Abstract
It is estimated that most colon cancers can be attributed to dietary causes. We have hypothesized that diet influences the health of the colonic mucosa through interaction with the microbiota and that it is the milieu interior that regulates mucosal proliferation and therefore cancer risk. To validate this further, we compared colonic contents from healthy 50- to 65-y-old people from populations with high and low risk, specifically low risk Native Africans (cancer incidence <1:100,000; n = 17), high risk African Americans (risk 65:100,000; n = 17), and Caucasian Americans (risk 50:100,000; n = 18). Americans typically consume a high-animal protein and -fat diet, whereas Africans consume a staple diet of maize meal, rich in resistant starch and low in animal products. Following overnight fasting, rapid colonic evacuation was performed with 2 L polyethylene glycol. Total colonic evacuants were analyzed for SCFA, vitamins, nitrogen, and minerals. Total SCFA and butyrate were significantly higher in Native Africans than in both American groups. Colonic folate and biotin content, measured by Lactobacillus rhamnoses and Lactobacillus plantarum ATCC 8014 bioassay, respectively, exceeded normal daily dietary intakes. Compared with Africans, calcium and iron contents were significantly higher in Caucasian Americans and zinc content was significantly higher in African Americans, but nitrogen content did not differ among the 3 groups. In conclusion, the results support our hypothesis that the microbiota mediates the effect diet has on colon cancer risk by their generation of butyrate, folate, and biotin, molecules known to play a key role in the regulation of epithelial proliferation.
Introduction
Population studies have shown wide variations in the risk of colon cancer. Differences in inherited susceptibility characteristics and related gene-environment interactions are some of the possible explanations. Diet-gene interactions have been the most extensively studied and are among the most widely accepted explanations for the variation. Colon cancer is the second leading cause of cancer death in Americans, and African Americans are at the highest risk of developing and dying from colon cancer. However, our recent studies have highlighted the paradox that Africans living in Africa rarely get this disease (1,2). The observation that colon cancer is more common in developed than in developing nations suggests that the westernized way of life may be responsible for the increased risk of the disease (3). The aspect that has attracted the most attention is diet, as experimental studies have provided robust evidence for the ability of nutrients to modify cancer risk. Some dietary factors, such as fresh fruit and vegetables, fiber, antioxidants, and calcium and vitamin D suppress risk, whereas others such as red meat and saturated animal fat increase risk (3,4). Based on our own investigations, we have concluded that high intakes of red meat may be responsible for the increased risk in African Americans, whereas the low incidence in Africans may be a consequence of their high intakes of maize (corn) meal and low consumption of meat and animal fat (1). A novel aspect of our investigations was the identification of major differences in colonic bacterial metabolism. In African Americans, undigested carbohydrate was chiefly metabolized by hydrogen-producing bacteria, whereas in Africans it was mainly fermented by methane-producing bacteria (1). Our conclusion that the colonic microbiota was different was backed up by our detection of higher rates of colonization with secondary bile acid-producing bacteria in African Americans and Lactobacilli species in Africans. It is established that a diet high in animal fat stimulates the growth of secondary bile salt-producing bacteria and further studies have shown that secondary bile salts are cytotoxic and carcinogenic (5). A diet rich in red meat also stimulates the growth of sulfate-reducing bacteria, which produce hydrogen sulfide, which experimentally is known to be genotoxic (6). In contrast, Lactobacilli promote mucosal health (7,8), as do butyrate-producing Firmicutes. There is extensive experimental evidence that the SCFA butyrate is essential for colonic mucosal health. It is the preferred energy source for colonocytes and it suppresses epithelial proliferation, which is a recognized biomarker for cancer risk. Finally, the microbiota synthesize essential vitamins and recent studies have shown that transport mechanisms for their absorption exist in the colon. Two of these, folate and biotin, are intimately involved with epigenetic regulation of epithelial proliferation.
Amalgamating these findings, we developed the hypothesis that the differences in cancer risk may be attributed to differences in the colonic milieu, which represents the microbial interface between the external environment (namely the diet) and the colonic mucosa (9). Here, we examine differences in the constituents of the colonic milieu that have been shown experimentally to influence neoplastic change from high and low risk populations.
