Abstract
Microtubule-severing enzymes are critical for the biogenesis and maintenance of complex microtubule arrays in axons, spindles and cilia where tubulin detyrosination, acetylation and glutamylation are abundant. These modifications exhibit stereotyped patterns suggesting spatial and temporal control of microtubule functions. Using human engineered and differentially modified microtubules we find that glutamylation is the main regulator of the hereditary spastic paraplegia microtubule severing enzyme spastin. Glutamylation acts as a rheostat and tunes microtubule severing as a function of glutamate number added per tubulin. Unexpectedly, glutamylation is a nonlinear biphasic tuner and becomes inhibitory beyond a threshold. Furthermore, the inhibitory effect of localized glutamylation propagates across neighboring microtubules, modulating severing in trans. Our work provides the first quantitative evidence for a graded response to a tubulin posttranslational modification and a biochemical link between tubulin glutamylation and complex architectures of microtubule arrays such as those in neurons where spastin deficiency causes disease.
eTOC
Glutamylation acts as a rheostat that tunes microtubule severing as a function of the number of glutamate molecules added per tubulin to maintain the complex architectures of microtubule arrays.
Graphical Abstract
INTRODUCTION
Microtubule-severing enzymes are critical for the generation and maintenance of microtubule arrays with complex architectures like those in neurons, spindles and cilia (Roll-Mecak and McNally, 2010). They produce internal breaks in the polymer thereby generating new microtubule ends that can either depolymerize or grow to amplify microtubule numbers (Lindeboom et al., 2013; Ribbeck and Mitchison, 2006; Roll-Mecak and Vale, 2006; Srayko et al., 2006). Spastin is a microtubule severing enzyme critical in neurogenesis (Sherwood et al., 2004; Trotta et al., 2004; Yu et al., 2008), axonal regeneration (Stone et al., 2012), nuclear envelope breakdown (Vietri et al., 2015), mitosis (Zhang et al., 2007) and cytokinesis (Guizetti et al., 2011). Spastin is mutated in 40% of patients with hereditary spastic paraplegias (HSP), neurological disorders characterized by lower extremity weakness (Hazan et al., 1999). HSP spastin mutants are impaired in microtubule severing (Evans et al., 2005; Roll-Mecak and Vale, 2005, 2008) and loss of spastin severing activity leads to disorganized neuronal microtubule arrays and axonopathy (Sherwood et al., 2004; Trotta et al., 2004). Not surprisingly, the microtubule severing activity of these enzymes is stringently regulated and spastin or katanin hyperactivity can be highly deleterious to the cell (Cummings et al., 2009; Lu et al., 2004; Sherwood et al., 2004; Stone et al. 2012). Thus, an important question in understanding the cellular mechanism of microtubule severing enzymes is how their activities are spatially and temporally restricted.
Microtubule severing enzymes act on the intrinsically disordered C-terminal tails of tubulin (McNally and Vale, 1993; Roll-Mecak and Vale, 2005) that decorate the microtubule surface and are hotspots for conserved and chemically diverse posttranslational modifications (reviewed in Garnham and Roll-Mecak, 2012). These modifications vary between cell types and their intracellular distribution patterns are stereotyped suggesting spatial and temporal control of microtubule effectors as the tubulin tails are binding sites for motors and microtubule-associated proteins (MAPs). Thus, these modifications are thought to constitute a cell positioning and navigation system for microtubule effectors or a “tubulin code” (Verhey and Gaertig, 2007; Yu et al., 2015), analogous to the “histone code” (Jenuwein and Allis, 2001). The enzymes that introduce these modifications are important for normal development and alterations in tubulin modifications are linked to cancers, chemotherapy resistance and neurodegenerative disorders (reviewed in Garnham and Roll-Mecak, 2012). Glutamylation, detyrosination and acetylation are enriched on microtubule subpopulations with long lifetimes that are resistant to drug-induced depolymerization (Gundersen et al., 1984; Schulze et al., 1987; Webster et al., 1987). They are especially abundant in the complex microtubules arrays of spindles, axons and cilia where the function of microtubule severing enzymes is critical. Kinetochore and interpolar microtubules are detyrosinated, astral microtubules are tyrosinated, midbody and axonal microtubules are detyrosinated, acetylated and glutamylated and cilia microtubules have high levels of glutamylation and acetylation (Audebert et al., 1993; Bobinnec et al., 1998; Gundersen et al., 1984; Lacroix et al., 2010; Wolff et al., 1992; Yu et al., 2015).
Glutamylation is the most abundant tubulin modification in the adult mammalian brain where it increases during postnatal neuronal maturation (Audebert et al., 1993; Ikegami et al., 2006; Redeker, 2010). It involves the reversible addition of glutamates, either singly or sequentially in chains, to the intrinsically disordered tubulin C-terminal tails. The number of glutamates on tubulin tails in neurons is distributed in stereotyped patterns, and disruption of glutamylation levels and patterns leads to neuronal pathologies and defective regeneration of damaged axons (Ghosh-Roy et al., 2012; Lee et al., 2012; Rogowski et al., 2010). While highly dynamic growth cone microtubules are not glutamylated and the soma contains mostly non-glutamylated or small numbers of glutamylated microtubules with short (<2) glutamate chains, the axon is enriched in stable microtubules with longer glutamate chains (Audebert et al., 1993; Edde et al., 1992; Janke and Kneussel, 2010; Regnard et al., 1999; Wolff et al., 1992). Mass spectrometric analyses of tubulin purified from brain tissue reveals a preponderance of glutamylated tubulin with 3–6 glutamates on each tail, with as many as 11 and seven detected on α- and β-tubulin tails, respectively (Redeker, 2010). Axonemal microtubules are extensively glutamylated with as many as 21 glutamates per tubulin protomer detected (Geimer et al., 1997; Schneider et al., 1998). It is likely that longer glutamate chains are found in vivo, but are not detectable due to the difficulty of mass spectrometric analysis of these highly electronegative tubulin tail peptides (Redeker et al., 2005).
Efforts to decipher the effects of tubulin modifications on cellular effectors have been hampered by the unavailability of unmodified microtubules and microtubules with well-defined posttranslational modifications. The overwhelming majority of studies use tubulin purified from brains by repeated cycles of depolymerization and polymerization, which has two consequences: (1) the topographical information originally encoded in the distribution of tubulin posttranslational modifications in cells is lost so that modifications present in the original microtubules become incorporated in random mosaic patterns of tubulin polymerized from them; (2) the purified tubulin is highly heterogeneous as it is comprised of a randomized mixture of isoforms bearing multiple, chemically distinct and quantitatively varied posttranslational modifications, including acetylation, phosphorylation, detyrosination and glutamylation (Sullivan, 1988). While acetylation and detyrosination are monomodifications that can function as simple ON/OFF switches, glutamylation, by virtue of its variable and stereotyped extent (a range of one to 21 glutamates have been detected on tubulin tails, Geimer et al., 1997; Redeker, 2010; Schneider et al., 1998) has the potential for a graded quantitative regulation of microtubule effectors. Tests of this hypothesis require synthesis of microtubules with quantitatively defined extents of glutamylation. In addition to the need for an unmodified microtubule substrate, such efforts have been further hampered by the inherent challenges that stem from the physicochemical properties of glutamylation. This modification is heterogeneous and highly charged, making mass spectrometric analyses challenging. Recent in vitro studies of the tubulin code relied on chimeric Saccharomyces cerevisiae tubulin with grafted human tubulin tails. Comparison of multiple tail isoforms, detyrosinated α-tail, and tails with glutamate peptides attached via an unnatural linkage revealed modest effects on kinesin and dynein processivity and speed (Sirajuddin et al., 2014).
