severi G., Pizzitola M.R., Viscardi E. A phase II study of topotecan with vincristine and doxorubicin in children with recurrent/refractory neuroblastoma. Cancer. 2003;98(11):2488–2494. doi: 10.1002/cncr.11797. [DOI] [PubMed] [Google Scholar]
- 20.Kushner B.H., Kramer K., Modak S., Qin L.X., Cheung N.K. Differential impact of high-dose cyclophosphamide, topotecan, and vincristine in clinical subsets of patients with chemoresistant neuroblastoma. Cancer. 2010;116(12):3054–3060. doi: 10.1002/cncr.25232. [DOI] [PubMed] [Google Scholar]
- 21.Rubie H., Chisholm J., Defachelles A.S., Morland B., Munzer C., Valteau-Couanet D. Phase II study of temozolomide in relapsed or refractory high-risk neuroblastoma: a joint Societe Francaise des Cancers de l'Enfant and United Kingdom Children Cancer Study Group - new agents group study. J Clin Oncol. 2006;24(33):5259–5264. doi: 10.1200/JCO.2006.06.1572. [DOI] [PubMed] [Google Scholar]
- 22.Simon T., Langler A., Berthold F., Klingebiel T., Hero B. Topotecan and etoposide in the treatment of relapsed high-risk neuroblastoma: results of a phase 2 trial. Journal of pediatric hematology/oncology. 2007;29(2):101–106. doi: 10.1097/MPH.0b013e3180320b48. [DOI] [PubMed] [Google Scholar]
- 23.Simon T., Längler A., Harnischmacher U., Frühwald M.C., Jorch N., Claviez A. Topotecan, cyclophosphamide, and etoposide (TCE) in the treatment of high-risk neuroblastoma. Results of a phase-II trial. J Canc Res Clin Oncol. 2007;133(9):653–661. doi: 10.1007/s00432-007-0216-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mody R., Naranjo A., Van Ryn C., Yu A.L., London W.B., Shulkin B.L. Irinotecan-temozolomide with temsirolimus or dinutuximab in children with refractory or relapsed neuroblastoma (COG ANBL1221): an open-label, randomised, phase 2 trial. Lancet Oncol. 2017;18(7):946–957. doi: 10.1016/S1470-2045(17)30355-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moreno L., Rubie H., Varo A., Le Deley M.C., Amoroso L., Chevance A. Outcome of children with relapsed or refractory neuroblastoma: a meta-analysis of ITCC/SIOPEN European phase II clinical trials. Pediatr Blood Canc. 2017;64(1):25–31. doi: 10.1002/pbc.26192. [DOI] [PubMed] [Google Scholar]
- 26.London W.B., Frantz C.N., Campbell L.A., Seeger R.C., Brumback B.A., Cohn S.L. Phase II randomized comparison of topotecan plus cyclophosphamide versus topotecan alone in children with recurrent or refractory neuroblastoma: a Children's Oncology Group study. J Clin Oncol. 2010;28(24):3808–3815. doi: 10.1200/JCO.2009.27.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simon T., Berthold F., Borkhardt A., Kremens B., De Carolis B., Hero B. Treatment and outcomes of patients with relapsed, high-risk neuroblastoma: results of German trials. Pediatr Blood Canc. 2011;56(4):578–583. doi: 10.1002/pbc.22693. [DOI] [PubMed] [Google Scholar]
- 28.Thompson D., Vo K.T., London W.B., Fischer M., Ambros P.F., Nakagawara A. Identification of patient subgroups with markedly disparate rates of MYCN amplification in neuroblastoma: a report from the International Neuroblastoma Risk Group project. Cancer. 2016;122(6):935–945. doi: 10.1002/cncr.29848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simon T., Hero B., Schulte J.H., Deubzer H., Hundsdoerfer P., von Schweinitz D. 2017 GPOH guidelines for diagnosis and treatment of patients with neuroblastic tumors. Klin Pädiatr. 2017;229(3):147–167. doi: 10.1055/s-0043-103086. [DOI] [PubMed] [Google Scholar]
- 30.Board PDQPTE . National Cancer Institute (US); Bethesda (MD): 2002. Neuroblastoma treatment (PDQ(R)): health professional version. PDQ cancer information summaries. [Google Scholar]
- 31.Matthay K.K., Maris J.M., Schleiermacher G., Nakagawara A., Mackall C.L., Diller L. Neuroblastoma. Nat Rev Dis Primers. 2016;2:16078. doi: 10.1038/nrdp.2016.78. [DOI] [PubMed] [Google Scholar]
- 32.Corbacioglu S., Steinbach D., Lode H., Gruhn B., Fruehwald M., Broeckelmann M. The RIST design: a molecularly targeted multimodal approach for the treatment of patients with relapsed and refractory neuroblastoma. J Clin Oncol. 2013;31(15):10017. [Google Scholar]
- 33.A randomised phase IIb trial of bevacizumab added to temozolamide +/- Irinotecan for children with refractory/relapsed neuroblastoma. Clinical trials.gov NCT02308527 Trial protocol. University of Birmingham Cancer Research UK Cancer Research UK Clinical Trials Unit; 2015. 23/09/2015. Contract No.: Version 5.0a. [Google Scholar]
- 34.Kushner B.H., Modak S., Kramer K., Basu E.M., Roberts S.S., Cheung N.K. Ifosfamide, carboplatin, and etoposide for neuroblastoma: a high-dose salvage regimen and review of the literature. Cancer. 2013;119(3):665–671. doi: 10.1002/cncr.27783. [DOI] [PubMed] [Google Scholar]
- 35.Kushner B.H., Kramer K., Modak S., Cheung N.K. Irinotecan plus temozolomide for relapsed or refractory neuroblastoma. J Clin Oncol. 2006;24(33):5271–5276. doi: 10.1200/JCO.2006.06.7272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.