Methods
Study populations
As colon cancer predominantly occurs in middle life and after, colonic content samples from African American and Native African men and women aged 50–65 y were examined. As a western control, Caucasian Americans of a similar age range were included. The intention was to recruit by advertisement in public fora 20 participants of either sex from each population without history of gastrointestinal disease or surgery or colon polyps or cancer and without a history of antibiotic use during the previous 8 wk. This age group was selected, because the risk of cancer increases appreciably after age 50 y and screening colonoscopy is recommended as the standard of care. Americans were recruited predominantly from the Pittsburgh area of Pennsylvania and the Africans from semirural Pretoria (Garankua, Medical University of South Africa), central highlands) and from the rural regions of Limpopo Province, South Africa. The protocol was reviewed and approved by the University of Pittsburgh Institutional Review Board and General Clinical Research Center and by the Medical University of South Africa's Medical Ethics and Safety Committee. The protocol for collection of samples in South Africa was the same as that used at the University of Pittsburgh.
Experimental design
Participants consumed their normal diets until 2000 h and then fasted until the study was complete. At 0800 a blood sample (10 mL) was taken from fasting participants and then they were given 2 L of colonoscopy preparation fluid (polyethylene glycol 3550, GoLytely) to drink rapidly over 15–30 min. All stool passed during the following 3 h was collected in preweighed cans and kept on ice. Visual inspection showed that by this time, the residual effluent had little fecal contamination. The can was then reweighed to calculate the total weight of the stool passed. The container was sealed and emulsified by rigorous shaking for at least 30 min. The fluid was then carefully inspected to ensure there was no solid material remaining and 30-mL aliquots were extracted and frozen at −20°C to await transportation to the laboratory for analysis. Transportation from Africa to Pittsburgh was performed by packaging frozen samples on dry ice and by rapid airfreight transfer (<3 d). Visual inspection showed that samples were still frozen on arrival at the investigator's laboratory in Pittsburgh.
The results of the dietary analysis, colonoscopic findings, epithelial proliferation rates, and microbiota differences have been previously published (1).
Sample preparation
Colonic fluid for SCFA and vitamins.
Duplicates of ∼5 g were emulsified for 2 min and then centrifuged at 3800 × g for 30 min at 5°C. The supernatant was decanted, split in 4, and frozen at −20°C until analysis.
Colonic minerals.
Five grams of stool was dried thoroughly by centrifugation under vacuum (CentriVap Concentrator, Labconco) for analysis.
Other vitamins.
Twelve evacuant samples from each group were pooled, diluted with saline, centrifuged at 3800 × g for 5 min, and refiltered at 200 nm for a pilot investigation into the colonic fluid content of vitamin B-12, thiamine, and vitamin C.
Blood tests.
A 5-mL sample was collected in an EDTA tube for determining the complete blood count and another 5 mL collected without anticoagulant for measuring plasma folate concentrations.
Sample analysis
SCFA.
Concentrations were measured by GC (Agilent Technologies 6890N Network GC System with FID fitted with EC-1000 15-mL 0.53 mm × 1.2 μm ECONO-CAP capillary column) based on the method described by Scheppach et al. (10). A mixed SCFA standard solution was made up from reagents of the highest purity (>99%) (Sigma). 2,2-Dimethylbutyric acid at a concentration of 1 mmol/L was used as internal standard. SCFA values were computed using peak area ratio of the analyte to the internal standard based on the standard curve. There was a good linear correlation between the peak area ratio and the corresponding standard SCFA with r values > 0.99 for all SCFA. The inter-day and intra-day CV ranged from 2.4 to 3.9%.
Colonic folate.
Folate concentrations in fecal fluid were measured by 2 methods in a subset of 10 Africans and 10 African Americans by chemiluminescence folate affinity assay using a competitive binding assay on an automated Centaur Instrument (Siemens) in our hospital laboratory (i.e. the method used for blood folate analysis) and then by specific microbial bioassay (11) in Dr. O'Connor's laboratory to first assess the contribution of other fecal contaminants that register false positive values. Despite a poor correlation between colonic folate measured by the affinity assay and by the bioassay, the results were of a similar order of magnitude [mean ± SE 769 ± 153 μg vs. 412 ± 57 μg, respectively (folate 2.266 ng/ml = 1 nmol/L)]. Some samples gave very similar values, whereas others were 4× higher by the affinity assay. Consequently, the bioassay method (duplicate variation <1%) was used in the final analysis shown in the results section.