Here we use human engineered and differentially modified microtubules to investigate the effect of tubulin glutamylation, detyrosination and acetylation on the activity of the microtubule-severing enzyme spastin. Using preparations of recombinant and differentially modified microtubules, we show that glutamylation is the main modulator of spastin activity and not tubulin acetylation or detyrosination. Using a microtubule series with a range of attached glutamates per tubulin, we unexpectedly find that glutamylation biphasically regulates microtubule severing: spastin activity increases as the number of glutamates per tubulin rises from one to eight, but decreases beyond this glutamylation threshold. We demonstrate that this behavior reflects a linear increase in spastin-microtubule affinity with tubulin glutamylation counteracted by a non-linear decrease in enzyme specific activity caused by impaired force generation on the tubulin tails. Thus, glutamylation quantitatively tunes severing of a microtubule in response to its precise local modification status, consistent with the variable effects of microtubule-severing enzymes on different microtubule arrays in vivo as well as at different developmental stages (Lee, et al. 2009; Sharma et al., 2007; Stewart et al., 2012). The same biphasic response modulates spastin activity in trans, because densely glutamylated microtubules act as spastin sinks, thereby propagating the inhibitory effect of glutamylation across a microtubule array. Our results reveal how severing can be precisely controlled spatially and temporally within microtubule arrays and provide quantitative proof for the rheostat-like regulation of microtubule effectors previously hypothesized for tubulin glutamylation, thus furnishing strong support for the tubulin code hypothesis.
RESULTS
Biphasic Regulation by Tubulin Glutamylation
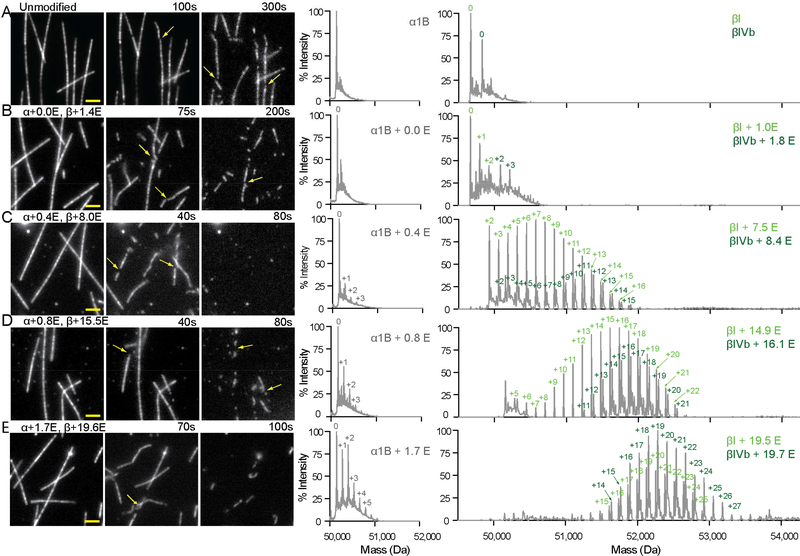
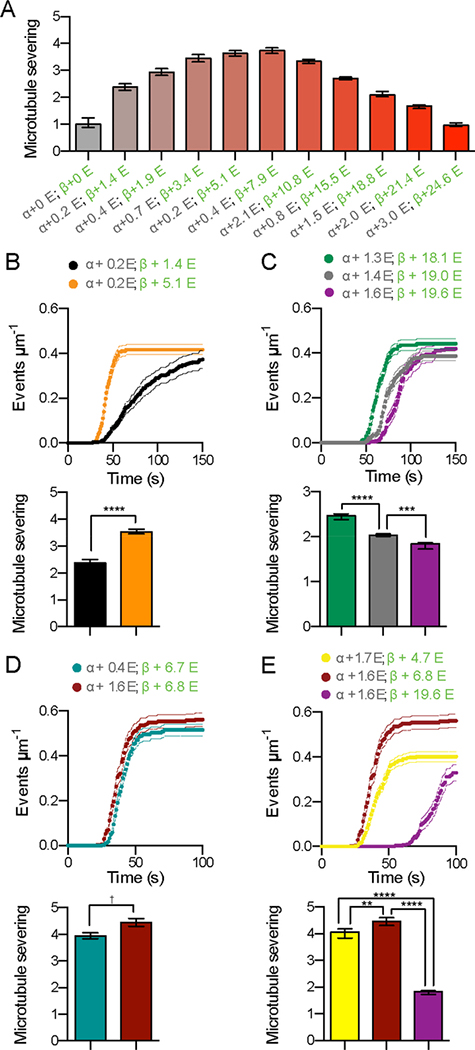
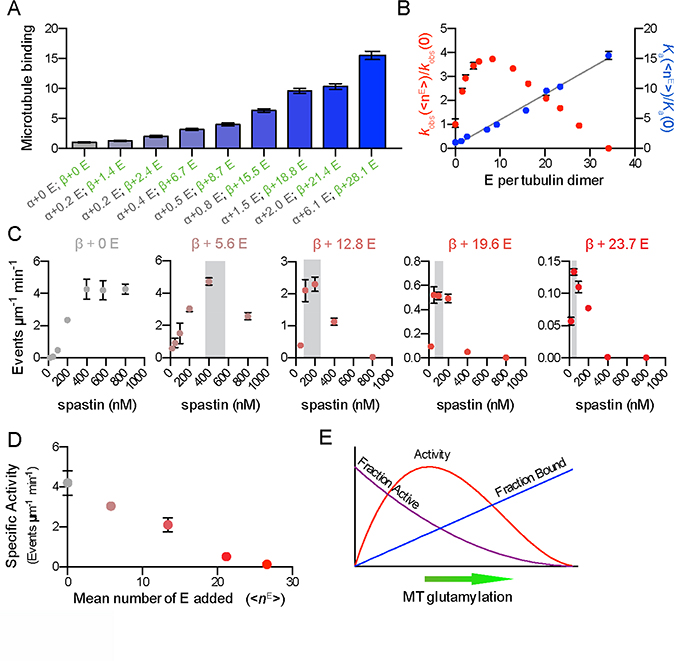
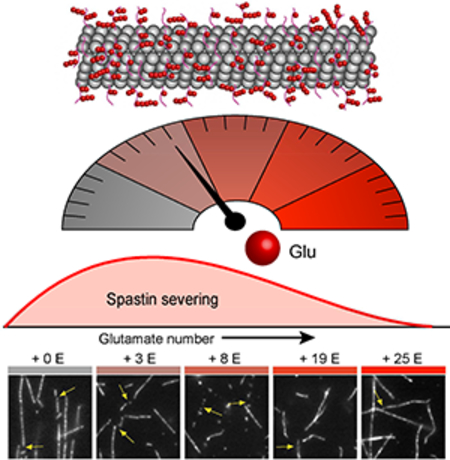
Glutamylation recruits spastin to microtubules in vivo (Zempel et al., 2013) and a qualitative enhancement of spastin severing activity by tubulin glutamylation has been observed in vitro and in vivo (Lacroix et al., 2010). To generate microtubules with defined glutamylation levels, we purified tubulin devoid of posttranslational modifications (Figure 1A and S1) and used TTLL7 (tubulin tyrosine ligase-like 7) glutamylase to modify it in vitro. TTLL7 is the most abundant neuronal tubulin glutamylase (Ikegami et al., 2006; van Dijk et al., 2007). It is critical for neurite outgrowth and is primarily responsible for the dramatic increase in β-tubulin glutamylation observed during neuronal maturation (Ikegami et al., 2006). TTLL7 both initiates and elongates glutamate chains on the C-terminal tubulin tails (Garnham et al., 2015; Mukai et al., 2009). Glutamylation was quantified using reversed-phase LC-MS (Figure 1, S1, and Experimental Procedures). Proteolytic digest followed by tandem MS analysis confirmed the presence of glutamylation only on the tubulin tails (Supplemental Experimental Procedures; data not shown). Using a microscopy-based assay to quantify spastin severing (Figure 1) we found that spastin activity gradually increases with glutamylation (Figures 1B, C), but unexpectedly decreases above a threshold (Figures 1D, 1E, 2A, S1A and Movie S1). The transition between the stimulatory and inhibitory effects occurs at a mean glutamate number (<nE>) of ~ 8 (Figure 2A). At <nE> ~ 8 severing is enhanced 3.8 fold over unmodified microtubules, with a nine fold increase in maximal rate (Figures 1 and S1A)
Figure 1. Graded regulation of spastin-catalyzed microtubule severing by tubulin glutamylation.
