This phase 3 randomized clinical trial assesses differences in health-related quality of life between men with low-risk prostate cancer who are treated with hypofractionated vs conventional radiotherapy.
Key Points
Question
Are there differences in health-related quality of life between men with low-risk prostate cancer who are treated with hypofractionated vs conventional radiotherapy?
Findings
In this phase 3 randomized clinical trial of 962 trial participants, treatment with hypofractionated radiotherapy was found to be noninferior to conventional radiotherapy in men with low-risk prostate cancer in terms of disease-free survival with no differences in prostate cancer–specific (eg, bowel, bladder, sexual) and general quality of life, as well as in anxiety and depression.
Meaning
Level 1 evidence affirms hypofractionated radiotherapy as a new practice standard for men with localized prostate cancer.
Abstract
Importance
Hypofractionated radiotherapy (HRT) would be more convenient for men with low-risk prostate cancer and cost less than conventional radiotherapy (CRT) as long as HRT is noninferior to CRT in terms of survival and quality of life (QOL) is not found to be worse.
Objective
To assess differences in QOL between men with low-risk prostate cancer who are treated with HRT vs CRT.
Design, Setting, and Participants
In this phase 3 randomized clinical trial, men with low-risk prostate cancer were enrolled from sites within the National Cancer Institute’s National Clinical Trials Network in the United States, Canada, and Switzerland.
Interventions
Random assignment to CRT (73.8 Gy in 41 fractions over 8.2 weeks) or to HRT (70 Gy in 28 fractions over 5.6 weeks).
Main Outcomes and Measures
Quality of life was assessed using the Expanded Prostate Index Composite questionnaire measuring bowel, urinary, sexual, and hormonal domains; the 25-item Hopkins Symptom Checklist measuring anxiety and depression; and the EuroQol–5 Dimension questionnaire measuring global QOL. All data were collected at baseline and 6, 12, 24, and 60 months. Change scores were compared between treatment arms using the Wilcoxon signed rank test. A significance level of .0125 to adjust for multiple comparisons was used for an overall 2-sided type 1 error of .05. Clinical significance was determined for the Expanded Prostate Index Composite change scores by an effect size of 0.5.
Results
Of 1092 patients analyzable for the primary end point, 962 (mean [SD] age, 66.6 [7.4] years) consented to the QOL component. No statistically significant differences with regard to baseline characteristics nor any of the QOL baseline domains were measured between arms. There were no differences in change score between arms with respect to any of the Expanded Prostate Index Composite questionnaire domain scores except at 12 months when the HRT arm had a larger decline than the CRT arm in the bowel domain (mean score, −7.5 vs −3.7, respectively; P<.001), but it did not reach clinical significance (effect size = 0.29). There were no differences between arms at any time point for the Hopkins Symptom Checklist nor EuroQol–5 Dimension questionnaire.
Conclusions and Relevance
Treatment with HRT is noninferior to CRT in men with low-risk prostate cancer in terms of disease-free survival and, as shown in the present study, in prostate cancer–specific (eg, bowel, bladder, sexual) and general QOL, as well as in anxiety and depression. This study provides evidence to affirm that HRT is a practice standard for men with low-risk prostate cancer.
Trial Registration
ClinicalTrials.gov identifier: NCT00331773
Introduction
In a study in which traditional outcomes of survival and progression are hypothesized to be similar, the outcomes of adverse events (AEs), health-related quality of life (QOL), and resources gain in importance. These latter outcomes play a significant role in patient, clinician, and policy considerations of a study. The NRG Oncology RTOG 0415 study (Supplement 1) is a randomized phase 3 noninferiority trial that compares conventional radiotherapy (CRT) of 73.8 Gy in 41 fractions over 8.2 weeks with hypofractionated radiotherapy (HRT) of 70 Gy in 28 fractions over 5.6 weeks for patients with low-risk prostate cancer. Briefly, the study has reported similar 5-year disease-free survival of about 85% in both arms and has met per-protocol criteria for noninferiority. However, late physician-reported grade 2 and 3 gastrointestinal and genitourinary Common Terminology Criteria Adverse Events (CTCAEs) were increased in patients treated with HRT.
