Abstract
Objectives:
Resolvins have been shown to attenuate inflammation, while NETosis, the process of neutrophils releasing neutrophil extracellular traps (NETs), produces increased inflammation. It is hypothesized that treatment of animals with resolvin D1 (RvD1) would reduce abdominal aortic aneurysm (AAA) formation by inhibiting NETosis.
Methods:
Wild-type (WT) 8–12-week old C57BL/6 male mice (n=47) and apolipoproteinE deficient (ApoE-/) mice (n=20) were used in two models to demonstrate the effects of RvD1 on AAA growth. In the topical elastase AAA model, WT mice were divided into three groups: (1) Deactivated elastase control group: sham surgery was performed using deactivated elastase and mice were intravenously injected with phosphate buffered saline (PBS) once a day until harvest. (2) Elastase group: active elastase was used to induce AAA and mice were injected with PBS daily until harvest. (3) RvD1 treated group: AAA was induced and mice were injected with RvD1 daily until harvest. In the angiotensin II (Ang II) induced AAA model, ApoE−/− mice were fed high fat diet and implanted with osmotic infusion pumps containing Ang II (1000ng/kg/min). The Ang II model was divided into two groups: (1) Ang II control group: Ang II was delivered and mice were injected with PBS daily until harvest. (2) RvD1 treated group: Ang II was delivered and mice were injected with RvD1 daily until harvest. On postoperative days 3, 14, or 28, aortic and blood samples were collected for western blot, histology, cytokine array, enzyme-linked immunosorbent assay, and gelatin zymography after aortic diameter measurement.
Results:
Day 14 RvD1 treated group demonstrated 42% reduced AAA diameter compared to elastase group (p<0.001). On postoperative day 3, RvD1 treated group showed decreased levels of NETosis markers citrullinated histone H3 (p=0.04) and neutrophil elastase (p=0.002) compared to the Elastase group. Among important cytokines involved in AAA formation, interleukin (IL) 1β was down-regulated (p=0.02), while IL-10, a protective cytokine, was upregulated (p=0.01) in the RvD1 treated group. Active matrix metalloproteinase 2 (MMP2) also decreased in the RvD1 treated group (p= 0.03). The RvD1 treated group in the Ang II AAA model, a second model, demonstrated reduced AAA diameter compared to Ang II control group on day 28 (p<0.046). RvD1 treated group showed decreased levels of citrullinated histone H3 on day 3 (p=0.002). Cytokines INF-γ, IL-1β, CXCL-10, MCP-1, and RANTES were all decreased on day 28 (p<0.05).
Conclusion:
Resolvin D1-mediated inhibition of NETosis may represent a future medical treatment for the attenuation of AAA growth.
Keywords: Resolvin D1, RvD1, Aneurysm, AAA, NETosis, Citrullinated Histone H3
INTRODUCTION
Many factors have been shown to contribute to the mechanism of AAA disease. One such pathway of recent interest has been NETosis.3 First reported in 2004, NETosis is the process of nuclear chromatin extracellular traps released by neutrophils (NETs) to engulf pathogens and cause cell death in a pathway distinct from apoptosis and necrosis.4, 5 As neutrophils are the first responders to infection, experimental evidence has shown that NETosis can both inhibit and upregulate sterile inflammation for many chronic diseses, including atherosclerosis and gout.6,7 Previous studies from our laboratory demonstrated that neutrophil depletion reduces aortic aneurysm diameter by 60% in mice on day 14 in the elastase perfusion model 8 and that neutrophils peak on day 3 before maximum AAA growth in the elastase perfusion model. 9 By inhibiting NETosis, which is produced by early responding neutrophils, AAA inflammation could be decreased.
RvD1 is one of the D-series resolvins that makes up the resolvin family. Resolvins are naturally occurring lipid-derived pro-resolving mediators that act upon the resolution phase of inflammation.10 It has been reported that RvD1 acts closely with neutrophils to resolve inflammation.11 Inhibiting NETosis therefore represents a possible pathway that RvD1 could be used as a medical therapy to treat AAA. In this study, it is hypothesized that RvD1 resolves AAA formation by targeting and inhibiting an early neutrophil inflammatory response, NETosis.
METHODS
Animal Housing
8 week-old WT C56BL/6 male mice (Jackson Laboratory, Bar Harbor, ME) were housed and maintained at 70°F, 50% humidity, in 12-hour light dark cycles per institutional animal protocols. Mice were provided drinking water and fed either a minimal phytoestrogen diet (2017 Teklad Global 16% Protein Rodent Diet, Harlan Labs, Inc., Frederick, MD) for the elastase-induced AAA model or a high fat diet for the Ang II induced AAA model (D12079B, Research Diets Inc., New Brunswick, NJ). We chose male mice because clinically AAA is 4 times as likely to occur in males compared to females and because there is a higher incidence of aortic aneurysms in male mice compared to female mice in previous AAA mouse models12–16. All experiments were conducted in accordance with the standards approved by the University of Virginia Animal Care and use committee (#3848).
Topical Elastase Surgery, Treatment Plan, and Harvest
Deactivated elastase control group (n=19):
Mice underwent sham AAA induction surgery, where 5µl of heat deactivated (100°C for 30 minutes) elastase, instead of active elastase, was applied topically to the aorta. The rest of the surgical procedure is identical to the other two groups. Depending on the day of harvest, PBS was injected intravenously (IV) once a day from the day of the surgery (day 0) to the day of harvest (days 3 or 14).
Elastase group (n=18):
The methods for induction of anesthesia, surgical procedure for AAA formation, and postoperative analgesia have been previously described.17 In short, after the abdominal aorta is circumferentially dissected from approximately 2mm below the left renal vein to the bifurcation, a fine tip pipette is then used for topical application of 5µl of elastase (Sigma-Aldrich; 10.1mg protein/ml, 19U/mg protein) to the exposed aortic adventitia for 5 minutes. After 5 minutes, the aorta is dried with a cotton tip applicator, the intestines are returned to the abdominal cavity, and the laparotomy is closed in layers. Depending on the day of harvest, PBS was injected daily from the day of the surgery (day 0) to the day of harvest (days 3 or 14).
