Abstract
Users of electronic cigarettes (e-cigs) are exposed to particles and other gaseous pollutants. However, major knowledge gaps on the physico-chemical properties of such exposures and contradictory data in published literature prohibit health risk assessment. Here, the effects of product brand, type, e-liquid flavoring additives, operational voltage, and user puffing patterns on emissions were systematically assessed using a recently developed, versatile, e-cig exposure generation platform and state-of-the-art analytical methods. Parameters of interest in this systematic evaluation included two brands (A and B), three flavors (tobacco, menthol, and fruit), three types of e-cigs (disposable, pre-filled, and refillable tanks), two puffing protocols (4 s and 2 s/puff), and four operational voltages (2.2 V to 5.7 V). Particles were generated at a high number concentration (106 to 107 particles/cm3). The particle size distribution was bi-modal (~200 nm and 1 μm). Furthermore, organic species (humectants propylene glycol and glycerin, nicotine) that were present in e-liquid and trace metals (potassium and sodium) that were present on e-cig heating coil were also released into the emission. In addition, combustion-related byproducts, such as benzene and toluene, were also detected in the range of 100 – 38,000 ppbv/puff. Parametric analyses performed in this study show the importance of e-cig brand, type, flavor additives, user puffing pattern (duration and frequency), and voltage on physico-chemical properties of emissions. This observed influence is indicative of the complexity associated with the toxicological screening of emissions from e-cigs and needs to be taken into consideration.
Keywords: Electronic cigarette, particulate matter, smoking, vaping, exposure assessment, inhalation toxicology
INTRODUCTION
Understanding the risks from electronic cigarette (e-cigs) exposures has become imperative because of the increasing use and the potential toxicological implications from such exposures (King et al. 2015). E-cig global sales reached $6 billion in 2014 and is projected to reach $32 billion in 2021 (Beige 2016; EUROMONITOR RESEARCH 2015). Influenced by heavy advertising, the public generally holds a positive perspective towards e-cigs compared to conventional tobacco smoking and the risk perception is that they are less harmful. However, major knowledge gaps exist both on the physico-chemical and toxicological properties of e-cig exposures (Coleman et al. 2016; Kim et al. 2014; Pearson et al. 2012).
There is a growing need to understand, in a systematic way, the effect of e-cig product brand and type, e-liquid flavor additives, operational parameters, and user puffing patterns on the physico-chemical properties of e-cig exposures (El-Hellani et al. 2018; Zhao et al. 2016). The e-cig market contained a total of 466 brands and 7,700 flavors in 2014, and is expected to grow further in the years ahead (Zhu et al. 2014). Unlike conventional cigarettes, e-cigs are highly customizable, allowing the user to adjust operational voltage to enhance personal satisfaction. Furthermore, it has been also documented in studies that e-cig users may use different puffing patterns compared to conventional smokers, with longer puffing duration (Farsalinos et al. 2013).
Furthermore, there is a limited number of studies on e-cig emission physico-chemical and morphological (PCM) characterization, which takes into account the effect of both operational parameters, user puffing patterns, product type, brand, and flavor additives. It is also worth noting that published data is inconsistent in terms of PCM properties of the e-cig exposures and there is a lack of a standardized methodology to generate and characterize such real-world e-cig exposures under the various operational scenarios and product/flavor variability. For example, literature agreed that e-cig exposure contained large amounts of particles (Fuoco et al. 2014; Marini et al. 2014; Zhao et al. 2016) and a complex mixture of chemicals, including propylene glycol, glycerin, nicotine, carbonyls, volatile organic compounds (VOCs) and metals (Allen et al., 2016; Goniewicz et al., 2012; Herrington & Myers, 2015; Pellegrino et al., 2012; Trehy et al., 2011; Westenberger,2009). However, large discrepancies were reported on e-cig exposure physico-chemical properties, e.g. particle size distribution (Ingebrethsen et al. 2012; Schober et al. 2014), particle number concentration (McAuley et al. 2012; Zhao et al. 2016), nicotine (Cobb et al. 2010; Trehy et al. 2011), VOCs (Goniewicz et al. 2014; Schripp et al. 2013) and trace metals (Mikheev et al. 2016; Williams et al. 2013). Such PCM discrepancies were largely due to the randomlyselected operational, user, and flavor parameters used in the generation of e-cig exposures. This included e-cig brand (Goniewicz et al. 2014; Pellegrino et al. 2012), e-cig type (McAuley et al. 2012; Zhao et al. 2016), e-liquid flavor (Allen et al. 2016; Zhao et al. 2016), operational voltage ranging from 3.2 to 5.0 V (Jensen et al., 2015; Kosmider et al., 2014), and puffing duration ranging from 1.8 to 8 s (Allen et al., 2016; Goniewicz et al., 2012). Without an adequate understanding of the role of these e-cig exposure generation parameters on e-cig emission PCM and toxicological properties, public health assessors cannot accurately derive potential risks from e-cigs. Also, one cannot explain the contradiction in the literature or establish e-cig emission profiles and adverse health effects from e-cig usage.
In our previous study, a versatile e-cig exposure generation system was developed to allow us to generate e-cig exposures in a systematic manner. The aforementioned operational parameters (i.e. brand/type, flavors, puffing patterns) can be adjusted in order to study their effects on the PCM properties of generated exposures (Zhao et al., 2016). In this study, this versatile e-cig exposure generation platform was used to perform a systematic investigation, linking exposure generation parameters to PCM properties of generated e-cig exposures using state-of-the-art analytical methods and real-time particle size characterization. The chemical analysis of the e-cig exposures included propylene glycol, glycerin, nicotine, VOCs, aldehydes, and trace metals.
METHODS
Generation of e-cig exposure
E-cig exposure generation system (E-cig-EGS)
We utilized our recently developed E-cig-EGS platform, which enabled generation of real-world e-cig exposures under precisely controlled generation parameters (Zhao et al. 2016). Figure 1 shows the E-cig-EGS system. In brief, a fully programmable single port e-cig generator (ECAG, e~Aerosols, LLC, Central Valley, NY) was used to control puffing patterns, operational voltage, and generate e-cig exposures. This e-cig generator can be used with all three types of e-cigs (disposable, pre-filled tank, and refillable tank). The generated e-cig emission and the dilution air were introduced into a 7-L, cylindricallyshaped environmental mixing chamber through two separate ports and mixed. The residence time of the environmental chamber was set to 60 s to mimic human-smoker lung “washout time” during active smoking (Invernizzi et al. 2007). E-cig-EGS was connected with real-time monitoring and time integrated aerosol sampling instrumentations for physico-chemical sampling and characterizations.
Figure 1.