(A–E). Left, spastin-catalyzed microtubule severing of unmodified microtubules (A) and microtubules with increasing glutamylation levels (B–E). Scale bar, 2 μm. Right, reversed-phase LC-MS of microtubules used in severing assays shown on the left (Experimental Procedures). There are one detectable α (α1Β) and two β (βI and βIVb) isoforms in these preparations. Throughout, numbers of glutamates added to α and β-tubulin isoforms are indicated in grey and green, respectively. The weighted mean of the number of glutamates (<nE>) added to α- and β-tubulin (overall and separately for the two β isoforms) are denoted α+<nE> and, β+<nE>, respectively (Experimental Procedures). See also Figure S1.
Figure 2. Spastin-catalyzed microtubule severing displays a biphasic response to tubulin glutamylation levels.
(A) Spastin-catalyzed microtubule severing varies with the number of glutamates on tubulin tails. <nE> on α and β-tubulin, indicated in grey and green, respectively. Error bars, S.E.M. (n > 23 microtubules from multiple chambers). Severing rates normalized to that of unmodified microtubules (Experimental Procedures).
(B-C) Modulation of β-tubulin glutamylation levels is sufficient to increase (B) or decrease (C) microtubule severing activity while α-tubulin glutamylation levels are kept constant (for mass spectra, see Figures S2A and S2B). Error bars, S.E.M. (n = 23 microtubules from multiple chambers for both B and C). **** p < 0.0001; *** p < 0.001. Top panels, progress curves of severing reactions; bottom panels, severing rates as in (A).
(D) Microtubule severing activity is highly sensitive to β-tubulin glutamylation levels (for mass spectra, see Figure S2C). Error bars, S.E.M. (n = 23 microtubules from multiple chambers). † p > 0.01 Top panels, progress curves of severing reactions; bottom panels, severing rates as in (A).
(E) An increase in β-tubulin glutamylation is sufficient to induce a biphasic response in microtubule severing when α-tubulin glutamylation is constant (for mass spectra, see Figure S2D). Error bars, S.E.M. (n = 24 microtubules from multiple chambers). **** p < 0.0001; ** p < 0.01. Top panels, progress curves of severing reactions; bottom panels, severing rates as in (A).
The observed stimulatory and inhibitory effects on microtubule severing are mostly due to β-tail glutamylation. Severing of microtubules with essentially unmodified α-tails, but with either 1.4 or 5.1 glutamates on their β-tails, is enhanced by 49% in the latter (Figures 2B and S2). Severing decreases stepwise as <nE> on β-tubulin increases from 18.1 to 19.0 to 19.6 (Figure 2C). Consistent with the dominant role of β-tail glutamylation, microtubules with near identical β-tubulin glutamylation levels (<nE> of 6.7 or 6.8), but a four-fold difference in α-tubulin glutamylation (<nE> of 0.4 versus. 1.6) are severed similarly by spastin (Figure 2D). In contrast, a similar increase in β-tubulin glutamylation from 0 to 1.4 enhances severing 2.4 fold (Figure 2A). Furthermore, constant α-tubulin glutamylation levels (<nE> of 1.6, 1.7, and 1.6) with increasing glutamate numbers on β-tubulin (<nE> of 4.7, 6.8, and 19.6, respectively) recapitulate the biphasic response to glutamylation (Figure 2E). These results indicate that β-tubulin glutamylation suffices to modulate spastin-severing activity.
The α-tubulin Tail Is Dispensable for Spastin-Mediated Severing
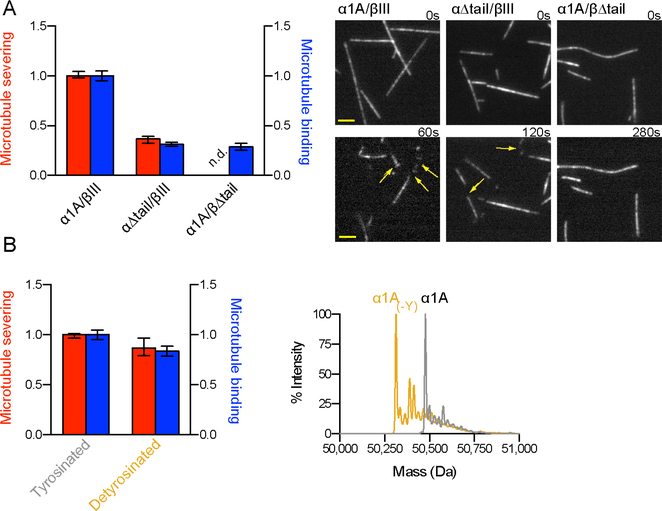
The critical role of the β-tail for spastin function is supported by experiments with engineered human tubulin. We used total internal reflection fluorescence (TIRF) microscopy and analyzed the association of DyLight 488-labeled spastin (the labeled enzyme retains full severing activity; Figure S3 and Experimental Procedures) with engineered microtubules missing either their α- or β-tails (Figures 3, S3A and Experimental Procedures). Our experiments demonstrate that both tubulin tails make contributions to spastin microtubule binding affinity: removal of the α- or β-tail leads to 69% and 71% reduction in binding, respectively (Figure 3A). However, microtubules without β-tails are resistant to severing, while microtubules without α-tails are still severed, albeit with 63% lower activity (Figure 3A). Microtubules missing their β-tails are not severed even at spastin concentrations as high as 1 μM (not shown). Thus, the β-tubulin tail is the major regulator for spastin severing. We obtained similar results with microtubules where β-tails were removed by partial proteolysis: removal of ~90% of the β-tails abrogates severing and leads to a 52% reduction in microtubule binding affinity (Figures S3B and S3C). Complete removal of the β-tails leads to a 79% reduction (no binding is detected in the absence of both α- and β-tails under these conditions). Microtubules that retain ~10% of their full-length β-tails are weakly severed by spastin, but only at high spastin concentrations (1 μM vs. 50 nM) and at a rate ~100-fold lower than microtubules with intact β-tails (Figures S3B and S3D). Microtubules that retain ~ 3% of their full-length β-tubulin tails are completely resistant to severing even at these high spastin concentrations (Figure S3D). Thus, while both tails contribute to microtubule binding and severing, the β-tubulin tail is necessary and sufficient for severing.
Figure 3. Spastin-mediated microtubule severing requires the β-, but not the α-tubulin C-terminal tail.
(A) Left, normalized microtubule severing rates (left axis) and binding affinity (right axis) for human recombinant microtubules missing either the α- or β-tail (Figure S3A). Error bars, S.E.M. (n > 34 or 70 microtubules from multiple chambers for severing and binding assays, respectively). Right, engineered human microtubules severed by spastin. Arrows indicate severing sites. Scale bar, 2 μm. See also Figure S3.
(B) Left, normalized microtubule severing rates (left axis) and binding affinity (right axis) for human recombinant tyrosinated and detyrosinated microtubules. Error bars, S.E.M. (n > 15 microtubules for severing assays; n > 96 microtubules for binding assays, from multiple chambers). Right, overlaid reversed-phase LC-MS of tyrosinated (gray) and detyrosinated (yellow) microtubules corresponding to the α-tubulin peak.
Tubulin Detyrosination and Acetylation Are not Major Spastin Modulators
Given the contribution of the α-tail to spastin microtubule binding, we examined whether detyrosination is a spastin modulator. Detyrosination involves the reversible removal of the genetically encoded C-terminal tyrosine of α-tubulin. The tyrosine is added back to soluble tubulin as part of the detyrosination/tyrosination cycle (Raybin and Flavin, 1975; Szyk et al., 2011). The C-terminal tyrosine is an ON/OFF signal for the recruitment of microtubule regulators such as plus-end-binding proteins and motors (Peris et al., 2009; reviewed in Garnham and Roll-Mecak, 2012; Gouveia and Akhmanova, 2010). Experiments with recombinant human tubulin with or without the α-tubulin C-terminal tyrosine show that neither microtubule binding nor severing is significantly affected by detyrosination (Figure 3B). Thus, detyrosination is not a strong modulator of spastin function and the downregulation in microtubule binding and severing seen with the α-tail deletion is primarily due to interactions with the rest of the α-tail.
