ABSTRACT
Background: Studies of the role of dietary factors in epithelial ovarian cancer (EOC) development have been limited, and no specific dietary factors have been consistently associated with EOC risk.
Objective: We used a nutrient-wide association study approach to systematically test the association between dietary factors and invasive EOC risk while accounting for multiple hypothesis testing by using the false discovery rate and evaluated the findings in an independent cohort.
Design: We assessed dietary intake amounts of 28 foods/food groups and 29 nutrients estimated by using dietary questionnaires in the EPIC (European Prospective Investigation into Cancer and Nutrition) study (n = 1095 cases). We selected 4 foods/nutrients that were statistically significantly associated with EOC risk when comparing the extreme quartiles of intake in the EPIC study (false discovery rate = 0.43) and evaluated these factors in the NLCS (Netherlands Cohort Study; n = 383 cases). Cox regression models were used to estimate HRs and 95% CIs.
Results: None of the 4 dietary factors that were associated with EOC risk in the EPIC study (cholesterol, polyunsaturated and saturated fat, and bananas) were statistically significantly associated with EOC risk in the NLCS; however, in meta-analysis of the EPIC study and the NLCS, we observed a higher risk of EOC with a high than with a low intake of saturated fat (quartile 4 compared with quartile 1; overall HR: 1.21; 95% CI: 1.04, 1.41).
Conclusion: In the meta-analysis of both studies, there was a higher risk of EOC with a high than with a low intake of saturated fat.
Keywords: nutrition, ovarian cancer, saturated fat, serous, prospective cohort, diet
INTRODUCTION
Diet is an important modifiable factor that has been shown to influence cancer risk in general (1); however, it is uncertain whether dietary factors may be useful for the prevention of epithelial ovarian cancer (EOC).48 The identification of modifiable dietary factors for the primary prevention of EOC is important because there are currently no strategies for early detection, and consequently most patients are diagnosed with advanced-stage disease that has a low 5-y survival rate (40% in Europe) (2).
A systematic meta-analysis by the World Cancer Research Fund (3) and a systematic literature review (4) focusing on dietary factors and EOC risk in prospective cohort studies observed that the current evidence was too limited, and no specific dietary factors were consistently associated with EOC risk. Inconsistent results across studies of dietary factors and EOC risk may be due to insufficient sample sizes and/or dietary measurement error (4) or the possibility that there may be no effect.
To systematically evaluate the role of dietary factors in relation to EOC risk, we used a nutrient-wide association study (NWAS) that is similar to methods that are routinely used in genetics studies; this involved the assessment of an extensive list of foods/food groups and nutrients in relation to EOC risk while accounting for multiple testing by estimating the false discovery rate (FDR) (5), followed by the evaluation of the statistically significant results in an independent study. The NWAS method has been used to identify novel dietary risk associations for diabetes and blood pressure (6, 7). We recently conducted a NWAS of endometrial cancer that involved the investigation of foods/nutrients in the EPIC (European Prospective Investigation into Cancer and Nutrition) study, estimation of the FDR, and confirmation of selected study findings in the Nurses’ Health Study and the Nurses’ Health Study II and observed that most dietary factors were not associated with endometrial cancer risk; however, we highlighted an inverse association between coffee intake and endometrial cancer risk (8). The current study describes the use of the NWAS approach to evaluate an extensive list of dietary factors in relation to EOC risk in 2 prospective European cohort studies.
METHODS
This NWAS involved the investigation of intakes of 28 foods/food groups and 29 nutrients in relation to risk of EOC in the EPIC study, identification of statistically significant associations when comparing participants who were classified in quartile 4 with those in quartile 1 of dietary intake amounts, estimation of the associated FDR, and investigation of selected dietary factors in an independent validation cohort, the NLCS (Netherlands Cohort Study). To compute the overall effect estimates for the selected foods/nutrients in relation to EOC risk, we used a random-effects meta-analytic method (Supplemental Figure 1). The current study focused on EOC because there are no consistent dietary associations; therefore, the NWAS approach may be particularly useful because it involves the systematic investigation and reporting of risk associations for a range of foods/nutrients in relation to EOC risk.