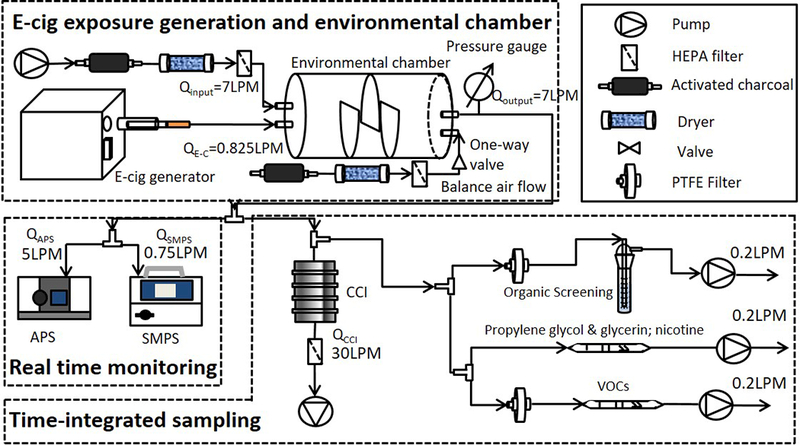
E-cig exposure generation system (E-cig-EGS, adapted from Zhao et al. (2016)) for sampling and characterization of e-cig emission physico-chemical properties
Aerosol characterization
The particulate matter (PM) number concentration and size distribution were monitored by real-time instruments. A scanning mobility particle sizer spectrometer (SMPS, Model 3910, TSI Inc., Shoreview, MN) was used to measure mobility diameter in the range of 10 to 420 nm. The measured mobility diameters were converted and reported as aerodynamic diameters (Figure 2A, 3A, 4A, 5A and 6A). An aerodynamic particle sizer (APS, Model 3321, TSI Inc., Shoreview, MN) was used to measure aerodynamic diameter in the range of 0.5 to 20 μm. For both SMPS and APS, single measurement length was set as 60 s, the same as the environmental chamber residence time.
Figure 2.
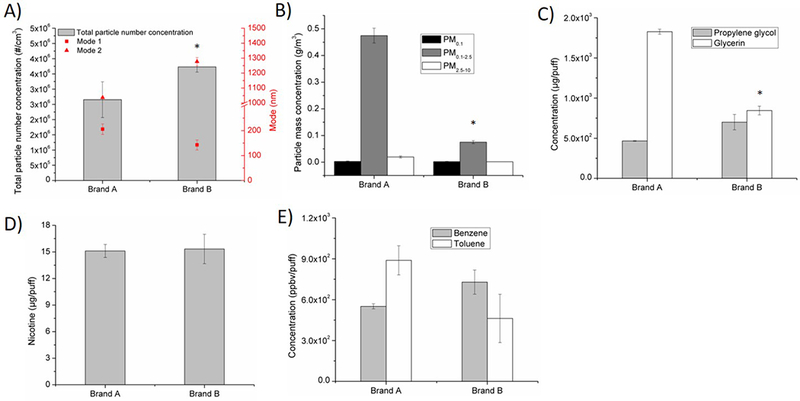
Effects of e-cig brand on A) particle number concentration and size distribution, B) particle mass concentration on three size-fractions: PM0.1, PM 0.1–2.5, PM2.5–10, C) humectants propylene glycol and glycerin, D) nicotine, E) VOCs (benzene, toluene). Baseline scenario parameters: Brand A, tobacco flavor, refillable tank, MPP, 3.7 V. In this investigation, Brand A was replaced by Brand B, while all other parameters were kept the same as in baseline scenario. *p < 0.05.
Figure 3.
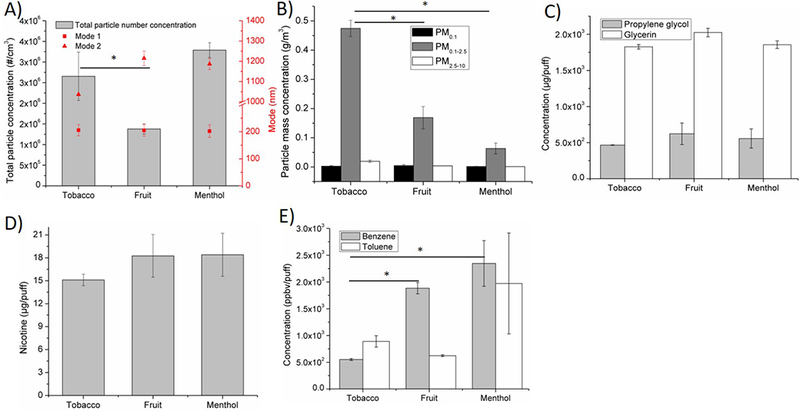
Effects of e-liquid flavor on A) particle number concentration and size distribution, B) particle mass concentration on three size- fractions: PM0.1, PM 0.1–2.5, PM2.5–10, C) humectants propylene glycol and glycerin, D) nicotine, E) VOCs (benzene, toluene). Baseline scenario parameters: Brand A, tobacco flavor, refillable tank, MPP, 3.7 V. In this investigation, tobacco flavor was replaced by menthol or fruit, while all other parameters were kept the same as in baseline scenario. *p < 0.05.
Figure 4.
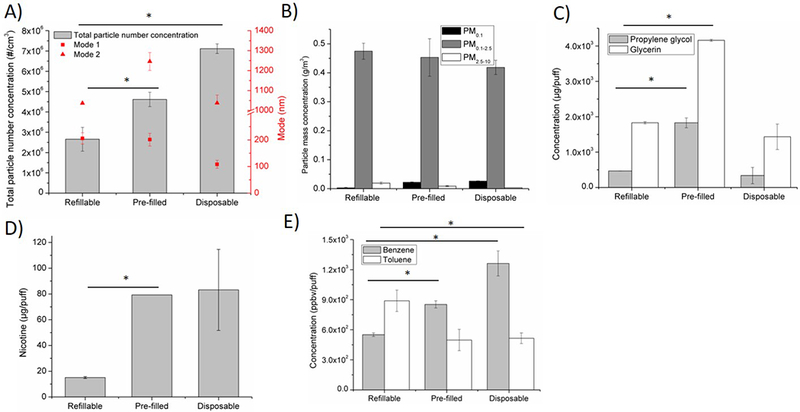
Effects of e-cig type on A) particle number concentration and size distribution, B) particle mass concentration on three size-fractions: PM0.1, PM 0.1–2.5, PM2.5–10, C) humectants propylene glycol and glycerin, D) nicotine, E) VOCs (benzene, toluene). Baseline scenario parameters: Brand A, tobacco flavor, refillable tank, MPP, 3.7 V. In this investigation, refillable tank was replaced by pre-filled tank or disposable, while all other parameters were kept the same as in baseline scenario. *p < 0.05.
Figure 5.

Effects of puffing protocol on A) particle number concentration and size distribution, B) particle mass concentration on three size- fractions: PM0.1, PM 0.1–2.5, PM2.5–10, C) humectants propylene glycol and glycerin, D) nicotine, E) VOCs (benzene, toluene). Baseline scenario parameters: Brand A, tobacco flavor, refillable tank, MPP, 3.7 V. In this investigation, MPP was replaced by FTC protocol, while all other parameters were kept the same as in baseline scenario. *p < 0.05.
Figure 6.
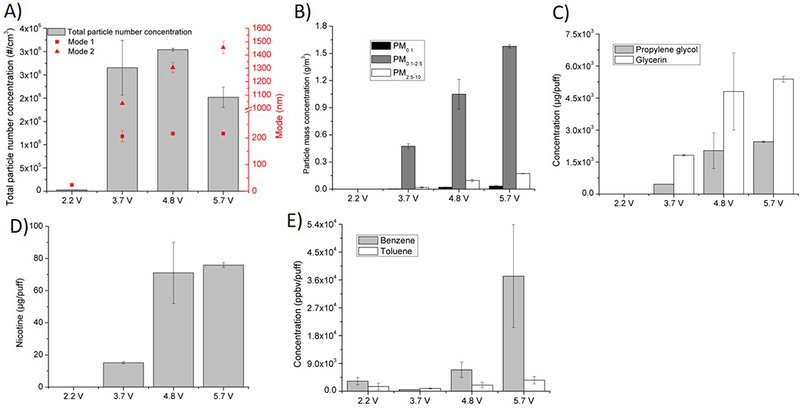
Effects of operational voltage on A) particle number concentration and size distribution, B) particle mass concentration on three size- fractions: PM0.1, PM 0.1–2.5, PM2.5–10, C) humectants propylene glycol and glycerin, D) nicotine, E) VOCs (benzene, toluene). Baseline scenario parameters: Brand A, tobacco flavor, refillable tank, MPP, 3.7 V. In this investigation, 3.7 V was replaced by 2.2 V, 4.8 V and 5.7 V, while all other parameters were kept the same as in baseline scenario.