Microbial bioassay.
Aliquots of fecal fluid fraction supernatants were diluted with 1% sodium ascorbate in potassium phosphate buffer (pH 7) to protect labile folates, heated (100°C for 5 min) to remove folate from binders, and after cooling, folates were converted to their microbiologically assayable form using rat serum folate conjugase as previously described (12). The folate concentrations in the resultant supernatants were then determined by microbiological assay described by Molloy and Scott (11) using the test organism Lactbacillus rhamnoses (ATCC 7469, American Type Tissue Culture Collection).
Colonic biotin.
An improved agar plate method of biotin bioassay using Lactobacillus plantarum ATCC 8014 and bromocresol purple was established to determine biotin levels in body fluids, foods, and feces (13). Samples were treated with 4.5 mol/L H2SO4 to liberate free biotin, autoclaved for 1 h, and neutralized by 4.5 mol/L NaOH, then 10 mL was added to wells in each plate. The intra-assay CV were 3.2 (n = 20) and 1.3% (n = 23), respectively.
Thiamine.
Total thiamine contents were determined as sum of thiamine and thiamine diphosphate. Trichloroacetic acid (5%) was added to the sample and supernatant of the mixture was used for measurement. Thiamine and thiamine diphosphate were determined by the HPLC-post labeled fluorescence method as previously (14).
Ascorbic acid.
Reduced and oxidized ascorbic acid and 2,3-diketogluconic acid were chemically converted to ascorbic acid derivative and the compound was determined by the HPLC method (15).
Vitamin B-12.
The assay was run on the Bayer Centaur 1556 (Advia Centaur, Siemens) with calibration lot CC12 and reagent lot 191. The assay is a competitive solid phase immune assay with chemiluminescence detection (16). The assay was repeatable to within 5% in the sample range (reagent lot 191; calibration lot CC13).
Colonic minerals.
Following sample digestion with nitric acid and treatment with hydrogen peroxide and hydrochloric acid (17), calcium, iron, zinc, and sulfur were measured in the resulting digestion solution by sequential inductively coupled plasma optical emission spectrometry (17) (Perkin-Elmer Optima 2000 DV ICP-OES, PerkinElmer Life And Analytical Sciences) utilizing VHG multi-element aqueous standard SM35A-500, trace-metal grade ammonium sulfate (Sigma-Aldrich), and Standard Reference Material 1577b (National Institute of Standards and Technology). Nitrogen was determined by the Kjeldahl method (17).
RBC folates.
EDTA-preserved whole blood samples (50 μL) were used for analysis of folate concentrations following hemolysis with ascorbic acid (1 mL) in the hospital laboratory using a similar method (chemiluminescence folate-affinity assay) described above for colonic samples. Measurements were converted to RBC concentrations after correction for hematocrit using the formula:
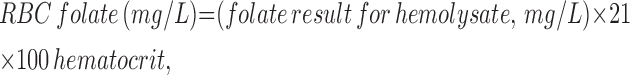 |
where 21 is the dilution factor (conversion factor: 2.266 ng/ml = 1 nmol/L).
Statistical analysis
As the data derived from the stool analysis were normally distributed, significant differences among the 3 population groups were first assessed by 1-way ANOVA. If there was an overall difference among the colonic contents of the 3 different population groups, then comparisons were made using Fischer's post hoc protected least significant difference test and the adjusted significance value for multiple testing. Regression analysis was used to assess the association between dietary intakes and nutrient colonic contents. Values in the text are means ± SEM. Differences were considered significant at P < 0.05.
Results
Dietary intakes.
Dietary intake results have been previously published (1) (Table 1). Native Africans consumed a relatively impoverished diet, significantly lower in protein, vitamins, and minerals than in both other groups.
TABLE 1.
