Abstract
There is considerable interest in the impact of (n-3) long-chain PUFA in mitigating the morbidity and mortality caused by chronic diseases. In 2002, the Institute of Medicine concluded that insufficient data were available to define Dietary Reference Intakes (DRI) for eicosapentaenoic acid (EPA) or docosahexaenoic acid (DHA), noting only that EPA and DHA could contribute up to 10% toward meeting the Adequate Intake for α-linolenic acid. Since then, substantial new evidence has emerged supporting the need to reassess this recommendation. Therefore, the Technical Committee on Dietary Lipids of the International Life Sciences Institute North America sponsored a workshop on 4–5 June 2008 to consider whether the body of evidence specific to the major chronic diseases in the United States—coronary heart disease (CHD), cancer, and cognitive decline—had evolved sufficiently to justify reconsideration of DRI for EPA+DHA. The workshop participants arrived at these conclusions: 1) consistent evidence from multiple research paradigms demonstrates a clear, inverse relation between EPA+DHA intake and risk of fatal (and possibly nonfatal) CHD, providing evidence that supports a nutritionally achievable DRI for EPA+DHA between 250 and 500 mg/d; 2) because of the demonstrated low conversion from dietary ALA, protective tissue levels of EPA+DHA can be achieved only through direct consumption of these fatty acids; 3) evidence of beneficial effects of EPA+DHA on cognitive decline are emerging but are not yet sufficient to support an intake level different from that needed to achieve CHD risk reduction; 4) EPA+DHA do not appear to reduce risk for cancer; and 5) there is no evidence that intakes of EPA+DHA in these recommended ranges are harmful.
Introduction and background
The (n-3) PUFA and foods from which they are derived have long been recognized as beneficial to human health. There is considerable interest in the potential ability of both the short-chain [α-linolenic acid (ALA)13 18:3(n-3)] and the long-chain [eicosapentaenoic acid (EPA) 20:5(n-3); docosahexaenoic acid (DHA) 22:6(n-3)] (n-3) PUFA to mitigate risk for chronic diseases, particularly those of the cardiovascular (CV) system and brain. In 2002, the Institute of Medicine (IOM) concluded that insufficient data were available to set a recommended dietary allowance or an adequate intake (AI) for EPA and/or DHA (1). The IOM report reviewed the available data on the physiology of absorption, metabolism, and excretion of (n-3) PUFA as well as evidence for deficiency and impact on 2 clinical outcomes: growth and neural development. An estimated average requirement was not established because of the lack of data in support of an (n-3) PUFA requirement for healthy individuals and for adequate growth and neural development. The IOM concluded that an AI for ALA could be set based on current population ALA intake in the apparent absence of dietary deficiency and that up to 10% of the AI for ALA may be provided by EPA+DHA. Again, the conclusion reflected current intakes of long-chain (LC) (n-3) PUFA. The IOM report formed the foundation for the 2005 Dietary Guidelines for Americans (2).
Since the publication of the IOM report, substantial new evidence has emerged to warrant a reassessment of the data for effects of EPA+DHA on health, particularly with respect to reducing risk for chronic diseases. To address this issue, the Technical Committee on Dietary Lipids of the International Life Sciences Institute (ILSI) North America sponsored and held a workshop on June 4–5, 2008 in Washington, DC. This workshop examined the rationale and process that governed the development of dietary reference intakes (DRI) for (n-3) LCPUFA in 2002 and considered whether the body of evidence specific to cardiovascular disease (CVD), cancer, and cognitive decline produced since that time justifies reconsideration of a DRI for EPA+DHA. An overview, rather than a thorough review, of the potential role of these fatty acids in human neurodevelopment was also included. The workshop also addressed the state of the science specific to the methods used to assess both intake and exposure, and identified research needs critical for DRI establishment.
EPA+DHA and health outcomes
Cardiovascular disease.
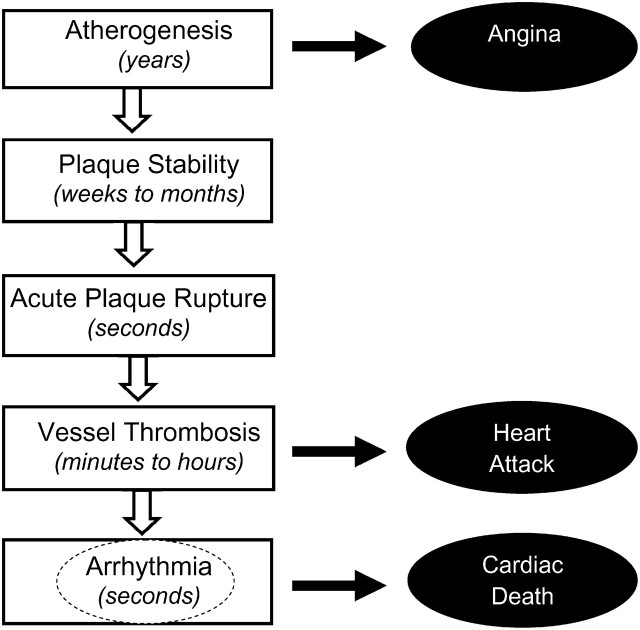
Since the 2002 IOM report on the DRI for macronutrients (1), substantial new evidence has accumulated on the health effects of EPA+DHA with respect to coronary heart disease (CHD) and CVD. CHD is not a distinct single event but a complex and multifaceted set of processes and clinical outcomes (Fig. 1). The first manifestation is the slow development and chronic progression of atherosclerotic plaques in response to chronic arterial injury. In contrast to this slow progression of plaque burden, characteristics related to plaque stability can change over weeks to months, increasing vulnerability to acute rupture, which can be followed by acute thrombosis. In up to half of plaque rupture cases, the resulting myocardial ischemia or injury can rapidly induce ventricular arrhythmia, which is nearly always fatal. Such fatal ventricular arrhythmias can be defined, depending upon clinical characteristics, as fatal myocardial infarction (MI), CHD death, or sudden cardiac death (SCD).
FIGURE 1 .
Development of coronary heart disease and potential sites of action of (n-3) fatty acids. At typical dietary doses (up to 1 g/d), the likely major site of action is the final pathway of ischemia-related cardiac arrhythmia (dotted circle), reducing the risk of cardiac death from fatal heart attack or sudden death. More modest effects on other pathways, such as atherosclerosis and plaque stability, may also reduce the risk of nonfatal myocardial infarctions (heart attacks) and acute coronary syndrome to a smaller extent.
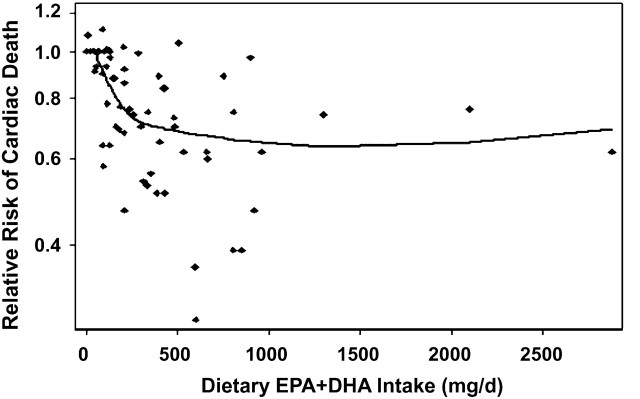
Thus, CHD involves a spectrum of biologic processes and related risk factors and mechanistic pathways, each of which can be affected in different ways by particular risk factors, treatments, and interventions, including (n-3) LCPUFA. The most consistent effect of (n-3) LCPUFA consumption is to reduce cardiac death, based on strongly concordant evidence from numerous prospective cohort studies in generally healthy populations (3–20), a retrospective case-control study of SCD (21), 4 large randomized controlled trials with fish or fish oil in patients with and without known heart disease (22–25), and numerous in vivo and animal experimental studies (26,27). Based on a previously reported pooled analysis of observational studies in adults (primary prevention) and of randomized controlled trials in patients with and without established CHD (primary and secondary prevention), this effect is seen at relatively low doses and is nonlinear (28). Effects of EPA+DHA in generally healthy individuals would be most relevant for setting population DRI. Thus, a new pooled analysis was performed and is presented in Figure 2. This analysis of prospective cohort studies of CHD death including only generally healthy individuals without established CHD (3–18) supported an intake of at least 250 mg/d of EPA+DHA to materially reduce risk for CHD. A recent meta-analysis (29) limited only to U.S. prospective studies (9,12,13,21,30,31) identified intakes of ∼500 mg/d as affording the greatest protection. One notable exception to these positive trials was 1 in men with angina that reported no benefit of recommending increased fish consumption (32).
