Abstract
Objective:
To examine the direct relationship between nutrient intake and cervical remodeling.
Design:
Longitudinal descriptive design.
Setting:
Maternal-fetal medicine clinic in a Midwestern urban city.
Participants:
Forty-seven pregnant African American women.
Methods:
Participants completed the Block brief food frequency questionnaire at 19 to 24 weeks and 27 to 29 weeks gestation and had quantitative ultrasound attenuation estimates at 19 to 21 weeks, 23 to 25 weeks, 27 to 29 weeks, 31 to 33 weeks, and 35 to 37 weeks gestation.
Results:
Trajectory mixture models identified two subpopulations within our sample: at-risk (n = 36) and less-risk (n = 11) for premature cervical remodeling. More participants in the less-risk group consumed the dietary reference intake (DRI) for calcium, vitamin A, folate, vitamin E, zinc, and vitamin D than in the at-risk group. The percentage of participants in the less-risk group who consumed the recommended DRI for vitamin E was twice the percentage of women in the at-risk group (82% and 44%, respectively, p=.004). Mean intake of calcium was almost 1.3 times more (p = 0.05) and for zinc was 1.5 times more (p =0.04) in the less-risk group than in the at-risk group.
Conclusions:
Practitioners can inform women that certain nutrients, particularly zinc, calcium, and vitamin E, could be important to the health of the cervix and inhibit premature cervical remodeling, which in turn may help to prevent preterm birth.
Keywords: Pregnancy, cervical remodeling, nutrition, African American
Precis
Pregnant African American women with poor nutritional intake of zinc, calcium, or vitamin E were at higher risk for premature cervical remodeling.
Although there has been a steady decline in rates of preterm birth among all racial and ethnic groups in the United States, African American women continue to have approximately twice the rate of preterm birth than White women (Martin, Hamilton, Osterman, Curtin, & Matthews, 2015). It is unknown why this disparity exists, but evidence suggests that maternal nutrient deficiency is one of several possible factors (Dunlop, Kramer, Hogue, Menon, & Ramakrishan, 2011). Decreased intake of multivitamins, zinc, calcium, iron, fat, carbohydrates, and protein has been associated with preterm labor and birth (Catov, Bodnar, Ness, Markovic, & Roberts, 2007; Hennessy, Volpe, Sammel, & Gennaro, 2010; Hofmeyr, Duley, & Atallah, 2007; Siega-Riz et al., 2006). Researchers have indicated that pregnant African American women in the United States consume fewer nutrient dense foods (Siega-Riz, Bodnar, & Savitz, 2002) and have higher rates of nutrient deficiencies (Siega-Riz & Popkin, 2001) than pregnant White women.
Women who give birth preterm have higher concentrations of matrix metalloproteinases (MMPs), specifically MMP-2, −3, −8 and −9 in cervical mucus and placenta tissue than women who give birth at term (Becher, Hein, Danielsen, & Uldbjerg, 2010; D. Sundrani, Chavan-Gautam, Pisal, Mehendale, & Joshi, 2013). The MMPs are calcium-dependent, zinc-containing endopeptidases (Verma & Hansch, 2007) that have a crucial role in remodeling the extracellular matrix (ECM) of cervical tissue during pregnancy (Sundrani, Chavan Gautam, Mehendale, & Joshi, 2011). The MMPs degrade extracellular matrix constituents, including collagen, elastin, gelatin, casein, and non-matrix proteins (Sundrani et al., 2011; Verma & Hansch, 2007), which contribute to cervical tissue remodeling in pregnancy (Yan & Boyd, 2007). Under pathological conditions and conditions of nutrient deficiency, MMP activity increases, which results in tissue degradation (Verma & Hansch, 2007).
CALLOUT 1
Although several researchers examined the associations between nutrient deficiencies and MMP activity (Sundrani et al., 2011; Verma & Hansch, 2007), we did not find any studies on relationship between nutrient intake and cervical remodeling. Serial ultrasonic attenuation assessments of the cervix provide a measure of tissue integrity, including collagen organization and water content (Labyed, Bigelow, & McFarlin, 2011; McFarlin, Balash, et al., 2015). As pregnancy progresses, collagen becomes disorganized, and ultrasonic attenuation decreases as the cervix remodels to allow passage of the fetus (McFarlin, Kumar, et al., 2015). The aim of this study was to examine the relationship between nutrient intake and cervical remodeling as measured by ultrasonic attenuation among a sample of pregnant African American women. We hypothesize that those women with nutrient deficiencies will have greater cervical remodeling.
Methods
Recruitment
This study is a secondary analysis of a larger pilot study examining multiple pathways to preterm birth. Approval for the study was received from the institutional review board of a city in the Midwest. Medical records of potential research participants were pre-screened for eligibility. Before 19 weeks gestational age, qualified women from the maternal-fetal medicine clinic at a medical center in the Midwest were approached during their clinic appointments. If the woman agreed to participate, an intake appointment was scheduled between 19 – 24 weeks gestation. At the intake visit, the study team obtained written informed consent from the participant in a face-to-face private meeting. A total of 54 participants were recruited with a total of 47 women with complete data for analysis.
Eligibility
Eligible participants were less than 19 weeks pregnant with singleton pregnancies, they self-identified as African American, and lived in Chicago or the surrounding suburbs. Participants were also at least 15 years old and able to read and write English. Participants were excluded if they had major fetal anomalies, autoimmune disorder (e.g., HIV, type I diabetes, lupus, Graves disease), were receiving steroid treatments (including inhalers for asthma), or had a cervical cerclage.
Measures
Demographic and clinical characteristics.
Participants completed a demographic questionnaire that included age, gender, employment status, monthly household income and receipt of public assistance. Weight, medical history, current and past obstetric history and birth data were collected by accessing the participants’ medical records.
Nutrient intake.
Participants completed the Block brief food frequency questionnaire (FFQ) (Block et al., 1986). This questionnaire is designed to provide estimates of usual dietary intake and includes 70 food items. Participants were asked to estimate their usual dietary intake during the past 3 months. Pictures were provided so participants could estimate food and beverage portion sizes. The food list was created from the National Health and Nutrition Examination Survey (NHANES) III dietary recall data. The nutrient database was based on the United States Department of Agriculture (USDA) Nutrient Database for Standard Reference. The FFQ was self-administered and collected between 19 to 24 weeks and again at 27 to 29 weeks gestation.