Dietary intakes assessed by 3-d recalls in African Americans, Caucasian Americans, and native Africans12
| Native Africans | African Americans | Caucasian Americans | |
|---|---|---|---|
| n | 17 | 17 | 18 |
| Protein, g/d | 58 ± 4 | 94 ± 9* | 108 ± 10* |
| Meat protein, g/d | 26 ± 2 | 65 ± 7** | 77 ± 8*** |
| Folate, μg/d | 201 ± 23 | 481 ± 47** | 526 ± 50*** |
| Calcium, mg/d | 228 ± 27 | 834 ± 100 | 1049 ± 112 |
| Iron, mg/d | 7.1 ± 0.5 | 18.3 ± 2.0*** | 18.9 ± 1.8*** |
| Zinc, mg/d | 6.7 ± 0.5 | 14 ± 1.5** | 15.4 ± 1.3*** |
Values are means ± SEM. Asterisks indicate different from Native Africans: *P < 0.05, **P < 0.005.
Reproduced from (1) with permission.
Colonic evacuants.
Total colonic evacuant volumes were slightly, but significantly, higher in African Americans compared with Africans, but not compared with Caucasian Americans (Table 2). Consequently, the differences in colonic mineral content between African Americans and native Africans would be smaller if fecal concentrations alone were examined, whereas the differences in SCFA would be higher.
TABLE 2.
Colonic evacuant contents of selected nutrients and the RBC folate concentration in African Americans, Caucasian Americans, and Native Africans1
| Native Africans | African Americans | Caucasian Americans | |
|---|---|---|---|
| n | 17 | 17 | 18 |
| Colon contents | |||
| Weight, g | 1302 ± 108 | 1796 ± 181* | 1694 ± 226 |
| Calcium, mg | 683 ± 119 | 1084 ± 143 | 1944 ± 260** |
| Nitrogen, mg | 3131 ± 497 | 2459 ± 390 | 3054 ± 443 |
| Iron, mg | 28.9 ± 4.0 | 30.1 ± 6.2 | 39.2 ± 4.9* |
| Zinc, mg | 6.1 ± 1.4 | 20.4 ± 5.2* | 13.4 ± 5.2 |
| Folate, μg | 632 ± 95 | 699 ± 131 | 860 ± 129 |
| Biotin, μg | 91 ± 24 | 65 ± 21 | 183 ± 68 |
| Blood | |||
| Hb,2g/L | 131 ± 10 | 138 ± 3 | 141 ± 3 |
| MCV, fL | 85 ± 4 | 90 ± 2 | 93 ± 2* |
| RBC folate, ng/mL | 181.3 ± 20.0 | 328.2 ± 34.2** | 341.4 ± 41.4** |
Values are means ± SEM, n = 10–14 randomly selected individuals from each group. Asterisks indicate different from Native Africans: *P < 0.05, **P < 0.005.
Hb, Hemoglobin; MCV, mean corpuscular volume.
Colonic minerals and nitrogen.
The calcium and iron contents were significantly higher in Caucasian Americans, whereas that of zinc was significantly higher in African Americans than in Native Africans (Table 2). Unfortunately, assessment of normal basal fecal levels of sulfur could not be made, because the colonic evacuant used, GoLytely, contained 1280 mg/L elemental sulfur. Colonic nitrogen contents were similar despite the considerably higher dietary protein intakes in Americans.
Colonic SCFA.
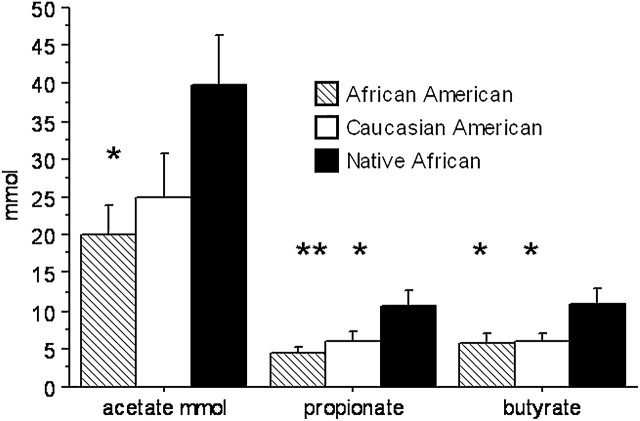
The quantities of all 3 major SCFA, namely acetate, propionate, and butyrate, were all higher in native Africans than in African Americans and Caucasian Americans, with the exception of acetate, which tended to be lower in Caucasian Americans than in native Africans (P = 0.07) (Fig. 1). There was no significant difference between Colonic SCFA contents in African Americans and Caucasian Americans did not differ. Because colonic volume was lower in Africans, the concentrations of SCFA in colonic effluent were even more elevated (P < 0.001) in Africans compared with both American groups (data not shown). Overall, there were no significant correlations between colonic butyrate and total dietary carbohydrate and fiber intake.