PM mass was size-fractionated and sampled using the Harvard compact cascade impactor (CCI; Demokritou et al. 2004) and characterized for mass concentration by gravimetric analysis. Three substrates (Teflon filter for PM0.1 and polyurethane foams[PUF] with designated sizes for PM0.1–2.5 and PM2.5–10) were used to collect particles. Before pre- and post-sampling weighing, the PUFs and filters were conditioned in a controlled environment under constant 24 °C and 40% relative humidity for 48 h. They were then weighed using a Mettler Toledo XPE analytical microbalance (Mettler-Toledo LLC, Columbus, OH). The weight difference was calculated and normalized to determine the PM mass concentration.
Chemical characterization of e-cig emission
Nicotine
Nicotine vapors were collected on sorbent tubes (XAD®−4, Catalog number 226–93, SKC Inc. Eighty-Four, PA) at a flow rate of 0.2 l/min for five minutes. The XAD-4 traps were ultrasonically extracted with 1 ml of ethyl acetate (HPLC grade, Fisher Scientific) for 30 min. All samples were analyzed on a Shimadzu-17A gas chromatography system equipped with flame thermionic detector on a 5% diphenyl/95% dimethylpolysiloxane Rtx-5 capillary column (30 m x 0.32 mm i.d. x 0.50 μmthickness; Restek Corp. Bellefonte, PA). The temperature ramp was splitless injection at 200 °C, oven temperature at 60 °C, which was then increased to 290 °C at a rate of 20 °C/min.
Glycols
For propylene glycol and glycerin, sorbent tubes with glass fiber filter (XAD®−7 OVS, SKC Inc. Eighty-Four, PA) were used for five minutes of sampling at a flow rate of 0.2 l/min. Traps were ultrasonically extracted with 1 ml of methanol for 30 min. All samples were analyzed on a Shimadzu-17A gas chromatography system equipped with a flame ionization detector on a 35% diphenyl/65% dimethylpolysiloxane Rtx-35 capillary column (30 m x 0.53 mm i.d. x 1.00 μm thickness; Restek Corp. Bellefonte, PA). The temperature ramp was splitless injection at 250 °C, oven temperature at 40 °C, which was then increased to 290 °C at a rate of 80 °C /min, and FID temperature at 290 °C.
Benzene and toluene
Benzene and toluene were collected on coconut charcoal sorbent tubes (SKC Inc. Eighty-Four, PA) for 7.5 minutes at a flow rate of 0.2 l/min. The charcoal traps were eluted with 10 ml of CS2 (Fisher Scientific). An aliquot of the extract (1 μL) was analyzed on an Agilent Technologies 6850A gas chromatography system equipped with a flame ionization detector on a 100% dimethylpolysiloxane Agilent J&W HP-1 capillary column (30 m x 0.321 mm i.d. x 0.25 um thickness). The temperature was splitless injection at 250 °C, oven temperature at 70 °C, which was then increased to 300 °C at a rate of 60 °C/min, and FID temperature at 250 °C.
Materials and quality assurance
Pure solvents (HPLC grade) and standard compounds (Fisher Scientific) were used for extraction and identification of compounds. Glassware used in extraction and analysis was pre-cleaned at 450 °C for 12 h. Filter blanks were analyzed using the same method as the samples. The identification and quantification of nicotine, propylene glycol, glycerin, benzene, and toluene were performed using external reference standards (Fisher Scientific). Five-point external calibrations were run prior to analysis and one calibration check was run at the end of the analysis. If the response of individual compound was more than 10% off, the system was recalibrated.
Trace metals
E-cig emissions were sampled using CCI with PUFs and PTFE filters (see above for details). The sampling flow rate was 30 l/min for 10 minutes. Since the majority of PM mass was collected on PM0.1–2.5 and PM0.1 size-fractions, only these two samples were analyzed. Field blanks were collected with the same set-up without e-cig emissions. The filter and PUF-collected PM was solubilized and determined by high-resolution magnetic-sector inductively-coupled plasma mass spectrometry (SF-ICPMS, Thermo-Finnigan Element 2). Quantitative recovery was verified through inclusion of several Certified Reference Materials in the analytical batch. Details of these methods were published (Hu et al. 2009; Okuda et al. 2014; Shafer et al. 2012).
Parametric investigation of the effects of e-cig brand, e-liquid flavor, e-cig type, puffing protocol and operational voltage on emission physico-chemical properties
Various generation scenarios were used to investigate the effects of e-cig brand, e-liquid flavor, e-cig type, puffing protocol, and operational voltage on emission physico-chemical properties.
Baseline scenario: Tobacco flavor (10 mg/ml nicotine) e-liquid from a popular e-cig Brand A was used to fill the refillable tank and operated at 3.7 V. The modified puffing protocol (MPP; puff volume: 55 ml, puff duration: 4 s and puff interval: 30 s) was applied (Farsalinos et al. 2013; Lee et al. 2015).
In order to investigate the effects of the generation parameters on the PCM properties of e-cig emissions, one out of five parameters, namely e-cig brand, e-liquid flavor, e-cig type, puffing protocol and operational voltage, was modified at one at a time. The physico-chemical properties of the generated e-cig emission were characterized in the same manner as in baseline scenario (as described above) and compared. For the effect of the brand, another popular e-cig brand B was used. For the effect of flavor, menthol and fruit flavors (10 mg/ml nicotine) were used. For the effect of e-cig type, disposable and pre-filled tank types were used. For the effect of puffing pattern, another one protocol, namely the Federal Trade Commission protocol (FTC; puff volume: 35 ml; puff duration: 2 s; puff interval: 60 s), which was the standard puffing protocol for conventional cigarettes, was used (Standardization 2012). For the effect of voltage, 2.2, 4.8, and 5.7 V were used.
RESULTS
Baseline scenario: physico-chemical characterization of e-cig emission
A total of 2.7 × 106 particles/cm3 with bi-modal diameters at 205.7 nm and 1.04 μm was observed (Figure 2A). The total particle mass concentration was 0.50 g/m3 (Figure 2B). Propylene glycol and glycerin in both particle and gas phases were found to be 466 and 1827 μg/puff, respectively (Figure 2C). Total nicotine from both particle and gas phases was found at 15.11 μg/puff (Figure 2D). Benzene and toluene in the gas phase were detected as 551 and 889 ppbv/puff, respectively (Figure 2F).
Effects of generation parameters on physico-chemical properties of e-cig emissions
Effects of e-cig brand
Figure 2 shows e-cig emission profile changes between brands A and B used in the study. Total particle number concentration was higher by approximately 40% for brand B. Additionally, a change in modal diameters was also observed (Figure 2A). Total PM mass concentration was lower for brand B by 84% (Figure 2B). Propylene glycol was higher by 50% for brand B while glycerin was lower by 54% (Figure 2C). Nicotine concentration did not change significantly with brand (Figure 2D). For VOCs, benzene concentration was higher by 32% while toluene concentration decreased 48% compared to brand A (Figure 2E).