FIGURE 2 .
Meta-analysis of estimated dietary EPA+DHA consumption from seafood and risk of cardiac death in generally healthy populations of individuals without known heart disease. The pooled analysis includes 4473 cardiac deaths in 326,572 generally healthy individuals in 16 prospective cohort studies (3–18) from multiple countries in Europe, the United States, China, and Japan, using methods described by Mozaffarian and Rimm (28).
In comparison, magnitudes of benefit and overall evidence for effects of (n-3) LCPUFA on other CV events, such as atherosclerosis progression and nonfatal acute coronary syndrome (ACS)/MI, are more modest. The much stronger effects on cardiac death, together with consistent antiarrhythmic effects of EPA+DHA from in vivo studies in rats, dogs, and primates and in vitro experiments (26,27,33), indicate that, at least for dietary doses (<1 g/d), the main cardiovascular effect of EPA+DHA is to reduce risk of fatal CHD, likely by reducing cardiac arrhythmias. Thus, based on the pathophysiology of CHD (Fig. 1), the likely major mode of action of (n-3) LCPUFA is on development of ischemia-related cardiac arrhythmia, with lesser effects on earlier processes (i.e., atherosclerosis progression, plaque instability, or thrombosis).
Largely on the basis of studies utilizing estimated dietary intake from questionnaires (Fig. 2), cardiac mortality is reduced ∼35% by modest EPA+DHA consumption (∼250–500 mg/d), an effect at least as great, for example, as that of statin therapy (34,35). Given random errors (and thus bias toward the null) inherent in dietary questionnaire estimates of EPA+DHA consumption (36), tissue levels of EPA+DHA (e.g., in erythrocytes, plasma, phospholipids, whole blood, or adipose) are more objective biomarkers of exposure that provide more precise estimates of effects. In both retrospective and prospective biomarker studies among generally healthy individuals without known CHD, compared with individuals in the highest quartile of EPA+DHA tissue levels, individuals in the lowest quartile had 10-fold higher risk of SCD independent of other known risk factors. This suggests that findings based on dietary questionnaires alone substantially underestimate the true benefits of (n-3) LCPUFA on SCD. Furthermore, the magnitudes of risk reduction seen with biomarkers of EPA+DHA are rarely seen in epidemiology—comparable to associations of smoking or asbestos with incidence of lung cancer—and are nearly impossible to explain solely by residual confounding by other factors.
As mentioned, numerous studies have evaluated potential effects of (n-3) LCPUFA on nonfatal CV outcomes, with more mixed results than those for cardiac death. These include studies showing equivocal effects of fish oil on atherosclerosis progression (37–40), and observational studies (12,13,18,41,42), and a large randomized controlled open-label trial [Japan Eicosapentaenoic Acid Lipid Intervention Study (JELIS)] (24) that suggest a modest reduction in risk of nonfatal ACS/MI. Evidence for effects on stroke is also mixed. Meta-analyses indicate a 30% lower risk of ischemic stroke with fish ≥1/wk compared with <1/mo (43) but no effect of fish oil consumption on stroke risk (44). Conversely, a post-hoc analysis from the JELIS trial found that EPA supplementation reduced recurrent stroke in individuals with a history of stroke (45). Overall, a growing body of evidence suggests that EPA+DHA consumption may benefits CV outcomes other than cardiac death, but the magnitudes of effect and breadth and concordance of evidence are not yet definitive compared with the clear reduction in cardiac death.
In randomized controlled trials, short-term EPA+DHA consumption at pharmacologic levels (>1–2 g/d) favorably affects many physiological measures of CV risk including blood pressure, resting heart rate, triglyceride levels, and possibly heart rate variability (28,46,47). For most of these risk factors, particularly those related to cardiac function, concordant findings have been seen in observational studies with habitual dietary EPA+DHA consumption at lower levels (<1 g/d) (48–52), suggesting that such lower levels of consumption may provide benefits in regard to these risk factors long-term. Because modest effects of dietary EPA+DHA on several of these risk factors could produce the moderate reductions in risk of nonfatal MI/ACS and ischemic stroke seen in some observational studies and controlled trials, these studies provide plausible physiological mechanisms to support the observed benefits of EPA+DHA on nonfatal cardiovascular events.
Unfortunately, effects of EPA+DHA on arrhythmic risk are difficult to quantify in a physiological study, as no widely accepted single modifiable physiological measure of arrhythmic risk exists. Three randomized trials in patients with implanted cardioversion defibrillator (ICD) devices have produced mixed results, with 2 trials (53,54) reporting no effects, and 1 reporting a benefit (55). Post-hoc subgroup analyses in 1 trial (53) suggested potential adverse effects in patients with a diagnosis of ventricular tachycardia who received the ICD, but this finding was not confirmed in the 2 larger studies (33,54). Established CHD risk factors such as plasma cholesterol levels, blood pressure, and C-reactive protein only modestly predict risk of arrhythmic cardiac death (55), and these risk factors are not strongly affected by dietary EPA+DHA. Resting heart rate, the QT interval, and heart rate variability each predict risk of fatal cardiac arrhythmia, and each has been favorably affected by fish oil consumption (47,49,50). The underlying variability in these markers makes them relatively insensitive for routine clinical evaluation of physiological effects on arrhythmic risk. Perhaps the best biochemical measure of arrhythmic risk is the tissue level of EPA+DHA, which is associated with 10-fold differences in risk of SCD across quartiles of tissue levels, as discussed previously (19,21). The omega-3 index, a measure of tissue (erythrocyte) EPA+DHA level, is discussed further below.
Both EPA and DHA are present in seafood and all fish oil supplements, although the ratio between the 2 typically differs, with fish usually having more DHA and supplements more EPA (56). Nearly all observational studies and randomized clinical trials of disease outcomes have evaluated the summed effects of combined EPA+DHA. Although some studies indicate that DHA may be preferentially antiarrhythmic (26), many experimentally characterized effects of EPA and DHA are similar (27,33,57), and the JELIS trial, which used pure EPA, reduced major coronary events by 19% (P = 0.01) (24). Thus, current evidence does not allow strong conclusions about whether EPA or DHA, or a specific ratio of the 2, is most beneficial. Based on this uncertainty, both should be consumed (in ratios between ∼1:2 to 2:1) to maximize cardiac health.
The evidence for a benefit of ALA in CV risk is much less well established than that for EPA+DHA (16,58,59). Although several cohort studies support such a connection (60), especially in the context of a low-linoleic acid (LA) diet (16), the lack of convincing data from randomized, controlled trials (61) leaves the role of ALA in cardioprotection unresolved. Hence, at present, ALA should not be considered as a replacement for EPA+DHA in reducing risk of cardiac death or other CVD.
In conclusion, evidence from randomized controlled trials of disease outcomes, prospective observational studies, retrospective case-control studies of disease outcomes, and randomized controlled trials of physiological measures, along with mechanistic insights from in vitro and in vivo animal-experimental studies, indicates that modest EPA+DHA consumption markedly reduces the risk of cardiac death. The quality, strength, and concordance of this evidence are remarkable, meeting and indeed generally exceeding those for any other dietary factor for which a DRI has been set based on reducing risk for chronic disease, including saturated fat, dietary cholesterol, salt, and dietary fiber. For primary prevention of cardiac death, the leading cause of death in both men and women in the United States and nearly all other developed nations, current evidence supports a DRI for EPA+DHA between 250 and 500 mg/d.
Cognitive development.
The brain and retina contain relatively large quantities of PUFA, specifically DHA and arachidonic acid (AA) (62,63). The accumulation of these fatty acids in central nervous system (CNS) tissue occurs during fetal development, primarily through placental transfer from maternal sources (64,65) and, in infancy and toddlerhood, largely through dietary sources (66,67). A consistent literature indicating that mammalian diets deficient in (n-3) PUFA adversely affect the development of visual acuity (68) and learning (69–75) and that replacing DHA in these diets prevents or corrects these behavioral and functional deficits implies a direct and specific role for DHA in normal neurological development.