Study populations
The EPIC study includes 521,330 male and female participants aged 25–70 y at enrollment (1991–2000) (9). From 367,903 women in the EPIC study, individuals were excluded if they reported a prevalent cancer except nonmelanoma skin cancer (n = 19,853), were missing follow-up information (n = 2898), had a bilateral oophorectomy (n = 10,404), did not complete a dietary questionnaire (n = 3217), were classified in the top or bottom 1% of energy intake to energy requirement (n = 6502) (to reduce the effect of implausible extreme values on the analysis), were missing a lifestyle questionnaire (n = 22), or had outlying values for specific nutrient intakes (n = 3); 325,004 participants remained in the current study. Informed consent was provided by all participants, and ethical approval for the study was obtained from the internal review board of the International Agency for Research on Cancer and from local ethics committees in each participating country.
Incident ovarian cancers in the EPIC study were identified through population-based cancer registries or active follow-up, and mortality data were obtained from cancer or mortality registries (9). Ovarian cancers were classified as ovarian, fallopian tube, and primary peritoneal cancers based on the third revision of the International Classification of Diseases for Oncology codes C56.9, C57.0 and C48, respectively. From 325,004 study participants, 1293 first-incident ovarian cancer cases were identified, and cases were censored if they were nonepithelial (n = 77), missing tumor behavior (n = 25), or tumors of borderline malignancy (n = 96); 1095 invasive EOCs were evaluated in the current study.
The NLCS was established in September 1986 and includes 62,573 women from the general population, aged 55–69 y, who resided in 204 municipalities with computerized population registries (10). At the start of the study, participants completed a self-administered questionnaire on diet, lifestyle factors, medical histories, and other putative cancer risk factors. A case-cohort approach was used for reasons of efficiency in questionnaire processing and follow-up. Cancer cases were identified from the entire cohort, whereas the accumulated person-years of the entire cohort were calculated from a random subcohort of 2589 women who were selected immediately after baseline. Information about new cancer diagnoses was collected annually by using record linkage to the Netherlands cancer registry and a pathology registry. For cases and subcohort members, we excluded participants with a prevalent cancer other than nonmelanoma skin cancer at baseline (n = 52) and women who reported an oophorectomy (n = 33). After a maximum follow-up of 17.3 y, 427 incident, invasive EOCs were identified. Participants with incomplete or inconsistent dietary data (11) were also excluded (n = 227 total; 44 cases, including one case who was a subcohort member, and 183 noncase subcohort members); this left 383 invasive EOC cases (including 17 cases who were subcohort members) and 2199 noncase subcohort members in the current analysis. The NLCS was approved by the institutional review boards of the Nederlandse Organisatie voor Toegepast Natuurwetenschappelijk Onderzoek (TNO) Quality of Life research institute (Zeist, Netherlands) and Maastricht University (Maastricht, Netherlands).
Dietary assessment
The habitual diet of the EPIC participants at enrollment was assessed by using country-specific or study center-specific dietary questionnaires or food records (9), and for this study, we evaluated foods/food groups and nutrients that were available in all 10 countries. The country and center-specific dietary questionnaires have been validated mostly by using monthly 24-h recall interviews (12). The EPIC Nutrient Database was used to calculate standardized nutrient intake for the 10 countries, and all standardized priority nutrients were analyzed; nutrients were prioritized according to their availability in national databases for countries participating in the EPIC study, their relative comparability, completeness, and relevance to cancer etiology (13).
The NLCS participants completed a 150-item semiquantitative food-frequency questionnaire at baseline that estimated the mean frequency and amounts of foods and beverages consumed in the previous 12 mo. The food-frequency questionnaire has been validated and tested for reproducibility (11, 14). Nutrient intakes were calculated by multiplying the frequency of intake by the nutrient content of specified portions based on the Dutch food composition table (15).