RvD1 treated group (n=18):
The mice underwent the same AAA induction procedure as previously described above17. Depending on the day of harvest, RvD1 (300ng/kg in 300µl volume solution, Cayman Chemicals, Ann Arbor, MI, USA) was injected daily from the day of the surgery (day 0) to the day of harvest (days 3 or 14).
Mice from each group were euthanized under anesthesia by overdose and exsanguination. The abdominal aorta, from below the left renal vein to the bifurcation was dissected. The external aortic adventitia diameter was measured at its maximum diameter and at the intact self-control portion just below the left renal vein using video microscopy with NIS-Elements D.3.10 software attached to the microscope (Nikon SMX-800, Melville, NY).17 Aortic dilation percentage was determined using (maximal AAA diameter − self-contr ol aortic diameter)/maximal AAA diameter × 100% 17. A dilation of ≥100% was considered to be positive for AAA.17 On postoperative days 3 or 14, after diameter measurement, serum and aneurysm tissue were collected for cytokine analyses (n=6/group/day 3), gelatin zymography (n=6–9/group/day 3), Western blot (n=3–6/group/day 3), and enzyme-linked immunosorbent assay (n=10/group/day 3). Aortic tissue was also collected for histology (n=4–5/group/day3 & day14).
Angiotensin II Osmotic Infusion Pump Surgery, Treatment Plan, and Harvest
Osmotic infusion pump (Alzet® 1004, Durect Corp., C upertino, California) containing 1000 ng/kg/min of Ang II was implanted subcutaneously into 20 ApoE −/− male mice (Jackson Laboratory, Bar Harbor, ME) on a high fat diet introduced one week prior to the day of surgery as previously described.16,18–20 In the Ang II control group, mice (n=28) received PBS intravenously once a day from the day of surgery (day 0) to the day of harvest (day 3 or day 28). In the RvD1 treated group, mice (n=25 mice) received RvD1 once a day from the day of the surgery (day 0) to the day of harvest (days 3 or day 28).
Mice were euthanized under anesthesia by overdose and exsanguination. The abdominal aorta was fully dissected and video micrometry measurements of the maximum aortic diameter were performed using NIS-Elements D.3.10 software attached to the microscope (Nikon SMX- 800, Melville, NY). On postoperative days 3 or 28, aortic samples were then flash-frozen and used for cytokine analysis (n=9–10/group/day28). Any mouse that died prior to the study endpoint underwent autopsy to determine cause of death. One day 3 mouse in the RvD1 treated group died during the injection process and one day 28 mouse in the Ang II group died of aortic rupture.
Cytokine array
Using isolated protein from murine abdominal aortas, cytokine arrays (R&D Systems, 7 Minneapolis, MN) were completed according to manufacturer instructions, on mice harvested on 8 days 3 and 28 (n=4–6/group). Protein samples from each group were pooled for analysis, and all 9 samples were run in duplicate, and the mean value was used.21
Enzyme-Linked Immunosorbent Assay
An enzyme-linked immunosorbent assay (ELISA) analysis was performed for interleukin 14 (IL) 1β according to manufacturer instructions (BD Biosciences Pharmingen, San Diego, CA). 15 Protein samples (10 µg/well) isolated from the mouse abdominal aorta (n=10/group) were used 16 for the IL-1β ELISA. A RvD1 ELISA kit (Cayman Chemicals, Ann Arbor, MI) was used to 17 measure the Resolvin D1 levels in the elastase AAA induced mice groups (n=4/group) according 18 to manufacturer instructions.
Western Blot
Citrullinated histone H3 has previously been shown as a marker for NETosis.22,23 Therefore, by determining that citrullinated histone H3 levels are decreased in day 3 RvD1 treated mice, it would link treatment with RvD1 to possibly inhibiting NETosis. WT mice (C57BL/6 male mice) pre-treated with RvD1 were harvested on day 3 and the aortas underwent citrullinated histone 3 (cit H3, Anti-citrullinated histone H3-Cit 2 + 8 + 17 antibody, Adcam, Cambridge, MA) protein western blot, according to manufacturer instructions (n=3–6/group).
Histology
Aortic tissue was fixed in 4% buffered formaldehyde for 24 hours, transferred to 70% ethanol, and embedded in paraffin. Day 3 harvested (n=4–5/group) cross-sections were prepared with Verhoeff-VanGieson (VVG) for elastin 24; and immunohistochemical staining for citrullinated histone H3 (ab5103, 1:100 dilution, Abcam, Cambridge, MA), non-citrullinated histone H3 (H3) (ab1791, 1:300 dilution, Abcam, Cambridge, MA), neutrophils 24, neutrophil elastase (NE) (ab21595, 1:200 dilution, Abcam, Cambridge, MA), and smooth muscle α-actin.18 For grading, the positive staining area of the entire aortic tissue sample was selected and measured using integrated optical density of each section. Day 14 harvested (n=4/group) cross-sections were prepared with VVG, and immunohistochemical staining for neutrophils.24
Gelatin Zymography
Gelatin zymography was performed using isolated protein from the abdominal aortic samples (n=6–9/group) to measure pro and active MMP 2 and 9 activity. Precast zymography gels (10%, Invitrogen, Carlsbad, CA) were loaded with 3µg of aortic protein from each sample. Samples were diluted into 2x Tris-glycine SDS sample buffer and electrophoretically separated under non-reducing conditions. The gels were renatured for 30 minutes in renaturing buffer (Invitrogen) and incubated in developing buffer (Invitrogen, Carlsbad, CA) for 24 hours at 37°C while rocking. The gels were then stained in Simply Blue Safe Stain (Invitrogen, Carlsbad, CA). Pro and active forms of MMP2 and 9 appeared as clear band against the blue background. Quantification was determined according the optical density using Bio-Rad Image Lab Software version 4.0.17
Statistical analysis
Graph pad prism 7 (Graphpad Software, La Jolla, CA) software was used for all statistical analyses. AAA diameter, RvD1 ELISA, Western blot, histology, and cytokine analysis were determined using an unpaired t-test. Zymography comparisons were determined by a Mann-Whitney Test. The data is presented as mean values and standard error, with an alpha of less than 0.05 considered statistically significant.