It has been well documented in previous radiotherapy (RT) trials that for more subjective parameters (eg, sexual function, anxiety, depression) or parameters with a subjective component not easily observable by clinicians (eg, diarrhea, bloating), patient-reported outcomes are more reflective of the patient experience and sometimes identify more bothersome symptoms than CTCAE data. Thus, to supplement the CTCAE data, the present study was planned to compare the treatment arms for differences in prostate cancer–specific and general QOL outcomes, anxiety, and depression. Results of these outcomes may determine whether alternative RT regimens advance to wider use, because adoption has been limited in prostate cancer and other cancer settings despite multiple supportive trial results.
Methods
In RTOG 0415, the NRG Oncology Statistics and Data Management Center randomized patients to receive CRT or HRT by using permuted block randomization in a parallel fashion 1:1. Sites within the National Cancer Institute’s National Clinical Trials Network in the United States, Canada, and Switzerland enrolled participants. This study was approved by each site’s institutional review board, and all patients signed written informed consent. A total of 1092 men, 542 patients in the CRT arm and 550 in the HRT arm, were randomized and eligible for analysis with a median follow-up of 5.8 years. Disease-free survival, the primary end point, at 5 years was 85.3% (95% CI, 81.9%-88.1%) in the CRT arm and 86.3% (95% CI, 83.1%-89.0%) in the HRT arm, which met per-protocol criteria for noninferiority. Late physician-reported grade 2 and 3 gastrointestinal and genitourinary CTCAEs were increased (hazard ratio, 1.3-1.6) in the HRT arm. Eligible patients had organ-confined, biopsy-proven Gleason scores of 2 to 6 and prostate-specific antigen of less than 10 ng/mL. Patients were ineligible if they had metastatic disease, had a Zubrod performance status score greater than 1, used antiandrogens, or had prior therapy for prostate cancer.
All patients in the protocol were offered the opportunity to participate in the QOL component, measuring secondary end points; 962 consented to the QOL assessment (478 in the CRT arm and 484 in the HRT arm) and were required to have baseline measures administered prior to start of per-protocol therapy. All QOL-measuring instruments were also administered at 6, 12, 24, and 60 months, and included the comparison of disease-specific, health-related QOL, measured by the Expanded Prostate Cancer Index Composite (EPIC) questionnaire; anxiety and depression, measured by the 25-item Hopkins Symptom Checklist (HSCL-25); and health utilities, measured by the EuroQol–5 Dimension (EQ–5D) questionnaire.
Measures
Prostate cancer–specific QOL was measured by the reliable and valid patient-reported EPIC. EPIC is a 50-item measure designed to evaluate patient function and bother after prostate cancer treatment by using a Likert-like scale with responses transformed to a scale of 0 to 100. Higher scores indicate better QOL. The instrument includes 4 separately validated domains: urinary, bowel, sexual, and hormonal. Each domain requires at least 80% of the items to be completed for inclusion in analysis.
Anxiety and depression were assessed with the HSCL-25, a 25-item, patient-reported instrument. It has demonstrated reliability and validity across a variety of general and medical populations, including cancer. The total score of the HSCL-25 is calculated as the sum of all 25 items and requires all items to be completed for analysis. Scores range from 25 to 100, with higher scores indicating worse symptoms. Two cutoff scores are used in the literature (a conservative and more commonly used cutoff of 44 and above, and a more liberal cutoff of 39 and above) to indicate a positive screen (caseness) for anxiety or distress. This study assesses both.
The EQ–5D is a 2-part self-assessment questionnaire. The first part consists of 5 items covering 5 dimensions: mobility, self care, usual activities, pain or discomfort, and anxiety or depression. Each dimension can be graded on 3 levels: (1) no problems, (2) moderate problems, and (3) extreme problems. The second part is a visual analogue scale valuing current health state, measured on a 20-cm, 10-point interval scale. Worst imaginable health state is scored as 0 at the bottom of the scale, and best imaginable health state is scored as 100 at the top.