Effects of e-liquid flavor
Figure 3 shows the effects of e-liquid flavors after switching from tobacco flavor (baseline scenario) to menthol and fruit flavors. Among the three flavors, the menthol flavor generated the highest particle number concentration (3.3 million particles/cm3) and the fruit flavor generated the lowest (1.4 million particles/cm3;Figure 3A). Tobacco flavor had the highest PM mass concentration (0.50 g/m3) followed by fruit flavor (0.18 g/cm3) and menthol flavor (0.07 g/m3; Figure 3B). No significant changes in propylene glycol, glycerin, and nicotine concentrations were observed among the three flavors (Figure 3C, D). Compared to tobacco flavor, menthol flavor generated 330% more benzene and 120% more toluene; fruit flavor generated 240% more benzene and 30% less toluene (Figure 3E).
Effects of e-cig type
Figure 4 shows the effects of refillable, disposable, and pre-filled tank types. Among the three types, disposable e-cigs generated the highest particle number concentration (7.1 million particles/cm3), followed by pre-filled types (4.6 million particles/cm3) and refillable types (2.7 million particles/cm3; Figure 4A). However, the modal diameters of the particle size distribution generated from disposable e-cig were the smallest (108 nm and 1.04 μm) compared to pre-filled e-cigs (201 nm and 1.25 μm) and refillable e-cigs (206 nm and 1.04 μm; Figure 4A). No significant differences in PM mass concentration were observed among the types (Figure 4B). Pre-filled e-cigs generated 290% more propylene glycol and 130% more glycerin than refillable and disposable e-cigs, which had similar levels (Figure 4C). Both disposable and pre-filled e-cigs generated more than 400% nicotine compared to refillable e-cigs (Figure 4D). Both disposable and pre-filled e-cigs generated more benzene compared to refillable e-cig, 130% and 50% respectively (Figure 4E). However, they both generated 40% less toluene compared to refillable e-cigs (Figure 4E).
Effects of puffing protocol
Figure 5 shows that the FTC protocol, with shorter and less frequent puffs, generated fewer particles and gases compared to the MPP protocol, except for toluene (similar amount was detected for both protocols). For the FTC protocol, the total particle number concentration decreased by 84% compared to the MPP protocol (Figure 5A). One modal diameter decreased from 206 nm to 121 nm, while the other modal diameter was unchanged at 1.04 μm (Figure 5A). Total PM mass concentration also decreased 98% (Figure 5B) compared to the MPP protocol. The concentration of propylene glycol, glycerin, nicotine, and benzene for the case of the FTC protocol was lower by 95%, 81%, 99%, and 87%, respectively compared to the MPP protocol (Figure 5C-E).
Effects of operational voltage
Figure 6 shows the effects of operational voltage on the e-cig emission profile. With the increase from 2.2 V to 4.8 V, the total particle number concentration increased from 0.03 to 3 million particles/cm3. It is worth noting that for the 5.7 V case, the particle number concentration decreased to 2 million particles/cm3 (Figure 6A). The modal diameters kept increasing as the operational voltage increased: 23 nm and 0.54 μm for 2.2 V, 206 nm and 1.04 μm for 3.7 V, 216 nm and 1.31 μm for 4.8 V and 216 nm and 1.46 μm for 5.7 V (Figure 6A). Total PM mass concentration also increased with the increase in voltage from 0.001 to 1.79 g/m3 (Figure 6B). Similarly, the concentrations of propylene glycol, glycerin, and nicotine increased with the increase in voltage from 2.2 V (1.18, 1.99, and 0.03 μg/puff) to 5.7 V (2456.51, 5387.60, and 75.91 μg/puff) by 2090, 2710, and 2370 times, respectively (Figures 6C, D). Among the four voltages, 3.7 V generated the least amount of benzene and toluene (551 and 889 ppbv/puff respectively), while the 5.7 V generated the most (37239 and 3573 ppbv/puff, respectively; Figure 6E).
Figure 7 shows the trace metal concentrations in both the e-liquid and e-cig emissions generated for the case of the tobacco flavor from Brand A and the refillable tank operated at 5.7 V under the MPP protocol. E-liquid contained a total of 51100 ng trace metal/ml, in which K contributed approximately 70% (Figure 7A). For the PM0.1–2.5 size fraction, a total of 1.43 ng trace metal/mg of collected PM mass was observed, in which boron contributed more than 90% (Figure 7B). For the PM0.1 size fraction, a total of 109.8 ng trace metals/mg of collected PM mass was observed, in which Na, Al, Cu, and Zn contributed more than 50% (Figure 7C).
Figure 7.
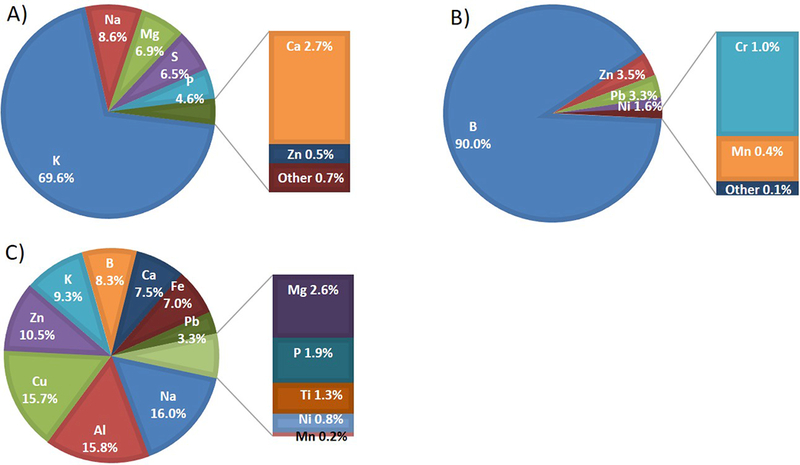
Trace metal concentration in e-liquid and e-cig emission generated at 5.7 V. A) E-liquid contained a total of 51100 ng trace metals/ml, in which K contributed to approximately 70%, B) In the stage of PM0.1–2.5, a total of 1.43 ng trace metals/mg of collected PM mass was observed, in which B contributed to more than 90%, C) In the stage of PM0.1, a total of 109.8 ng trace metals/mg of collected PM mass was observed, in which Na, Al, Cu, and Zn contributed to more than 50%.
DISCUSSION
E-cigs expose users to a large number of particles and a variety of chemicals
It is worth noting that, as described in detail in our published study on the development of the e-cig generation platform (Zhao et al. 2016), the particle concentration depended on both the type of the e-cig, and the operational conditions (e.g voltage and the topography of the user [puffing pattern]). In this study, both the user topography (breathing patterns) as well as other operational conditions, which were listed in the methods section, represented a variety of real-world scenarios related to actual human vaping. More research is needed to define the human vaping topography in more detail, and investigate its effects on emission characteristics and toxicological properties. Regardless of “generation” parameters, a large number of particles were generated, with total particle number and mass concentrations in the range of 0.03 – 7.1 × 106 particles/cm3 and 0.001 – 1.79 g/m3 respectively. Furthermore, a bi-modal size distribution was observed (modal diameters at 23 – 216 nm and 0.54 – 1.46 μm), with the majority (69% - 96%) of particles in the PM0.1–2.5 size-fraction. Organic species that were major ingredients of the e-liquid were also found in e-cig emissions at elevated concentration levels (propylene glycol:1.18 – 2456.51 μg/puff, glycerin: 1.99 – 5387.60 μg/puff and nicotine: 0.03 – 83.16 μg/puff). Combustion-related organic byproducts such as benzene and toluene were also detected in the e-cig emissions, in the range of 71.10 – 37238.63 ppbv/puff and 462.15 – 3572.78 ppbv/puff, respectively. The level of xylenes was below the detection limit and no ethylbenzene was detected. Our results were in agreement with previous exposure assessment studies (Cobb et al. 2010; Goniewicz et al. 2014; Mikheev et al. 2016; Zhao et al. 2016). It is worth noting that the systematically investigated e-cig “generation” parameters affected the physico-chemical composition of the emissions drastically. This has important implications in the toxicological characterization of e-cig exposures and makes the case that a number of “generation” scenarios need to be investigated and reported in such studies.