Initial studies, both observational (76) and experimental (77–78), examining the impact of (n-3) LCPUFA supplementation, showed that (n-3) LCPUFA levels were related to the development of visual acuity in human infants. Reviews of research on the effects of (n-3) LCPUFA on visual function in human infants (79–82) indicate that supplementing infant formula with (n-3) LCPUFA improves visual development of preterm infants, although perhaps not cognitive development (83). The evidence for benefit in full-term infants is more equivocal.
Further evidence for the effects of (n-3) LCPUFA on cognitive development comes from studies of early attention and recognition memory in infancy. Research using measures of attention in infancy (expressed in terms of duration of looking) is fairly consistent, showing shorter looking times (consistent with more mature cognitive development) during the first year with (n-3) LCPUFA-supplemented infants (84–87) or infants from mothers with higher erythrocyte (n-3) LCPUFA levels (88). In addition, longer looking and greater attention during free play has been reported in a separate analysis of the same sample (88). Although a few studies have reported better visual recognition memory in infants supplemented with (n-3) LCPUFA (89), most studies have not (85,87,90–92).
Measures of receptive language (ability to perceive, process, and understand various aspects of language), communicative development, and vocabulary have also been used as outcomes. The (n-3) LCPUFA status in breast-fed infants was found to be greatly correlated with retention of the ability to discriminate a nonnative speech contrast (valid speech sounds not used in the individual's native language) at 9 mo (93) and with receptive and expressive vocabulary (words actually spoken) at 14 mo (89). Recently, it was reported that blood levels of DHA were highly positively correlated with performance on the Peabody Picture Vocabulary Test (94). The domain of language has yielded the only negative findings to date with respect to (n-3) LCPUFA status (95), although the negative outcome (slightly lower vocabulary in supplemented infants) did not persist in follow-up evaluations (95,96).
Results of studies using standardized indices of cognitive status as outcome variables in observational and experimental studies of (n-3) LCPUFA status are equivocal (89,90,97–102). These studies were recently reviewed and noted to vary across many parameters that could account for differences (103).
Dosage has not generally been evaluated in a comparable manner across studies (90,96,98,104,105). Although findings from these studies may not allow for extrapolation to daily intake, they do suggest that the current maximum for DHA (0.35%) in infant formula may actually represent a minimum. A consensus document in 2007 recommended at least 200 mg/d of DHA for pregnant women (80).
Cognitive decline.
There is wide variability in age-related cognitive decline in the population, a large portion of which represents various stages of dementia (106,107). Dementia is defined as the loss of cognition of sufficient severity to interfere with everyday function. The primary form of dementia is Alzheimer's disease (AD), which accounts for ∼60–80% of the cases and affects 10–13% of the population aged 65 y and older (108). To date there is no cure for AD, no widely accepted prevention strategy, and no effective treatment. The economic impact is enormous, with annual costs exceeding US$100 billion.
AD is characterized by a gradual decline in memory and at least 1 other cognitive domain (109). A primary theory of AD is that oxidative and inflammatory processes damage neuronal cells, causing severe disruption in their functioning, which leads to DNA damage, the accumulation of amyloid plaques, and loss of neurons and synapses (110). The established risk factors of AD include older age, apolipoprotein E-ε4 genotype, and low education (111), but risk factors common to CHD, including a high intake of saturated fat, low consumption of fish and (n-3) LCPUFA, hyperlipidemia, and hypertension, may also be implicated. Cerebral infarcts more than double the risk of dementia and AD (112).
Interest in fish consumption and the (n-3) LCPUFA as risk factors for dementia and AD is fairly recent, stemming mostly from the literature on DHA and neurocognitive development (113). DHA is concentrated in the most metabolically active areas of the brain, such as the cerebral cortex, synaptosomes, and mitochondria (114). Studies with aging animals have found that DHA composition of the brain decreases with age (115), and that loss of DHA is associated with decreases in antioxidant enzymes, scavengers of amyloid protein, hippocampal nerve growth (116), fluidity of synaptic membranes (117), and with increased oxidation of lipid membranes (118), ischemic damage (119), synaptic loss (120), and amyloid burden (121).
The epidemiological literature on (n-3) LCPUFA and dementia is fairly limited. Among 4 prospective studies (122–125) that examined fish consumption and the risk of developing dementia, 3 observed statistically significant decreased risks of 30–70% with consumption of 1 fish meal/wk after adjustment for age, sex, educational level, and various factors. The Chicago Health and Aging Project (CHAP) is 1 of the largest studies to date on the effects of fish and (n-3) LCPUFA intake on risk of incident AD and cognitive decline. In a sample of 815 CHAP participants, there was a 60% reduction in the risk of developing incident AD over 3.9 y among persons who consumed 1 fish meal/wk compared with persons who never or rarely consumed fish after adjustment for age, sex, race, apolipoprotein E-ε4, education, and total energy intake (125). The CHAP study also investigated the risk of incident AD by type of (n-3) LCPUFA consumed. Intake of EPA was not associated with AD risk. However, persons in quintiles 3 through 5 (median DHA intakes, 0.06 and 0.10 g/d, respectively) had a 60–80% lower risk compared with persons in quintile 1. Only 1 other prospective study (126) examined (n-3) LCPUFA intake and risk of dementia and found no evidence of an association. Studies reporting (n-3) LCPUFA levels in plasma or brain in subjects with various degrees of dementia have not shown a consistent relation between low DHA levels and clinical evidence of cognitive decline or dementia (127–129).
Four cohort studies have reported a protective effect against cognitive decline with higher (n-3) LCPUFA intakes (130–133). In a 4-y follow-up of 246 participants of the EVA study, a 1 standard deviation increase in erythrocyte total (n-3) LCPUFA and DHA were associated with a statistically significant 40% reductions in cognitive decline [as measured by a 2-point drop of on the Mini-Mental Status Examination (MMSE)] (130). In the CHAP study of 3718 persons, the annual rate of cognitive decline over 6 y was greatly reduced by 10% and 13%, respectively, for persons who consumed 1 and 2 fish meals/wk when compared with the rate among persons who consumed fish less often (131). Compared with the fish consumers among 210 participants of the Zutphen Elderly Study, nonconsumers had 4 times the risk of a 2-point decline in MMSE score over 5 y (132). Plasma phospholipid levels of (n-3) LCPUFA were closely associated with slower decline in verbal fluency among 2251 participants of the Atherosclerosis Risk in Communities study, but there was no association with levels and measures of verbal fluency, perceptual speed, or a global measure of cognitive function (133).
To date, there is only 1 published large randomized clinical trial of (n-3) LCPUFA and cognitive decline in which 204 patients with AD were randomized to daily intake of 1.7 g DHA and 0.6 g EPA or placebo for 6 mo. Whereas there was no overall effect on MMSE score or the Alzheimer Disease Assessment Scale, in post-hoc analyses, positive effects were observed in a small group of patients with very mild AD (134).
In summary, the available evidence for protective effects of (n-3) LCPUFA from fish on risk of dementia is promising but limited. Epidemiological evidence is positive for benefits of just 1 fish meal/wk on risk of AD, dementia, and cognitive decline. Further, the animal evidence and biologic plausibility support a protective relation of (n-3) LCPUFA on neurodegeneration of the brain with aging. However, tissue DHA levels do not seem to be consistently lower in AD or any form of cognitive decline associated with aging (127). Several primary and secondary prevention trials are in progress in the United States and Europe that will greatly inform the field. Further research is necessary to examine the effects of different dose levels, benefits of DHA, EPA, and ALA, and the importance of relative intakes of (n-3) vs. (n-6) fatty acids.
Cancer.
The preponderance of evidence for anticarcinogenic effects of EPA+DHA in humans is weak despite persuasive data from animal models and cultured tumor cell lines. An Agency for Healthcare and Research Quality review (135) and a meta-analysis reviewing 10 randomized controlled trials [relative risk (RR) 1.07, 95% CI 0.88–1.30] and 7 cohort studies (44) each concluded that there was little evidence for an effect of dietary (n-3) LCPUFA intake on cancer incidence (44,135). In a more recent meta-analysis examining (n-3) LCPUFA and colorectal cancer risk, the pooled RR from 14 studies comparing highest and lowest intakes was 0.88 (95% CI 0.78–1.00) (136). No significant associations were found between total fish intakes and breast cancer risk in the multicenter European Prospective Investigation into Cancer and Nutrition study (RR 1.01, 95% CI 0.99–1.02, P = 0.28) (137) or colon cancer risk in the Norwegian Women and Cancer study (n = 63,914) (138).