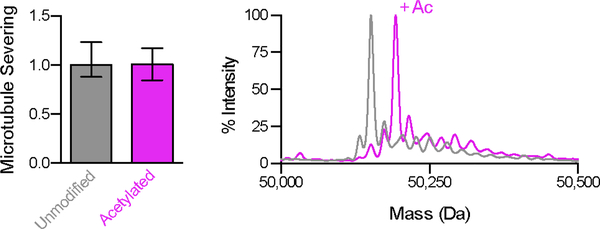
We also examined the effects of α-tubulin acetylation on spastin mediated microtubule severing as the closely related microtubule-severing enzyme katanin was reported to preferentially sever acetylated microtubules in vivo (Sudo and Baas, 2010). Unlike detyrosination and glutamylation that alter the intrinsically disordered C-terminal tubulin tails that decorate the microtubule exterior, α-tubulin acetylation occurs on Lys40 in the microtubule lumen and was proposed to affect the strength of lateral contacts in the microtubule lattice as well as recruit intraluminal MAPs (Cueva et al., 2012). Microtubule severing assays with unmodified and acetylated microtubules generated by in vitro enzymatic modification using tubulin acetyltransferase show that acetylation has no effect on spastin activity (Figure 4 and Supplemental Experimental Procedures), consistent with previous in vivo studies (Sudo and Baas, 2010). It remains to be established whether the effect of acetylation on katanin is direct. Thus, of the three major tubulin modifications enriched in stable microtubules arrays where spastin function is important, glutamylation is the key modulator of spastin-mediated microtubule severing.
Figure 4. α-tubulin acetylation does not affect spastin-mediated microtubule severing.
(A) Left, normalized spastin severing activity with unmodified and acetylated human microtubules. Error bars, S.E.M. (n = 50 and 27 for unmodified and acetylated microtubules, respectively, from multiple chambers). Right, overlaid reversed-phase LC-MS of unmodified (gray) and acetylated (magenta) α-tubulin showing the 42 Da mass shift corresponding to the acetylated species. No mass change is detected in β-tubulin (not shown).
Glutamylation Is a Linear Affinity Tuner
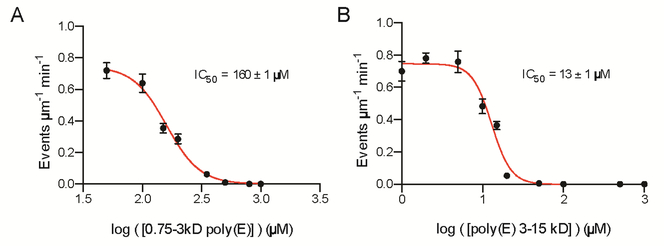
To understand the molecular basis for the modulation of spastin activity by tubulin glutamylation, we investigated the effects of this modification on microtubule binding. We found that spastin microtubule binding affinity increases monotonically with glutamylation (Figure 5, S4). Consistent with this, spastin severing is inhibited by free polyglutamic acid in a manner proportional to its chain length (IC50 of 160 ± 1 μM for poly(E) of 6 to 23 glutamates; IC50 of 13 ± 1 μM for poly(E) of 23 to 115 glutamates; Figure 6 and Experimental Procedures). Spastin belongs to the family of AAA ATPases (ATPases associated with various cellular activities) and microtubule severing requires ATP hydrolysis (Evans et al., 2005; Roll-Mecak and Vale, 2005). We investigated the effects of soluble polyglutamic acid on spastin catalyzed ATP hydrolysis. We find that spastin ATPase is not inhibited by soluble polyglutamic acid (Figure S5), indicating that the inhibitory effect is not due to ATPase inhibition and most likely to direct competition with the tubulin tails for spastin binding. Remarkably, binding affinity rises linearly with <nE> (slope of ~0.43 per glutamate added to tubulin) indicating that glutamylation acts as a linear affinity tuner (Figure 5B). The strong contribution of electrostatic interactions to spastin microtubule binding is consistent with the salt dependence of microtubule severing (Eckert et al., 2012a). The mean free energy of binding per added glutamate is 0.06 kcal/mol, indicating weak interactions. The linearity for the affinity also indicates a stochastic binding mechanism i.e., the binding affinity is enhanced by increasing local glutamate concentration and not by a cooperative mechanism whereby increasing numbers of glutamates are simultaneously engaged by the enzyme. As motors and MAPs contain positively charged patches critical for microtubule binding, it is likely that glutamylation acts as an affinity tuner for other microtubule regulators (Roll-Mecak, 2015) as suggested by early qualitative experiments with MAP1B, MAP2 and tau (Bonnet et al., 2001; Boucher et al., 1994; Larcher et al., 1996).
Figure 5. Competition between affinity and specific activity gives rise to the biphasic regulation of spastin-catalyzed microtubule severing by tubulin glutamylation.
(A) Spastin affinity for microtubules increases with the number of glutamates on tubulin tails. Spastin binding on modified microtubules normalized to that for unmodified microtubules). <nE> on α and β-tubulin are indicated as in Figure 1. Error bars, S.E.M. (n = 50 microtubules from multiple chambers for each <nE>).
(B) Spastin microtubule affinity increases linearly with glutamate numbers on tubulin (R2 = 0.99). Left, microtubule severing rates, kobs, right, microtubule association constants, Ka for various <nE> normalized to those for unmodified microtubules. Error bars represent S.E.M.
(C) Microtubule severing rates as a function of spastin concentration for microtubules with various glutamylation levels. Increased glutamylation induces earlier and more abrupt onset of anti-cooperativity of microtubule severing. Error bars, S.E.M. (n > 21 microtubules from multiple chambers for each <nE>). Grey shading, region of transition from cooperative to anti-cooperative behavior.
(D) Microtubule severing activities for different glutamylation levels at constant number of bound spastin molecules per tubulin. The specific activity of bound spastin decreases with increased glutamylation. Error bars, S.E.M. (n > 20 microtubules from multiple chambers for each <nE>).
(E) Competition between glutamylation-induced increase in microtubule affinity and decrease in enzyme specific activity yields a biphasic response of spastin severing to microtubule glutamylation levels. See also Figure S4.
Figure 6. Free poly-glutamic acid inhibits spastin microtubule severing.
(A) Microtubule severing rates in the presence of 0.75 – 3 kDa poly-glutamic acid (6 to 23 glutamates). Error bars, S.E.M. (n > 24 microtubules from multiple chambers)
(B) Microtubule severing rates in the presence of 3 – 15 kDa poly-glutamic acid (23 to 115 glutamates). Error bars, S.E.M. (n > 24 microtubules from multiple chambers). See also Figure S5.
Glutamylation Lowers Spastin Mechanochemical Coupling
Spastin exhibits cooperative severing of microtubules that varies with tubulin glutamylation (Figure 5C). Cooperative assembly on the microtubule was proposed as an activation mechanism for katanin (Hartman and Vale, 1999). Spastin exhibits cooperative severing of unmodified microtubules (Hill coefficient = 3.1, Figure 5C, left panel). Surprisingly, as the glutamate number per tubulin increases, a new behavior emerges wherein the cooperativity peaks at progressively lower spastin concentrations (indicated by shading in Figure 5C) and drops precipitously thereafter. Beyond the peak, severing becomes anti-cooperative. At <nE> of 5.6, maximal severing is observed at 400 nM spastin, while at 12.8, it peaks at 100 nM and drops in half at 400 nM spastin.
To determine whether tubulin glutamylation alters the specific activity of microtubule-bound spastin, we assayed a series of progressively glutamylated microtubules at concentrations that result in equivalent spastin densities on the microtubule (Figure 5D and Experimental Procedures). We find that the specific activity of microtubule-bound spastin declines steeply and non-linearly with glutamylation (Figure 5D). Since spastin ATPase varies modestly with microtubule glutamylation (Figure S4), it suggests that the mechanochemical coupling of the enzyme (Eckert et al., 2012b) is progressively weakened with increasing glutamylation despite the concomitant increase in microtubule binding affinity; i.e., the enzyme gradually shifts to a non-productive tubulin binding mode with increased glutamylation. Therefore, the combination of the linear increase in affinity with the non-linear decrease in severing specific activity gives rise to the overall biphasic response to microtubule glutamylation (Figures 5B and 5E). Thus, spastin gradually transitions from a microtubule-severing enzyme to a microtubule stabilizing protein as a function of glutamylation. Inactive spastin and katanin mutants crosslink microtubules (McNally and McNally, 2011; Roll-Mecak and Vale, 2008) and katanin crosslinking activity is important for normal spindle morphology (McNally and McNally, 2011). Our work suggests that the glutamylation status of the microtubule could control a switch between these two opposing activities.