Effects of e-cig brand, type, e-liquid flavor, user puffing patterns, and operational voltage on PCM properties of emissions
E-cig emissions generated by Brands A and B contained different amounts of particles, humectants (propylene glycol and glycerin), and VOCs. This was likely due to the differences in manufacturer recipes. Limited data was available on the brand effect on emission profiles. Two studies analyzing the chemical composition of pure e-liquids from different manufacturers showed differences in liquid composition (Bahl et al. 2012; Behar et al. 2014). They also both observed differences in cytotoxicity after cellular exposure to the e-liquids, not the emissions, and attributed them to the differences in e-liquid ingredients.
Furthermore, the tobacco flavor e-liquid generated more than seven times more particle mass than the menthol flavor. Interestingly, the menthol flavor e-liquid generated four times more benzene and two times more toluene than the tobacco flavor. The effects of flavors on e-cig emissions were likely due to the different flavoring additives, such as aldehydes, ketones, esters. These flavoring chemicals can be vaporized into emissions and form particles. They can also go through chemical reactions under high temperature/pyrolysis to form VOCs and other free radicals and molecules. Toxicological studies also found that flavoring additives in e-liquids can play a role in cytotoxicity. Bahl et al. (2012) tested the cytotoxicity of pure e-liquids and found toxicity correlated with the number and concentration of flavoring chemicals. Behar et al. (2014) identified the toxicants in a cinnamon-flavored e-liquid and found the cinnamon flavorings linked to cytotoxicity. Putzhammer et al. (2016) also tested popular flavored e-liquids and found herbal flavor seemed to be the most toxic compared to other flavors. Our study is one of the few studies to make the link between additives/flavors and the physico-chemical properties of the emissions pointing to differential effects on their toxicological profiles. Similar toxicological findings driven by additives/flavors were reported by Cervellati et al. (2014).
In addition, our findings clearly demonstrate that e-cig type also affects the e-cig emission profile. Disposable e-cigs generated larger number of particles with smaller sizes, compared to the pre-filled and refillable tank types. More importantly, the highest amount of benzene generation was observed for the disposable e-cig type. Such type-dependent effects may be attributed to the differences in e-cig manufacturing, including heating wire material, conductivity, and capacity, which can affect the heating/vaporization and pyrolysis process. It is worth noting that the voltage for the three types used here were the same 3.7 V. However, the influence of e-cig type in exposure and toxicological screening of e-cig emissions was often neglected in previous studies, and e-cig type was often not reported. For example, McAuley et al. (2012) used only disposable e-cigs while Schripp et al. (2013) used only refillable tanks. Therefore, the difference in particle number concentration and size distribution observed in those studies may be attributed to the different e-cig types used. Only one study was found to have used all three e-cigs types and reported type-dependent cytotoxicity in vitro (Putzhammer et al. 2016).
Furthermore, longer and more frequent puffing generated more particles and higher concentrations of organic species. Since a battery was activated when puffing was initiated, longer puffing duration led to higher heating coil temperature and more electric energy being transformed to heat, which was then used to vaporize and generate e-cig emissions. This was demonstrated in our previous study by measuring heating material temperatures under MPP (195 °C) and FTC (148 °C) protocols (Zhao et al. 2016). More vaporization led to more releases of chemicals (propylene glycol, glycerin and nicotine) from the e-liquid. Higher temperatures also caused more combustion-related product generation (e.g. benzene) as we observed. It was worth noting that puffing protocols used in exposure and toxicological screening studies were not standardized and a variety of protocols were used in literature, with puffing duration ranging from 1.8 – 8 seconds (Allen et al. 2016; Goniewicz et al. 2014; McAuley et al. 2012). Our findings reinforce the notion that standardization in puffing protocols is needed for e-cig studies because the puffing protocol was a modifier of physico-chemical and toxicological properties of emissions.
Finally, we demonstrated that increasing operational voltage led to more emission generation. As the voltage increased from 2.2 to 5.7 V, 1500 times more particle mass, 2000 times more propylene glycol, 2700 times more glycerin, 2300 times more nicotine, 11 times more benzene, and 2 times more toluene were generated. As the voltage increased from 2.2 to 5.7 V, the operating temperature increased from 106.8 to 265.8 °C, which may explain the variations in properties of emissions (Rowell and Tarran 2015; Zhao et al. 2016). Another observation was that larger particles tended to be generated at higher voltages (Figure 6A). This phenomenon may be attributed to the rapid temperature change upon contact with the cold e-cig mouthpiece. Including the effects of operational voltage in toxicological screening of e-cig emissions is imperative because the majority of e-cig users are currently using advanced e-cigs that allow users to adjust voltage (Dawkins et al. 2013; Giovenco et al. 2014; Yingst et al. 2015). Moreover, the World Health Organization warned that voltage variation can result in considerable variability one-cig emission profile, including nicotine and other chemical concentration, and may contribute to toxicant formation (World Health Organization 2014). Our findings were in agreement with the limited number of publications on this matter. Zhao et al. (2016) showed higher CO2 and VOC concentrations in e-cig emissions at higher voltage. Higher carbonyl generation associated with the increase in voltage (or expressed as heating power) was also reported in three other studies (Farsalinos et al. 2015; Jensen et al. 2015; Kosmider et al. 2014). It is also worth noting that higher levels of reactive oxygen species were also found in the emissions under higher voltage (Zhao et al, 2018). This profound influence of operational voltage on e-cig emission profiles demonstrated here indicates the need to investigate voltage influence on e-cig exposure toxicological outcomes.
The majority of the e-cig emission profiles from all scenarios were organic in nature. Chemical analyses demonstrated high concentrations of propylene glycol, glycerin, and nicotine in e-cig emission, which matched the e-liquid ingredient. These nearly identical chemical fingerprints provide evidence that e-liquid ingredients dominate the chemical composition of e-cig emissions. Another important finding was trace metals, such as B, Cu and Al, were not present in e-liquids, but were detected in e-cig emissions. These elements matched the heating wire metal material, confirming that heating elements under high temperatures can release ions into the emission. This is in agreement with previous research (Williams et al. 2013). Detected total metal concentrations from e-liquid 51100 ng/ml cannot be directly compared with the metal concentrations from particles from the PM0.1 and PM0.1–2.5 stages, because e-liquids may not be the main source of metals in e-cig emissions (Figure 7). We also recommend that further metal characterization on larger particles (PM2.5–10) stage should be conducted in future study. VOCs that were not part of e-liquid were also detected. Their presence was attributed to the pyrolysis effects. Our parametric analysis showed e-cig type, puffing protocol and voltage greatly influenced such potential pyrolytic reactions.