A review of hormone-related cancers (prostate and breast) also revealed no evidence for an effect (beneficial or harmful) of fish and marine fatty acids (139–141). Although some studies suggested that intakes or blood levels of marine-derived (n-3) PUFA may be inversely associated with risk for prostate cancers, particularly at advanced, late, and/or metastatic stages than at earlier stages (142,143), others have reported the opposite (144). Unfortunately, the stage of cancer is not usually taken into account by systematic reviews (44,139,145).
Recently, there has been some concern that dietary ALA might increase the risk of prostate cancer, particularly advanced and late stage (140,145,146). Although this was supported by a meta-analysis (60), more recent studies with other cohorts found no association (141,147). Nevertheless, because ALA is the predominant dietary (n-3) PUFA, clarifying the potential for interactions between dietary ALA and (n-3) LCPUFA and advanced prostate cancer risk merits further investigation.
The discordant results between animal and human studies for cancer effects have yet to be reconciled. This is an area of concern, particularly because the data generated from animal models and cell culture experiments have traditionally had preclinical utility as supporting evidence for the generation of DRI (1). Issues, separately or combined, that could explain the differential effects observed between clinical trials and experimental models include the inability to accurately assess (n-3) LCPUFA intakes in epidemiological studies, the supraphysiological doses often used in animal studies, the lack of established guidelines for background diets in animal studies (148), and the greater potential tissue need for DHA in rodents compared with humans (149).
In summary, there are no clear relations between dietary intakes of EPA and DHA and risk for cancer. The epidemiological data, and particularly prospective studies, are relatively consistent on this issue. Inconsistencies in dose and duration could be problematic as evidenced by the improvement in symptoms in animals that already have the disease and that are fed very high levels of (n-3) LCPUFA. Generating a standard dietary protocol that results in human-equivalent tissue levels of (n-3) LCPUFA will be essential to maximize the preclinical value of experimental animal models (148).
Total mortality.
Higher intakes of (n-3) LCPUFA have been associated with a reduced risk for death from any cause, largely related to their benefit on CHD mortality. In 3 prospective cohort studies that provided data on EPA+DHA consumption (11,30,150), significant reductions in all-cause mortality were associated with higher intakes; this, however, was not observed in a study in women initially free of heart disease (15). In a meta-analysis of the effects of treatments for dyslipidemia (diets and drugs), Studer et al. (151) reported that total mortality was reduced by only 2 interventions: (n-3) PUFA and statins. In 2 large randomized trials in cardiac patients (23,25), treatment with EPA+DHA reduced risk for death from any cause. Finally, in a meta-analysis of randomized controlled trials, fish oil treatment greatly reduced total mortality (28).
The conversion of ALA to EPA+DHA
As addressed by the IOM Panel on Macronutrients (1), the efficiency of conversion of ALA to EPA and on to DHA is a complex yet critical question. Uncertainty surrounds the extent of conversion in humans of the parent (n-3) PUFA, ALA, to its long-chain products, the potential health effects of ALA per se, and how ALA compares to stearidonic acid [SDA, 18:4(n-3)] as a source of EPA, docosapentaenoic acid (DPA, 22:5(n-3)], or DHA.
With the rare exception of genetic anomalies such as Zellweger syndrome, humans have a functional desaturation-chain elongation pathway in the liver (as well as the gut and brain) that can convert some ALA or LA to the respective LCPUFA. This has been established by several groups using in vitro assays in cultured human cells or assays of semipurified liver microsomal preparations of the Δ6 and Δ5 desaturases (152–155). Various model systems have been used to elucidate the generally acknowledged features of this desaturation-chain elongation pathway (156), which unequivocally exists in humans.
The relative health effects of the various (n-3) LCPUFA hinge not on the existence of desaturation-chain elongation activity but rather on its capacity. Two research approaches have been used in humans to study the conversion of the 18-carbon PUFA substrates to their 20- and 22-carbon products: tracer studies and dietary supplementation studies in which changes in levels of the LCPUFA in serum lipids are assessed. There are ∼18 papers reporting 1 of these approaches, and with few exceptions, they broadly agree that ALA conversion to DHA is no greater than ∼0.5% (156).
Given these data, the in vitro performance of the purified desaturases cannot be directly linked to their functional capacity in humans. In fact, because the capacity of the desaturation-chain elongation pathway in healthy, nonvegetarian humans is <0.5%, [perhaps somewhat greater in women than men (157)], even large amounts of dietary ALA have a negligible effect on plasma DHA, an effect paralleled by the (n-6) PUFA pathway, where dietary LA has only a negligible effect on plasma AA levels (158). Data on SDA or EPA conversion to DHA are extremely limited, but they confirm the inefficiency of DHA synthesis in healthy, adult, nonvegetarian humans (159).
It has also been reported that higher background intakes of LA can reduce plasma content of EPA (160). Whether this is a result of reduced EPA synthesis from ALA or of increased competition for acylation sites on plasma lipids is not clear. In addition, desaturation-chain elongation depends on several enzymes the activity of which can be inhibited by inadequate intakes of several cofactor nutrients including iron, zinc, and pyridoxine (161). The ability of ALA to slightly raise EPA and DPA (but not DHA) levels may partly explain the inverse associations between ALA and CHD noted earlier. Clearly, raising EPA alone can reduce risk for CHD (24). In addition, the presence in tissues of more DPA than EPA suggests that the former, as well as their precursor ALA, may have an as yet undefined physiological role (162).
Assessing (n-3) PUFA intake and exposure
What are we eating?.
Dietary sources of the plant-derived (n-3) PUFA, ALA, include canola and soybean oils, walnuts, and flaxseed oil, the latter being 1 of the most concentrated sources of ALA known (56). Although flaxseed oil is much richer in ALA than soybean oil (55 vs. 8% of fatty acids), the latter is the largest contributor of ALA to the U.S. diet, accounting as it does for more than two-thirds of the edible oil consumed (163).
Oils found naturally in fish are the richest dietary sources of EPA and DHA. There is typically more DHA than EPA in fish oils, but their ratios can vary widely across species (and in fish oil supplements). Even though U.S. fish consumption has increased slightly in recent years, intake is still quite low compared with other countries. In addition, many of the more popular varieties (e.g., shrimp, pollock, catfish, cod, clams, crabs, scallops, and tilapia) are not particularly good sources of (n-3) LCPUFA. The U.S. PUFA intake has been estimated using NHANES data, for which respondents provide dietary intake information over a 24-h period. According to the 2005–2006 survey, total PUFA [(n-6) and (n-3)] intake in adults was ∼19 g/d (164), of which ∼9% came from (n-3) PUFA, 8.2% (∼1.5 g/d) from ALA, and 0.7% (∼135 mg/d) from EPA+DHA. These values have changed little over the last 6 y (164,165). Fortified foods and fish oil supplements are large market growth areas by commercial sales figures; however, there are no reliable data indicating the amounts of EPA and DHA these products contribute to the American diet.
Per capita food disappearance data indicate that fish intake in the United States constitutes ∼6% of total meat consumption (166,167). In the context of a very low national fish intake, terrestrial meats (e.g., beef, pork, mutton, poultry, game) may actually contribute significant amounts of DHA and DPA. In the Australian diet, it has been estimated that terrestrial meats contribute almost 50% of the (n-3) LCPUFA, primarily in the form of DPA (168). Assessment of the impact of terrestrial meat consumption on (n-3) LCPUFA intake in the United States has been limited by the lack of complete data on these fatty acids in some terrestrial meats in food composition databases before 2008. The contribution of (n-3) LCPUFA from meat in the Australian diet may be higher than that in the U.S. diet because Australian cattle are more often fed grass (which contains some ALA), whereas U.S. cattle are more commonly finished on corn (very low in ALA).