Total nutrient amounts were summed across food and supplement sources and averaged across both data collection time-points. Valid FFQs had estimated kilocalories between 500 and 5,000 and five or fewer missing responses to food intake questions.
Cervical remodeling assessment.
The process of cervical (collagen) remodeling during pregnancy was examined using ultrasonic attenuation estimates. Ultrasonic attenuation is defined as a quantity that refers to a reduction in the strength (or energy) of an ultrasonic signal and quantified in deciBel (dB). When attenuation is normalized to distance (centimeter [cm]) and ultrasonic frequency (megahertz [MHz]), it is termed ultrasonic coefficient and quantified in dB/cm-MHz.
The participants underwent between one and five transvaginal ultrasound examinations to estimate ultrasonic attenuation and cervical length over the course of pregnancy. Ultrasound data were collected up to five times: 19 to 21 weeks, 23 to 25 weeks, 27 to 29 weeks, 31 to 33 weeks, and 35 to 37 weeks gestation. Immediately after each cervical scan, using the same ultrasound system settings, a scan of the Gammex (Gammex Inc., Middleton, WI) tissue-mimicking phantom was obtained to use as a reference scan for data processing. The Gammex phantom had a known ultrasonic attenuation of 0.6 dB/cm-MHz. There were two sonographers for this study. One sonographer was a registered diagnostic medical sonographer (RDMS) and the other sonographer was RDMS eligible.
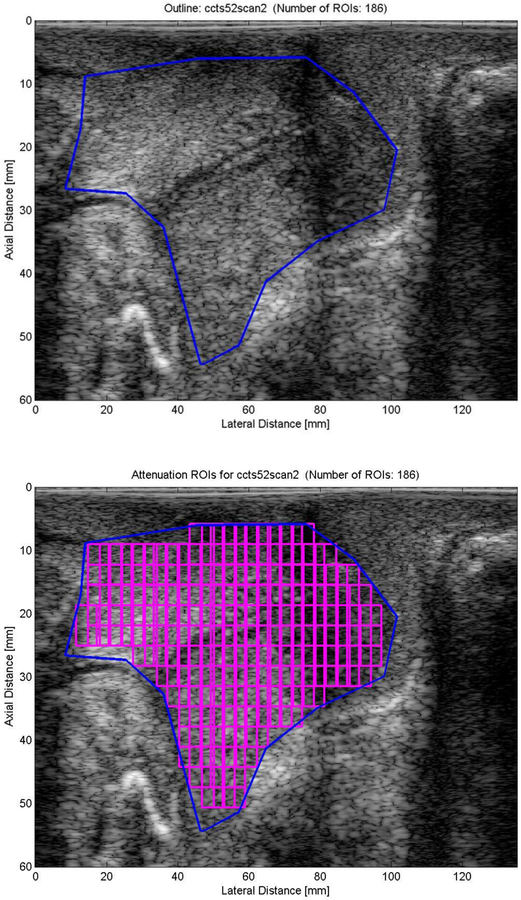
Raw ultrasonic data were obtained from z.one, Zonare ultrasound system (ZONARE Medical Systems, Inc., Mountain View, CA), saved on a flash drive and converted to IQ (In-phase Quadrature) data. The IQ data contain scans of the cervix and phantom for a center frequency of 5 MHz. These IQ data were then converted to radio frequency (RF) data. The RF data were then segmented into smaller regions of interest (ROI) (Figure 1) to estimate the ultrasonic attenuation throughout the entire cervix.
Figure 1.
Example of the ultrasonic attenuation processing of a transvaginal ultrasound examination of the cervix. Transvaginal ultrasound image of the pregnant cervix where the cervix is outlined. The same image of the cervix where regions of interest (ROIs) are segmented. Ultrasonic attenuation will be calculated for each individual ROI. Finally, a mean attenuation value is calculated from all of the ROIs in the image.
For this study, the spectral log difference (SLD) method was used to estimate ultrasonic attenuation (Labyed et al., 2011). This method was selected because among the different algorithms for ultrasonic attenuation estimation, the SLD method is one of the least susceptible to heterogeneity in the tissue as observed in previous studies (Bigelow, Labyed, McFarlin, Sen-Gupta, & O’Brien, 2011). A minimum of 15 echoes in the lateral direction and 12 pulse lengths (PLs) in the axial direction are optimal for the least amount of error in ultrasonic attenuation estimates when using the SLD method (Bigelow et al., 2011). The correlation coefficient was used to find the number of uncorrelated lines in each ROI. Laterally every 3rd echo was found to be independent; to obtain at least 15 independent echoes per ROI we acquired 45 echoes in total for each ROI spanning a range of 19 degrees. For axial resolution, we had 40 points for each pulse length, 4 PLs per window and 5 windows per ROI making the axial resolution equal to 12 PLs (7.39 mm).
After windowing the data into the different ROIs, the Fourier transform of the windowed data within each ROI was obtained. The windows were then averaged with 99% overlap in both axial and lateral directions. The noise value in decibels was determined from the spectral plot, which gave us the usable frequency range. The spectral values were averaged over the overlapping usable frequency range and then divided by the reference spectrum. The spectral log difference method was used to estimate the ultrasonic attenuation by calculating the slope of the straight line that fits the log ratio (difference between log spectra) of the two power spectra from the proximal and the distal segments of the ROI. The ultrasonic attenuation map was then overlaid on the B mode image. We evaluated the inter-rater reliability of ultrasonic attenuation mapping of the cervix by having 50% of the scans mapped and processed independently by a second examiner and found an inter-rater reliability r = 0.97, p < 0.001.
Statistical Analyses
We were interested in the trajectory of ultrasonic attenuation of the cervix over time to see how women progress during the course of their pregnancy, knowing that ultrasonic attenuation is lowest when the cervix remodels before the time of birth (McFarlin, Balash, et al., 2015). While a hierarchical model or latent growth curve approach could be used to describe the variation in ultrasonic attenuation trajectory, we selected a mixture model to assess if unobserved heterogeneity was present in the population, i.e., that subpopulations exist that have different trajectory parameters. This semi-parametric approach describes the data by identifying subgroups as well as their intercept, slope, quadratic terms, etc., using the SAS TRAJ procedure (Jones & Nagin, 2007; Jones Nagin,, & Roeder, 2001). In order to determine the number of subgroups and the form of their trajectories, models were compared using the Bayesian Information Criteria (BIC), a log-likelihood type estimate adjusted for sample size and number of model parameters (Jones et al., 2001; Schwarz, 1978). The change in BIC from complex to simpler models (multiplied by 2) approximates the log form of the Bayes factor where established criteria can be used to assess the evidence supporting a more complex model. Initially, polynomial models of degree 2 having one, two, and three subgroups were tested sequentially to determine the number of subgroups, selecting the maximum number of subgroups supported by the data (Nagin, 2005).