Potential health implications from e-cig exposures
The detailed physico-chemical characterization data of e-cig exposures under the various generation scenarios raised a number of concerns in terms of potential health implications. E-cig usage resulted in high-level particle and gas exposures with complex organic species and trace metals, which potentially posed toxicological threats. Preliminary in vitro and in vivo studies observed such toxicity effects from both pure e-liquid and e-cig emissions (Bahl et al. 2012; Scheffler et al. 2015). Moreover, previous studies documented adverse pulmonary, cardiovascular, nervous, and gastrointestinal effects from such e-cig exposures (Chen 2013; Czogala et al. 2014; Meo and Asiri 2014; Monroy et al. 2012). However, large discrepancies exist in toxicological studies largely due to the variation in un-standardized e-cig emission generation and characterization. Despite the rising evidence on potential toxicological implications, mechanistic pathways still remain unknown and more research is needed.
More specifically, the total particle concentration in e-cig emissions observed in this study was in the range of millions particles/cm3 with the peak at approximately 200 nm. This concentration was close to what was observed in tobacco cigarettes, depending on the generation parameters and dilution factors (McAuley et al. 2012), and is far higher compared to other occupational engineered nanomaterial exposure scenarios, including nano-enabled printers (Pirela et al. 2016, 2014, 2015), heavily polluted highways (Padró-martínez et al. 2012), and even welding fumes (Chang et al. 2013; Graczyk et al. 2015). Given the abundant evidence of particle deposition in the respiratory tract causing biological effects (Bakand et al. 2012; Strak et al. 2012), the high concentrations and small sizes of the particles observed in e-cig emissions raise concerns.
Furthermore, propylene glycol and glycerin are the major ingredients in e-liquid. As shown in this study, high amounts of propylene glycol and glycerin were vaporized and released into e-cig emissions with the highest observed concentration up to 5000 μg/puff. It was worth noting that these two species were not found in conventional tobacco smoke and their toxicological relevance needed to be assessed in the light of other synergistic co-pollutants present in e-cig emissions.
In addition, as expected, up to 100 μg/puff nicotine in e-cig emissions was detected. There is an ongoing debate on the long-term toxicological implications of inhaled nicotine, which is known to be highly addictive (Picciotto and Corrigall 2002; Condorelli et al. 2013), promote cancer cell growth (Catassi et al. 2008), and reduce cancer response to chemotherapy (Banerjee et al. 2013; Dinicola et al. 2013).
Moreover, the presence of VOCs found in this study raised concerns. In previous studies, VOC exposure caused adverse biological effects on the pulmonary and cardiovascular systems, including respiratory inflammation and heart rate variability (Wang et al. 2013; Weichenthal et al. 2012). We are currently conducting analysis on other VOCs, namely carbonyls, and the results will be reported in future manuscripts.
Finally, the metal presence in e-cig emissions observed in this study, which was likely attributed to the heating elements, was also concerning because metal exposure can induce oxidative stress, initiate/increase lung inflammation, decrease cell viability, and have other adverse effects (Kumar and Nagesha 2013; Moschini et al. 2013; Shrivastava et al. 2014). Metal oxide can lead to cellular DNA damage at low doses (5 μg/ml;Watson et al. 2014).
CONCLUSIONS
In summary, the comprehensive physico-chemical characterization of e-cig emissions provides abundant evidence that e-cig emissions contain large numbers of particles (millions/cm3) and gases with complex organic and inorganic compositions. For the first time, a systematic investigation demonstrated the profound influence of e-cig brand and type, e-liquid flavoring additives, operational voltage, and user puffing patterns on e-cig exposure profiles is reported. It has become apparent that these aforementioned e-cig generation parameters largely define the physico-chemical and toxicological properties of e-cig emissions and thus should be taken into consideration by risk assessors. This proposed integrated methodology can be used in developing standardized methodologies linking exposures to toxicology.
ACKNOWLEDGEMENTS
This study was funded by NIEHS Grant (ES-000002). Jiayuan Zhao would like to thank the Swiss National Science Foundation for the Early Postdoc Mobility Fellowship (P2LAP3_161808).
REFERENCES
- Allen JG, Flanigan SS, LeBlanc M, Vallarino J, MacNaughton P, Stewart JH, et al. 2016. Flavoring chemicals in e-cigarettes: Diacetyl, 2,3-pentanedione, and acetoin in a sample of 51 products, including fruit-, candy-, and cocktail-flavored e-cigarettes. Environ. Health Perspect 124:733–739; doi: 10.1289/ehp.1510185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahl V, Lin S, Xu N, Davis B, Wang YH, Talbot P. 2012. Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reprod. Toxicol 34:529–537; doi: 10.1016/j.reprotox.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Bakand S, Hayes A, Dechsakulthorn F. 2012. Nanoparticles: a review of particle toxicology following inhalation exposure. Inhal. Toxicol 24:125–135; doi: 10.3109/08958378.2010.642021. [DOI] [PubMed] [Google Scholar]
- Banerjee J, Al-Wadei HAN, Schuller HM. 2013. Chronic nicotine inhibits the therapeutic effects of gemcitabine on pancreatic cancer in vitro and in mouse xenografts. Eur. J. Cancer 49:1152–1158; doi: 10.1016/j.ejca.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar RZ, Davis B, Wang Y, Bahl V, Lin S, Talbot P. 2014. Identification of toxicants in cinnamon-flavored electronic cigarette refill fluids. Toxicol. Vitr 28:198–208; doi: 10.1016/j.tiv.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Beige BMI. 2016. Global Vape Market (e-Cigarette and Vaporizer) – Strategic Assessment and Forecast – Till 2021.
- Catassi A, Servent D, Paleari L, Cesario A, Russo P. 2008. Multiple roles of nicotine on cell proliferation and inhibition of apoptosis: Implications on lung carcinogenesis. Mutat. Res. - Rev. Mutat. Res 659:221–231; doi: 10.1016/j.mrrev.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Chang C, Demokritou P, Shafer M, Christiani D. 2013. Physicochemical and toxicological characteristics of welding fume derived particles generated from real time welding processes. Environ. Sci. Process. Impacts 15:214–24; doi: 10.1039/C2EM30505D. [DOI] [PubMed] [Google Scholar]
- Chen IL. 2013. FDA summary of adverse events on electronic cigarettes. Nicotine Tob. Res 15:615–616; doi: 10.1093/ntr/nts145. [DOI] [PubMed] [Google Scholar]
- Cobb NK, Byron MJ, Abrams DB, Shields PG. 2010. Novel Nicotine Delivery Systems and Public Health: The Rise of the E-Cigarette”. Am. J. Public Heal 100:2340–2342; doi: 10.2105/AJPH.2010.199281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman BN, Johnson SE, Tessman GK, Tworek C, Alexander J, Dickinson DM, et al. 2016. “It’s not smoke. It’s not tar. It’s not 4000 chemicals. Case closed”: Exploring attitudes, beliefs, and perceived social norms of e-cigarette use among adult users. Drug Alcohol Depend. 159:80–85; doi: 10.1016/j.drugalcdep.2015.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condorelli RA, La Vignera S, Giacone F, Iacoviello L, Vicari E, Mongioi L, et al. 2013. In vitro effects of nicotine on sperm motility and bio-functional flow cytometry sperm parameters. Int. J. Immunopathol. Pharmacol 26: 739–746. [DOI] [PubMed] [Google Scholar]
- Czogala J, Goniewicz ML, Fidelus B, Zielinska-Danch W, Travers MJ, Sobczak A. 2014. Secondhand exposure to vapors from electronic cigarettes. Nicotine Tob. Res 16:655–662; doi: 10.1093/ntr/ntt203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawkins L, Turner J, Roberts A, Soar K. 2013. “Vaping” profiles and preferences: An online survey of electronic cigarette users. Addiction 108:1115–1125; doi: 10.1111/add.12150. [DOI] [PubMed] [Google Scholar]
- Demokritou P, Lee SJ, Ferguson ST, Koutrakis P. 2004. A compact multistage (cascade) impactor for the characterization of atmospheric aerosols. J. Aerosol Sci 35: 281–299. [Google Scholar]
- Dinicola S, Morini V, Coluccia P, Proietti S, D’Anselmi F, Pasqualato A, et al. 2013. Nicotine increases survival in human colon cancer cells treated with chemotherapeutic drugs. Toxicol Vitr. 27:2256–2263; doi: 10.1016/j.tiv.2013.09.020. [DOI] [PubMed] [Google Scholar]
- EUROMONITOR RESEARCH. 2015. Vapour Devices and e-Cigarettes in the Global Tobacco Market.