Continual improvement of food composition databases is critical to accurate assessment of (n-3) LCPUFA intake. These databases form the ultimate foundation for U.S. food policy. Food composition databases, especially the USDA National Nutrient Database for Standard Reference (SR) (56), catalogue the (n-3) LCPUFA content of foods. The SR is used to estimate intakes in studies based on both 24-h recall and FFQ methods. Under the National Food and Nutrient Analysis Program, the USDA, NIH, and FDA are currently collaborating to expand the (n-3) LCPUFA composition data for foods (seafood as well as other foods, such as terrestrial meats and eggs) and dietary supplements in the SR (169). The new analytical data will be released in ∼2010. Although these efforts will greatly improve the accuracy of (n-3) LCPUFA intake estimates in the future, studies currently in the published literature (all of which used earlier versions of the database) probably underestimated intakes.
Even with a perfect database, all methods of estimating food consumption are limited by participant recall error, difficulties in describing amounts and composition of foods eaten, and assessment-induced changes in dietary patterns. Although the 24-h recall is less likely to accurately capture occasional foods (e.g., fish), FFQ are limited by crude categorization of fish species and portion size. Together, these factors can lead to inaccurate intake estimates, which when used in cohort studies of disease endpoints, can result in substantial underestimation of the true magnitude of benefit or risk (36). The need for validated biomarkers of (n-3) LCPUFA exposure is critical and is discussed further below.
What should we be eating?.
Recommendations for (n-3) LCPUFA intake have been put forth by several organizations globally (Table 1) (170–178). Most focus on primary prevention of CHD, whereas others aim only to prevent nutritional deficiencies. (The extent to which premature CHD can be considered a manifestation of EPA+DHA deficiency remains a point of discussion.) Some (e.g., American Heart Association), recommend foods (i.e., oily fish), whereas others (e.g., IOM) deal with nutrients. Whether the recommendation is for fish or for EPA+DHA, the resulting EPA+DHA values typically fall between 200 and 600 mg/d.
TABLE 1.
Recommendations for fish and/or EPA+DHA intakes for healthy adults from government and health organizations worldwide
| Organization | Year | Recommendation |
|---|---|---|
| Eurodiet Conference (170) | 2000 | 200 mg/d |
| Agence Française de Sécurité Sanitaire des Aliments, Centre national de coordination des études et recherches sur la nutrition et l'alimentation, and Centre national de la recherche scientifique (France) (171) | 2001 | 500 mg/d |
| UK Scientific Advisory Committee on Nutrition (172) | 2004 | Fish twice/wk, 1 serving of which should be oily, minimum intake 450 mg/d |
| International Society for the Study of Fatty Acids and Lipids (173) | 2004 | 500 mg/d |
| Australia Department of Health and Ageing (Australia and New Zealand) (174) | 2005 | 442 mg/d for men, 318 mg/d for women* |
| American Heart Association (175) | 2006 | 2 servings/wk of (preferably oily) fish |
| Health Council of the Netherlands (176) | 2006 | Fish twice/wk, 1 serving of which should be oily, to achieve the DRI of 450 mg/d of (n-3) LCPUFA |
| Superior Health Council of Belgium (177) | 2006 | Minimum of 0.3% energy for adults (∼667 mg/d) |
| American Dietetic Association/ Dietitians of Canada (178) | 2007 | Fish twice/wk, both servings oily or 500 mg/d |
The Australia and New Zealand recommendation is for EPA+DHA+DPA. The portion provided by EPA+DHA alone is given here per Howe et al. (168).
An AI of ALA was defined as ∼0.6% of energy by the IOM Panel on Macronutrients (1). This translates into ∼0.5 g/d for infants, 1.1 g/d for women, and 1.6 g/d for men. The AI is based on the median intake of ALA in the United States, according to the Continuing Survey of Food Intakes by Individuals (CSFII) 1994–1996 and 1998 (now replaced by NHANES), and concomitant lack of apparent deficiency in the population. It should be appreciated that the IOM Panel did not make a recommendation for EPA and DHA, per se. Rather, the Panel concluded that, because roughly 10% of the total (n-3) PUFA intake currently comes from EPA and DHA, 10% of the AI for ALA could be met by these 2 (n-3) LCPUFA.
Assessing (n-3) status.
Given the uncertainties surrounding accurate assessment of (n-3) LCPUFA intakes, a biomarker-based approach to defining target intakes may be more rational. In other words, if a certain blood level of (n-3) LCPUFA can be clearly linked to an important health benefit (e.g., reduced risk for CHD), then that blood level could be considered a surrogate endpoint, and the intakes needed to achieve that blood level may be recommended. The omega-3 index (55) is an example of a marker of (n-3) LCPUFA biostatus. The omega-3 index is the sum of EPA+DHA in erythrocyte membranes and is expressed as a percentage of total erythrocyte fatty acids (179). This marker has been validated against dietary intake (180) and has been shown to correlate strongly with reduced risk for mortality from CHD (as described above). The choice of membrane (n-3) LCPUFA levels, as opposed to plasma (n-3) LCPUFA composition or concentration, as a biomarker of intake is based on the fact that the effects of these fatty acids on basic cellular function appear to arise primarily from their effects on and in membranes. Still, whole plasma (and plasma phospholipid and cholesteryl ester) levels do correlate closely with erythrocyte levels (55). The omega-3 index is strongly associated with human cardiac membrane EPA+DHA levels (r = 0.81, P < 0.0001), and in cardiac transplant patients supplemented with (n-3) LCPUFA, both cardiac and RBC EPA+DHA increase to the same extent (162). Depending on the dose, it takes 3 to 6 mo for a new steady-state omega-3 index to be established (181,182). In addition, the omega-3 index has been shown to reflect the intake of both oily fish and fish oil supplements and to be less variable than plasma EPA+DHA levels (183).
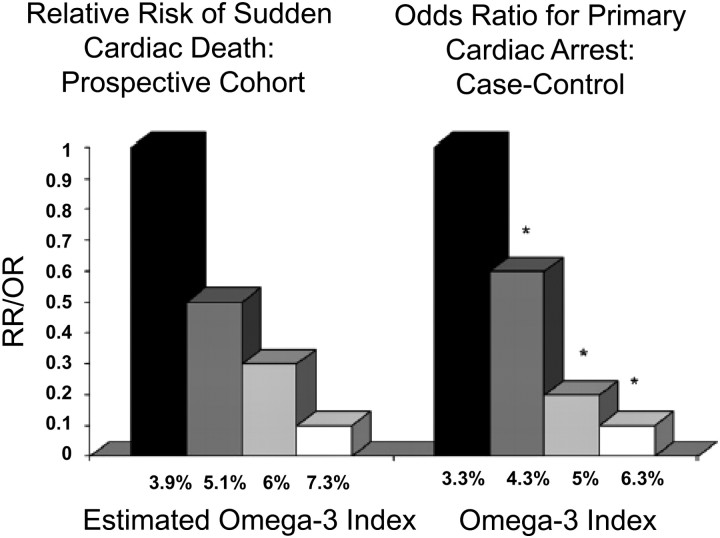
The blood content of (n-3) LCPUFA [which is highly correlated with the omega-3 index (179)] may actually be a better predictor of risk for SCD than traditional CHD risk factors. This may be seen by comparing 2 reports from the Physicians' Health Study (Fig. 3) (184). Albert et al. (185) published the relative risk for SCD across quartiles of C-reactive protein (CRP), total cholesterol (TC), LDL- and HDL-cholesterol, triglycerides, homocysteine and the TC/HDL ratio. In the second article (19), the relations between blood (n-3) LCPUFA content and the same endpoints were reported. Only 2 risk factors demonstrated statistically significant relations with risk for SCD after controlling for age and smoking status: CRP and (n-3) LCPUFA. Blood (n-3) LCPUFA content was related to risk in a dose-dependent manner, unlike CRP, which was more of a dichotomous variable. In addition, the risk reduction at the highest levels of the omega-3 index (90%) was greater than that associated with the lowest levels of CRP (65%). Therefore, in the case of SCD [which is responsible for about half of all CHD deaths (186)], the omega-3 index may be more informative than other currently accepted risk factors.
FIGURE 3 .