Statistical Analysis
Pretreatment characteristics were compared between arms for analyzable patients using Fisher exact test. Patients with missing and completed assessments at each time point were compared with respect to pretreatment characteristics. All eligible patients consenting to the QOL component of the study were analyzed according to the arm in which they were randomized.
For the EPIC analysis, change scores, calculated as follow-up score minus baseline score, at 6, 12, 24, and 60 months from the initiation of RT were compared between treatment arms using a t test. A negative change score indicated a decline in QOL. A significance level of .0125 to adjust for multiple comparisons with respect to the 4 EPIC domain scores was used to maintain an overall 2-sided type 1 error of 0.05. In addition, an a priori test of clinical significance for EPIC change scores was determined using an effect size of 0.5 standard deviations. A maximum likelihood estimation (both full and reduced) model using backward selection by hand was run for each domain. A significance level of .10 was required to remain in the reduced model. In addition to time, treatment, time by treatment interaction, and baseline score, other pretreatment characteristics were considered. Variables that were found to be significantly different between patients with missing and completed assessments were forced into reduced models to account for any biases owing to missing data. The primary end point sample size provided sufficient statistical power to conduct these secondary end point analyses. Specifically, 194 patients per treatment arm would provide 90% statistical power to compare the change scores from baseline between treatment arms for the 4 EPIC domains, each with a type 1 error rate of 0.0125 (after a Bonferroni adjustment) if the completion rate was only 60%.
For the HSCL-25 analysis, a comparison between arms of total score at baseline and at follow-up time points was conducted using a Wilcoxon signed rank test. A paired analysis of baseline with follow-up total scores was conducted using a paired t test. Significance was set at .05. A mixed-effects (both full and reduced) model using maximum likelihood estimation assessed the effect of time, treatment arm, stratification factors, and other pretreatment characteristics on total score while controlling for baseline HSCL-25 total score. Covariates in the model were tested using a t test. Time, treatment arm, and covariates significant at the .10 level remained in the reduced model. Caseness was determined using 2 different cutoff points (39 and 44) as previously described, and χ2 tests were used to compare differences in caseness at each time point between arms. Visual analogue scale and index scores from the EQ–5D were compared at each time point using a t test.
Results
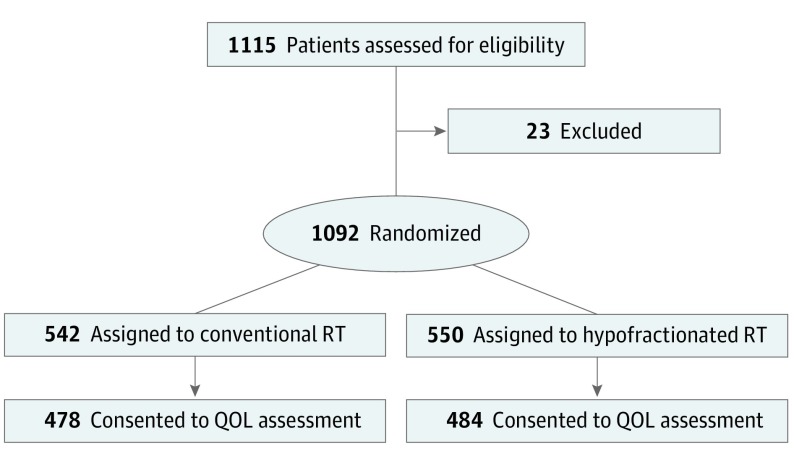
There were 1115 patients randomized from April 2006 to December 2009 when the target accrual was met; of these patients, 1092 were analyzable for the primary end point analysis. Of analyzable patients, 962 consented to the secondary end point QOL assessment (478 in the CRT arm and 484 in the HRT arm) (Figure and Table 1). Pretreatment characteristics are summarized in Table 2 and were similar between arms. At baseline, patients missing assessments and with completed assessments were similar with respect to these characteristics (differences at 6, 12, 24, and 60 months are summarized in the eTable in Supplement 2).
Figure. CONSORT Flowchart.
EPIC indicates the Expanded Prostate Cancer Index Composite questionnaire; EQ–5D, EuroQol–5 Dimension questionnaire; HSCL-25, Hopkins Symptom Checklist; QOL, quality of life; RT, radiotherapy.