FIGURE 1 .
Colonic evacuant contents of the primary SCFA in African Americans, Caucasian Americans, and Native Africans. Values are means ± SE, n = 17 or 18. Asterisks indicate different from Native Africans: *P < 0.05, **P < 0.005.
Colonic folate.
The colonic folate content was not lower in Africans despite the lower dietary intake (201 ± 23 μg/d) than African Americans (481 ± 47 μg/d) (P < 0.001) and Caucasian Americans (526 ± 50 μg/d) (P < 0.0001) and lower RBC concentrations than in native Africans (Table 2). The low RBC concentrations in Africans were not, however, associated with functional evidence of deficiency as indicated by anemia or macrocytosis. There was a weak overall association between dietary carbohydrate and dietary fiber intake and colonic folate content (r2 = 0.18, P = 0.02; and r2 = 0.17, P = 0.04). The association within the 2 American groups for folate and dietary carbohydrate was stronger (r2 = 0.26; P = 0.01).
Colonic biotin.
Large quantities of biotin were also recovered from colonic effluents in all 3 groups (Table 2). The colonic content in Caucasian Americans (183 ± 68 μg) was similar to the U.S. recommended dietary intake (RDA) of 150–300 μg/d, but the quantity measured in native Africans exceeded considerably the measured dietary intake levels, i.e. 91 ± 24 μg compared with only 19 ± 4 μg/d (1). Colonic biotin concentrations correlated positively with colonic folate concentrations (r = 0.56; P < 0.0001).
Other vitamins.
In the pooled group colonic samples (12 from each group), we found that the colonic content of thiamine [2.1 μg in AA, 1.6 μg in Africans; RDA 1.5 mg/d] and vitamin C (347.2 μg in AA, 1355.3 μg in Africans; RDA 75–90 mg/d) were insignificant, whereas vitamin B-12 (AA 2.5 μg, Africans 2.1 μg; RDA 1 μg/d) was high, measuring ∼50% of normal dietary intake levels.
Discussion
The results of our analyses of colonic contents support the proposed role of the microbiota in the maintenance of colonic mucosal health and determination of cancer risk (9). Perhaps the most important mediator of epithelial function and regulation is the SCFA, butyrate. There is substantial experimental animal and human evidence that butyrate not only maintains colonic mucosal health as the preferred fuel for respiration (18) but also has potent antiinflammatory, antiproliferative, and therefore antineoplastic properties (19–22). Butyrate does not come from the diet but is produced by specific colonic bacteria, predominantly Clostridia clusters XIVa and IV of Firmicutes (23), from food residues such as dietary fiber or resistant starch. Our results show that the colonic content of butyrate, and all of the chief SCFA, were significantly higher in native Africans, the group that has the lowest colon cancer risk, i.e. < 1:100,000, compared with 65:100,000 in African Americans and 50:100,000 in Caucasian Americans (1). Our results also illustrate the potentially vital role the colonic microbiota may play in vitamin synthesis in undernourished communities, such as the rural Africans included in this study who had higher quantities of folate and biotin in their colons than in their usual diet. Despite the fact that participants had fasted 12 h, we cannot conclude that the total quantities of vitamins recovered from the colon were microbiota-derived, because dietary vitamin absorption by the small intestine is never 100%. The ability of the microbiota to synthesize vitamins has been known for many years, but this was considered unimportant for nutritional health, because it was assumed they were malabsorbed and lost in stools unless copraphagia was practiced. However, the recent demonstration of specific transporters for folate (24), biotin (25), thiamine (26), riboflavin (27), and pyridoxine (28) in the colonic mucosa has forced us to reconsider our view.
The observation of higher levels of SCFA in the colons of Africans suggests that their diet contains more resistant starch, because their intake of total carbohydrate and fiber was not higher than the 2 American groups. The likely explanation is their staple diet of maize meal, which is consumed communally throughout the day (1,2,4). We (1) and others (29) have shown that between 10 and 20% of the average daily intake of 300 g of maize-derived carbohydrate is resistant to small intestinal digestion and absorption. Thus, in addition to 10–20 g dietary fiber, 30–60 g of resistant starch will be delivered to the colonic microbiota each day to be fermented to SCFA.