Relation between the omega-3 index and risk for primary cardiac arrest (right) and the estimated omega-3 index and SCD (left). The former were derived from a population-based case-control study (21), and the latter from a case-control study nested in the prospective Physicians' Health Study (P-trend = 0.001) (19). [The omega-3 index was estimated from the whole-blood (n-3) LCPUFA content using equations described by Harris and von Schacky; *P < 0.05 vs. quartile 1.] Reproduced with permission from William S. Harris (162).
In proposing that the omega-3 index may serve as a new risk marker for CHD mortality, Harris and von Schacky (179) estimated what a “healthy” omega-3 index target might be by extrapolating from studies of other (n-3) LCPUFA biomarkers (e.g., whole blood, plasma phospholipids) and of (n-3) LCPUFA intakes. They arrived at a proposed cardioprotective target level of 8% of erythrocyte fatty acids as EPA+DHA. An omega-3 index of 4% or less was associated with the greatest risk for CHD death. These values have recently been shown to distinguish between ACS patients and controls (41). One of the major obstacles to defining target cardioprotective levels of (n-3) LCPUFA is the lack of standardized methods for their assessment. In the 1980s, the National Heart Lung and Blood Institute and CDC undertook to establish the Lipid Standardization Program, which has led to worldwide uniformity in lipid and lipoprotein testing. A similar effort is needed for blood fatty acid testing.
Obstacles to setting a DRI
Overarching issues.
One of the major goals of the workshop was to understand the obstacles preventing the establishment of a DRI for (n-3) LCPUFA. In 2002, the IOM Committee concluded that there was insufficient evidence available to set a DRI for (n-3) LCPUFA (1). Some of the concerns were as follows:
Because ALA can be converted to (n-3) LCPUFA, the latter could not strictly be considered as essential nutrients.
EPA and DHA are distinct chemical species, not 1 nutrient (like ascorbic acid), and absent data showing a specific need for 1 or the other (or a defined ratio of the 2), it was unclear how a combined recommendation could be made.
The evidence linking higher intakes of (n-3) LCPUFA to reduced CVD risk was not based on studies with these fatty acids per se but on population estimates of fish intake, which provides multiple other nutrients and displaces other foods associated with higher CVD risk.
The trial evidence for cardioprotection was derived from patients with CHD, not from the general population, so the application of these intakes to primary prevention was unclear.
The extent to which increased intakes of (n-3) LCPUFA were safe (particularly with respect to risks for bleeding) was poorly defined.
Six years later, many of these concerns have been addressed (if not resolved). To summarize:
The rate of conversion of ALA to EPA is low (∼5%), and to DHA even lower (∼0.5%). This degree of conversion is insufficient to achieve tissue levels of (n-3) LCPUFA that will protect against premature CHD.
Although specific or optimal ratios among the (n-3) LCPUFA cannot yet be defined, both their natural ratios in fish (typically DHA > EPA) and in the prescription (n-3) product used in the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico (GISSI) studies (23,25) (EPA > DHA) appear to provide substantial protection against fatal CHD. Although EPA and DHA have different physiological roles (187) that will need to be more precisely defined, EPA:DHA ratios ranging from ∼1:2 to 2:1 are expected to be effective. In addition, there is significant precedent for treating macronutrients as complex mixtures of multimolecular species, the individual components of which often have differing physiological effects (e.g., dietary fiber, total fat, saturated fat, carbohydrate, protein). Despite the inability to define the “optimal” composition of each one, a DRI has been set for each of these macronutrient complexes. Consequently, concerns that the “right” ratio of EPA and DHA is not yet precisely known or that all of their physiological effects have not been discovered should not be an obstacle to defining a DRI.
The effects on CHD first observed with fish intake [e.g., in the Diet and Reinfarction Trial (22)] have now been replicated in 3 major clinical trials using only (n-3) LCPUFA (23–25). Hence, although fish provides many nutrients, the (n-3) LCPUFA content is clearly a major mediator of the observed CHD benefits of fish seen in healthy populations (primary prevention).
The wealth of data from multiple observational cohort studies in numerous countries strongly support a primary prevention benefit for (n-3) LCPUFA on fatal CHD. Furthermore, JELIS (24), the first large prevention trial to include a majority of patients without established CHD, demonstrated a 19% reduction in major coronary events with EPA. Although the trial was not powered to separately detect benefits in both the primary and secondary prevention arms, subgroup analyses indicated very similar risk reduction estimates in these 2 groups. It is questionable, in the face of similar results from both observational studies of hundreds of thousands of generally healthy individuals and randomized trials of patients with and without established CHD, to conclude that secondary prevention data does not inform primary prevention. Indeed, contention that secondary prevention data cannot support efficacy in primary prevention may be challenged by the fact that there has never been a treatment regimen in CVD that, after being documented to be effective in the secondary setting, failed to be effective when ultimately tested in the primary setting. The basic pathophysiology of CVD is the same, whether for the first heart attack or the second. Concerns regarding the extrapolation of secondary to primary prevention efficacy are quite appropriate when evaluating drugs whose benefits must outweigh potential side effects. These are of considerably less concern with agents such as (n-3) LCPUFA, which have a strong safety profile. Finally, the requirement that a nutrient be shown to reduce clinical events in primary prevention, randomized trials before the assigning of a DRI has not been applied to other nutrients or food components. Neither increases in dietary fiber (discussed in more detail below) nor reductions in total fat, saturated fat, or cholesterol have ever been shown, in single-variable, placebo-controlled, prospective trials, to reduce risk for CHD events in either primary or secondary populations, and yet DRI exist for them all. Because the requirement for definitive evidence of primary prevention has not been applied to other nutrients, it is not clear why it should be required for establishing a DRI for (n-3) LCPUFA, nutrients for which the highest levels of evidence for benefit have been assembled.
With regard to safety, JELIS (24), GISSI-Prevenzione (23), and GISSI-Heart Failure (25) reported no clinically relevant adverse effects in over 35,000 individuals. In addition, a review of the effects of pharmacologic doses (3 g/d or greater, even in combination with anticoagulants) on clinically significant bleeding found no reason for concern (188). Over 10 y ago, the FDA determined that intakes of EPA+DHA of up to 3 g/d are safe for the general population (189). Hence, there should be no concerns about the safety of targeting an EPA+DHA intake of up to 500 mg/d.
The dietary fiber precedent.
The 2002 IOM report on macronutrients included a DRI for dietary fiber (1), which provides a useful precedent for EPA+DHA. An AI for adults was established based on CHD risk reduction, as observed from 3 prospective observational studies of substantial scientific quality. The basis for selecting the AI was, “[T]he AI of 14 g of fiber per 1000 kcal was calculated using the average intake of fiber in the ‘protected group’ in each of the studies (i.e., the highest quintile of fiber intake)” (1). The same principles may be applied to EPA+DHA, for which a reduction in CHD was documented in several prospective observational studies in North America (9,12,13,19,21,30). The average intake in the highest quintile in these studies was 566 mg/d (29). In addition, there are 4 randomized controlled trials (22–25) with hard CHD endpoints for (n-3) LCPUFA and none for fiber. Hence, the evidence for a CHD benefit with EPA+DHA is considerably stronger than that for fiber.
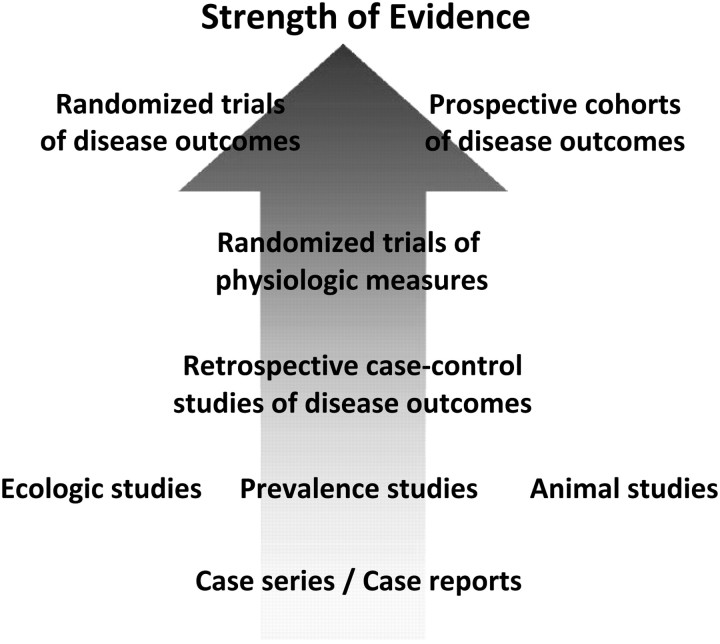
Evidence supporting a CHD benefit for increased intakes of EPA+DHA is available from studies across the hierarchy of trial designs and qualities (Fig. 4). Since the 2002 IOM report was issued, over 50 studies addressing the relations between CHD and EPA+DHA have been published (Table 2). It is largely on the strength of this dataset that the health organizations listed in Table 1 have made recommendations for EPA+DHA to reduce risk for CHD. In the view of the authors, a literature of this depth should be sufficient to support a reevaluation of the need to establish a U.S. DRI for EPA+DHA.