RESULTS
Day 14 aneurysm formation decreased with RvD1 treatment in a topical elastase model
Previous work done by Pope and others showed how D series resolvins, RvD2 and RvD1, successfully inhibit aneurysm in the elastase perfusion treated mouse model; 21 therefore, it was important to determine whether RvD1 could decrease AAA formation in the topical elastase model. Mice treated with RvD1 showed significantly decreased abdominal aortic diameter on day 14 compared to the elastase group in the topical elastase model (84.9% ± 8.4 vs. 136.5% ± 6.9, p=0.0002, Fig.1A&B). There was an average day 14 abdominal aorta maximum diameter of 0.38mm for the deactivated group, 0.87mm for the RvD1 group, and 1.09mm for the elastase group. Histology at 10x with VVG staining verified preserved elastin with RvD1 treatment on day 14 (p=0.01, Fig. 1C&D), with RvD1 treated groups more closely resembling deactivated elastase control group, expressing significantly more elastin preservation compared to the elastase group.
Fig. 1.
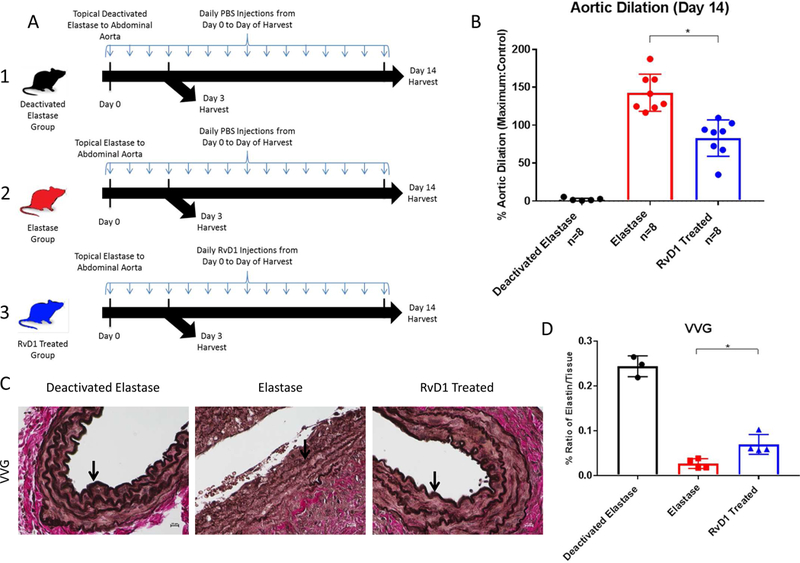
A. Topical elastase treatment design. 1. Deactivated Elastase control group: sham AAA induction surgery was performed using deactivated elastase and mice were injected with PBS once a day until harvest on day 3 or 14. 2. Elastase group: active elastase was used to induce AAA and mice were injected with PBS once a day until harvest on day 3 or 14. 3. RvD1 Treated group: AAA was induced and mice were injected with RvD1 (300ng in 300µl volume solution) once a day until harvest on day 3 or 14.
B. Day 14 topical elastase AAA model phenotype. AAA percent dilation is decreased in the RvD1 treated group vs. the elastase group (84.9% vs. 136.5%, p<0.001, n=8/group).
C&D. VVG histology images and quantification. Elastin is preserved in the RvD1 treated group. The ratios of the elastin/tissue levels in the RvD1 treated group are increased compared to the elastase group (0.028% vs. 0.070%, p=0.01, n=4–5/group).
Day 3 citrullinated histone H3 is lower in the RvD1 treated group, while RvD1 levels are higher in AAA the elastase group
As previously stated, determining that citrullinated histone H3 (a marker for NETosis22, 23) levels are decreased in day 3 RvD1 treated mice would link RvD1 to possibly inhibiting NETosis. RvD1-treated mice harvested on day 3 had citrullinated histone H3 levels significantly lower than the elastase group, as shown by Western blot (0.83± 0.1 vs. 1.23± 0.1 densitometry units, p=0.04, Fig. 2A&B). The deactivated elastase group also had significantly lower citrullinated histone H3 volume intensities compared to the elastase group (0.63± 0.05 vs 1.23± 0.1 densitometry units, p<0.001, Fig. 2A&B). The difference between the deactivated elastase and the RvD1 treated group was not significant, showing how citrullinated histone H3 levels in the RvD1 treated group more closely resemble the deactivated elastase group rather than the elastase group (p>0.05, Fig. 2A&B). RvD1 levels were significantly higher in elastase AAA induced serum compared to the deactivated elastase group (183.3 ±27.5 pg/ml vs. 75.5 ±14.2 pg/ml, p=0.01).
Fig. 2. A&B. Day 3 citrullinated histone H3 Western blot accompanied by quantification.