Table 1. Patient Completion of Quality-of-Life Assessment Measurements.
| Quality-of-Life Measurement | CRT Arm, No. (%) (n = 478) | HRT Arm, No. (%) (n = 484) |
|---|---|---|
| EPIC | ||
| Baseline | 423 (88.5) | 440 (90.9) |
| 6 Months | 328 (68.6) | 373 (77.1) |
| 12 Months | 306 (64.0) | 329 (68.0) |
| 24 Months | 259 (54.2) | 275 (56.8) |
| 60 Months | 286 (59.8) | 299 (61.8) |
| HSCL-25 | ||
| Baseline | 378 (79.1) | 398 (82.2) |
| 6 Months | 298 (62.3) | 337 (69.6) |
| 12 Months | 288 (60.3) | 308 (63.6) |
| 24 Months | 237 (49.6) | 250 (51.7) |
| 60 Months | 271 (56.7) | 281 (58.1) |
| EQ–5D | ||
| Baseline | 418 (87.4) | 439 (90.7) |
| 6 Months | 324 (67.8) | 371 (76.7) |
| 12 Months | 303 (63.4) | 322 (66.5) |
| 24 Months | 245 (51.3) | 259 (53.5) |
| 60 Months | 277 (57.9) | 287 (59.3) |
Abbreviations: CRT, conventional radiotherapy; EPIC, Expanded Prostate Cancer Index Composite questionnaire; EQ–5D, EuroQol–5 Dimension questionnaire; HRT, hypofractionated radiotherapy; HSCL-25, Hopkins Symptom Checklist.
Table 2. Patient and Tumor Characteristics for Patients Who Consented to the Quality-of-Life Assessment.
| Characteristics | CRT Arm, No. (%) (n = 478) | HRT Arm, No. (%) (n = 484) | P Valuea |
|---|---|---|---|
| Age, y | .40 | ||
| ≤65 | 200 (41.8) | 216 (44.6) | |
| >65 | 278 (58.2) | 268 (55.4) | |
| Race | .94 | ||
| Other | 93 (19.6) | 96 (20.0) | |
| White | 381 (80.4) | 384 (80.0) | |
| Ethnicity | .71 | ||
| Hispanic or Latin American | 16 (3.6) | 13 (2.9) | |
| Not Hispanic or Latin American | 429 (96.4) | 431 (97.1) | |
| Zubrod performance status score | .13 | ||
| 0 | 450 (94.1) | 443 (91.5) | |
| 1 | 28 (5.9) | 41 (8.5) | |
| PSA, ng/mL | .63 | ||
| <4 | 93 (19.5) | 101 (20.9) | |
| 4 to <10 | 385 (80.5) | 383 (79.1) | |
| Gleason score | .25 | ||
| 2-4 | 2 (0.4) | 0 | |
| 5-6 | 476 (99.6) | 484 (100) | |
| T stage | .24 | ||
| T1 | 368 (77.0) | 388 (80.2) | |
| T2 | 110 (23.0) | 96 (19.8) | |
| N stage | .30 | ||
| N0 | 392 (82.0) | 409 (84.5) | |
| NX | 86 (18.0) | 75 (15.5) | |
| M stage | .62 | ||
| M0 | 476 (99.6) | 483 (99.8) | |
| MX | 2 (0.4) | 1 (0.2) | |
| RT modality | n = 470 | n = 482 | .93 |
| 3-Dimensional CRT | 89 (18.9) | 93 (19.3) | |
| IMRT | 381 (81.1) | 389 (80.7) |
Abbreviations: CRT, conventional radiotherapy; HRT, hypofractionated radiotherapy; IMRT, intensity-modulated radiation therapy; PSA, prostate-specific antigen; RT, radiotherapy.
P values from 2-sided Fisher exact tests.