In our earlier studies, we were puzzled by the observation that the diet of low-risk Africans was deficient in folate and calcium, 2 nutrients generally considered protective against colonic neoplasia (2). However, since that time, epidemiological studies have provided evidence that the relationship between dietary folate and colon cancer risk assumes a bell-shaped curve, with very low intakes increasing risk as well as very high intakes (30,31). Our findings of high quantities of folate in African colons despite their marginal dietary intakes may provide part of the explanation for this apparent paradox. Kim et al. (12) previously reported a large pool of utilizable folate in the colons of pigs, which have similar omnivorous dietary habits and colonic function to humans, and in human infants. They demonstrated that in infants, 57% of total fecal folate was monoglutamylated and therefore in the absorbable form. Although the primary mechanism for dietary folate absorption is its deconjugation to monoglutamates in the brush border of the small intestine by the enzyme glutamate carboxypeptidase II and its subsequent absorption by active transport carrier, there is robust evidence, as mentioned above, that a similar mucosal transport mechanism operates in the colon (23). It is probable that microbial folate production is higher in Africans, as folate is synthesized from glycolysis and tricarboxylic acid cycle intermediates (32), and studies have shown that increasing colonic carbohydrate, in the form of oligosaccharides or resistant starch, increases the production rate of folate by the microbiota (8,33). It is well recognized that some bacteria such as Bifidobacteria are net producers while others are net consumers of folate (32,33). However, even within 1 species, there is considerable variation in the ability of different strains to produce folate. For example, Pompei et al. (33) found that only 6 of the 76 strains of Bifidobacteria tested could produce folate in a folate-free medium. In a follow-up study, the same group demonstrated that supplementation of the diet of folate-deficient rats with 3 of these bacteria increased plasma and liver folate concentrations and that the effect was amplified by the further dietary supplementation with fructo-oligosaccharides, which functions similarly to resistant starch and fiber as a prebiotic (32).
There remains concern, however, that body folate status was marginal in Africans, because RBC levels, an accepted measure of body stores, were low, but not low enough to produce anemia and macrocytosis. We speculate that the rich topical source of folate in the colon may suppress neoplastic change as there is experimental evidence that localized folate deficiency plays a key role in premalignant changes in the epithelium (31). The importance of topical, or local, folate as opposed to systemic blood levels for mucosal health might also explain the other side of the coin: why rural Africans have one of the highest risks of squamous cell carcinoma of the esophagus in the world (34), whereas the cancer is rare in Americans.
Equally impressive was the colonic content of biotin, which is also a product of bacterial fermentation and therefore likely induced by dietary residues. Little attention has been paid to biotin's potential role in carcinogenesis. The vitamin was thought to be principally involved in carbohydrate metabolism as a coenzyme for carboxylases involved in fatty acid synthesis, amino acid catabolism, and gluconeogenesis (13). However, recent investigations have suggested that biotin may exert similar epigenetic effects as folate, because biotinylation, like methylation, of histones has been shown to regulate cell proliferation through its influence on DNA transcription, replication, and repair (35). Another similarity in function between folate and biotin is that marginal tissue deficiencies of both are associated with birth defects (36). Could it be that combined biotin and folate topical sufficiency in the colon suppresses colon cancer risk?
In summary, our study supports our hypothesis that colon cancer risk is determined by interactions between the diet and the colonic microbiota and that cancer risk can therefore be modified by dietary and microbial manipulation. The failure of short-term dietary interventions to prevent adenomatous polyp recurrence and cancer may be explained by the multiple protective mechanisms of the body to regulate DNA turnover and the fact that it takes a lifetime to develop environmentally induced cancers. Consequently, short-term dietary intervention studies need to first prove that biomarkers of cancer risk, e.g. epithelial proliferation, can be changed as proof of concept before longitudinal interventional programs are introduced to reduce cancer risk in high risk populations, such as African Americans.
Acknowledgments
S.O.K. designed and conducted the research; S.O.K., J.O., S.A., D.O.C., S.S., J.S., T.F., K.S., and T.W. analyzed the data; S.O.K. wrote the paper and had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Supported by the American Institute of Cancer Research.