FIGURE 4 .
Hierarchy of quality and strength of evidence of different study designs for evaluating causation of chronic disease. Studies of physiological endpoints may not fully account for all effects of an intervention, and thus, evidence is strongest when derived from well-conducted studies of disease endpoints. Well-controlled prospective observational studies and well-performed randomized clinical trials have different, and highly complementary, strengths and limitations. The major strength of randomized trials is to minimize residual confounding; limitations include potential lower generalizability to external populations, the inability to evaluate the effects of many risk factors of interest (e.g., smoking, biomarker levels) due to ethical or practical limitations, inadequacy of duration and/or dose tested, challenges in testing multiple doses, noncompliance, unblinding, differential loss to follow-up, and treatment crossover. The major limitation of prospective observational studies is potential residual confounding, whereas major strengths generally include the converse of each of the major limitations of randomized trials. Thus, evidence is most robust when studies of both designs provide concordant results, as is the case for EPA+DHA consumption and (especially fatal) CHD.
TABLE 2.
Examples of studies related to (n-3) LCPUFA and CHD published since the 2002 IOM Report on Macronutrients
| First author (reference) | Year | Study topic |
|---|---|---|
| Marchioli (196) | 2002 | GISSI Prevenzione update |
| Albert (19) | 2002 | Blood (n-3) FA and risk for SCD |
| Hu (12) | 2002 | Fish intake and CHD death: Nurse's Health Study |
| He (197) | 2002 | Fish intake and incident stroke |
| Bucher (198) | 2002 | (n-3) FA trials and death: meta-analysis |
| Nestel (199) | 2002 | (n-3) FA and systemic arterial compliance |
| Thies (200) | 2003 | (n-3) FA and plaque stability |
| Burr (32) | 2003 | Diet and Reinfarction Trial-II |
| Mozaffarian (13) | 2003 | (n-3) FA and CHD: Cardiovascular Health Study (CHS) |
| Erkkilä (201) | 2003 | Serum (n-3) FA and CHD death |
| Dallongeville (202) | 2003 | Fish intake and heart rate |
| Lemaitre (20) | 2003 | Serum (n-3) FA and CHD death: CHS |
| Pischon (203) | 2003 | (n-3) FA intake and inflammatory markers |
| Hino (204) | 2004 | (n-3) FA intake and carotid intima-media thickness (CIMT) |
| Mozaffarian (205) | 2004 | (n-3) FA and incident atrial fibrillation: CHS |
| He (5) | 2004 | Fish and CHD mortality: meta-analysis |
| Schrepf (206) | 2004 | Infusion of (n-3) FA and arrhythmic inducibility |
| Harris (162) | 2004 | Cardiac and RBC (n-3) FA levels in transplant patients |
| Harris (55) | 2004 | Omega-3 index as a CHD risk marker |
| He (43) | 2004 | Fish and incident stroke: meta-analysis |
| Erkkilä (37) | 2004 | Serum (n-3) FA and coronary artery disease (CAD) progression in women |
| Hooper (207) | 2004 | Cochrane (n-3) FA and CHD: meta-analysis |
| Mozaffarian (47) | 2005 | (n-3) FA and heart rate: meta-analysis |
| Mozaffarian (208) | 2005 | (n-3) FA and incident stroke: CHS |
| Raitt (53) | 2005 | (n-3) FA and ICD discharge: Portland |
| Leaf (33) | 2005 | (n-3) FA and ICD discharge: Boston |
| Studer (151) | 2005 | Antilipidemic agents and death: meta-analysis |
| Calò (209) | 2005 | (n-3) FA and atrial fibrillation post-coronary artery bypass graft |
| Iso (18) | 2006 | Japan Public Health Center: fish/(n-3) FA and CHD |
| Wang (210) | 2006 | (n-3) FA and CHD: systematic review |
| Mozaffarian (28) | 2006 | (n-3) FA risk/benefit review |
| Mozaffarian (50) | 2006 | (n-3) FA and electrocardiogram: CHS |
| Mozaffarian (51) | 2006 | (n-3) FA and cardiac structure and function: CHS |
| Brouwer (54) | 2006 | (n-3) FA and ICD discharge: Holland |
| Radaelli (211) | 2006 | (n-3) FA and baroreceptor reflex |
| Hjerkinn (212) | 2006 | Fish and (n-3) FA and pulse wave velocity and CIMT |
| Harris (213) | 2006 | (n-3) FA and heart rate in cardiac transplant patients |
| Yokoyama (24) | 2007 | JELIS: EPA and CHD events |
| Harris (179) | 2007 | (n-3) FA and bleeding risk |
| Harris (214) | 2007 | (n-3) FA biomarkers and CHD risk |
| Wennberg (215) | 2007 | (n-3) intake and stroke |
| Mita (216) | 2007 | EPA and CIMT: arterial compliance in type 2 diabetes mellitus |
| Harris (183) | 2007 | Fish vs. fish oil capsules on RBC (n-3) levels |
| Block (41) | 2008 | Blood cell (n-3) PUFA and ACS: case control |
| GISSI-HF (25) | 2008 | GISSI-Heart Failure |
| Harris (29) | 2008 | (n-3) FA intake for primary prevention |
| Sekikawa (217) | 2008 | Serum (n-3) FA and CAD: Japan vs. U.S. |
| Cohen (218) | 2008 | RBC (n-3) FA and depression in ACS |
| Aarsetoy (219) | 2008 | RBC (n-3) FA and post-MI ventricular fibrillation |
| Mozaffarian (49) | 2008 | Fish intake and heart rate variability: CHS |
| Sun (42) | 2008 | Serum (n-3) FA and nonfatal MI: Nurse's Health Study |
| Tanaka (45) | 2008 | JELIS-EPA and stroke |
| Walser (220) | 2008 | (n-3) FA effects on stroke volume |
| Wilhelm (221) | 2008 | Blood (n-3) FA and risk for ventricular arrhythmia |
| Hall (222) | 2008 | Acute effects of EPA on digital pulse volume |
| Yamagishi (223) | 2008 | (n-3) FA intakes and CV death in Japan |
Research needs
Additional areas of research that would help to further define optimal intakes for EPA+DHA are below:
Expand investigation into the impact of (n-3) LCPUFA on other CVD outcomes, such as atherosclerosis progression and plaque stability.
Further define the individual effects of ALA, SDA, EPA, DPA, and DHA.
Improve tools for dietary assessment, including food composition databases used to quantify the (n-3) LCPUFA content of foods, fortified foods, and dietary supplements (including lesser known sources, such as terrestrial animals, microorganisms, and technology-enhanced oils) and FFQ instruments with greater specificity for seafood varieties and preparations.
Validate biomarkers for exposure and health outcomes (e.g., the omega-3 index), establish a dose-response relation between dietary intakes and biomarker levels, and standardize methods for laboratory analysis.
Further document the safety of (n-3) LCPUFA and identify populations susceptible to potential adverse effects.