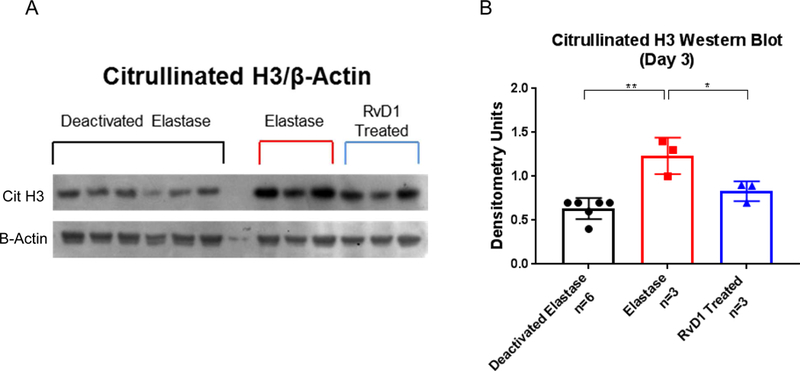
Day 3 Citrullinated histone H3 levels are decreased in the RvD1 treated group vs. Elastase group (0.83% vs. 1.23% densitometry units, p=0.04, n=3/group). The Deactivated Elastase group is also decreased vs. the elastase group (0.63% vs 1.23%, p<0.001, n=3–6/group). The RvD1 treated group shows no statistical difference from the Deactivated Elastase group (p>0.05, n=3–6/group).
IL-1β is down regulated, while IL-10 is upregulated in the RvD1 treated group
After showing a significant decrease in citrullinated histone H3 levels with RvD1 treatment 3 days following elastase application, inflammatory cytokine levels known to be important in AAA formation were examined during AAA formation in RvD1 treated mice. The day 3 RvD1 treated mice demonstrated decreased IL-1β cytokine levels in aortic tissue via ELISA compared to the elastase group (p=0.02, Fig. 3A). Decreased IL-1β levels demonstrate that RvD1 is affecting a cytokine important to aneurysm progression as early as 3 days into treatment therapy.8, 25, 26 Another cytokine known to be important in AAA, IL-6, trended down as well (p=0.18). Pooled animal aortic tissue harvested on day 3 from the RvD1 treated group also showed significantly increased levels of IL-10, a protective cytokine, via cytokine array compared to the elastase group (p=0.01, Fig. 3B).
Fig. 3.
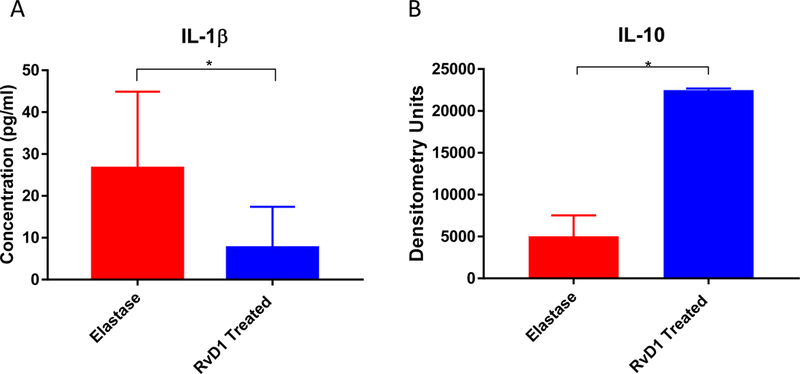
A. Day 3 IL-1β ELISA results. Day 3 IL-1β cytokine level is down regulated in the RvD1 treated group compared to the Elastase group (7.56pg/ml vs. 26.6pg/ml, p=0.0097, n=10/group).
B. IL-10 cytokine array results. Day 3 IL-10 cytokine levels are up regulated in the RvD1 treated group compared to the Elastase group (22,485 vs. 5,032 densitometry units, p=0.01, n=8 pooled/group).
RvD1 treatment shows decreased staining for NETosis markers
Immunohistochemical staining at 10x (Fig. 4A) substantiated the impact of RvD1 on day 3. There was no statistical difference regarding neutrophil (PMN) infiltration and non- citrullinated histone H3 between the RvD1 treated group and the elastase group (p<0.05, Fig. 4B&C). Confirming the previously mentioned western blot results, there were significantly decreased levels of citrullinated histone H3 in the RvD1 treated group vs the elastase group (p=0.03, Fig. 4D). Neutrophil Elastase (NE) staining was also significantly decreased in the RvD1 treated group compared to the elastase group (p=0.002, Fig 4E). Additionally, the RvD1 treated group expressed significantly more elastin preservation compared to the elastase group (p=0.037).
Fig. 4. A–E. Day 3 immunohistochemically stained images and quantification.
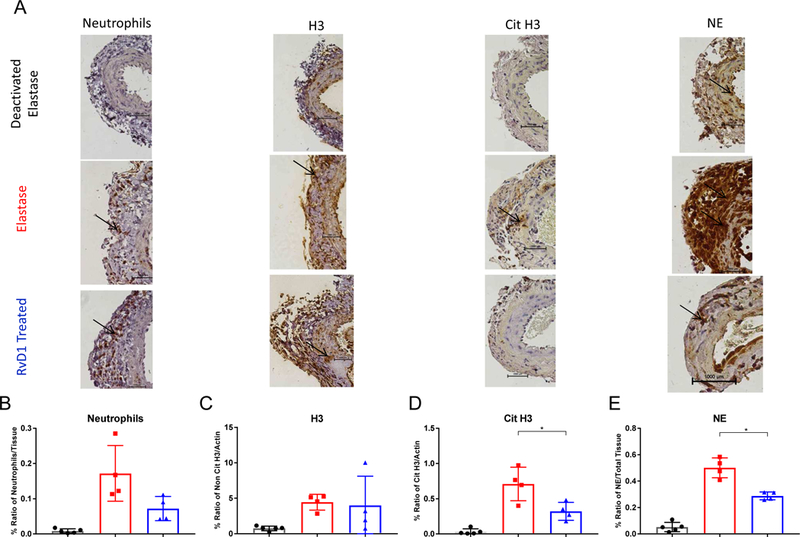
A&B. The ratios of neutrophils/tissue levels in the RvD1 treated group are not statistically different compared to the Elastase group (p>0.05, n=4–5/group). A&C. The ratios of the H3/actin levels in the RvD1 treated group are not statistically different compared to the Elastase group (p>0.05, n=4–5/group). A&D. The ratios of the cit H3/actin levels in the RvD1 treated group are decreased compared to the Elastase group (0.3214% vs. 0.7092%, p=0.03, n=4–5/group). A&E. The ratios of neutrophil elastase/tissue levels in the RvD1 treated group are decreased compared to the Elastase group (0.2885% vs. 0.5008%, p=0.002, n=4–5/group).