Following that, the subgroup trajectory shapes were explored and simplified to the most parsimonious models where parameters were significantly different from zero and models performed similarly based on the Bayes factor criteria. Based on the mixture model, we assigned the latent trajectory class according to the highest posterior probability and conducted bivariate associations of class with micronutrients using Student’s t-tests and general linear models. Micronutrients were assessed for normality using the Shapiro-Wilk test and power transformed as needed based on the optimal lambda suggested by the Box-Cox method (Box & Cox, 1964).
Results
Sample Characteristics
A convenience sample of 54 pregnant African American women was enrolled (August 2011 to April 2013), and seven participants were excluded for invalid FFQ data, for a final sample size of 47. All of these participants had ultrasound scans at 19 to 21 weeks and/or 23 to 25 weeks gestation, which was important to ensure the ultrasonic attenuation trajectory clusters were comprised of women with scans taken during early pregnancy. Nineteen participants (40%) had all five scans, 12 (26%) had four, six (13%) had three, two (4%) had two, and eight (17%) had one scan.
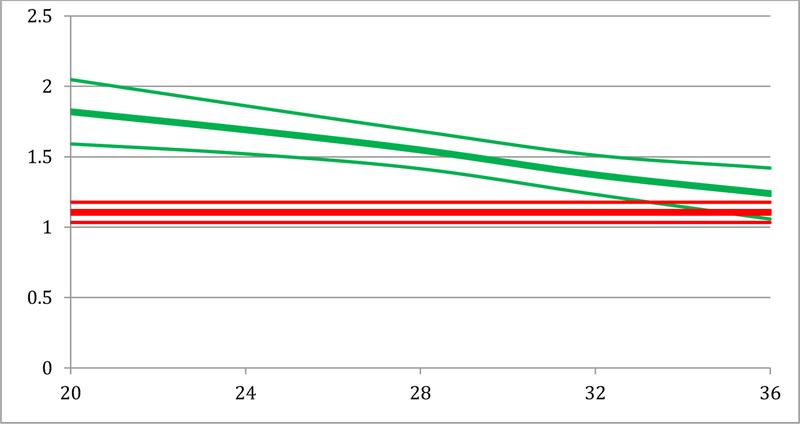
Trajectory mixture models were tested iteratively to determine the number of subgroups and the optimal form for each trajectory, evaluated by comparison of model BICs. The at-risk for cervical remodeling group had 36 individuals (74.3%) and was best fit by an intercept-only trajectory, that is, their ultrasonic attenuation value was 1.09 dB/cm-MHz (95% CI: 1.01, 1.18) from weeks 20 to 36 of gestation. A less-risk for cervical remodeling group of 11 (25.7%) individuals was best described by greater ultrasonic attenuation at 20 weeks (1.75 dB/cm-MHz, 95% CI: 1.49, 2.01) and a linearly decreasing trajectory, slope = −0.03 (95% CI: −0.05, −0.01) through 36 weeks (Figure 2). These findings indicate that participants in the less-risk group had less cervical remodeling throughout the pregnancy than those in the at-risk group.
Figure 2.
Model attenuation trajectories and 95% confidence intervals for at-risk (red) and less-risk (green) groups for cervical attenuation scores (y-axis) and weeks gestation (x-axis). The at-risk group has lower attenuation scores and greater cervical remodeling throughout pregnancy while the less-risk group has higher attenuation scores and less cervical remodeling throughout pregnancy.
Demographic and clinical characteristics are presented in Table 1. The majority of participants were single (85%) and not currently employed (67%). The mean age of the sample was 28 years, and birth occurred at a mean of 36 weeks gestation. Women in both trajectory groups were similar with regard to preterm birth and gestational age at birth. All women in the less-risk for cervical remodeling group had a previous abortion or miscarriage.
Table 1.
Participant Characteristics
| Total Mean (SD) (n = 47) |
At-risk group Mean (SD) (n = 36) |
Less-risk group Mean (SD) (n = 11) |
p-value | ||||
|---|---|---|---|---|---|---|---|
| Age (years) | 28.15 (6.67) | 27.78 (6.35) | 29.36 (7.84) | 0.496 | |||
| Gestational age at delivery (weeks) | 36.38 (4.98) | 36.3 (4.8) | 36.64 (5.78) | 0.699 | |||
| Birth weight (grams) | 2,742 (970) | 2,670 (947) | 2,999 (1,050) | 0.236 | |||
| Prepregnancy BMI (kg/m2) | 32.57 (11.6) | 32.51 (12.05) | 32.73 (10.56) | 0.958 | |||
| na | % | na | % | na | % | ||
| Previous full term births | 0.201 | ||||||
| 0 | 25 | 53.2 | 21 | 58.3 | 4 | 36.4 | |
| 1+ | 22 | 46.8 | 15 | 41.7 | 7 | 63.6 | |
| Previous preterm births | 0.953 | ||||||
| 0 | 26 | 55.3 | 20 | 55.6 | 6 | 54.5 | |
| 1+ | 21 | 44.7 | 16 | 44.4 | 5 | 45.5 | |
| Previous abortion/miscarriage | 0.001 | ||||||
| No | 20 | 42.6 | 16 | 44.4 | 0 | 0 | |
| Yes | 27 | 57.4 | 36 | 100 | 11 | 100 | |
| Living children | 0.139 | ||||||
| 0 | 17 | 37 | 15 | 42.9 | 2 | 18.2 | |
| 1+ | 29 | 63 | 20 | 57.1 | 9 | 81.8 | |
| Progesterone this pregnancy | 0.483 | ||||||
| No | 30 | 63.8 | 22 | 61.1 | 8 | 72.7 | |
| Yes | 17 | 36.2 | 14 | 38.9 | 3 | 27.3 | |
| Preterm birth this pregnancy | 0.934 | ||||||
| No | 33 | 71.7 | 25 | 71.4 | 8 | 72.7 | |