Measurement of other covariates
The following covariates were selected a priori and were adjusted for in all multivariate models: total energy intake (kcal, continuous) and established risk factors of EOC, oral contraceptive use [never use (reference), use <5 y, use 5+ y, ever use (unknown duration), missing], menopausal status [premenopausal (reference), postmenopausal, dubious or unknown menopause], and the number of full-term pregnancies [0 (reference), 1–2, 3–4, >4, parous (unknown number), missing]. In the EPIC study, we further adjusted the multivariate models for BMI, physical activity, smoking status, and educational attainment, and the risk estimates were very similar and therefore these covariates were not included in the final models. All models were stratified by the participant’s age at recruitment (continuous) and the study center (EPIC only).
Statistical methods
Individual foods/food groups and nutrients were related one at a time to the risk of EOC by using Cox proportional hazards regression to estimate the HRs and 95% CIs. In the EPIC study, age was the underlying time metric for Cox regression, with the subjects’ age at recruitment as the entry time and their age at cancer diagnosis, death, emigration, or last follow-up, whichever occurred first, as the exit time. Dietary factors were modeled in all of the EPIC countries together, and to control for differing follow-up procedures, questionnaire design, and other differences across study centers, we stratified all models by study center as well as the age at recruitment (continuous).
In the NLCS, the total person-years at risk were estimated from the subcohort, and Prentice-weighted Cox proportional hazards regression models for case-cohort designs with robust SE estimates (16) were used to estimate HRs and 95% CIs. In both studies, the proportional hazards assumption was verified by using the method described by Grambsch and Therneau (17). Nutrient intakes were energy adjusted by using the regression residual method (18) separately for the entire EPIC cohort and the NLCS, and participants were classified into quartiles of consumption unless stated otherwise. In the EPIC study, dietary intake amounts were divided into quartiles based on the distribution in the entire cohort, and in the NLCS, quartiles were based on the subcohort set. The P value for the test of linear trend was calculated by modeling a continuous term. To test for heterogeneity between countries in the EPIC study, we conducted data analyses separately within each country and pooled them by using a random-effects meta-analytic method with the restricted maximum likelihood estimator (19).
To account for multiple comparisons in the EPIC study, we estimated the FDR for each food/nutrient, which is the ratio of the expected number of false positives to the total number of positive associations, or the percentage of findings drawn from the null distribution at a given significance level (5). To compute the FDR, we used an analytic method that estimates the number of false-positive results by creating a null distribution of regression test statistics; this was accomplished by randomly assigning both the case status and time to event, running the Cox proportional hazards model, and collecting the associated P value over 1000 permutations (6, 7). Foods/nutrients with a P value <0.05 for the comparison of quartile 4 and quartile 1 of intake were selected for validation studies. Last, to increase the power and provide a global estimate of the effect size, we computed overall estimates for food/nutrient intake and EOC risk associations for the EPIC study and the NLCS by combining coefficients from each study by using random-effects meta-analysis. Cox proportional hazards regression was performed by using the survival (20) package and random-effects meta-analysis was performed by using the metafor (21) and rmeta (22) packages in R (version 3.0.2; R Core Team) (23).
RESULTS
In total, 1522 incident invasive EOCs were evaluated, including 1095 cases from the EPIC study, with a mean ± SD follow-up of 11.0 ± 2.7 y, and 383 cases from the NLCS, with a mean ± SD subcohort follow-up of 15.6 ± 3.7 y. In comparisons of the study population characteristics at baseline, we observed that women in the NLCS subcohort were older (mean age: 61.4 y for NLCS compared with 50.1 y for EPIC) and less likely to ever use oral contraceptives (25% of NLCS participants used oral contraceptives compared with 59% in the EPIC study) (Table 1).
TABLE 1.
Age-standardized characteristics of the EPIC study and the NLCS1
| EPIC | NLCS2 | |
| Participants, n | 325,004 | 2216 |
| Age at enrollment, y | 50.1 ± 9.83 | 61.4 ± 4.3 |
| Parous, % | 85 | 82 |
| Ever use of OCs, % | 59 | 25 |
| Postmenopausal, % | 45 | 100 |
| Children,4 n | 2.3 ± 1.0 | 3.4 ± 2.0 |
| Duration of OC use,5 y | 7.8 ± 7.3 | 7.4 ± 5.4 |
| BMI, kg/m2 | 24.9 ± 4.4 | 24.3 ± 5.8 |
All variables except age were age standardized in 5-y age groups according to the age distribution of the study population. EPIC, European Prospective Investigation into Cancer and Nutrition; NLCS, Netherlands Cohort Study; OC, oral contraceptive.