EPIC compliance rates ranged from 89.7% at baseline, 66.0% at 12 months, 60.8% at 24 months, and 55.5% at 60 months. These rates were similar for the HSCL-25 and EQ–5D. An EPIC assessment was considered complete if at least one of the domains was scored. There were no differences in change score between arms with respect to any of the EPIC domain scores at 6, 24, or 60 months. At 12 months, the HRT arm experienced a larger decline than the CRT arm in the bowel domain only (mean score, −7.5 vs −3.7, respectively; P<.001; Table 3). However, the effect size of the change scores (mean differences between arms divided by the pooled standard deviation) was 0.29 and thus did not meet the a priori threshold of 0.5 for clinical significance.
Table 3. EPIC Change Scores at 12 Months.
| Domain | 3-Dimensional/IMRT | P Valuea | P Valueb | |
|---|---|---|---|---|
| CRT Arm (n = 306) | HRT Arm (n = 328) | |||
| Bowel c | ||||
| Patients, No. | 274 | 296 | .51 | <.001 |
| Mean (SD) | −3.7 (11.5) | −7.5 (14.5) | ||
| Median | −1.8 | −3.6 | ||
| Urinary | ||||
| Patients, No. | 276 | 301 | .99 | .11 |
| Mean (SD) | −0.3 (10.4) | −1.8 (13.3) | ||
| Median | 0.0 | 0.0 | ||
| Sexual | ||||
| Patients, No. | 262 | 273 | .99 | .91 |
| Mean (SD) | −8.2 (19.7) | −8.4 (20.0) | ||
| Median | −6.4 | −5.8 | ||
| Hormonal | ||||
| Patients, No. | 264 | 290 | .99 | .99 |
| Mean (SD) | −1.6 (8.5) | −1.6 (9.4) | ||
| Median | 0.0 | 0.0 | ||
Abbreviations: CRT, conventional radiotherapy; EPIC, Expanded Prostate Cancer Index Composite; HRT, hypofractionated radiotherapy; IMRT, intensity-modulated radiation therapy.
P value from 1-sided t test with pooled variances for test of H0: μ73.8Gy–μ70Gy≤δ. δ = 4 for bowel domain, δ = 6 for urinary domain, δ = 13 for sexual domain, and δ = 5 for hormonal domain.
P value from 2-sided t test with pooled variances for test of H0: μ73.8Gy–μ70Gy = 0.
Effect size for bowel domain (Cohen’s d), 0.29.
Tests of the treatment for each domain were conducted for the reduced longitudinal model (Table 4). When tested against the moderate effect size (4 points for bowel and 6 points for urinary), the bowel and urinary domains were not clinically meaningful (bowel estimate, −1.97; standard error, 0.78; P = .99; and urinary estimate, −1.96; standard error, 0.71; P = .99). Treatment arm was not associated with EPIC sexual score or hormonal score.
Table 4. Longitudinal Models for EPIC Bowel and Urinary Domainsa.
| Outcome | Effect (Reference) | Estimate (SE) | P Valueb |
|---|---|---|---|
| EPIC bowel domain | Intercept | 30.42 (5.02) | <.001 |
| Time | −0.0002 (0.01) | .98 | |
| Treatment arm (CRT arm) | −2.00 (0.78) | .01 | |
| Baseline EPIC bowel score | 0.66 (0.04) | <.001 | |
| Zubrod performance status score (0) | 2.46 (1.48) | .10 | |
| RT modality (3-dimensional CRT) | −0.09 (0.98) | .92 | |
| Ethnicity (Hispanic or Latin American) | 0.36 (2.53) | .89 | |
| Age (≤65 y) | −0.29 (0.80) | .71 | |
| Race (other) | −3.13 (1.07) | .004 | |
| Test of H0: μ73.8Gy–μ70Gy≤4 | −1.97 (0.78) | .99 | |
| EPIC urinary domain | Intercept | 30.59 (3.90) | <.001 |
| Time | −0.02 (0.01) | .01 | |
| Treatment arm (3-dimensional/IMRT CRT) | −1.96 (0.71) | .006 | |
| Baseline EPIC urinary score | 0.60 (0.03) | <.001 | |
| N stage (N0) | 1.79 (0.96) | .06 | |
| RT modality (3-dimensional CRT) | 0.11 (0.89) | .90 | |
| Ethnicity (Hispanic or Latin American) | 3.28 (2.39) | .17 | |
| Age (≤65 y) | 0.50 (0.73) | .49 | |
| Race (other) | 1.08 (0.96) | .26 | |
| Test of H0: μ73.8Gy–μ70Gy≤6 | −1.96 (0.71) | .99 |
Abbreviations: CRT, conventional radiotherapy; EPIC, Expanded Prostate Cancer Index Composite; HRT, hypofractionated radiotherapy; IMRT, intensity-modulated radiation therapy; SE, standard error.