| Yes | 13 | 28.3 | 10 | 28.6 | 3 | 27.3 | |
| Birth weight < 2,500 grams | 0.464 | ||||||
| No | 33 | 73.3 | 24 | 70.6 | 9 | 81.8 | |
| Yes | 12 | 26.7 | 10 | 29.4 | 2 | 18.2 | |
| Marital Status | 0.517 | ||||||
| Single | 39 | 84.8 | 29 | 82.9 | 10 | 90.9 | |
| Married/Divorced/Separated | 7 | 15.2 | 6 | 17.1 | 1 | 9.1 | |
| Employed | 0.665 | ||||||
| No | 31 | 67.4 | 23 | 65.7 | 8 | 72.7 | |
| Yes | 15 | 32.6 | 12 | 34.3 | 3 | 27.3 | |
| WIC | 0.794 | ||||||
| No | 14 | 30.4 | 11 | 31.4 | 3 | 27.3 | |
| Yes | 32 | 69.6 | 24 | 68.6 | 8 | 72.7 | |
| Public aid | 0.494 | ||||||
| No | 13 | 28.3 | 9 | 25.7 | 4 | 36.4 | |
| Yes | 33 | 71.7 | 26 | 74.3 | 7 | 63.6 | |
| Interpregnancy interval | 0.665 | ||||||
| Primiparous or >24 months | 31 | 68.9 | 24 | 70.6 | 7 | 63.6 | |
| ≤24 months | 14 | 31.1 | 10 | 29.4 | 4 | 36.4 | |
| UTI | 0.106 | ||||||
| No | 33 | 71.7 | 23 | 65.7 | 10 | 90.9 | |
| Yes | 13 | 28.3 | 12 | 34.3 | 1 | 9.1 | |
| Alcohol during pregnancy | 0.377 | ||||||
| No | 44 | 95.7 | 34 | 97.1 | 10 | 90.9 | |
| Yes | 2 | 4.3 | 1 | 2.9 | 1 | 9.1 | |
Note. Missing data were present for some variables; percentages based on complete data, BMI = body mass index, UTI = urinary tract infection, WIC = Special Supplemental Nutrition Program for Women, Infants and Children.
CALLOUT 2
The majority of the sample received support from the Special Supplemental Nutrition Program for Women, Infants and Children and public aid (70–72%), but there were no differences between the at-risk and less-risk cervical ultrasonic attenuation groups on these variables. The majority of women did not consume alcohol (96%) during the pregnancy, and no differences in alcohol intake were found between the two groups.
Nutrient Intake and Cervical Remodeling
When examining the dietary reference intakes by ultrasonic attenuation risk group, a greater percentage of participants in the less-risk group consumed the DRI for pregnancy for calcium, vitamin A, folate, vitamin E, zinc, and vitamin D compared to the at-risk group. The percentage of participants who consumed the recommended daily allowance for vitamin E in the less-risk group was twice that of those in the at-risk group (Table 2).
Table 2.
Mean Nutrient Intake by Attenuation Risk Group
| Total n = 47 Mean (SD) |
At-risk n =36 Mean (SD) |
Less-risk n =11 Mean (SD) |
p-value | |
|---|---|---|---|---|
| Calories (Kcal) | 2,496 (962) | 2,459 (861) | 2,616 (1,282) | 0.641 |
| Protein (g) | 91.62 (33.57) | 91.39 (31.24) | 92.39 (42.02) | 0.932 |
| Calcium (mg) | 957.52 (379.76) | 897.78 (327.04) | 1,153.06 (483.89) | 0.031 |
| Iron (mg) | 33.77 (25.49) | 33.65 (24.39) | 34.15 (30.09) | 0.779 |
| Vitamin A (IU) | 13,003.4 (12,387.4) | 12,121.15 (8,748.66) | 15,890.86 (20,639.82) | 0.692 |
| Thiamin B1 (mg) | 2.59 (2.10) | 2.66 (2.33) | 2.35 (1.13) | 0.520 |
| Riboflavin B2 (mg) | 2.75 (2.21) | 2.82 (2.43) | 2.55 (1.31) | 0.606 |
| Niacin (mg) | 30.94 (23.11) | 32.07 (25.69) | 27.24 (11.28) | 0.410 |
| Vitamin C (mg) | 258.81 (156.66) | 246.03 (144.02) | 300.64 (194.39) | 0.380 |
| Folate (μg) | 569.84 (249.01) | 548.59 (259.51) | 639.39 (206.3) | 0.216 |
| Vitamin E (IU) | 27.5 (29.8) | 22 (22.56) | 45.5 (42.86) | 0.044 |
| Zinc (mg) | 16.63 (9.14) | 14.95 (6.85) | 22.13 (13.27) | 0.049 |
| Vitamin B6 (mg) | 2.85 (1.63) | 2.84 (1.65) | 2.86 (1.64) | 0.825 |
| Magnesium (mg) | 342.92 (131.33) | 335.47 (103.43) | 367.27 (202.59) | 0.603 |
| Beta-carotene (μg) | 5,635.63 (5,878.56) | 4,797.66 (3,700.03) | 8,378.09 (10,012.37) | 0.332 |
| Vitamin D (IU) | 295.33 (204.79) | 273.15 (186.85) | 367.91 (251.32) | 0.210 |
| Vitamin B12 (μg) | 7.05 (5.08) | 6.75 (4.41) | 8.02 (7.02) | 0.988 |
Note. p-values adjusted for calorie density.
In general, participants in the less-risk cervical remodeling group consumed greater amounts of nutrients compared to women in the at-risk cervical remodeling group (Table 3). In the less-risk group, the mean intake of vitamin E was 2 times more (p = 0.037), for calcium almost 1.3 times more (p = 0.050), and for zinc 1.5 times more (p = 0.042) compared with the at-risk group. All statistical conclusions were obtained from multivariate models adjusted for daily kilocalorie intake.
Table 3.