Values refer to the NLCS subcohort.
Mean ± SD (all such values).
Among parous women.
Among OC ever users.
Of the 57 foods/nutrients that were evaluated in the EPIC study, 4 were statistically significantly associated with EOC risk (comparing quartile 4 and quartile 1) and had a FDR of 0.43 (Figure 1, Supplemental Table 1). In comparisons of participants who reported high with those who reported low dietary intake, cholesterol, polyunsaturated fat, and saturated fat were associated with a higher risk of EOC, whereas banana consumption was associated with a lower risk of EOC. We observed a dose-response for polyunsaturated fat intake (P-trend = 0.04) but not for cholesterol, saturated fat, or banana intake. Heterogeneity across the EPIC countries for the 57 foods/nutrients that were assessed was rare [P value for heterogeneity (P-heterogeneity) ≤ 0.04 for calcium, retinol, wine, and potatoes]; all analyses were therefore carried out in the entire EPIC cohort. Apart from cholesterol, polyunsaturated and saturated fat, and banana intake, the remaining foods and nutrients that were assessed did not meet the statistical significance cutoff (Supplemental Table 2).
FIGURE 1.
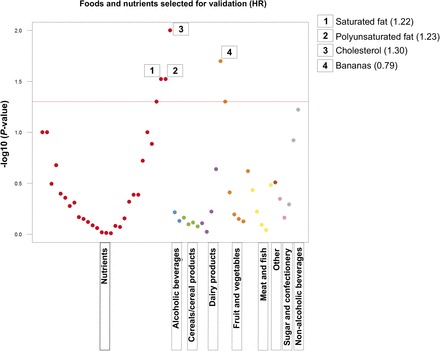
“Manhattan plot” showing results from the nutrient-wide association study method to evaluate the association between dietary intake of various foods and nutrients and epithelial ovarian cancer risk in the EPIC study. The y axis shows the −log10P values from the multivariate-adjusted Cox proportional hazards regression coefficient for the comparison of the highest with the lowest quartiles of dietary intake (red horizontal line indicates P = 0.05). Each x axis label represents a dietary category (for visualization purposes only; each dietary factor was analyzed one at a time), and within each category, dietary items were ordered left to right according to the lowest to highest HR. The 4 dietary factors that were selected for confirmation in the NLCS were labeled with the HR from the EPIC study for the comparison of the highest and the lowest quartiles of dietary intake in relation to risk of epithelial ovarian cancer. EPIC, European Prospective Investigation into Cancer and Nutrition; NLCS, Netherlands Cohort Study.
In the NLCS, we then evaluated the 4 foods/nutrients that were identified previously. Compared with the EPIC cohort, the NLCS subcohort participants consumed on average higher amounts of polyunsaturated and saturated fat and had a lower intake of banana and total energy intake (Supplemental Table 3). None of the 4 foods/nutrients were independently associated with EOC risk in the NLCS (Supplemental Table 4); however, we observed similar risk estimates when comparing participants with the highest and lowest quartiles of saturated fat (1.22 and 1.19) intake in the EPIC study and the NLCS, respectively, and meta-analysis of these studies highlighted a statistically significant positive association with saturated fat intake (quartile 4 compared with quartile 1; overall HR: 1.21; 95% CI: 1.04, 1.41) (Figure 2). Because all of the NLCS participants were postmenopausal, we carried out a meta-analysis of postmenopausal EPIC study participants and the NLCS and observed that the suggestive positive association with saturated fat intake remained (quartile 4 compared with quartile 1; overall HR: 1.18; 95% CI: 0.99, 1.41) (Supplemental Figure 2, Supplemental Table 5).
FIGURE 2.