Time, treatment arm, and significant covariates (P < .10) remained in the model, and variables that were significantly different between men with missing and completed EPIC assessments (eTable in the Supplement) were included except radiotherapy received due to the high correlation with planned radiotherapy.
P value from t test.
The HSCL-25 median scores at baseline for the CRT and HRT arms were 29.0 (range, 25.0-68.0) and 29.0 (range, 25.0-76.0), respectively (P = .05), with little change over time. There were no significant differences observed in caseness as defined by a score of 44 or more at any time point. The only significant difference between arms in caseness was when using the more liberal cutoff point of 39 at 12 months. More patients in the HRT arm had caseness as compared with the CRT arm (56 of 302 [18.2%] patients vs 34 of 288 [11.8% ] patients, respectively; absolute difference, 6.4%; 95% CI, 4.4%-8.4%; relative difference, 54.2%; 95% CI, 50.2%-58.2%; P = .03). However, the median HSCL-25 scores were similar for men with caseness (44.5 [range, 39.0-89.0] for the HRT arm vs 44.5 [range, 39.0-81.0] for the CRT arm), and there were no differences in this cutoff at subsequent time points. There were no differences in EQ–5D follow-up in visual analogue scale or index scores between the arms at any time point.
Discussion
The RTOG 0415 study demonstrated HRT to be noninferior to CRT in men with low-risk prostate cancer. This follows on a preponderance of data showing HRT as noninferior to CRT in both low- and intermediate-risk prostate cancer. Before practice standards are revised, it is important to demonstrate that not only is tumor control and disease-free survival noninferior, but also that QOL is not diminished with a shorter fractionation schedule with a higher dose per treatment session. Physician-reported AEs showed a slightly higher late grade 2 gastrointestinal CTCAE rate of 18.3% in the HRT arm compared with 11.4% in the CRT arm (relative risk, 1.59; 95% CI, 1.22-2.06; P = .002). There were no significant differences in physician-reported grade 3 or higher gastrointestinal or any grade genitourinary AEs.
In the present study, there was no difference in patient-reported urinary symptom scores between arms at any time point. There were also no differences in patient-reported bowel symptom scores between arms at 6, 24, or 60 months. At 12 months, the HRT arm had a mean 3.8-point and median 1.6-point greater decline on the bowel domain score than the CRT arm. Although these differences were statistically significant, the effect size of the change scores did not meet the a priori threshold for clinical significance. Clinical significance is an important metric when assessing the issue of interpretability of patient-reported outcomes. It helps us understand what changes in score correspond to small, moderate, or large patient benefit or decrement. For example, if a person improves by 5 points on a 100-point scale on sexual function, will he be more satisfied with his sexual experiences or perceived as having better sexual function by his partner? In this context, the present study shows that the single time point the bowel score was statistically significantly worse in the HRT arm was of small consequence as perceived by the patient. A limitation of the study is the declining compliance with the patient-reported outcome metrics over time, a common issue in large clinical trials. However, at 24 months with a 61% compliance rate with EPIC, these findings are consistent with the recent CHHiP (conventional or hypofractionated high dose intensity modulated radiotherapy for prostate cancer) trial, which found no difference in EPIC bowel or urinary scores after HRT compared with after CRT up to 24 months posttreatment. This may increase confidence in and reproducibility of the data.