Long-Range Effects of Glutamylation
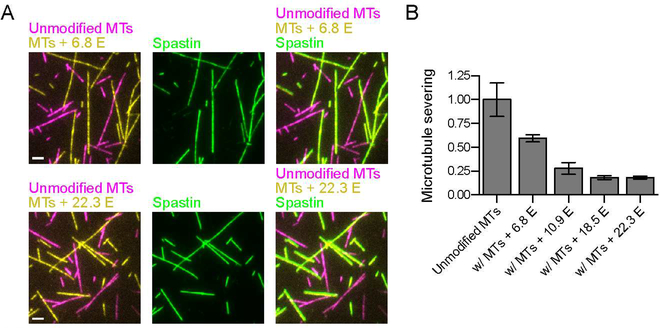
Our work shows that glutamylation gives rise to microtubule-autologous regulation of severing by spastin whereby the precise local glutamylation status of the microtubule itself quantitatively controls spastin activity. The simultaneous divergent increase in spastin microtubule affinity and decrease in severing specific activity in response to glutamylation levels has an important physiological corollary. Because high glutamylation results in a marked increase in spastin microtubule affinity (Figures 5A and 5B), but a decrease in severing activity above a certain threshold (Figures 2A, 5B and S1A), the modified microtubule can act as a sink for spastin (Figure 7A), thereby inhibiting severing of a less modified microtubule in trans in addition to the microtubule-autologous (cis) regulation (Figure 7B). Indeed, severing of unmodified microtubules decreases by 41% in the presence of equimolar amounts of microtubules with <nE> ~ 7 and progressively decreases in the presence of microtubules with <nE> of 11 or higher (Figure 7B). Because spastin microtubule affinity increases linearly with glutamylation, this effect will be present (in the correspondingly muted form) even at low glutamate numbers.
Figure 7. Spastin-catalyzed severing activity is modulated by the glutamylation levels of neighboring microtubules.
(A) Spastin association with unmodified and glutamylated microtubules (top, panels <nE> ~ 6.8 E, bottom panels, <nE> ~ 22.3 E). Magenta, unmodified microtubules; yellow, glutamylated microtubules (<nE> of 6.8 or 22.3); green, DyLight 488 labeled spastin. Scale bar, 2 μm.
(B) Spastin severing activity in chambers with mixed microtubule populations. Severing activity for unmodified microtubules in isolation or in the presence of equimolar concentrations of microtubules with defined glutamylation levels (<nE> of 6.8, 10.9, 18.5 or 22.3). Error bars, S.E.M. (n > 17 microtubules from multiple chambers).
DISCUSSION
Our work reveals how the fate of a microtubule can be reversibly controlled by posttranslationally modifying an intrinsically disordered region (IDR) of tubulin not involved in interfaces essential for its polymerization into microtubules. The control of spastin severing by tubulin glutamylation levels enables substrate-regulated, spatially-controlled severing. As glutamylation increases up to <nE> ~ 8, microtubule severing is enhanced autologously and repressed in less glutamylated neighboring microtubules because of the linear increase in spastin recruitment with microtubule glutamylation. As <nE> becomes larger than ~ 8, severing is progressively inhibited autologously, and this inhibitory effect of high glutamylation levels propagates through spatially adjacent microtubules. This response to glutamylation could be a mechanism to control spastin-mediated midbody microtubule severing during cytokinesis, limit severing of highly glutamylated centriolar microtubules or quantitatively control microtubule severing during neuronal development when β-tubulin glutamylation levels increase progressively. Spastin mutations are responsible for 40% of HSPs, characterized by progressive axonopathy. The graded control of microtubule severing reveals how precise spatial regulation of microtubule severing can be achieved in a neuron where growth cone microtubules are not glutamylated (and thus are poor spastin substrates) and axonal microtubules are enriched in glutamylation and more susceptible to spastin. The differential susceptibility of these microtubule populations could have relevance for HSP patients with spastin mutations.
We propose that the closely related microtubule-severing enzyme katanin exhibits a similar response to glutamylation, consistent with the in vivo observation that katanin severs the glutamylated B-tubule of the axonemal doublet, but leaves the adjacent unmodified A-tubule untouched (Sharma et al., 2007). The response to glutamate numbers could be different for the two enzymes and this could potentially specialize them for microtubule arrays with different glutamylation ranges. Interestingly, glutamylation levels that enhance spastin induced microtubule severing in cells, lead to recruitment of katanin to these glutamylated microtubules, where it induces mostly bundling and rarely severing (Lacroix et al., 2010).
The biphasic response to glutamylation could be used as a mechanism to initially activate severing enzymes in a microtubule array to equalize microtubule lengths or amplify their numbers (Loughlin et al., 2011; Srayko et al., 2006), and then gradually dampen their activity through the autologous biphasic response as well as the “in trans” regulation as the array reaches homeostasis. Newly grown microtubules from severed seeds would have low glutamylation levels and thus be initially protected from severing, allowing the formation of a new dynamic array. The inhibitory effect of higher glutamylation levels could also be a mechanism to locally inactivate and retain a pool of compartmentalized microtubule severing enzymes that are available for rapid activation on microtubules with lower glutamylation levels. This mechanism could be operational at the centrosome (enriched in highly glutamylated microtubules) where spastin and katanin localize and microtubules are released by severing (Zhang et al., 2007).
Spastin forms a hexameric ring when bound to ATP and is thought to destabilize the microtubule by pulling on the tubulin C-terminal tails through a positively charged central pore, by analogy with the mechanism of AAA ATPases such as ClpX and the proteasome (Roll-Mecak and Vale, 2008). Our data suggest that the posttranslationally added glutamates on the C-terminal tails provide additional unproductive binding sites and cause slippage of the tail when engaged by spastin, leading to a reduction in severing efficiency. Thus, even though more spastin molecules are recruited to the microtubule at higher glutamylation, because their specific activity is decreased due to impaired mechanochemical coupling, spastin molecules spend more of their microtubule-bound cycle in a state that is unable to remove tubulin and thus act to stabilize the microtubule, counteracting the action of the molecules in the productive part of their mechanochemical cycles, thus the anticooperative behavior observed at higher spastin densities. As tails with different glutamate numbers coexist on the same microtubule, the biphasic response that we uncovered could also be elicited by having a sharp transition between stimulation and inhibition at a particular glutamylation level. We favor rheostatic control over a threshold model because there is no species common to all the inhibitory microtubule samples (<nE> above ~ 8). Future structural studies will shed light on the mechanism of tubulin tail engagement by microtubule severing enzymes.
The intrinsically disordered tubulin tails are hubs of posttranslational modifications. Posttranslational modifications of IDRs are ubiquitously used to assemble complex signaling platforms (Wright and Dyson, 2015). Recent studies revealed involvement of the tubulin tails in diverse cellular networks (Aiken et al., 2014). Here we show that glutamylation, the most prevalent tubulin tail modification and most abundant in the nervous system, quantitatively tunes the activity of a microtubule regulator. This is reminiscent of the regulatory mechanism by additive phosphorylation on IDRs that can operate both by a rheostatic or threshold response (Wright and Dyson, 2015). For example, the unstructured N-terminus of the p53 transcription factor is subject to multisite phosphorylation that tunes its affinity for the CREB-binding protein CBP/p300 and recruits it away from other transcription factors in response to genotoxic stress (Lee et al., 2010). Similarly, the DNA binding affinity of the Ets-1 (V-Ets Avian Erythroblastosis Virus E26 Oncogene Homolog 1) transcription factor is tuned by additive phosphorylation of an IDR in its sequence (Pufall et al., 2005). The activation of the cyclin-dependent kinase inhibitor Sic1 is achieved via a sharp threshold response to multisite phosphorylation (Nash et al., 2001). In these cases, as for the tubulin tail, the intrinsically disordered nature of the regulatory region in the protein makes it accessible to modification enzymes as well as readout by cellular effectors, be it another protein or nucleic acid.