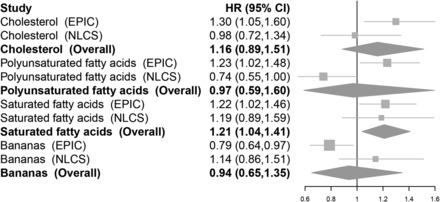
Forest plots showing multivariate HRs and 95% CIs for comparisons of the highest and lowest categories of intake of 4 foods and nutrients in relation to epithelial ovarian cancer risk in the EPIC study and the NLCS. Foods and nutrients were evaluated if they had a P value <0.05 for the comparison of extreme quartiles of dietary intake in the EPIC study based on results from multivariate-adjusted Cox proportional hazards regression. Overall risk estimates were estimated from random-effects meta-analysis. P-heterogeneity comparing the EPIC study and the NLCS were ≥0.14 with the following exceptions: PUFAs (P-heterogeneity = 0.01) and bananas (P-heterogeneity = 0.04). Multivariate models were adjusted for total energy intake, oral contraceptive use, menopausal status, and parity and were stratified by age (both studies) and study center (EPIC only). Contrasts and median intake values were cholesterol (EPIC: quartile 4, 334.7 mg/d compared with quartile 1, 148.5 mg/d; NLCS: quartile 4, 292.3 mg/d compared with quartile 1, 164.1 mg/d), polyunsaturated fat (EPIC: quartile 4, 14.5 g/d compared with quartile 1, 7.0 g/d; NLCS: quartile 4, 21.1 g/d compared with quartile 1, 7.9 g/d), saturated fat (EPIC: quartile 4, 29.6 g/d compared with quartile 1, 17.4 g/d; NLCS: quartile 4, 34.3 g/d compared with quartile 1, 22.0 g/d), and bananas (EPIC: quartile 4, 62.4 g/d compared with quartile 1, 0 g/d; NLCS: highest, 32.1 g/d compared with lowest, 0 g/d). EPIC, European Prospective Investigation into Cancer and Nutrition; NLCS, Netherlands Cohort Study.
Serous tumors accounted for 582 (53.2%) and 186 (48.6%) of the EPIC and NLCS cases, respectively. In meta-analysis of serous tumors in the EPIC study and the NLCS, we observed no association with saturated fat intake (quartile 4 compared with quartile 1; overall HR: 1.11; 95% CI: 0.86, 1.44) (Supplemental Figure 3). Nonserous histologic subtypes of EOC were not evaluated separately because of the small number of cases; for example, there were 118 and 31 endometrioid EOC cases in the EPIC and NLCS studies, respectively.
DISCUSSION
In the current study, we used a novel NWAS approach to evaluate dietary intake amounts of 57 foods/food groups and nutrients in the EPIC study and identified 4 dietary factors for which the highest compared with the lowest intake amounts were associated with a statistically significantly higher risk (cholesterol, polyunsaturated fat, saturated fat) or lower risk (bananas) of EOC. Using the criterion that a statistically significant association in the same direction is required in the independent cohort (NLCS) for validation, none of the dietary items were confirmed.
It has been suggested that a high intake of saturated and/or animal fat or total fat may stimulate extraovarian estrogen production (24), which may lead to an increased risk of developing EOC (25). Our data were not consistent with this hypothesis because in the EPIC study, there was no association between animal and total fat intake when comparing participants classified into the highest with those in the lowest quartiles of intake with EOC risk. In contrast to reports of no association between saturated fat intake and EOC risk in a pooled analysis of 12 cohorts (including the NLCS) (26) and the NIH-AARP study (27), we observed a higher EOC risk with a high intake than with a low intake of saturated fat. However, this finding is consistent with our earlier report in the NLCS of a statistically significant positive association with saturated fat intake on a continuous scale and the nonsignificant higher risk of invasive EOC observed when comparing the highest with the lowest quintiles of intake (28). This study focused on fat and meat consumption and did not use the NWAS method. Further studies are warranted first to evaluate whether a high fat intake is associated with higher circulating endogenous estrogen concentrations because results to support this mechanistic link have been inconsistent (29–31), and it is possible that the reported significant associations may be due to chance. Second, if the link between fat intake and circulating estrogen concentrations is confirmed, it may be of interest to investigate the association between fat intake and risk of endometrioid EOC because a recent study of women during pregnancy observed that higher estradiol concentrations were associated with a higher risk to develop endometrioid but not serous EOC (32). We were unable to evaluate endometrioid tumors in the current study because of the small number of cases. Of the other dietary factors that were investigated but not confirmed in the current study, consumption of polyunsaturated fat and cholesterol (26) and bananas (33) has been evaluated in previous pooled analyses or meta-analyses of prospective cohort studies, and consistent with our final conclusions, these nutrients and foods did not appear to be associated with EOC risk.