Active matrix metalloproteinase 2 is down regulated in the day 3 RvD1 treatment group
After noting elastin protection in the day 3 RvD1 treated group, gelatin zymography on day 3 was used to see if RvD1 decreased the degradation of the extracellular matrix proteins that occur in AAA formation. Zymography demonstrated decreased active MMP2 activity in the RvD1 treated group compared to the elastase group (62,048 vs. 176,859 densitometry units, p=0.03, Fig. 5A&B). Active MMP2 in the deactivated elastase group was also significantly lower compared with the elastase group (p<0.001). Pro MMP2, as well as pro and active MMP 2, were not significantly different between the RvD1 group and elastase groups (p>0.05).
Fig. 5. A&B. Gelatin zymography with quantification.
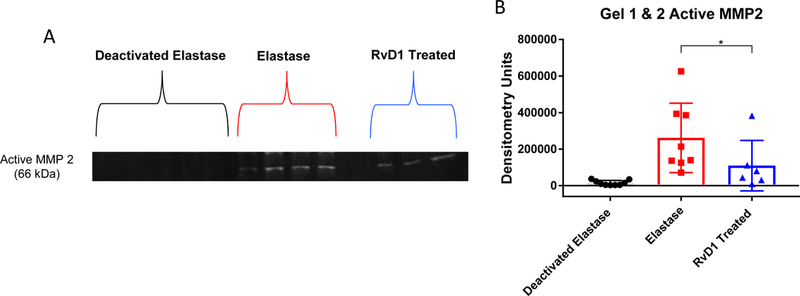
The active form of MMP2 was decreased in the RvD1 treated day 3 group compared to the Elastase group (8,976 vs. 62,048 densitometry units, p=0.03, n=6–9/group).
RVD1 decreased AAA formation and citrullinated h3 in the angiotensin II mouse model
After RvD1 was found to lower citrullinated h3 and attenuate AAA in the topical elastase model, a second model was needed to confirm the results. Mice treated with RvD1 showed significantly decreased abdominal aortic diameter on day 28 compared to the Ang II group in the Angiotensin II infusion AAA model (0.97mm ± 0.057 vs. 1.65 ± 0.25, p=0.016, Fig. 6A&B). There were no significant changes in death rates due to rupture (p>0.05). Cytokine array showed decreased levels of IL-1β, INF-γ, CXCL-10, MCP-1, and RANTES on day 28 harvested mice in RvD1 treated animals (p<0.05, Fig. 6C). RvD1-treated mice aortic tissue harvested on day 3 had citrullinated histone H3 levels significantly lower than the Ang II group, as shown by western blot (0.133± 0.014 vs. 0.211± 0.005 densitometry un its, p=0.002, Fig. 7A&B). IL-10 levels were increased in RvD1 treated tissue of day 3 harvested mice via cytokine array (p=0.01, Fig. 7C). Staining showed no significant difference in the number of neutrophils between the Ang II group and RvD1 treated group (p=.8935).
Fig. 6.
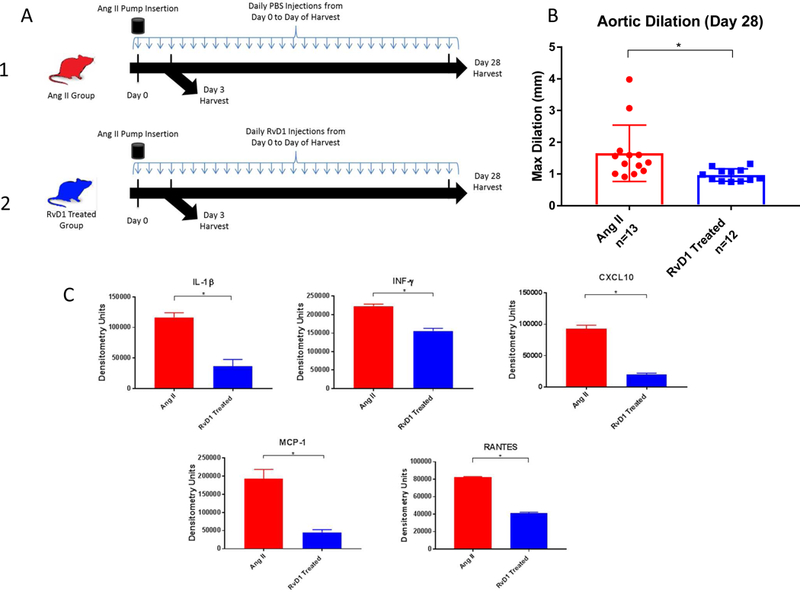
A. Angiotensin II treatment design. 1. Ang II control group: Ang II was delivered by subcutaneous osmotic pumps and mice were injected with PBS once a day until harvest on day 3 or 28. 2. RvD1 Treated group: Ang II was delivered by subcutaneous osmotic pumps and mice were injected with RvD1 once a day until harvest on day 3 or 28.
B. Day 28 angiotensin II AAA model phenotype. AAA maximum diameter is decreased in the RvD1 treated group vs. the Ang II group (0.97mm vs. 1.65mm, p=0.016, n=12–13/group).
C. Day 28 cytokine array results. Day 28 IL-1β, INF-γ, CXCL-10, MCP-1, and RANTES cytokine levels are down regulated in the RvD1 treated group compared to the Ang II group (all p<0.05, n=4–5 pooled/group).