- EL-Hellani A, Salman R, El-Hage R, Talih S, Malek N, Baalbaki R, Karaoghlanian N, Nakkash R, Shihadeh A, Saliba NA. 2018. Nicotine and Carbonyl Emissions From Popular Electronic Cigarette Products: Correlation to Liquid Composition and Design Characteristics. NICTOB. 20:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos K, Voudris V, Poulas K. 2015. Response to Shihadeh et al. (2015): E-cigarettes generate high levels of aldehydes only in “dry puff” conditions. Addiction 110:1862–1864; doi: 10.1111/add.13078. [DOI] [PubMed] [Google Scholar]
- Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Voudris V. 2013. Evaluation of electronic cigarette use (Vaping) topography and estimation of liquid consumption: Implications for research protocol standards definition and for public health authorities’ regulation. Int. J. Environ. Res. Public Health 10:2500–2514; doi: 10.3390/ijerph10062500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuoco FC, Buonanno G, Stabile L, Vigo P. 2014. Influential parameters on particle concentration and size distribution in the mainstream of e-cigarettes. Environ. Pollut 184:523–529; doi: 10.1016/j.envpol.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Giovenco DP, Lewis MJ, Delnevo CD. 2014. Factors associated with e-cigarette use: A national population survey of current and former smokers. Am. J. Prev. Med 47:476–480; doi: 10.1016/j.amepre.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, et al. 2014. Levels of selected carcinogens and toxicants in vapor from electronic cigarettes. Tob Control 23:133–139; doi: 10.1136/tobaccocontrol-2012-050859.Levels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graczyk H, Lewinski N, Zhao J, Concha-Lozano N, Riediker M. 2015. Characterization of Tungsten Inert Gas (TIG) Welding Fume Generated by Apprentice Welders. Ann. Occup. Hyg 60:205–219; doi: 10.1093/annhyg/mev074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Herner JD, Shafer M, Robertson W, Schauer JJ, Dwyer H, et al. 2009. Metals emitted from heavy-duty diesel vehicles equipped with advanced PM and NOX emission controls. Atmos. Environ 43:2950–2959; doi: 10.1016/j.atmosenv.2009.02.052. [DOI] [Google Scholar]
- Ingebrethsen BJ, Cole SK, Alderman SL. 2012. Electronic cigarette aerosol particle size distribution measurements. Inhal. Toxicol 24:976–984; doi: 10.3109/08958378.2012.744781. [DOI] [PubMed] [Google Scholar]
- Invernizzi G, Ruprecht A, De Marco C, Paredi P, Boffi R. 2007. Residual tobacco smoke: measurement of its washout time in the lung and of its contribution to environmental tobacco smoke. Tob. Control 16:29–33; doi: 10.1136/tc.2006.017020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P, Luo W, Pankow JF, Strongin RM, Peyton DH. 2015. Hidden formaldehyde in E-Cigarette Aerosols. N. Engl. J. Med 372:392–394; doi: 10.1056/NEJMc1414731. [DOI] [PubMed] [Google Scholar]
- Kim AE, Arnold KY, Makarenko O. 2014. E-cigarette advertising expenditures in the U.S., 2011–2012. Am. J. Prev. Med 46:409–412; doi: 10.1016/j.amepre.2013.11.003. [DOI] [PubMed] [Google Scholar]
- King BA, Patel R, Nguyen KH, Dube SR. 2015. Trends in awareness and use of electronic cigarettes among US adults, 2010–2013. Nicotine Tob. Res 17:219–227; doi: 10.1093/ntr/ntu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosmider L, Sobczak A, Fik M, Knysak J, Zaciera M, Kurek J, et al. 2014. Carbonyl compounds in electronic cigarette vapors: Effects of nicotine solvent and battery output voltage. Nicotine Tob. Res 16:1319–1326; doi: 10.1093/ntr/ntu078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Nagesha DK. 2013. Size-Dependent Study of Pulmonary Responses to Nano- sized Iron and Copper Oxide Nanoparticles. In Oxidative Stress and Nanotechnology, Vol. 1028 of, pp. 247–264. [DOI] [PubMed] [Google Scholar]
- Lee YH, Gawron M, Goniewicz ML. 2015. Changes in puffing behavior among smokers who switched from tobacco to electronic cigarettes. Addict. Behav 48:1–4; doi: 10.1016/j.addbeh.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini S, Buonanno G, Stabile L, Ficco G. 2014. Short-term effects of electronic and tobacco cigarettes on exhaled nitric oxide. Toxicol. Appl. Pharmacol 278:9–15; doi: 10.1016/j.taap.2014.04.004. [DOI] [PubMed] [Google Scholar]
- McAuley TR, Hopke PK, Zhao J, Babaian S. 2012. Comparison of the effects of e-cigarette vapor and cigarette smoke on indoor air quality. Inhal. Toxicol 24:850–7; doi: 10.3109/08958378.2012.724728. [DOI] [PubMed] [Google Scholar]
- Meo S, Asiri SAA. 2014. Effects of electronic cigarette smoking on human health. Eur. Rev. Med. Pharmacol. Sci 18:3315–3319; doi:8033 [pii]. [PubMed] [Google Scholar]
- Mikheev VB, Brinkman MC, Granville CA, Gordon SM, Clark PI. 2016. Real-Time Measurement of Electronic Cigarette Aerosol Size Distribution and Metals Content Analysis. Nicotine Tob. Res 1895–1902; doi: 10.1093/ntr/ntw128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroy AE, Hommel E, Smith ST, Raji M. 2012. Paroxysmal atrial fibrillation following electronic cigarette use in an elderly woman. Clin. Geriatr 20: 28–32. [Google Scholar]
- Moschini E, Gualtieri M, Colombo M, Fascio U, Camatini M, Mantecca P. 2013. The modality of cell-particle interactions drives the toxicity of nanosized CuO and TiO2 in human alveolar epithelial cells. Toxicol. Lett 222:102–116; doi: 10.1016/j.toxlet.2013.07.019. [DOI] [PubMed] [Google Scholar]
- Okuda T, Schauer JJ, Shafer MM. 2014. Improved methods for elemental analysis of atmospheric aerosols for evaluating human health impacts of aerosols in East Asia. Atmos. Environ 97:552–555; doi: 10.1016/j.atmosenv.2014.01.043. [DOI] [Google Scholar]
- Padró-martínez LT, Patton AP, Trull JB, Zamore W, Brugge D, Durant JL. 2012. Mobile monitoring of particle number concentration and other traffic-related air pollutants in a near-highway neighborhood over the course of a year. Atmos. Environ 61:253–264; doi: 10.1016/j.atmosenv.2012.06.088.Mobile. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson JL, Richardson A, Niaura RS, Vallone DM, Abrams DB. 2012. E-cigarette awareness, use, and harm perceptions in US adults. Am. J. Public Health 102:1758–1766; doi: 10.2105/AJPH.2011.300526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino RM, Tinghino B, Mangiaracina G, Marani a, Vitali M, Protano C, et al. 2012. Electronic cigarettes: an evaluation of exposure to chemicals and fine particulate matter (PM). Ann. Ig 24: 279–288. [PubMed] [Google Scholar]