Advantages of the NWAS approach were the ability to systematically evaluate an extensive list of dietary factors in relation to EOC risk while accounting for multiple testing by estimating the FDR, and by examining the associations between selected foods/nutrients and EOC risk in the NLCS, this provided further confidence in our findings. For most foods/nutrients that were evaluated in the EPIC study, we observed no association with EOC risk; this observation emphasizes the importance of the NWAS method because it necessitates the reporting of all results and therefore addresses the issue of the selective reporting of statistically significant findings (34, 35). For example, it appears that much of the published literature has reported findings where the P value is <0.05 (35). Possible limitations of this study included the single assessment of diet at the study baseline and the use of a self-reported dietary assessment, which could lead to some amount of misclassification of dietary intake and may attenuate the risk estimates toward the null. Thus, complementary methods of dietary assessment such as biomarker studies would be useful to corroborate the current results. In the NLCS cohort, we observed null results for the dietary factors that were examined; this may be because of the smaller number of cases, and/or limiting the validation study to a single national cohort with typical dietary habits may introduce an element of chance when validating dietary associations. It is also possible that other participant characteristics, such as differences in dietary intake amounts, could explain why the dietary associations were not confirmed; thus, meta-analyses including a large number of cohort studies would complement results from this NWAS. Because we were unable to evaluate the less common histologic subtypes of EOC because of the small numbers in the current study, larger pooled studies or consortium efforts are needed to investigate dietary risk associations for the rarer histologic subtypes of EOC. Dietary associations for EOC may also exist for specific foods/nutrients that were not included in the current analysis. Finally, as with any observational study, concern remains about residual confounding, even though we controlled for parity, oral contraceptive use, and menopausal status in the models.
In summary, these results represent the use of a novel NWAS method to assist in the search for modifiable dietary risk factors that may be of importance for the prevention of EOC. We evaluated dietary intake of 57 foods/food groups and nutrients in the EPIC study, and the 4 dietary factors that met the significance threshold were investigated in the NLCS. In the combined results from the EPIC study and the NLCS, we observed a higher EOC risk with a high intake than with a low intake of saturated fat, although there was no evidence of a dose-response relation. The positive associations with high compared with low saturated fat intake and risk of EOC observed in the EPIC study and NLCS could be due to chance, and it remains to be determined whether the association with saturated fat intake will be of clinical importance. Additional studies are needed to confirm the possible positive association between saturated fat intake and EOC risk.
Supplementary Material
Acknowledgments
We thank Razvan Sultana for assistance with programming. Sacha van de Crommert provided important assistance with data management of the NLCS.
The authors’ responsibilities were as follows—MAM, IT, and MJG: designed this research, including the development of the overall research plan; MAM, IT, and MJG: analyzed the data or provided study oversight; MAM: wrote the manuscript under the guidance of MJG; MAM and MJG: assumed primary responsibility for the final manuscript content; and all authors: conducted the research, including the data collection; provided essential materials such as databases necessary for this research; and critically evaluated, edited, read, and approved the final manuscript. The authors declared no conflicts of interest.
ABBREVIATIONS
- EOC
epithelial ovarian cancer
- EPIC
European Prospective Investigation into Cancer and Nutrition
- FDR
false discovery rate
- NLCS
Netherlands Cohort Study
- NWAS
nutrient-wide association study.
REFERENCES
- 1. World Cancer Research Fund/American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a global perspective [Internet]. 2007. [cited 2014 Sep 12]. Washington (DC): American Institute for Cancer Research; 2007. Available from: http://www.dietandcancerreport.org/cancer_resource_center/downloads/Second_Expert_Report_full.pdf.