Fig. 7.
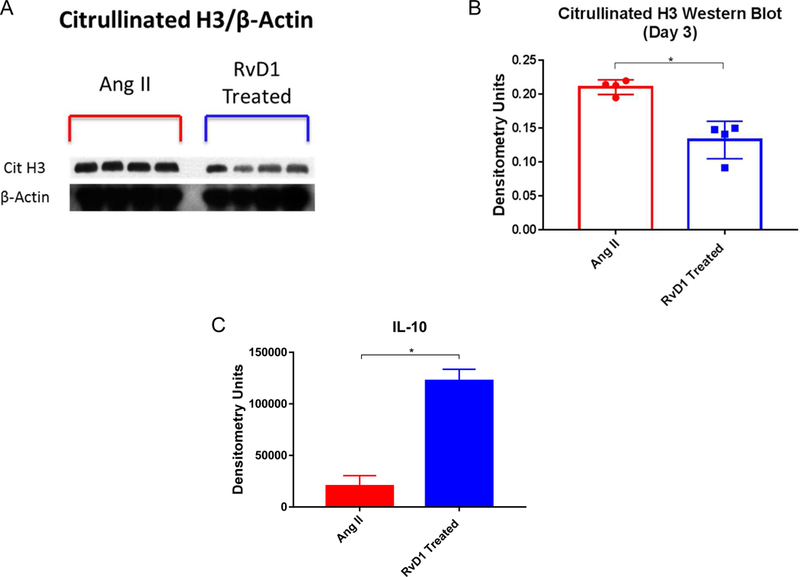
A&B. Day 3 citrullinated histone H3 western blot accompanied by quantification. Day 3 Citrullinated histone H3 levels are decreased in the RvD1 treated group vs. the Ang II group (0.133% vs. 0.211% densitometry units, p=0.002, n=2–3 pooled/group).
C. Day 3 IL-10 cytokine array results. Day 3 IL-10 cytokine levels are up regulated in the RvD1 treated group compared to the Ang II group (122,294 vs. 20,286, p=0.01, n=4–5 pooled/group).
DISCUSSION
In the present study, RvD1 treatment is associated withinhibition of NETosis as shown by decreased levels of citrullinated histone H3 and neutrophil elastase at early time points. Histology and ELISA results also support both RvD1’s effectiveness in attenuating AAA and its link to inhibiting NETosis. In association with NETosis inhibition, RvD1 reproducibly inhibited AAA formation in two murine models, down regulating pro-inflammatory cytokines, up regulating anti-inflammatory cytokines, and decreasing MMP levels all known to be important in AAA formation.
Resolvins are also “strategically positioned” to im pact neutrophils because they are generated between the initiation phase and resolution phase of inflammation.27 To show how RvD1 was mechanistically impacting AAA growth, it was imperative that we study earlier time points (day 3 when neutrophils peak) 9 more closely connected to neutrophil inflammatory recruitment. Neutrophils are the first inflammatory cells recruited to the site of infection6, 28 and when AAA is induced9, reaching their peak 3 days post-surgery.29 Akagi et al. documented the connection between resolvins and neutrophils, with RvD2 reducing neutrophil infiltration by 48% and Maresin 1 (another family of resolvins) reducing neutrophil infiltration by 56% in a carotid ligation model.30 Immediate effects of RvD1, specifically on neutrophils, are seen using the 3 day models as RvD1 levels in the serum of elastase treated mice are higher than the deactivated elastase mice on day 3. This suggests that RvD1 is present during the early inflammatory period and could inhibit inflammation by affecting neutrophils. Decreased elastin breaks in the RvD1 group compared to the Elastase group on day 3 also suggest that RvD1 contributes to reducing AAA formation in the early stages of aneurysm inflammation.
Resolvins have previously been shown to mitigate inflammation by decreasing macrophage16 and smooth muscle infiltration.31 In this paper, RvD1 inhibits AAA growth as shown in mice using the 14 day topical elastase model and the 28 day Ang II model. The decrease in INF-γ, IL-1β, CXCL-10, MCP-1, and Rantes cytokines in the RvD1 treated 28 day group and the decrease of elastin breaks in the RvD1 treated day 14 group further demonstrates RvD1’s effectiveness in attenuating AAA.
The early effects of RvD1 were shown in a significant decrease in the pro-inflammatory cytokines IL-1β and IL-6 on day 3 also, which when lowered have been shown to prevent aortic aneurysm formation.25, 32 These cytokines are also increased in other diseases where NETosis is thought to be a key player, like gout.33 Paradoxically, the anti-inflammatory cytokine IL-10 level increased in the day 3 RvD1 treated group in both the elastase and Ang II AAA model. This suggests that resolvins fight inflammation by increasing the amount of the anti-inflammatory cytokines,21 as well as decreasing pro-inflammatory cytokines. Additionally, decreased active MMP2 on day 3 further links RvD1 to a common AAA inflammatory process, but more investigation is needed to explain how inhibiting NETosis can affect the mechanism of MMP 2 and MMP 9 activity
Mechanistically, decreased levels of citrullinated histone H3 in RvD1 treated mice using western blot and immune-histochemical staining offers a possible mechanism as to how RvD1 is lowering inflammation by acting on neutrophils. Citrullination is the process of deamination that charges the amino acid arginine into the uncharged side chain, citrulline.6 Because H3 citrullination is associated with rapid intracellular decondensation of nuclear chromatin6 and inhibiting citrullination has been shown to lower NET formation34, citrullinated histone H3 has been linked in humans and mice as a marker for NETosis.7,22, 23 This study reveals that RvD1 is possibly inhibiting NETosis through lowering citrullination.