- Picciotto MR, Corrigall WA. 2002. Neuronal systems underlying behaviors related to nicotine addiction: neural circuits and molecular genetics. J. Neurosci 22:3338–3341; doi:20026360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirela SV, Lu X, Miousse I, Sisler JD, Qian Y, Guo N, et al. 2016. Effects of intratracheally instilled laser printer-emitted engineered nanoparticles in a mouse model: A case study of toxicological implications from nanomaterials released during consumer use. NanoImpact 1:1–8; doi: 10.1016/j.impact.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirela SV, Pyrgiotakis G, Bello D, Thomas T, Castranova V, Demokritou P. 2014. Development and characterization of an exposure platform suitable for physico-chemical, morphological and toxicological characterization of printer-emitted particles (PEPs). Inhal. Toxicol 26:400–8; doi: 10.3109/08958378.2014.908987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirela SV, Sotiriou GA, Bello D, Shafer M, Bunker KL, Castranova V, et al. 2015. Consumer exposures to laser printer-emitted engineered nanoparticles: A case study of life-cycle implications from nano-enabled products. Nanotoxicology 9:760–8; doi: 10.3109/17435390.2014.976602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putzhammer R, Doppler C, Jakschitz T, Heinz K, F??rste J, Danzl K, et al. 2016. Vapours of US and EU market leader electronic cigarette brands and liquids are cytotoxic for human vascular endothelial cells. PLoS One 11:1–15; doi: 10.1371/journal.pone.0157337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell TR, Tarran R. 2015. Will Chronic E-Cigarette Use Cause Lung Disease? Am. J. Physiol. Lung Cell. Mol. Physiol ajplung.00272.2015; doi: 10.1152/ajplung.00272.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffler S, Dieken H, Krischenowski O, Förster C, Branscheid D, Aufderheide M. 2015. Evaluation of e-cigarette liquid vapor and mainstream cigarette smoke after direct exposure of primary human bronchial epithelial cells. Int. J. Environ. Res. Public Health 12:3915–3925; doi: 10.3390/ijerph120403915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober W, Szendrei K, Matzen W, Osiander-Fuchs H, Heitmann D, Schettgen T, et al. 2014. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int. J. Hyg. Environ. Health 217:628–637; doi: 10.1016/j.ijheh.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Schripp T, Markewitz D, Uhde E, Salthammer T. 2013. Does e-cigarette consumption cause passive vaping? Indoor Air 23:25–31; doi: 10.1111/j.1600-0668.2012.00792.x. [DOI] [PubMed] [Google Scholar]
- Shafer MM, Toner BM, Overdier JT, Schauer JJ, Fakra SC, Hu S, et al. 2012. Chemical speciation of vanadium in particulate matter emitted from diesel vehicles and urban atmospheric aerosols. Environ. Sci. Technol 46:189–195; doi: 10.1021/es200463c. [DOI] [PubMed] [Google Scholar]
- Shrivastava R, Raza S, Yadav A, Kushwaha P, Flora SJS. 2014. Effects of sub acute exposure to TiO2 ZnO and Al2O3 nanoparticles on oxidative stress and histological changes in mouse liver and brain. Drug Chem. Toxicol 37:336–347; doi: 10.3109/01480545.2013.866134. [DOI] [PubMed] [Google Scholar]
- Standardization IOF. 2012. ISO 3308: routine analytical cigarette-smoking machine - definitions and standard conditions.
- Strak M, Janssen NAH, Godri KJ, Gosens I, Mudway IS, Cassee FR, et al. 2012. Respiratory health effects of airborne particulate matter: The role of particle size, composition, and oxidative potential-the RAPTES project. Environ. Health Perspect. 120:1183–1189; doi: 10.1289/ehp.1104389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trehy ML, Ye W, Hadwiger ME, Moore TW, Allgire JF, Woodruff JT, et al. 2011. Analysis of Electronic Cigarette Cartridges, Refill Solutions, and Smoke for Nicotine and Nicotine Related Impurities. J. Liq. Chromatogr. Relat. Technol 34:1442–1458; doi: 10.1080/10826076.2011.572213. [DOI] [Google Scholar]
- Wang F, Li C, Liu W, Jin Y, Guo L. 2013. Effects of Subchronic Exposure to Low-Dose Volatile Organic Compounds on Lung Inflammation in Mice. Environ. Toxicol 29:1089–1097; doi: 10.1002/tox. [DOI] [PubMed] [Google Scholar]
- Watson C, Ge J, Cohen J, Pyrgiotakis G, Engelward BP, Demokritou P. 2014. High-Throughput Screening Platform for Engineered Nanoparticle-Mediated Genotoxicity Using CometChip Technology. ACS Nano 8: 2118–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichenthal S, Kulka R, Bélisle P, Joseph L, Dubeau A, Martin C, et al. 2012. Personal exposure to specific volatile organic compounds and acute changes in lung function and heart rate variability among urban cyclists. Environ. Res 118:118–123; doi: 10.1016/j.envres.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Williams M, Villarreal A, Bozhilov K, Lin S, Talbot P. 2013. Metal and Silicate Particles Including Nanoparticles Are Present in Electronic Cigarette Cartomizer Fluid and Aerosol. PLoS One 8:1–11; doi: 10.1371/journal.pone.0057987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. 2014. Electronic nicotine delivery systems: Report by WHO. WHO Framew. Conv. Tob. Control 13–18; doi: 10.1161/CIRCULATIONAHA.114.008545.7. [DOI] [Google Scholar]
- Yingst JM, Veldheer S, Hrabovsky S, Nichols TT, Wilson SJ, Foulds J. 2015. Factors Associated With Electronic Cigarette Users’ Device Preferences and Transition From First Generation to Advanced Generation Devices. Nicotine Tob. Res 17:1242–1246; doi: 10.1093/ntr/ntv052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Pyrgiotakis G, Demokritou P. 2016. Development and characterization of electronic-cigarette exposure generation system (Ecig-EGS) for the physico-chemical and toxicological assessment of electronic cigarette emissions. Inhal. Toxicol 0:1–12; doi: 10.1080/08958378.2016.1246628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Zhang Y, Sisler JD, Shaffer J, Leonard SS, Morris AM, Qian Y, Dhimiter B, Demokritou P. 2018. Assessment of reactive oxygen species generated by electronic cigarettes using acellular and cellular approaches. J Hazard Mater. 344:549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S-H, Sun JY, Bonnevie E, Cummins SE, Gamst A, Yin L, et al. 2014. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob. Control 23:iii3–9; doi: 10.1136/tobaccocontrol-2014-051670. [DOI] [PMC free article] [PubMed] [Google Scholar]