- 2. Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang XS, Bannon F, Ahn JV, Johnson CJ, Bonaventure A, et al. . Global surveillance of cancer survival 1995–2009: analysis of individual data for 25 676 887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 2015;385:977–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Cancer Research Fund/American Institute for Cancer Research. Continuous update project report: food, nutrition, physical activity, and the prevention of ovarian cancer [Internet]. 2014 [cited 2015 Apr 3]. Washington (DC): American Institute for Cancer Research; 2014. Available from: http://www.dietandcancerreport.org/cup/cup_resources.php.
- 4. Crane TE, Khulpateea BR, Alberts DS, Basen-Engquist K, Thomson CA.. Dietary intake and ovarian cancer risk: a systematic review. Cancer Epidemiol Biomarkers Prev 2014;23:255–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Benjamini Y, Hochberg Y.. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc, B 1995;57:289–300. [Google Scholar]
- 6. Patel CJ, Bhattacharya J, Butte AJ.. An environment-wide association study (EWAS) on type 2 diabetes mellitus. PLoS One 2010;5:e10746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tzoulaki I, Patel CJ, Okamura T, Chan Q, Brown IJ, Miura K, Ueshima H, Zhao L, Van HL, Daviglus ML, et al. . A nutrient-wide association study on blood pressure. Circulation 2012;126:2456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Merritt MA, Tzoulaki I, Tworoger SS, De VI, Hankinson SE, Fernandes J, Tsilidis KK, Weiderpass E, Tjonneland A, Petersen KE, et al. . Investigation of dietary factors and endometrial cancer risk using a nutrient-wide association study approach in the EPIC and Nurses’ Health Study (NHS) and NHSII. Cancer Epidemiol Biomarkers Prev 2015;24:466–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Riboli E, Hunt KJ, Slimani N, Ferrari P, Norat T, Fahey M, Charrondiere UR, Hemon B, Casagrande C, Vignat J, et al. . European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr 2002;5:1113–24. [DOI] [PubMed] [Google Scholar]
- 10. van den Brandt PA, Goldbohm RA, van't Veer P, Volovics A, Hermus RJ, Sturmans F.. A large-scale prospective cohort study on diet and cancer in the Netherlands. J Clin Epidemiol 1990;43:285–95. [DOI] [PubMed] [Google Scholar]
- 11. Goldbohm RA, van den Brandt PA, Brants HA, van’t Veer P, Al M, Sturmans F, Hermus RJ.. Validation of a dietary questionnaire used in a large-scale prospective cohort study on diet and cancer. Eur J Clin Nutr 1994;48:253–65. [PubMed] [Google Scholar]
- 12. Sieri S, Krogh V, Ferrari P, Berrino F, Pala V, Thiebaut AC, Tjonneland A, Olsen A, Overvad K, Jakobsen MU, et al. . Dietary fat and breast cancer risk in the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr 2008;88:1304–12. [DOI] [PubMed] [Google Scholar]
- 13. Slimani N, Deharveng G, Unwin I, Southgate DA, Vignat J, Skeie G, Salvini S, Parpinel M, Moller A, Ireland J, et al. . The EPIC nutrient database project (ENDB): a first attempt to standardize nutrient databases across the 10 European countries participating in the EPIC study. Eur J Clin Nutr 2007;61:1037–56. [DOI] [PubMed] [Google Scholar]
- 14. Goldbohm RA, van’t Veer P, van den Brandt PA, van’t Hof MA, Brants HA, Sturmans F, Hermus RJ.. Reproducibility of a food frequency questionnaire and stability of dietary habits determined from five annually repeated measurements. Eur J Clin Nutr 1995;49:420–9. [PubMed] [Google Scholar]
- 15.Nevo table: Dutch food composition table 1986–1987. The Hague (Netherlands): Voorlichtingsbureau voor de voeding; 1986.
- 16. Prentice RL.. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika 1986;73:1–11. [Google Scholar]
- 17. Grambsch P, Therneau T.. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994;81:515–26. [Google Scholar]
- 18. Willett W, Stampfer M.. Implications of total energy intake for epidemiologic analyses In:Willett W, editor. Nutritional epidemiology. 2nd ed.Oxford (United Kingdom): American Society for Nutrition; 1998. p. 273–301. [Google Scholar]
- 19. Viechtbauer W.. Bias and efficiency of meta-analytic variance estimators in the random-effects model. J Educ Behav Stat 2005;30:261–93. [Google Scholar]
- 20. Therneau T.. A package for survival analysis in S [Internet]. 2014. [cited 2014 Sep 12]. Available from: http://CRAN.R-project.org/package=survival.