In this study, there was no clinically meaningful difference between arms in mental health status defined by anxiety or depression. Men receiving either treatment had mean anxiety or depression scores well below caseness and, compared with a study screening for depression and anxiety in a cohort of patients beginning RT for any cancer, had scores slightly better, with a mean 3.5 points lower using the cutoff of 44, and almost exactly the same score as a study of patients with prostate cancer from the Netherlands using the cutoff of 39 (mean, 31.7).
Limitations
One limitation of this study is that it did not specify a single time point for the primary QOL EPIC end point. Also, the design did not fully account for the 16 multiple comparisons examined (4 time points within each of the 4 domains), though post-hoc power calculations indicate sufficient power (>90%) to detect effect sizes of 0.5 with 534 patients.
Conclusions
A study to identify factors men consider important when choosing RT over radical prostatectomy for localized prostate cancer found the advantages many men cited for choosing were evidence in favor of RT and less incontinence. The most frequently patient-cited disadvantage of RT was the long duration of therapy. Thus, evidence-based rationale for shorter duration of therapy, as documented in this study, seems in keeping with patient preferences. Therapy that has no more AEs for a course of 5.6 weeks compared with 8.2 weeks would have obvious implications for patient decision making and resource savings. A recent editorial discussing the 3 recent CRT vs HRT trials establishing the noninferiority of HRT concluded that “The evidence is clear, the value to patients is obvious, and the potential cost savings are substantial. It is time to use moderate hypofractation for patients with intact prostate cancer.”(721) It should be noted that most patients who qualified for this trial would also be eligible for active surveillance, and thus better QOL than RT or surgery, yet many men choose treatment.
Studies show the physician’s recommendation as one of the most influential decisional factors for men facing prostate cancer treatment options. Other reasons for choosing treatment over active surveillance include perceptions of treatment efficacy and adverse effects, which are mainly derived from physicians’ descriptions with occasional anecdotal experiences of family and friends, as well as information gleaned indiscriminately from the Internet. Thus, if men are to choose treatment, and most men do, the current study adds further evidence supporting HRT as a new standard of care, and it counters arguments that reduced treatment duration is offset by clinically meaningful increased AEs and decreased QOL.
Given the QOL findings and the treatment noninferiority of HRT, there should be no deterrents to its adoption, and HRT does not require new or additional technology or training. A calculation of technical plus professional Medicare reimbursement for 28 vs 41 fractions in this setting suggests an estimated health care cost savings of $7700 per patient treated with HRT. Overall savings in medical resource use and in societal and patient medical expense, increased convenience, and decreased time away from work and activities of daily living is clear. Metrics and incentives for value-based care are increasing, and regimens of HRT align well with this trend. Professional organizations now have sufficient evidence to deliberate cogently on refinements to practice guidelines for patients with localized prostate cancer. In conjunction with the results of the efficacy analysis of RTOG 0415, which demonstrated that moderate HRT is not inferior to CRT in terms of therapeutic efficacy, the current data add to the soundness of a conclusion that there is also no between-arm clinically meaningful differences in AEs nor quality of life, the last lingering concern of HRT.
Trial Protocol
eTable. Significant Patient and Tumor Characteristics by EPIC Completion Status at 6, 12, 24, and 60 Months
Data Sharing Statement
References
- 1.Lee WR, Dignam JJ, Amin MB, et al. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol. 2016;34(20):2325-2332. doi: 10.1200/JCO.2016.67.0448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Movsas B, Scott C, Langer C, et al. Randomized trial of amifostine in locally advanced non-small-cell lung cancer patients receiving chemotherapy and hyperfractionated radiation: radiation therapy oncology group trial 98-01. J Clin Oncol. 2005;23(10):2145-2154. doi: 10.1200/JCO.2005.07.167 [DOI] [PubMed] [Google Scholar]
- 3.Watkins-Bruner D, Scott C, Lawton C, et al. RTOG’s first quality of life study—RTOG 90-20: a phase II trial of external beam radiation with etanidazole for locally advanced prostate cancer. Int J Radiat Oncol Biol Phys. 1995;33(4):901-906. doi: 10.1016/0360-3016(95)02002-5 [DOI] [PubMed] [Google Scholar]
- 4.Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56(6):899-905. doi: 10.1016/S0090-4295(00)00858-X [DOI] [PubMed] [Google Scholar]
- 5.Hough R, Landsverk J, Stone J, et al. Comparison of Psychiatric Screening For Primary Care Patients, final report on contract No. 278-81-0036 (DB). Bethesda, MD: National Institute of Mental Health ; 1995.