Participants That Met Recommended Dietary Reference Intake (DRI) by Attenuation Risk Group
| At-risk n = 36 |
Less-risk n = 11 |
p-value |
DRI in pregnancy | |
|---|---|---|---|---|
| Calcium (mg) | 36% | 64% | 0.131 | 1,000 UL = 2,500 |
| Iron (mg) | 11% | 9% | 0.809 | 27 UL = 45 |
| Vitamin A (IU) | 47% | 55% | 0.707 | 2,565 UL = 10,000 |
| Thiamin B1 (mg) | 83% | 82% | 0.683 | 1.4 UL = ND |
| Riboflavin B2 (mg) | 92% | 73% | 0.052 | 1.4 UL = ND |
| Niacin (mg) | 56% | 55% | 0.974 | 18 UL = 35 |
| Vitamin C (mg) | 94% | 91% | 0.550 | 85 UL = 2,000 |
| Folate (μg) |
28% | 45% | 0.264 | 400 UL = ND |
| Vitamin E a-TE | 44% | 82% | 0.045 | 15 UL = 1,000 |
| Zinc (mg) | 69% | 82% | 0.496 | 11 UL = 40 |
| Vitamin B6 (mg) | 78% | 73% | 0.530 | 1.9 UL = 100 |
| Magnesium (mg) | 67% | 64% | 0.885 | 360 UL = 350 |
| Vitamin D (IU) | 56% | 82% | 0.109 | 200 UL = 2,000 |
| Vitamin B12 (μg) | 92% | 73% | 0.052 | 2.6 UL = ND |
Note. UL = upper limit; ND = not determined.
Discussion
We examined the relationship between nutrient intake and cervical remodeling. We found lower intake of zinc, calcium, and vitamin E to be significantly related to assignment to the at-risk cervical remodeling group. The under-consumption of these particular nutrients (zinc, calcium, and vitamin E) has been associated with preterm birth (Hofmeyr et al., 2007). These nutrients are essential for the regulation of the MMP pathway, which is primarily responsible for cervical remodeling in pregnancy (Sundrani et al., 2011). During conditions of nutrient deficiency, MMP activity is increased, which causes tissue and collagen degradation and results in premature cervical remodeling during pregnancy (Verma & Hansch, 2007). Tissue inhibitor of metalloproteinases (TIMP) inhibits the activity of MMP and sub-optimal nutrient intake may change the regulation of both MMP and TIMP concentrations. Altering the regulation of MMP and TIMP concentrations may cause the process of ECM cervical tissue remodeling in pregnant women to function irregularly (Sundrani et al., 2011), thus increasing risk for preterm birth.
Although we did not find statistically significant differences for total calories, protein, iron, vitamin A, thiamin, riboflavin, niacin, vitamin C, folate, vitamin B6, magnesium, beta-carotene, vitamin D, and vitamin B12 between the at-risk and less-risk for cervical remodeling groups, the proportion of participants meeting the DRI for these nutrients was greater in the less-risk ultrasonic attenuation group compared to the at-risk ultrasonic attenuation group, and greater amounts of nutrients were consumed by women in the less-risk group than in the at-risk cervical remodeling group. The sample for this study was small and may not have been adequately powered to observe differences in all of the above listed nutrients by ultrasonic attenuation group. Future research with a larger sample might better address the potential role of these nutrients in cervical remodeling and ultrasonic attenuation.
CALLOUT 3
The present findings suggest that low intake of zinc, calcium, and vitamin E may play important roles in influencing preterm birth. Specifically, zinc intake was lower in the at-risk ultrasonic attenuation group compared to the less-risk ultrasonic attenuation group. Zinc supplementation has been shown to decrease the incidence of preterm birth when administered early in pregnancy (Zahiri Sorouri, Sadeghi, & Pourmarzi, 2015), and lower intake of dietary zinc early in pregnancy has been associated with an increase in preterm birth [risk ratio (RR) = 3.46, 95% CI = 1.04 – 11.47] (Scholl et al., 1993). In a systematic review of 13 randomized controlled trials (6,854 women), investigators examined zinc supplementation during pregnancy and found a significant reduction in preterm birth in those that were supplemented (RR = 0.8, 95% CI = 0.7 – 0.9) (Mahomed, Bhutta, & Middleton, 2007). However, African American women with low plasma zinc concentrations supplemented with zinc later in pregnancy (19 weeks or greater) did not experience a significant reduction in preterm birth (10.2% vs. 13.3%, p = 0.25) (Goldenberg et al., 1995). Together, these results suggest that zinc has a role in preventing prematurity, but that zinc supplementation in early pregnancy or pre-conception supplementation may be necessary to have positive effects in reducing preterm birth risk.
Zinc is involved in numerous aspects of cellular metabolism and has a role in immune function, wound healing, DNA synthesis, protein synthesis and cell division (Institute of Medicine (U.S.). Panel on Micronutrients. & Institute of Medicine (U.S.). Food and Nutrition Board., 2001). In addition, zinc has a role in stabilizing cell membranes (Shankar & Prasad, 1998), maintaining the integrity of mucosal membranes (Wintergerst, Maggini, & Hornig, 2007), and maintaining collagen. Zinc is an integral component to the TIMP/MMP pathway. Tissue inhibitor of metalloproteinases inhibits MMP activity by inserting its edge into the zinc binding-catalytic site and substrate binding groove (Brew, Dinakarpandian, Nagase, 2000). Through crystallographic analyses, TIMP has been shown to bind with a zinc ion in the catalytic site of the MMPs (Murphy, 2011). Once this bond occurs, MMPs decrease in activity, thereby inhibiting cervical remodeling in preparation for labor. Therefore, adequate zinc may be necessary for decreasing MMP production and preventing premature cervical remodeling.
Mean plasma zinc levels are lower for African American women compared to their Caucasian counterparts (Neggers et al., 1996). In a longitudinal study, African American women consistently had lower mean plasma zinc concentrations compared to Caucasian women. This occurrence was observed at less than 12 weeks (11.6 ± 2.4 μmol/L vs. 12.2 ± 2.2 μmol/L), 12 to 19 weeks (10.7 ± 2.2 μmol/L vs. 11.1 ± 2.4 μmol/L), and greater than 20 weeks (9.6 ± 2.1 μmol/L vs. 10.1 ± 2.4 μmol/L) gestation (Neggers et al., 1996). Given the important role that zinc has in maintenance of collagen and cervical remodeling, it is especially important that pregnant African American women consume adequate amounts.
Similar to zinc, our study demonstrates that calcium intake was significantly lower in the at-risk group compared to the less-risk group. Calcium is the most abundant mineral in the body, is important to bone health, and is required for proper intracellular signaling, muscle function, and hormonal secretion (IOM, 2011). Calcium is associated with the activation of MMPs and is essential for folding of the molecule for substrate recognition and catalysis (Meraz-Cruz et al., 2006). Lower concentrations of calcium activate the MMP complex, and calcium concentrations above 2 mM have been shown to stabilize MMP activity (Meraz-Cruz et al., 2006). Thus, adequate calcium levels are likely needed to stabilize MMP activity and prevent premature cervical tissue remodeling.