- 21. Viechtbauer W.. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010;36:1–48. [Google Scholar]
- 22. Lumley T. rmeta: meta-analysis [Internet]. 2012. [cited 2015 Aug 27]. Available from: http://CRAN.R-project.org/package=rmeta.
- 23. R Core Team. R: a language and environment for statistical computing [Internet]. 2014 [cited 2014 Sep 12]. Vienna (Austria): the R Foundation for Statistical Computing; 2014. Available from: http://www.R-project.org/.
- 24. Hill MJ, Goddard P, Williams RE.. Gut bacteria and aetiology of cancer of the breast. Lancet 1971;2:472–3. [DOI] [PubMed] [Google Scholar]
- 25. Lukanova A, Kaaks R.. Endogenous hormones and ovarian cancer: epidemiology and current hypotheses. Cancer Epidemiol Biomarkers Prev 2005;14:98–107. [PubMed] [Google Scholar]
- 26. Genkinger JM, Hunter DJ, Spiegelman D, Anderson KE, Beeson WL, Buring JE, Colditz GA, Fraser GE, Freudenheim JL, Goldbohm RA, et al. . A pooled analysis of 12 cohort studies of dietary fat, cholesterol and egg intake and ovarian cancer. Cancer Causes Control 2006;17:273–85. [DOI] [PubMed] [Google Scholar]
- 27. Blank MM, Wentzensen N, Murphy MA, Hollenbeck A, Park Y.. Dietary fat intake and risk of ovarian cancer in the NIH-AARP Diet and Health Study. Br J Cancer 2012;106:596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gilsing AM, Weijenberg MP, Goldbohm RA, van den Brandt PA, Schouten LJ.. Consumption of dietary fat and meat and risk of ovarian cancer in the Netherlands Cohort Study. Am J Clin Nutr 2011;93:118–26. [DOI] [PubMed] [Google Scholar]
- 29. Cui X, Rosner B, Willett WC, Hankinson SE.. Dietary fat, fiber, and carbohydrate intake and endogenous hormone levels in premenopausal women. Horm Cancer 2010;1:265–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu AH, Pike MC, Stram DO.. Meta-analysis: dietary fat intake, serum estrogen levels, and the risk of breast cancer. J Natl Cancer Inst 1999;91:529–34. [DOI] [PubMed] [Google Scholar]
- 31. Holmes MD, Spiegelman D, Willett WC, Manson JE, Hunter DJ, Barbieri RL, Colditz GA, Hankinson SE.. Dietary fat intake and endogenous sex steroid hormone levels in postmenopausal women. J Clin Oncol 2000;18:3668–76. [DOI] [PubMed] [Google Scholar]
- 32. Schock H, Surcel HM, Zeleniuch-Jacquotte A, Grankvist K, Lakso HA, Fortner RT, Kaaks R, Pukkala E, Lehtinen M, Toniolo P, et al. . Early pregnancy sex steroids and maternal risk of epithelial ovarian cancer. Endocr Relat Cancer 2014;21:831–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Koushik A, Hunter DJ, Spiegelman D, Anderson KE, Arslan AA, Beeson WL, van den Brandt PA, Buring JE, Cerhan JR, Colditz GA, et al. . Fruits and vegetables and ovarian cancer risk in a pooled analysis of 12 cohort studies. Cancer Epidemiol Biomarkers Prev 2005;14:2160–7. [DOI] [PubMed] [Google Scholar]
- 34. Patel CJ, Cullen MR, Ioannidis JP, Butte AJ.. Systematic evaluation of environmental factors: persistent pollutants and nutrients correlated with serum lipid levels. Int J Epidemiol 2012;41:828–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schoenfeld JD, Ioannidis JP.. Is everything we eat associated with cancer? A systematic cookbook review. Am J Clin Nutr 2013;97:127–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