- 6.Bolton P. Cross-cultural validity and reliability testing of a standard psychiatric assessment instrument without a gold standard. J Nerv Ment Dis. 2001;189(4):238-242. doi: 10.1097/00005053-200104000-00005 [DOI] [PubMed] [Google Scholar]
- 7.van Scheppingen C, Schroevers MJ, Smink A, et al. Does screening for distress efficiently uncover meetable unmet needs in cancer patients? Psychooncology. 2011;20(6):655-663. doi: 10.1002/pon.1939 [DOI] [PubMed] [Google Scholar]
- 8.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337-343. doi: 10.3109/07853890109002087 [DOI] [PubMed] [Google Scholar]
- 9.Barry MJ. Quality of life and prostate cancer treatment. J Urol. 1999;162(2):407. doi: 10.1016/S0022-5347(05)68571-0 [DOI] [PubMed] [Google Scholar]
- 10.Dearnaley D, Syndikus I, Mossop H, et al. ; CHHiP Investigators . Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17(8):1047-1060. doi: 10.1016/S1470-2045(16)30102-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Catton CN, Lukka H, Gu CS, et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol. 2017;35(17):1884-1890. doi: 10.1200/JCO.2016.71.7397 [DOI] [PubMed] [Google Scholar]
- 12.Guyatt GH, Osoba D, Wu AW, Wyrwich KW, Norman GR; Clinical Significance Consensus Meeting Group . Methods to explain the clinical significance of health status measures. Mayo Clin Proc. 2002;77(4):371-383. doi: 10.4065/77.4.371 [DOI] [PubMed] [Google Scholar]
- 13.Wagner LI, Pugh SL, Small W Jr, et al. Screening for depression in cancer patients receiving radiotherapy: feasibility and identification of effective tools in the NRG Oncology RTOG 0841 trial. Cancer. 2017;123(3):485-493. doi: 10.1002/cncr.29969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmboe ES, Concato J. Treatment decisions for localized prostate cancer: asking men what’s important. J Gen Intern Med. 2000;15(10):694-701. doi: 10.1046/j.1525-1497.2000.90842.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bekelman JE, Lee WR. Six questions to ask before we shorten radiation treatments for intact prostate cancer. Int J Radiat Oncol Biol Phys. 2017;97(4):718-721. doi: 10.1016/j.ijrobp.2016.11.038 [DOI] [PubMed] [Google Scholar]
- 16.Donovan JL, Hamdy FC, Lane JA, et al. ; ProtecT Study Group . Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375(15):1425-1437. doi: 10.1056/NEJMoa1606221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bayliss DR, Duff J, Stricker P, Walker K. Decision-making in prostate cancer—choosing active surveillance over other treatment options: a literature review. Urol Nurs. 2017;37(1):15-22. doi: 10.7257/1053-816X.2017.37.1.15 [DOI] [PubMed] [Google Scholar]
- 18.Xu J, Dailey RK, Eggly S, Neale AV, Schwartz KL. Men’s perspectives on selecting their prostate cancer treatment. J Natl Med Assoc. 2011;103(6):468-478. doi: 10.1016/S0027-9684(15)30359-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohideen N, Kavanagh BD, Beyer D, Madhani S, Steinberg ML. Radiation oncology: a perspective on health reform and value-based initiatives. J Oncol Pract. 2014;10(4):e212-e214. doi: 10.1200/JOP.2013.001337 [DOI] [PubMed] [Google Scholar]
- 20.Paravati AJ, Boero IJ, Triplett DP, et al. Variation in the cost of radiation therapy among Medicare patients with cancer. J Oncol Pract. 2015;11(5):403-409. doi: 10.1200/JOP.2015.005694 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable. Significant Patient and Tumor Characteristics by EPIC Completion Status at 6, 12, 24, and 60 Months
Data Sharing Statement