Literature Cited
- 1. O'Keefe SJ, Chung D, Mahmoud N, Sepulveda AR, Manafe M, Arch J, Adada H, van der Merwe T. Why do African Americans get more colon cancer than Native Africans? J Nutr. 2007; 137Suppl 1:S175–82. [DOI] [PubMed] [Google Scholar]
- 2. O'Keefe SJ, Kidd M, Espitalier-Noel G, Owira P. Rarity of colon cancer in Africans is associated with low animal product consumption, not fiber. Am J Gastroenterol. 1999;94:1373–80. [DOI] [PubMed] [Google Scholar]
- 3. Sharma S, O'Keefe SJD. Environmental influences on the high mortality from colorectal cancer in African Americans. Postgrad Med J. 2007;83:583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. O'Keefe SJ. The African way of life and colon cancer risk. Am J Gastroenterol. 2001;96:3220–1. [DOI] [PubMed] [Google Scholar]
- 5. Nagengast FM, Grubben MJ, Van Munster IP. Role of bile acids in colorectal carcinogenesis. Eur J Cancer. 1995;31A:1067–70. [DOI] [PubMed] [Google Scholar]
- 6. Attene-Ramos MS, Wagner ED, Gaskins HR, Plewa MJ. Hydrogen sulfide induces direct radical-associated DNA damage. Mol Cancer Res. 2007;5:455–9. [DOI] [PubMed] [Google Scholar]
- 7. Pochapin M. The effect of probiotics on C. difficile diarrhea. Am J Gastroenterol. 2000;95: Suppl:S11–3. [DOI] [PubMed] [Google Scholar]
- 8. Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–12. [DOI] [PubMed] [Google Scholar]
- 9. O'Keefe SJ. Nutrition and colonic health: the critical role of the microbiota. Curr Opin Gastroenterol. 2008;24:51–8. [DOI] [PubMed] [Google Scholar]
- 10. Scheppach WM, Fabian CE, Kasper HW. Fecal short-chain fatty acid (SCFA) analysis by capillary gas-liquid chromatography. Am J Clin Nutr. 1987;46:641–6. [DOI] [PubMed] [Google Scholar]
- 11. Molloy AM, Scott JM. Microbiological assay for serum, plasma, and red cell folate using cryopreserved, microtiter plate method. Methods Enzymol. 1997;281:43–53. [DOI] [PubMed] [Google Scholar]
- 12. Kim TH, Yang J, Darling PB, O'Connor DL. A large pool of available folate exists in the large intestine of human infants and piglets. J Nutr. 2004;134:1389–94.87. [DOI] [PubMed] [Google Scholar]
- 13. Sawamura H, Fukuwatari T, Shibata K. Effects of excess biotin administration on the growth and urinary excretion of water-soluble vitamins in young rats. Biosci Biotechnol Biochem. 2007;71:2977–84. [DOI] [PubMed] [Google Scholar]
- 14. Iwata H, Matsuda T, Tonomura H. Improved high-performance liquid chromatographic determination of thiamine and its phosphate esters in animal tissues. J Chromatogr. 1988;450:317–23. [DOI] [PubMed] [Google Scholar]
- 15. Kishida E, Nishimoto Y, Kojo S. Specific determination of ascorbic acid with chemical derivatization and high-performance liquid chromatography. Anal Chem. 1992;64:1505–7. [Google Scholar]
- 16. Morovat A, James TS, Cox SD, Norris SG, Rees MC, Gales MA, Taylor RP. Comparison of Bayer Advia Centaur immunoassay results obtained on samples collected in four different Becton Dickinson Vacutainer tubes. Ann Clin Biochem. 2006;43:481–7. [DOI] [PubMed] [Google Scholar]
- 17. Official Methods of Analysis of AOAC International Official method 990.08 and 984.13. 18th ed. Gaithersburg (MD): AOAC International; 2005. [Google Scholar]
- 18. Roediger WEW. Utilization of nutrients by the isolated epithelial cells of the rat colon. Gastroenterology. 1982;83:424–9. [PubMed] [Google Scholar]
- 19. Kim YS, Milner JA. Dietary modulation of colon cancer risk. J Nutr. 2007;137:S2576–9. [DOI] [PubMed] [Google Scholar]