To make sure RvD1 is not affecting total histone levels and instead is specific towards NETs, non-citrullinated H3 was also stained. Because the non-citrullinated histone H3 is not significantly different in the RvD1 group vs. the elastase group, it is hypothesized that the RvD1 is not generally decreasing the infiltration of neutrophils, but more specifically targeting the process of NETosis. The lower level of NE staining in the RvD1 treated group vs the elastase group also implies the specificity of RvD1 to NETs, 3 as does the non-significant difference of neutrophil infiltration at day 3 between the same groups and the non-significant difference in macrophage staining at day 3. The Ang 2 model also showed no significant differences in neutrophil depletion between the RvD1 treated group and the Ang 2 control group, while also showing depleted levels of citrullinated H3 in the RvD1 treated group. This data, in connection with the elastase model results, further shows the possibility for RvD1 affecting NETosis independently of neutrophils.
More studies are needed to further link NETosis and RvD1 with AAA formation. Even though our goal was to look at the role of RvD1 on early responding neutrophils undergoing NETosis, studies showing the ability for RvD1 to resolve established AAA would strengthen the translational power of resolvins. Using the D series 2 resolvin, Pope et al showed inhibition of AAA growth in formed AAA using a mouse model21. In vitro studies showing the direct inhibition of NETosis by RvD1 could also help further confirm that resolvins might specifically target NETosis. NETosis has been shown to be triggered by lipopolysaccharides in many other types of inflammatory diseases35, 36 and RvD1 can protect against lipopolysaccharides.36 It is also hypothesized that RvD1 works through Formyl Peptide Receptor 1 (FPR2) and studies show that NETosis in humans can be inhibited by a FPR2 agonists.37 The ligands binding to FPR2 can have both pro-inflammatory and pro-resolution effects.38
The results of this study show the beneficial effects of resolvins in inhibiting AAA growth. Although these results highlighted just a single resolvin, resolvins have been hypothesized to act open many different pathways.22, 28 Linking RvD1 to NETosis offers an example as to how certain resolvins might act to slow AAA growth and possibly lead to a medical treatment for early identified growing AAA in the future.
CONCLUSION
The present study demonstrates that resolvin treatment decreases AAA size. This occurs in association with the inhibition of NETosis by RvD1 as demonstrated by decreased citrullinated histone H3 and neutrophil elastase levels in two well established AAA models. Histological staining and cytokine profiles further support the anti-NETosis effects of RvD1. These results suggest that RvD1 could be a possible medical therapy for inhibition of AAA growth.
JVS-D-18-00094R1, Resolvin D1 Decreases Abdominal Aortic Aneurysm Formation by Inhibiting NETosis in a Mouse Model
Type of Research:
Experimental study using a mouse elastase model of abdominal aortic aneurysm (AAA)
Key Findings:
Resolvin D1 reduced AAA diameter and also reduced levels of the early neutrophil inflammatory response (NETosis) marker citrullinated histone H3
Take Home Message:
Strategies to increase resolvin D1 activity may be new mechanisms of medical treatment of AAAs.
Acknowledgements:
We thank Anthony Herring, Cindy Dodson, Sheila Hammond, Melissa Bevard, and Ciara Zagaja for their knowledge and technical expertise.
Source of funding:
The National Institutes of Health under Award Numbers T32HL007849, R01 HL132395 & R01 HL081629 supported research reported in this publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author conflict of interest: None
Presentation: Eastern Vascular Society, October 8th, 2017, Savannah, Georgia, Rapid Fire.
References
- 1.Harris LM, Faggioli GL, Fiedler R, Curl GR, Ricotta JJ. Ruptured abdominal aortic aneurysms: factors affecting mortality rates. J Vasc Surg 1991;14:812–8. [DOI] [PubMed] [Google Scholar]
- 2.Thompson SG, Ashton HA, Gao L, Scott RAP. Screening men for abdominal aortic aneurysm: 10 year mortality and cost effectiveness results from the randomised Multicentre Aneurysm Screening Study. BMJ 2009;338:b2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan H, Zhou HF, Akk A, Hu Y, Springer LE, Ennis TL, et al. Neutrophil Proteases Promote Experimental Abdominal Aortic Aneurysm via Extracellular Trap Release and Plasmacytoid Dendritic Cell Activation. Arteriosclerosis, Thrombosis, and Vascular Biology 2016;36(8):1660–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. A.Neutrophil extracellular traps kill bacteria. Science 2004;303:1532–1535. [DOI] [PubMed] [Google Scholar]
- 5.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 2007;176(2):231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Remijsen Q, Kuijpers TW, Wirawan E, Lippens S, Vandenabeele P, Vanden Berghe T. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death and Differentiation 2011;18(4):581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meher Akshaya K., Spinosa Michael, Davis John P., Pope Nicolas, Laubach Victor E., Su Gang, et al. Novel Role of IL (Interleukin)-1B in Neutrophil Extracellular Trap Formation and Abdominal Aortic Aneurysms. ATVB 2018; 117309897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eliason JL, Hannawa KK, Ailawadi G, Sinha I, Ford JW, Deogracias MP, et al. Neutrophil depletion inhibits experimental abdominal aortic aneurysm formation. Circulation 2005;112:232–240. [DOI] [PubMed] [Google Scholar]
- 9.Hannawa KK, Eliason JL, Woodrum DT, Pearce CG, Roelofs KJ, Grigoryants V, et al. L- selectin-mediated neutrophil recruitment in experimental rodent aneurysm formation. Circulation 2005;112:241–247. [DOI] [PubMed] [Google Scholar]