In a meta-analysis of 10 trials on calcium supplementation during pregnancy, investigators found a non-significant reduction in overall preterm birth (RR = 0.8, 95% CI = 0.6 – 1.0) but a significant reduction in preterm birth for women (4 trials, n = 578) at high-risk for preeclampsia (RR = 0.5, 95% CI = 0.2 – 0.8) when they were administered calcium (Hofmeyr et al., 2007). Similarly, other researchers found that calcium supplementation of two grams per day reduced spontaneous preterm birth in high-risk adolescents (6.4 % vs. 17.9%, p = 0.01) (Villar & Repke, 1990). Together, these investigators’ studies suggest that adequate dietary calcium intake and calcium supplementation may have a positive effect in preventing preterm birth in high-risk populations. This reduction in preterm birth may be related to calcium’s role in cervical remodeling via the MMP pathway.
Similar to zinc and calcium, we found that levels of vitamin E were significantly lower in the at-risk group than in the less-risk group. Other researchers who studied women during pregnancy found the average intake of vitamin E was least in African American women (6.0 ± 0.2 mg) compared to Hispanic (6.4 ± 0.3 mg) and White (6.6 ± 0.2 mg) women (Arab, Carriquiry, Steck-Scott, & Gaudet, 2003). Vitamin E is a fat-soluble vitamin with many biological functions, including its role as an antioxidant, that protects tissues and cells from the damaging effect of reactive oxygen species (ROS) (Traber, 2007). Reactive oxygen species react with cell molecules and reduce the tissue’s ability to repair itself. A close correlation exists between ROS and the activation of MMPs, which may be the pathway by which vitamin E affects cervical tissue remodeling. One group of investigators found that ROS accumulation in human pancreatic cells enhanced MMP-2 secretion (Liu et al., 2012). However, another group found the quenching of ROS by the potent antioxidant vitamin E normalizes ROS levels (Liu & Liu, 2012). Other researchers found that vitamin E reduced MMP expression and activity in human dermal cells (Hantke et al., 2002), and murine model vitamin E supplementation decreased the expression and activity of MMP-2 (Wang, Tao, Yuan, Shen, & Liu, 2010). Therefore, adequate concentrations of vitamin E during pregnancy may impede ROS accumulation, subsequently decrease MMP expression, and thereby decrease cervical tissue degradation and ultrasonic attenuation.
Limitations
The Block brief FFQ is a self-report measure and is a measure of usual intake. Food frequency questionnaires are limited in their ability to collect complex information and require categorized responses, which may not adequately capture variability in energy- or nutrient-dense foods (Kristal, Peters, & Potter, 2005).
Since many of these nutrients have antioxidant and/or anti-inflammatory properties, investigators should examine the relationships between nutrient intake, inflammatory markers, MMPs, and cervical ultrasonic attenuation. Examination of serum markers of some nutrients would provide a more accurate picture of the bioavailability of these nutrients during pregnancy and their potential effect on cervical remodeling and ultrasonic attenuation.
Since we exclusively sampled African American women, our results cannot be generalized to other populations. Investigators should examine these associations in a more diverse sample. In addition, this study was not designed or powered to determine whether nutrient intake was associated with pregnancy complications and was a secondary analysis of data on multiple factors that lead to preterm birth.
This study is a secondary analysis of a pilot study examining multiple pathways to preterm birth. Since the sample size is small and this is a secondary analysis with no a priori sample calculation, the results are preliminary and future studies should examine these variables in a larger sample.
Implications for Practice
Building healthy tissues takes time. Thus, consuming a nutritious diet and taking vitamins once pregnancy is determined may not be sufficient to optimize cervical tissue for the prevention of pregnancy complications, including preterm birth. Practitioners need to encourage their patients considering pregnancy and patients currently pregnant to consume a diet rich in zinc, calcium, and vitamin E. Zinc is found in a wide variety of foods, including oysters, red meat, poultry, beans, nuts, whole grains, fortified breakfast cereals, and dairy products (Institute of Medicine, Food and Nutrition Board, 2001). Milk, yogurt, and cheese are rich natural sources of calcium; nondairy sources of calcium include vegetables such as Chinese cabbage, kale, and broccoli, but calcium from these foods is less bioavailable (Institute of Medicine, Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, 1997). Nuts, seeds, and vegetable oils are the best sources of vitamin E, and significant amounts are available in green leafy vegetables and fortified cereals (Monsen, 2000). Practitioners should inform women, particularly those at greatest risk for preterm birth, that certain nutrients (such as zinc, calcium, and vitamin E) are important to the health of the cervix and therefore may prevent spontaneous preterm birth.
Callouts.
Nutrient intake may be an important predictor of cervical remodeling.
Pregnant African American women with poor nutritional intake of zinc, calcium, or vitamin E were at higher risk for premature cervical remodeling.
Practitioners should encourage women who are considering pregnancy or are currently pregnant to consume diets rich in zinc, calcium, and vitamin E.
Acknowledgement
Funded by NIH R21 HD062790, University of Illinois at Chicago Center for Clinical and Translational Sciences Pilot Grant number CCTS0811–02, the Irving Harris Foundation, and the Robert Wood Johnson Foundation Nurse Faculty Scholars. The project described was also supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR000050. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Biographies
Mary Dawn Koenig, PhD, RN, CNM, is an assistant professor in the Department of Women, Children, and Family Health Science, University of Illinois at Chicago, Chicago, IL.
Barbara L. McFarlin, PhD, CNM, RDMS, FACNM, is an associate professor and Head of the Department of Women, Children, and Family Health Science, College of Nursing, University of Illinois at Chicago, Chicago, IL.
Alana D. Steffen, PhD, is a research assistant professor in the Department of Health Systems Science, University of Illinois at Chicago, Chicago, IL.
Lisa Tussing-Humphreys, PhD, MS, RD, is an assistant professor in the Department of Medicine, University of Illinois at Chicago, Chicago, IL.
Carmen Giurgescu, PhD, RN, is an assistant professor in the College of Nursing, Wayne State University, Detroit, MI.
Christopher Engeland, PhD, is an assistant professor in the Department of Biobehavioral Health and College of Nursing, Pennsylvania State University, University Park, PA.