- 20. Nguyen KA, Cao Y, Chen JR, Townsend CM Jr, Ko TC. Dietary fiber enhances a tumor suppressor signaling pathway in the gut. Ann Surg. 2006;243:619–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rodríguez-Cabezas ME, Gálvez J, Lorente MD, Concha A, Camuesco D, Azzouz S, Osuna A, Redondo L, Zarzuelo A. Dietary fiber down-regulates colonic TNFα and NO production in trinitrobenzenesulfonic acid-induced colitic rats. J Nutr. 2002;132:3263–71. [DOI] [PubMed] [Google Scholar]
- 22. Watson AJM. An overview of apoptosis and the prevention of colorectal cancer. Crit Rev Oncol Hematol 2006;57:107–21. [DOI] [PubMed] [Google Scholar]
- 23. Louis P, Scott KP, Duncan SH, Flint HJ. Understanding the effects of diet on bacterial metabolism in the large intestine. J Appl Microbiol. 2007;102:1197–208. [DOI] [PubMed] [Google Scholar]
- 24. Dudeja PK, Torania SA, Said HM. Evidence for the existence of a carrier-mediated folate uptake mechanism in human colonic luminal membranes. Am J Physiol. 1997;272:G1408–15. [DOI] [PubMed] [Google Scholar]
- 25. Said HM, Ortiz A, McCloud E, Dyer D, Moyer MP, Rubin S. Biotin uptake by human colonic epithelial NCM460 cells: a carrier-mediated process shared with pantothenic acid. Am J Physiol. 1998;275:C1365–71. [DOI] [PubMed] [Google Scholar]
- 26. Said HM, Ortiz A, Subramanian VS, Neufeld EJ, Moyer MP, Dudeja PK. Mechanism of thiamine uptake by human colonocytes: studies with cultured colonic epithelial cell line NCM460. Am J Physiol Gastrointest Liver Physiol. 2001;281:G144–50. [DOI] [PubMed] [Google Scholar]
- 27. Said HM, Ortiz A, Moyer MP, Yanagawa N. Riboflavin uptake by human-derived colonic epithelial NCM460 cells. Am J Physiol Cell Physiol. 2000;278:C270–6. [DOI] [PubMed] [Google Scholar]
- 28. Said ZM, Subramanian VS, Vaziri ND, Said HM. Pyridoxine uptake by colonocytes: a specific and regulated carrier-mediated process. Am J Physiol Cell Physiol. 2008;294:C1192–7. [DOI] [PubMed] [Google Scholar]
- 29. Segal I, Walker ARP, Naik I, Riedel L, Daya B, de Beer M. Malabsorption of carbohydrate foods by urban blacks. S Afr Med J. 1991;80:543–5. [PubMed] [Google Scholar]
- 30. Mason JB. Diet, folate, and colon cancer. Curr Opin Gastroenterol. 2002;18:229–34. [DOI] [PubMed] [Google Scholar]
- 31. Kim YI. Role of folate in colon cancer development and progression. J Nutr. 2003;133:S3731–9. [DOI] [PubMed] [Google Scholar]
- 32. Pompei A, Cordisco L, Amaretti A, Zanoni S, Raimondi S, Matteuzzi D, Rossi M. Administration of folate-producing bifidobacteria enhances folate status in Wistar rats. J Nutr. 2007;137:2742–6. [DOI] [PubMed] [Google Scholar]
- 33. Pompei A, Cordisco L, Amaretti A, Zanoni S, Matteuzzi D, Rossi M. Folate production by Bifidobacteria as a potential probiotic property. Appl Environ Microbiol. 2007;73:179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mqoqi N, Kellet P, Sitas F, Jula M editors. The incidence of histologically diagnosed cancer in South Africa 1998–1999. Johannesburg (South Africa): The National Cancer Registry, National Health Laboratory Service; 2004. [Google Scholar]
- 35. Stanley JS, Griffin JB, Zempleni J. Biotinylation of histones in human cells: effects on cell proliferation. Eur J Biochem. 2001;268:5424–9. [DOI] [PubMed] [Google Scholar]
- 36. Mock DM. Marginal biotin deficiency is common in normal human pregnancy and is highly teratogenic in mice. J Nutr. 2009;139:154–7. [DOI] [PMC free article] [PubMed] [Google Scholar]