- 10.Krishnamoorthy S, Recchiuti A, Chiang N, Fredman G, Serhan CN. Resolvin D1 receptor stereoselectivity and regulation of inflammation and proresolving microRNAs. Am. J. Pathol 2012;180:2018–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nature Reviews Immunology 2008;8(5):349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hannawa KK, Eliason JL, Upchurch GR. Gender Differences in Abdominal Aortic Aneurysms. Vascular 2009;17(Suppl 1):S30–S39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lederle FA, Johnson GR, Wilson SE. Abdominal aortic aneurysm in women. J Vasc Surg 2001;34(1):122–126. [DOI] [PubMed] [Google Scholar]
- 14.Katz DJ, Stanley JC, Zelenock GB. Gender differences in abdominal aortic aneurysm prevalence, treatment, and outcome. J Vasc Surg 1997;25(3):561–568. [DOI] [PubMed] [Google Scholar]
- 15.Manning MW, Cassi LA, Huang J, Szilvassy SJ, Daugherty A. Abdominal aortic aneurysms: fresh insights from a novel animal model of the disease. Vasc Med 2002;7(1):45–54. [DOI] [PubMed] [Google Scholar]
- 16.Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest 2000;105(11):1605–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu G, Su G, Davis JP, Schaheen B, Downs E, Roy RJ, et al. A novel chronic advanced stage abdominal aortic aneurysm murine model. J Vasc Surg 2017;66(1):232–242.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salmon M, Johnston WF, Woo A, Pope NH, Su G, Upchurch GR Jr., et al. KLF4 regulates abdominal aortic aneurysm morphology and deletion attenuates aneurysm formation. Circulation 2013;128:S163–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daugherty A, Cassis LA. Chronic Angiotensin II Infusion Promotes Atherogenesis in Low Density Lipoprotein Receptor −/− Mice. Annals of the New York Academy of Sciences 1999;892:108–18. [DOI] [PubMed] [Google Scholar]
- 20.Saraff K, Babamusta F, Cassis LA, Daugherty A. Aortic dissection precedes formation of aneurysms and atherosclerosis in angiotensin II-infused, apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 2003;23:1621–1626. [DOI] [PubMed] [Google Scholar]
- 21.Pope NH, Salmon M, Davis JP, Chatterjee A, Su G, Conte MS, et al. D-series resolvins inhibit murine abdominal aortic aneurysm formation and increase M2 macrophage polarization. FASEB Journal 2016;30:4192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirose T, Hamaguchi S, Matsumoto N, Irisawa T, Seki M, Tasaki O, et al. Presence of neutrophil extracellular traps and citrullinated histone H3 in the bloodstream of critically ill patients. PLoS One 2014;9(11):e111755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Liu B, Fukudome EY, Lu J, Chong W, Jin G, et al. Identification of citrullinated histone H3 as a potential serum protein biomarker in a lethal model of lipopolysaccharide-induced shock. Surgery 2011;150(3);442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lau CL, Zhao Y, Kron IL, Stoler MH, Laubach VE, Ailawadi G, et al. The role of adenosine A2A receptor signaling in bronchiolitis obliterans. Ann Thorac Surg 2009;88(4):1071–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnston WF, Salmon M, Pope NH, Meher A, Su G, Stone ML, et al. Inhibition of interleukin-1β decreases aneurysm formation and progression in a novel model of thoracic aortic aneurysms. Circulation 2014;130:S51–S59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnston WF, Salmon M, Su G, Lu G, Stone ML, Zhao Y, et al. Genetic and Pharmacologic Disruption of Interleukin-1β Signaling Inhibits Experimental Aortic Aneurysm Formation. Arterioscler, Thromb, and Vasc Biol 2013;33(2):294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serhan CN, Petasis NA. Resolvins and Protectins in Inflammation-Resolution. Chemical reviews 2011;111(10):5922–5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol 2006;6(3):173–182. [DOI] [PubMed] [Google Scholar]
- 29.Pagano MB, Bartoli MA, Ennis TL, Mao D, Simmons PM, Thompson RW, et al. Critical role of dipeptidyl peptidase I in neutrophil recruitment during the development of experimental abdominal aortic aneurysms. Proc Natl Acad Sci USA 2007;104:2855–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akagi D, Chen M, Toy R, Chatterjee A, Conte MS. Systemic delivery of proresolving lipid mediators resolvin D2 and maresin 1 attenuates intimal hyperplasia in mice. FASEB Journal 2015;29:2504–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyahara T, Runge S, Chatterjee A, Chen M, Mottola G, Fitzgerald JM, et al. D-series resolvin attenuates vascular smooth muscle cell activation and neointimal hyperplasia following vascular injury. FASEB Journal 2013;27(6):2220–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pope NH, Salmon M, Johnston WF, Lu G, Lau CL, Upchurch GR, et al. Interleukin-6 Receptor Inhibition Prevents Descending Thoracic Aortic Aneurysm Formation. Annals of Thoracic Surgery 2015;100(5):1620–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitroulis I, Kambas K, Chrysanthopoulou A, Skendros P, Apostolidou E, Ioannis Kourtzelis, et al. Neutrophil extracellular trap formation is associated with IL-1β and autophagy-related signaling in gout. PLoS One 2011:6(12):e29318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol 2009;184:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu S, Su X, Pan P, Zhang L, Hu Y, Tan H, et al. Neutrophil extracellular traps are indirectly triggered by lipopolysaccharide and contribute to acute lung injury. Scientific Reports 2016;6:37252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang B, Gong X, Wan JY, Zhang L, Zhang Z, Li HZ, et al. Resolvin D1 protects mice from LPS-induced acute lung injury. Pulmonary Pharmacology Therapeutics 2011;24:434–441. [DOI] [PubMed] [Google Scholar]
- 37.Tibrewal S, Ivanir Y, Sarkar J, Nayeb-Hashemi N, Bouchard CS, Kim E, et al. Hyperosmolar stress induces neutrophil extracellular trap formation: implications for dry eye disease. Invest Ophthalmol Vis Sci 2014;55(12):7961–7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dorward DA, Lucas CD, Chapman GB, Haslett C, Dhaliwal K, Rossi AG. The role of formylated peptides and formyl peptide receptor 1 in governing neutrophil function during acute inflammation. Am J Pathol 2015;185:1172–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]