Michelle A. Kominiarek, MD, MS, is an associate professor for the Feinberg School of Medicine, Northwestern University, Chicago, IL.
Christina Ciezczak-Karpiel, BS, is a research assistant in the Department of Women, Children, and Family Health Science, University of Illinois at Chicago, Chicago, IL.
William D. O’Brien Jr., PhD, is a research professor and professor emeritus, Department of Electrical and Computer Engineering, University of Illinois, Urbana-Champaign, Urbana, IL.
Rosemary White-Traut, PhD, RN, is a professor emerita, University of Illinois at Chicago and Director of Nursing Research, Children’s Hospital of Wisconsin, Milwaukee, WI.
Footnotes
Disclosure
The authors report no conflict of interest or relevant financial relationships.
References
- Arab L, Carriquiry A, Steck-Scott S, & Gaudet MM (2003). Ethnic differences in the nutrient intake adequacy of premenopausal US women: results from the Third National Health Examination Survey. Journal of the American Dietetic Association, 103(8), 1008–1014. doi: 10.1053/jada.2003.50194 [DOI] [PubMed] [Google Scholar]
- Becher N, Hein M, Danielsen CC, & Uldbjerg N (2010). Matrix metalloproteinases in the cervical mucus plug in relation to gestational age, plug compartment, and preterm labor. Reproductive Biology and Endocrinology, 8, 113. doi: 10.1186/1477-7827-8-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigelow TA, Labyed Y, McFarlin BL, Sen-Gupta E, & O’Brien WDJ (2011). Comparison of algorithms for estimating ultrasound attenuation when predicting cervical remodeling in a rat model. Proceedings of the 2011 IEEE International Symposium on Biomedical Imaging, 883–886. [Google Scholar]
- Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, & Gardner L (1986). A data-based approach to diet questionnaire design and testing. American Jouranl of Epidemiology, 124(3), 453–469. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3740045 [DOI] [PubMed] [Google Scholar]
- Box GEP, & Cox DR (1964). An analysis of transformations. Journal of the Royal Statistical Society. Series B (Methodological), 26(2), 221–252. [Google Scholar]
- Brew K, Dinakarpandian D, & Nagase H (2000). Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochimica et Biophysica Acta, 1477(1–2), 267–283. [DOI] [PubMed] [Google Scholar]
- Catov JM, Bodnar LM, Ness RB, Markovic N, & Roberts JM (2007). Association of periconceptional multivitamin use and risk of preterm or small-for-gestational-age births. American Journal of Epidemiology, 166(3), 296–303. doi:kwm071[pii]10.1093/aje/kwm071 [DOI] [PubMed] [Google Scholar]
- Dunlop AL, Kramer M, Hogue CJ, Menon R, & Ramakrishan U (2011). Racial disparities in preterm birth: an overview of the potential role of nutrient deficiencies. Acta Obstetricia Et Gynecologica Scandinavica. doi: 10.1111/j.1600-0412.2011.01274.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg RL, Tamura T, Neggers Y, Copper RL, Johnston KE, DuBard MB, & Hauth JC (1995). The effect of zinc supplementation on pregnancy outcome. Journal of the American Medical Association, 274(6), 463–468. [DOI] [PubMed] [Google Scholar]
- Hantke B, Lahmann C, Venzke K, Fischer T, Kocourek A, Windsor LJ, . . . Tschesche H (2002). Influence of flavonoids and vitamins on the MMP- and TIMP-expression of human dermal fibroblasts after UVA irradiation. Photochemical Photobiological Sciences, 1(10), 826–833. [DOI] [PubMed] [Google Scholar]
- Hennessy MD, Volpe SL, Sammel MD, & Gennaro S (2010). Skipping meals and less walking among African Americans diagnosed with preterm labor. Journal of Nursing Scholarship, 42(2), 147–155. doi: 10.1111/j.1547-5069.2010.01345.x [DOI] [PubMed] [Google Scholar]
- Hofmeyr GJ, Duley L, & Atallah A (2007). Dietary calcium supplementation for prevention of pre-eclampsia and related problems: a systematic review and commentary. British Journal of Obstetrics & Gynaecology, 114(8), 933–943. doi: 10.1111/j.1471-0528.2007.01389.x [DOI] [PubMed] [Google Scholar]
- Institute of Medicine, Food and Nutrition Board. (2001). DRI, dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Retreived from http://www.nap.edu/read/10026/chapter/1#xiv
- Institute of Medicine, Standing Committee on the Scientific Evaluation of Dietary Reference Intakes (1997). Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- Jones B, & Nagin DS (2007). Advances in group-based trajectory modeling and an SAS procedure for estimating them. Sociological Methods & Research, 35, 542–571. [Google Scholar]
- Jones B, Nagin DS, Roeder K (2001). A SAS Procedure Based on Mixture Models for Estimating Developmental Trajectories. Sociological Methods & Research, 29, 374–393. [Google Scholar]
- Kristal AR, Peters U, & Potter JD (2005). Is it time to abandon the food frequency questionnaire? Cancer Epidemiology, Biomarkers & Prevention, 14(12), 2826–2828. doi: 10.1158/1055-9965.EPI-12-ED1 [DOI] [PubMed] [Google Scholar]
- Labyed Y, Bigelow TA, & McFarlin BL (2011). Estimate of the attenuation coefficient using a clinical array transducer for the detection of cervical ripening in human pregnancy. Ultrasonics, 51(1), 34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, & Liu A (2012). Administration of vitamin E prevents thymocyte apoptosis in murine sarcoma S180 tumor bearing mice. Cellular and Molecular Biology (Noisy-le-grand), 58(Suppl OL), 1671–1679. [PubMed] [Google Scholar]
- Liu J, Ben QW, Yao WY, Zhang JJ, Chen DF, He XY, . . . Yuan YZ (2012). BMP2 induces PANC-1 cell invasion by MMP-2 overexpression through ROS and ERK. Frontiers in Bioscience, 17, 2541–2549. [DOI] [PubMed] [Google Scholar]