Our approach outlines for the first time a general strategy for quantitative, in vitro characterization of the effects of informationally complex tubulin posttranslational modifications such as glutamylation on microtubule regulators. Currently, determination of the glutamylation status of microtubules is limited to immunofluorescence with antibodies that can only distinguish between chains of one versus greater than one or two glutamates (Lacroix et al., 2010; Magiera and Janke, 2013). The graded response of spastin-mediated severing to glutamylation and the switch between opposing biochemical activities as a function of glutamylation underscores the importance of devising new methods to determine the exact glutamylation status of individual microtubules in cells. Our approach for the generation and characterization of human microtubules with differential posttranslational modifications should motivate quantitative studies of the response to tubulin modifications of other microtubule regulators and serve as a quantitative paradigm for deciphering the biological consequences of the astonishing chemical complexity of cellular microtubules. While tubulin acetylation and detyrosination are monomodifications that can generate a binary response, glutamylation, by virtue of the variable number of glutamates added, can elicit a graded response by microtubule effectors. The combinatorial use of these signals can give rise to complex cellular responses of cytoskeletal regulators. Elucidating how differential tubulin posttranslational modifications modulate the activity of microtubule effectors is key to understanding how a single polymer can perform its diverse essential roles in cells. Furthermore, as glutamylation is found on many non-tubulin substrates (van Dijk et al., 2008) e.g., it regulates the histone chaperone activity of Nap-1 (nucleosome assembly protein Nap1; Miller and Heald, 2015), the precise graded regulation we have uncovered carries general implications for cell signaling via glutamylation.
EXPERIMENTAL PROCEDURES
Generation of glutamylated microtubules
Human unmodified tubulin was purified by the TOG affinity method (Widlund et al., 2012) using a TOG1 domain column (Vemu et al., 2014). Taxol-stabilized unmodified microtubules for severing assays were prepared by polymerizing purified unmodified human tubulin with 4% tetramethylrhodamine (TMR)- and 1% biotin-labeled brain tubulin. Microtubules were glutamylated by incubation with Xenopus tropicalis tubulin tyrosine ligase-like 7 (TTLL7) at a 1:10 molar ratio of enzyme to tubulin in 20 mM HEPES pH 7.0, 50 mM NaCl, 10 mM MgCl2, 1 mM Glu, 1 mM ATP, 10 μM taxol at room temperature for various incubation times (10 min for <nE> ~ 1, 20 min for <nE> ~ 2, 1 hr for <nE> ~ 5, 2.5 hrs for <nE> ~ 12, 5 hrs for <nE> ~ 16, 10 hrs for <nE> ~ 25, and 24 hrs for <nE> ~ 35). Control reactions were performed without glutamate. TTLL7 was removed by addition of 0.3 M KCl and sedimentation through a 60% glycerol cushion at 100,000 × g for 12 minutes at 30° C. Microtubules were stored in BRB80 (80 mM PIPES, 1 mM MgCl2, 1 mM EGTA, pH 6.8), 14.3 mM β-mercaptoethanol, 10 μM taxol. The number of glutamates added to α- and β-tubulin was determined by LC-MS (Supplemental Experimental Procedures). The spectra display the characteristic distribution of masses with peaks separated by 129 Da corresponding to one glutamate (Figure 1). The extent of tubulin glutamylation on α or β-tubulin was determined by calculating the weighted average of peak intensities for each tubulin species present. Experiments were performed on different days with at least two independent preparations. The mass spectrometric analyses are reproducible within 0.1 <nE> between at least three independent measurements (Figure S1).
Generation of acetylated microtubule substrates and recombinant engineered human tubulin
A complete description can be found in Supplemental Experimental Procedures.
Microscopy based severing assays
Chambers were assembled as in (Szyk et al., 2014). Microscopy based microtubule severing assays were performed as described (Ziolkowska and Roll-Mecak, 2013). A complete description of data collection and analyses can be found in Supplemental Experimental Procedures.
TIRF microscopy microtubule-binding assays
DyLight 488-labeled spastin was perfused into chambers at a final concentration of 2 or 5 nM in severing reaction buffer (50 mM KCl, 2.5 mM MgCl2, 1 mM ATP, and 1% Pluronic F-127, 10 μM taxol and oxygen scavengers). Images were acquired using an inverted TIRF microscope. A complete description of image acquisition can be found in Supplemental Experimental Procedures.
ATPase assays
ATPase assays were performed as described in Supplemental Experimental Procedures.
Supplementary Material
HIGHLIGHTS.
Glutamylation of intrinsically disordered tubulin tails quantitatively tunes spastin
Spastin microtubule severing responds biphasically to glutamate number on tubulin
Localized glutamylation effects propagate to neighboring microtubules
Control by tubulin glutamylation echoes regulation by multisite phosphorylation
ACKNOWLEDGEMENTS
We thank C. P. Garnham and I. Yu, National Institute of Neurological Disorders and Stroke (NINDS) for purified TTLL7, A. Syzk (NINDS) for recombinant tubulin, Y. Li of the NINDS Mass Spectrometry facility for MS/MS, D.-Y. Lee and the National Heart, Lung and Blood Institute (NHLBI) Biochemistry facility for access to mass spectrometers, Y. He (NHLBI) for large-scale cultures. A. R.-M. thanks H. Bourne, A. Ferre-D’Amare, E. Giniger, S. Gottesman and M. Maurizi for helpful discussions; M.L.V. thanks C. C. Valenstein for feedback. This work was supported by a Searle Scholar award to A.R-M. and the intramural programs of NINDS and NHLBI.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures, five supplementary figures and a movie.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aiken J, Sept D, Costanzo M, Boone C, Cooper JA, and Moore JK (2014). Genome-wide analysis reveals novel and discrete functions for tubulin carboxy-terminal tails. Current biology : CB 24, 1295–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audebert S, Desbruyeres E, Gruszczynski C, Koulakoff A, Gros F, Denoulet P, and Edde B (1993). Reversible polyglutamylation of alpha- and beta-tubulin and microtubule dynamics in mouse brain neurons. Molecular biology of the cell 4, 615–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobinnec Y, Moudjou M, Fouquet JP, Desbruyeres E, Edde B, and Bornens M (1998). Glutamylation of centriole and cytoplasmic tubulin in proliferating non-neuronal cells. Cell Motil Cytoskeleton 39, 223–232. [DOI] [PubMed] [Google Scholar]
- Bonnet C, Boucher D, Lazereg S, Pedrotti B, Islam K, Denoulet P, and Larcher JC (2001). Differential binding regulation of microtubule-associated proteins MAP1A, MAP1B, and MAP2 by tubulin polyglutamylation. The Journal of biological chemistry 276, 12839–12848. [DOI] [PubMed] [Google Scholar]
- Boucher D, Larcher JC, Gros F, and Denoulet P (1994). Polyglutamylation of Tubulin as a Progressive Regulator of in-Vitro Interactions between the Microtubule-Associated Protein-Tau and Tubulin. Biochemistry 33, 12471–12477. [DOI] [PubMed] [Google Scholar]
- Cueva JG, Hsin J, Huang KC, and Goodman MB (2012). Posttranslational acetylation of alpha-tubulin constrains protofilament number in native microtubules. Current biology : CB 22, 1066–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings CM, Bentley CA, Perdue SA, Baas PW, and Singer JD (2009). The Cul3/Klhdc5 E3 ligase regulates p60/katanin and is required for normal mitosis in mammalian cells. The Journal of biological chemistry 284, 11663–11675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert T, Le DT, Link S, Friedmann L, and Woehlke G (2012a). Spastin’s microtubule-binding properties and comparison to katanin. PloS one 7, e50161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert T, Link S, Le DT, Sobczak JP, Gieseke A, Richter K, and Woehlke G (2012b). Subunit Interactions and cooperativity in the microtubule-severing AAA ATPase spastin. The Journal of biological chemistry 287, 26278–26290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edde B, Rossier J, Le Caer J-P, Promé J-C, Desbruyeres E, Gros F, and Denoulet P (1992). Polyglutmylated alpha-Tubulin Can Enter the Tyrsoination/Detyrosination Cycle. Biochemistry 31, 403–410. [DOI] [PubMed] [Google Scholar]
- Evans KJ, Gomes ER, Reisenweber SM, Gundersen GG, and Lauring BP (2005). Linking axonal degeneration to microtubule remodeling by Spastin-mediated microtubule severing. The Journal of cell biology 168, 599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnham CP, and Roll-Mecak A (2012). The chemical complexity of cellular microtubules: tubulin post-translational modification enzymes and their roles in tuning microtubule functions. Cytoskeleton 69, 442–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnham CP, Vemu A, Wilson-Kubalek EM, Yu I, Szyk A, Lander GC, Milligan RA, and Roll-Mecak A (2015). Multivalent Microtubule Recognition by Tubulin Tyrosine Ligase-like Family Glutamylases. Cell 161, 1112–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geimer S, Teltenkotter A, Plessmann U, Weber K, and Lechtreck KF (1997). Purification and characterization of basal apparatuses from a flagellate green alga. Cell Motil Cytoskeleton 37, 72–85. [DOI] [PubMed] [Google Scholar]
- Ghosh-Roy A, Goncharov A, Jin Y, and Chisholm AD (2012). Kinesin-13 and tubulin posttranslational modifications regulate microtubule growth in axon regeneration. Developmental cell 23, 716–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouveia SM, and Akhmanova A (2010). Cell and molecular biology of microtubule plus end tracking proteins: end binding proteins and their partners. Int Rev Cell Mol Biol 285, 1–74. [DOI] [PubMed] [Google Scholar]
- Guizetti J, Schermelleh L, Mantler J, Maar S, Poser I, Leonhardt H, Muller-Reichert T, and Gerlich DW (2011). Cortical constriction during abscission involves helices of ESCRT-III-dependent filaments. Science 331, 1616–1620. [DOI] [PubMed] [Google Scholar]
- Gundersen GG, Kalnoski MH, and Bulinski JC (1984). Distinct populations of microtubules: tyrosinated and nontyrosinated alpha tubulin are distributed differently in vivo. Cell 38, 779–789. [DOI] [PubMed] [Google Scholar]
- Hartman JJ, and Vale RD (1999). Microtubule disassembly by ATP-dependent oligomerization of the AAA enzyme katanin. Science 286, 782–785. [DOI] [PubMed] [Google Scholar]
- Hazan J, Fonknechten N, Mavel D, Paternotte C, Samson D, Artiguenave F, Davoine CS, Cruaud C, Durr A, Wincker P, et al. (1999). Spastin, a new AAA protein, is altered in the most frequent form of autosomal dominant spastic paraplegia. Nature genetics 23, 296–303. [DOI] [PubMed] [Google Scholar]
- Ikegami K, Mukai M, Tsuchida J, Heier RL, Macgregor GR, and Setou M (2006). TTLL7 is a mammalian beta-tubulin polyglutamylase required for growth of MAP2-positive neurites. The Journal of biological chemistry 281, 30707–30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke C, and Kneussel M (2010). Tubulin post-translational modifications: encoding functions on the neuronal microtubule cytoskeleton. Trends Neurosci 33, 362–372. [DOI] [PubMed] [Google Scholar]
- Jenuwein T, and Allis CD (2001). Translating the histone code. Science 293, 1074–1080. [DOI] [PubMed] [Google Scholar]
- Kormendi V, Szyk A, Piszczek G, and Roll-Mecak A (2012). Crystal structures of tubulin acetyltransferase reveal a conserved catalytic core and the plasticity of the essential N terminus. The Journal of biological chemistry 287, 41569–41575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix B, van Dijk J, Gold ND, Guizetti J, Aldrian-Herrada G, Rogowski K, Gerlich DW, and Janke C (2010). Tubulin polyglutamylation stimulates spastin-mediated microtubule severing. The Journal of cell biology 189, 945–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larcher JC, Boucher D, Lazereg S, Gros F, and Denoulet P (1996). Interaction of kinesin motor domains with alpha- and beta-tubulin subunits at a tau-independent binding site - Regulation by polyglutamylation. Journal of Biological Chemistry 271, 22117–22124. [DOI] [PubMed] [Google Scholar]
- Lee HH, Jan LY and Jan YN (2009). Drosophila IKK-related kinase Ik2 and katain p60-like 1 regulate dendrite pruning of sensory neuron during metamorphosis. Proc Natl Acad Sci U S A 106, 6363–6368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CW, Ferreon JC, Ferreon AC, Arai M, and Wright PE (2010). Graded enhancement of p53 binding to CREB-binding protein (CBP) by multisite phosphorylation. Proc Natl Acad Sci U S A 107, 19290–19295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Silhavy JL, Zaki MS, Schroth J, Bielas SL, Marsh SE, Olvera J, Brancati F, Iannicelli M, Ikegami K, et al. (2012). CEP41 is mutated in Joubert syndrome and is required for tubulin glutamylation at the cilium. Nature genetics 44, 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeboom JJ, Nakamura M, Hibbel A, Shundyak K, Gutierrez R, Ketelaar T, Emons AM, Mulder BM, Kirik V, and Ehrhardt DW (2013). A mechanism for reorientation of cortical microtubule arrays driven by microtubule severing. Science 342, 1245533. [DOI] [PubMed] [Google Scholar]
- Loughlin R, Wilbur JD, McNally FJ, Nedelec FJ and Heald R (2011). Katanin contributes to interspecies spindle scaling in Xenopus. Cell 147(6):1397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Srayko M, and Mains PE (2004). The Caenorhabditis elegans microtubule-severing complex MEI-1/MEI-2 katanin interacts differently with two superficially redundant beta-tubulin isotypes. Molecular biology of the cell 15, 142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magiera MM, and Janke C (2013). Investigating tubulin posttranslational modifications with specific antibodies. Methods Cell Biol 115, 247–267. [DOI] [PubMed] [Google Scholar]
- McNally KP, and McNally FJ (2011). The spindle assembly function of Caenorhabditis elegans katanin does not require microtubule-severing activity. Molecular biology of the cell 22, 1550–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KE, and Heald R (2015). Glutamylation of Nap1 modulates histone H1 dynamics and chromosome condensation in Xenopus. The Journal of cell biology 209, 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai M, Ikegami K, Sugiura Y, Takeshita K, Nakagawa A, and Setou M (2009). Recombinant mammalian tubulin polyglutamylase TTLL7 performs both initiation and elongation of polyglutamylation on beta-tubulin through a random sequential pathway. Biochemistry 48, 1084–1093. [DOI] [PubMed] [Google Scholar]
- Nash P, Tang X, Orlicky S, Chen Q, Gertler FB, Mendenhall MD, Sicheri F, Pawson T, Tyers M. (2001). Multisite phosphorylation of a CDK inhibitor sets a threshold for the onset of DNA replication.Nature. 414(6863):514–21. [DOI] [PubMed] [Google Scholar]
- Peris L, Wagenbach M, Lafanechere L, Brocard J, Moore AT, Kozielski F, Job D, Wordeman L, and Andrieux A (2009). Motor-dependent microtubule disassembly driven by tubulin tyrosination. The Journal of cell biology 185, 1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pufall MA, Lee GM, Nelson ML, Kang HS, Velyvis A, Kay LE, McIntosh LP, and Graves BJ (2005). Variable control of Ets-1 DNA binding by multiple phosphates in an unstructured region. Science 309, 142–145. [DOI] [PubMed] [Google Scholar]
- Raybin D, and Flavin M (1975). An enzyme tyrosylating alpha-tubulin and its role in microtubule assembly. Biochem Biophys Res Commun 65, 1088–1095. [DOI] [PubMed] [Google Scholar]
- Redeker V (2010). Mass spectrometry analysis of C-terminal posttranslational modifications of tubulins. Methods Cell Biol 95, 77–103. [DOI] [PubMed] [Google Scholar]