- Mahomed K, Bhutta Z, & Middleton P (2007). Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database of Systematic Reviews, 2, CD000230. doi: 10.1002/14651858.CD000230.pub3 [DOI] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Osterman MJ, Curtin SC, & Matthews TJ (2015). Births: Final data for 2013. National Vital Statistics Reports, 64(1), 1–65. [PubMed] [Google Scholar]
- McFarlin BL, Balash J, Kumar V, Bigelow TA, Pombar X, Abramowicz JS, & O’Brien WD Jr. (2015). Development of an ultrasonic method to detect cervical remodeling in vivo in full-term pregnant women. Ultrasound in Medicine & Biology, 41(9), 2533–2539. doi: 10.1016/j.ultrasmedbio.2015.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlin BL, Kumar V, Bigelow TA, Simpson DG, White-Traut RC, Abramowicz JS, & O’Brien WD Jr. (2015). Beyond cervical length: A pilot study of ultrasonic attenuation for early detection of preterm birth risk. Ultrasound in Medicine & Biology, 41(11), 3023–3029. doi: 10.1016/j.ultrasmedbio.2015.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraz-Cruz N, Ortega A, Estrada-Gutierrez G, Flores A, Espejel A, Hernandez-Guerrero C, & Vadillo-Ortega F (2006). Identification of a calcium-dependent matrix metalloproteinase complex in rat chorioallantoid membranes during labour. Molecular Human Reproduction, 12(10), 633–641. doi: 10.1093/molehr/gal072 [DOI] [PubMed] [Google Scholar]
- Monsen ER (2000). Dietary reference intakes for the antioxidant nutrients: vitamin C, vitamin E, selenium, and carotenoids. Journal of American Dietetic Association, 100(6), 637–640. doi: 10.1016/S0002-8223(00)00189-9 [DOI] [PubMed] [Google Scholar]
- Murphy G (2011). Tissue inhibitors of metalloproteinases. Genome Biology, 12, 233. doi: 10.1186/gb-2011-12-11-133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagin D (2005). Group-based modeling of development. Cambridge, MA: Harvard University Press. [Google Scholar]
- Neggers YH, Dubard MB, Goldenberg RL, Tamura T, Johnston KE, Copper RL, & Hauth JC (1996). Factors influencing plasma zinc levels in low-income pregnant women. Biological Trace Element Research, 55(1–2), 127–135. [DOI] [PubMed] [Google Scholar]
- Scholl TO, Hediger ML, Schall JI, Fischer RL, & Khoo CS (1993). Low zinc intake during pregnancy: its association with preterm and very preterm delivery. American Journal of Epidemiology, 137(10), 1115–1124. [DOI] [PubMed] [Google Scholar]
- Schwarz G (1978). Estimating the dimension of a model. Annals of Statistics, 6, 461–464. [Google Scholar]
- Shankar AH, & Prasad AS (1998). Zinc and immune function: the biological basis of altered resistance to infection. The American Journal of Clinical Nutrition, 68(2 Suppl), 447S–463S. [DOI] [PubMed] [Google Scholar]
- Siega-Riz AM, Bodnar LM, & Savitz DA (2002). What are pregnant women eating? Nutrient and food group differences by race. American Journal of Obstetrics and Gynecology, 186(3), 480–486. [DOI] [PubMed] [Google Scholar]
- Siega-Riz AM, Hartzema AG, Turnbull C, Thorp J, McDonald T, & Cogswell ME (2006). The effects of prophylactic iron given in prenatal supplements on iron status and birth outcomes: a randomized controlled trial. American Journal of Obstetrics and Gynecology, 194(2), 512–519. doi: 10.1016/j.ajog.2005.08.011 [DOI] [PubMed] [Google Scholar]
- Siega-Riz AM, & Popkin BM (2001). Dietary trends among low socioeconomic status women of childbearing age in the United States from 1977 to 1996: a comparison among ethnic groups. Journal of the American Medical Women’s Association, 56(2), 44–48, 72. [PubMed] [Google Scholar]
- Sundrani D, Chavan-Gautam P, Pisal H, Mehendale S, & Joshi S (2013). Matrix metalloproteinases-2, −3 and tissue inhibitors of metalloproteinases-1, −2 in placentas from preterm pregnancies and their association with one-carbon metabolites. Reproduction, 145(4), 401–410. doi: 10.1530/REP-12-0520 [DOI] [PubMed] [Google Scholar]
- Sundrani DP, Chavan Gautam PM, Mehendale SS, & Joshi SR (2011). Altered metabolism of maternal micronutrients and omega 3 fatty acids epigenetically regulate matrix metalloproteinases in preterm pregnancy: a novel hypothesis. Medical Hypotheses, 77(5), 878–883. doi: 10.1016/j.mehy.2011.08.001 [DOI] [PubMed] [Google Scholar]
- Traber MG (2007). Vitamin E regulatory mechanisms. Annual Review of Nutrition, 27, 347–362. doi: 10.1146/annurev.nutr.27.061406.093819 [DOI] [PubMed] [Google Scholar]
- Verma RP, & Hansch C (2007). Matrix metalloproteinases (MMPs): Chemical-biological functions and (Q)SARs. Bioorganic & Medicinal Chemistry, 15(6), 2223–2268. doi: 10.1016/j.bmc.2007.01.011 [DOI] [PubMed] [Google Scholar]
- Villar J, & Repke JT (1990). Calcium supplementation during pregnancy may reduce preterm delivery in high-risk populations. American Journal of Obstetrics and Gynecology, 163(4 Pt 1), 1124–1131. [DOI] [PubMed] [Google Scholar]
- Wang QL, Tao YY, Yuan JL, Shen L, & Liu CH (2010). Salvianolic acid B prevents epithelial-to-mesenchymal transition through the TGF-beta1 signal transduction pathway in vivo and in vitro. BMC Cell Biology, 11, 31. doi: 10.1186/1471-2121-11-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintergerst ES, Maggini S, & Hornig DH (2007). Contribution of selected vitamins and trace elements to immune function. Annals of Nutrition and Metabolism, 51(4), 301–323. doi: 10.1159/000107673 [DOI] [PubMed] [Google Scholar]
- Yan C, & Boyd DD (2007). Regulation of matrix metalloproteinase gene expression. Journal of Cellular Physiology, 211(1), 19–26. doi: 10.1002/jcp.20948 [DOI] [PubMed] [Google Scholar]
- Zahiri Sorouri Z, Sadeghi H, & Pourmarzi D (2015). The effect of zinc supplementation on pregnancy outcome: a randomized controlled trial. Journal of Maternal-Fetal & Neonatal Medicine, 1–5. doi: 10.3109/14767058.2015.1079615 [DOI] [PubMed] [Google Scholar]