- Redeker V, Levilliers N, Vinolo E, Rossier J, Jaillard D, Burnette D, Gaertig J, and Bre MH (2005). Mutations of tubulin glycylation sites reveal cross-talk between the C termini of alpha- and beta-tubulin and affect the ciliary matrix in Tetrahymena. The Journal of biological chemistry 280, 596–606. [DOI] [PubMed] [Google Scholar]
- Regnard C, Desbruyeres E, Denoulet P, and Edde B (1999). Tubulin polyglutmylase: isozymic variants and regulation during the cell cycle in HeLa cells. J Cell Sci 112, 4281–4289. [DOI] [PubMed] [Google Scholar]
- Ribbeck K, and Mitchison TJ (2006). Meiotic spindle: sculpted by severing. Current biology : CB 16, R923–925. [DOI] [PubMed] [Google Scholar]
- Rogowski K, van Dijk J, Magiera MM, Bosc C, Deloulme JC, Bosson A, Peris L, Gold ND, Lacroix B, Bosch Grau M, et al. (2010). A family of protein-deglutamylating enzymes associated with neurodegeneration. Cell 143, 564–578. [DOI] [PubMed] [Google Scholar]
- Roll-Mecak A (2015). Intrinsically disordered tubulin tails: complex tuners of microtubule functions? Seminars in cell & developmental biology 37, 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll-Mecak A, and McNally FJ (2010). Microtubule-severing enzymes. Current opinion in cell biology 22, 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll-Mecak A, and Vale RD (2005). The Drosophila homologue of the hereditary spastic paraplegia protein, spastin, severs and disassembles microtubules. Current biology : CB 15, 650–655. [DOI] [PubMed] [Google Scholar]
- Roll-Mecak A, and Vale RD (2006). Making more microtubules by severing: a common theme of noncentrosomal microtubule arrays? The Journal of cell biology 175, 849–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll-Mecak A, and Vale RD (2008). Structural basis of microtubule severing by the hereditary spastic paraplegia protein spastin. Nature 451, 363–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Plessmann U, Felleisen R, and Weber K (1998). Posttranslational modications of trichomonad tubulins; identication of multiple glutamylation sites. FEBS Letters 429, 399–402. [DOI] [PubMed] [Google Scholar]
- Schulze E, Asai DJ, Bulinski JC, and Kirschner M (1987). Posttranslational modification and microtubule stability. The Journal of cell biology 105, 2167–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Bryant J, Wloga D, Donaldson R, Davis RC, Jerka-Dziadosz M, and Gaertig J (2007). Katanin regulates dynamics of microtubules and biogenesis of motile cilia. The Journal of cell biology 178, 1065–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood NT, Sun Q, Xue M, Zhang B, and Zinn K (2004). Drosophila spastin regulates synaptic microtubule networks and is required for normal motor function. PLoS biology 2, e429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirajuddin M, Rice LM, and Vale RD (2014). Regulation of microtubule motors by tubulin isotypes and post-translational modifications. Nat Cell Biol 16, 335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srayko M, O’Toole E T, Hyman AA, and Muller-Reichert T (2006). Katanin disrupts the microtubule lattice and increases polymer number in C. elegans meiosis. Current biology : CB 16, 1944–1949. [DOI] [PubMed] [Google Scholar]
- Stewart A, Tsubouchi MM, A., Rolls MM, Tracey WD, and Sherwood NT (2012). Katanin p60-like1 promotes microtubules growth and terminal dendrite stability in the larval class IV sensory neurons of Drosophila. J. Neurosci, 32, 11631–11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone MC, Rao K, Gheres KW, Kim S, Tao J, La Rochelle C, Folker CT, Sherwood NT, and Rolls MM (2012). Normal spastin gene dosage is specifically required for axon regeneration. Cell Rep., 2, 1340–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo H, and Baas PW (2010). Acetylation of microtubules influences their sensitivity to severing by katanin in neurons and fibroblasts. The Journal of neuroscience : the official journal of the Society for Neuroscience 30, 7215–7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan KF (1988). Structure and utilization of tubulin isotypes. Annu Rev Cell Biol 4, 687–716. [DOI] [PubMed] [Google Scholar]
- Szyk A, Deaconescu AM, Piszczek G, and Roll-Mecak A (2011). Tubulin tyrosine ligase structure reveals adaptation of an ancient fold to bind and modify tubulin. Nat Struct Mol Biol 18, 1250–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyk A, Deaconescu AM, Spector J, Goodman B, Valenstein ML, Ziolkowska NE, Kormendi V, Grigorieff N, and Roll-Mecak A (2014). Molecular basis for age-dependent microtubule acetylation by tubulin acetyltransferase. Cell 157, 1405–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta N, Orso G, Rossetto MG, Daga A, and Broadie K (2004). The hereditary spastic paraplegia gene, spastin, regulates microtubule stability to modulate synaptic structure and function. Current biology : CB 14, 1135–1147. [DOI] [PubMed] [Google Scholar]
- van Dijk J, Miro J, Strub JM, Lacroix B, van Dorsselaer A, Edde B, and Janke C (2008). Polyglutamylation is a post-translational modification with a broad range of substrates. The Journal of biological chemistry 283, 3915–3922. [DOI] [PubMed] [Google Scholar]
- van Dijk J, Rogowski K, Miro J, Lacroix B, Edde B, and Janke C (2007). A targeted multienzyme mechanism for selective microtubule polyglutamylation. Molecular cell 26, 437–448. [DOI] [PubMed] [Google Scholar]
- Vemu A, Garnham CP, Lee DY, and Roll-Mecak A (2014). Generation of differentially modified microtubules using in vitro enzymatic approaches. Methods in enzymology 540, 149–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhey KJ, and Gaertig J (2007). The tubulin code. Cell Cycle 6, 2152–2160. [DOI] [PubMed] [Google Scholar]
- Vietri M, Schink KO, Campsteijn C, Wegner CS, Schultz SW, Christ L, Thoresen SB, Brech A, Raiborg C, and Stenmark H (2015). Spastin and ESCRT-III coordinate mitotic spindle disassembly and nuclear envelope sealing. Nature 522, 231–235. [DOI] [PubMed] [Google Scholar]
- Webster DR, Gundersen GG, Bulinski JC, and Borisy GG (1987). Differential turnover of tyrosinated and detyrosinated microtubules. Proc Natl Acad Sci U S A 84, 9040–9044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widlund PO, Podolski M, Reber S, Alper J, Storch M, Hyman AA, Howard J, and Drechsel DN (2012). One-step purification of assembly-competent tubulin from diverse eukaryotic sources. Molecular biology of the cell 23, 4393–4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff A, Denechaud B, Chillet D, Mazarguil H, Desbruyeres E, Audebert S, Edde B, Gros F, and Denoulet P (1992). Distribution of Glutamylated Alpha-Tubulin and Beta-Tubulin in Mouse-Tissues Using a Specific Monoclonal-Antibody, Gt335. Eur J Cell Biol 59, 425–432. [PubMed] [Google Scholar]
- Wright PE, and Dyson HJ (2015). Intrinsically disordered proteins in cellular signalling and regulation. Nature reviews Molecular cell biology 16, 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu I, Garnham CP, and Roll-Mecak A (2015). Writing and Reading the Tubulin Code. The Journal of biological chemistry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Qiang L, Solowska JM, Karabay A, Korulu S, and Baas PW (2008). The microtubule-severing proteins spastin and katanin participate differently in the formation of axonal branches. Molecular biology of the cell 19, 1485–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zempel H, Luedtke J, Kumar Y, Biernat J, Dawson H, Mandelkow E, and Mandelkow EM (2013). Amyloid-beta oligomers induce synaptic damage via Tau-dependent microtubule severing by TTLL6 and spastin. EMBO J 32, 2920–2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Rogers GC, Buster DW, and Sharp DJ (2007). Three microtubule severing enzymes contribute to the “Pacman-flux” machinery that moves chromosomes. The Journal of cell biology 177, 231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziolkowska NE, and Roll-Mecak A (2013). In vitro microtubule severing assays. Methods in molecular biology 1046, 323–334. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.