Potential impact of a DRI for EPA+DHA on food policy and industry
A defined nutrient recommendation can have an important impact on food policy. Potential avenues of policy implementation include Dietary Guidelines for Americans; MyPyramid; food labeling; fortification; and feeding programs (i.e., Women, Infants and Children). If a DRI for EPA+DHA were to be set, it would be important to consider the impact of such a recommendation on the ability of suppliers of (n-3) LCPUFA to meet the increased demand in a sustainable manner. Serious concerns about overfishing and aquaculture practices have been raised (190) and must be considered. Aquaculture has the potential to increase the global availability of seafood and, likewise, the intake of (n-3) LCPUFA. Although there are tonnage and dollar values for species raised through farming available through the Food and Agriculture Organization (191), data on fatty acid composition and actual consumption in the United States and other countries are quite limited. The extent to which aquaculture may influence (n-3) LCPUFA intake depends on many factors, not least of which is whether an increased availability of fish would generate an increased demand. If the demand for a food grows, systems and technologies needed to produce it will need to be developed that are both economically viable and sustainable. In addition to potential expansion of aquaculture and improved methods for sustainable wild catch fishing, other novel approaches have potential to increase availability of (n-3) LCPUFA to consumers. These include 1) the cultivation of single-celled organisms, with or without genetic modification, to produce EPA and/or DHA (192); 2) the creation of new plant cultivars that contain either more readily convertible precursors of LC (n-3) PUFA (159) or EPA and DHA (193,194); and 3) the use of deodorized and stabilized oils derived from inedible (n-3) PUFA-rich marine organisms (e.g., menhaden, krill) to produce a wide variety of fortified foods (195). In each case, the (n-3) LCPUFAs thus produced could be used either in human foods or to expand aquaculture. Nevertheless, challenges in implementing a nutrient recommendation should not deter federal agencies from setting a DRI if the scientific support and the potential health benefits are substantial. Additional sources of and new approaches to providing (n-3) LCPUFA will undoubtedly be found once a need is codified.
Conclusion
From this workshop the following conclusions were reached:
Based on the strength of the current literature (which includes over 50 additional observational and interventional studies published since the 2002 IOM review), there is a clear, inverse relation between EPA+DHA intake and risk of fatal (and possibly nonfatal) CHD. Accordingly, there is a compelling rationale for reconsidering the establishment of a nutritionally achievable DRI for EPA+DHA. Based on these studies, international recommendations have been made for intakes of ∼500 mg/d to reduce risk for CHD.
Because of the demonstrably low conversion of dietary ALA to EPA and especially to DHA, protective tissue levels of EPA+DHA can currently be achieved only through direct intake of these fatty acids.
Beneficial effects of EPA+DHA on cognitive decline are emerging, but the data are not yet sufficiently compelling to support an intake level different from that needed to achieve CHD risk reduction.
Higher intakes of EPA+DHA do not appear to reduce risk for cancer.
There is no evidence that intakes of EPA+DHA in the range recommended are harmful.
Abbreviations
- AA
arachidonic acid
- ACS
acute coronary syndrome
- AD
Alzheimer's disease
- AI
adequate intake
- ALA
α-linolenic acid)
- CAD
coronary artery disease
- CHAP
Chicago Health and Aging Project
- CHD
coronary heart disease
- CHS
Cardiovascular Health Study
- CIMT
carotid intima-media thickness
- CNS
central nervous system
- CRP
C-reactive protein
- CSFII
Continuing Survey of Food Intakes by Individuals
- CV
cardiovascular
- CVD
cardiovascular disease
- DHA
docosahexaenoic acid
- DPA
docosapentaenoic acid
- DRI
Dietary Reference Intakes
- EPA
eicosapentaenoic acid
- FA
fatty acids
- GISSI
Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico
- ICD
implantable cardioverter-defibrillator
- ILSI
International Life Sciences Institute
- IOM
Institute of Medicine
- JELIS
Japan Eicosapentaenoic Acid Lipid Intervention Study
- LA
linoleic acid
- LC
long-chain
- MI
myocardial infarction
- MMSE
Mini-Mental Status Examination
- RR
relative risk
- SCD
sudden cardiac death
- SDA
stearidonic acid
- SR
National Nutrient Database for Standard Reference
- TC
total cholesterol
Literature Cited
- 1. Institute of Medicine Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, DC: National Academy Press; 2002/2005. [DOI] [PubMed] [Google Scholar]
- 2. Report of the dietary guidelines advisory committee on the dietary guidelines for Americans Washington, DC: US Department of Agriculture and Department of Health and Human Services; 2005. [Google Scholar]
- 3. Kromhout D, Bosschieter EB, de Lezenne Coulander C.. The inverse relation between fish consumption and 20-year mortality from coronary heart disease. N Engl J Med. 1985;312:1205–9. [DOI] [PubMed] [Google Scholar]
- 4. Dolecek TA, Granditis G.. Dietary polyunsaturated fatty acids and mortality in the Multiple Risk Factor Intervention Trial (MRFIT). World Rev Nutr Diet. 1991;66:205–16. [DOI] [PubMed] [Google Scholar]
- 5. He K, Song Y, Daviglus ML, Liu K, Van Horn L, Dyer AR, Greenland P.. Accumulated evidence on fish consumption and coronary heart disease mortality: a meta-analysis of cohort studies. Circulation. 2004;109:2705–11. [DOI] [PubMed] [Google Scholar]
- 6. Kromhout D, Feskens EJ, Bowles CH.. The protective effect of a small amount of fish on coronary heart disease mortality in an elderly population. Int J Epidemiol. 1995;24:340–5. [DOI] [PubMed] [Google Scholar]
- 7. Daviglus ML, Stamler J, Orencia AJ, Dyer AR, Liu K, Greenland P, Walsh MK, Morris D, Shekelle RB.. Fish consumption and the 30-year risk of fatal myocardial infarction. N Engl J Med. 1997;336:1046–53. [DOI] [PubMed] [Google Scholar]
- 8. Mann JI, Appleby PN, Key TJ, Thorogood M.. Dietary determinants of ischaemic heart disease in health conscious individuals. Heart. 1997;78:450–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Albert CM, Hennekens CH, O'Donnell CJ, Ajani UA, Carey VJ, Willett WC, Ruskin JN, Manson JE.. Fish consumption and risk of sudden cardiac death. JAMA. 1998;279:23–8. [DOI] [PubMed] [Google Scholar]
- 10. Oomen CM, Feskens EJ, Rasanen L, Fidanza F, Nissinen AM, Menotti A, Kok FJ, Kromhout D.. Fish consumption and coronary heart disease mortality in Finland, Italy, and The Netherlands. Am J Epidemiol. 2000;151:999–1006. [DOI] [PubMed] [Google Scholar]
- 11. Yuan JM, Ross RK, Gao YT, Yu MC.. Fish and shellfish consumption in relation to death from myocardial infarction among men in Shanghai, China. Am J Epidemiol. 2001;154:809–16. [DOI] [PubMed] [Google Scholar]
- 12. Hu FB, Bronner L, Willett WC, Stampfer MJ, Rexrode KM, Albert CM, Hunter D, Manson JE.. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA. 2002;287:1815–21. [DOI] [PubMed] [Google Scholar]
- 13. Mozaffarian D, Lemaitre RN, Kuller LH, Burke GL, Tracy RP, Siscovick DS.. Cardiac benefits of fish consumption may depend on the type of fish meal consumed: the Cardiovascular Health Study. Circulation. 2003;107:1372–7. [DOI] [PubMed] [Google Scholar]
- 14. Osler M, Andreasen AH, Hoidrup S.. No inverse association between fish consumption and risk of death from all causes, and incidence of coronary heart disease in middle-aged, Danish adults. J Clin Epidemiol. 2003;56:274–9. [DOI] [PubMed] [Google Scholar]
- 15. Folsom AR, Demissie Z.. Fish intake, marine omega-3 fatty acids, and mortality in a cohort of postmenopausal women. Am J Epidemiol. 2004;160:1005–10. [DOI] [PubMed] [Google Scholar]
- 16. Mozaffarian D, Ascherio A, Hu FB, Stampfer MJ, Willett WC, Siscovick DS, Rimm EB.. Interplay between different polyunsaturated fatty acids and risk of coronary heart disease in men. Circulation. 2005;111:157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nakamura Y, Ueshima H, Okamura T, Kadowaki T, Hayakawa T, Kita Y, Tamaki S, Okayama A.. Association between fish consumption and all-cause and cause-specific mortality in Japan: NIPPON DATA80, 1980–99. Am J Med. 2005;118:239–45. [DOI] [PubMed] [Google Scholar]
- 18. Iso H, Kobayashi M, Ishihara J, Sasaki S, Okada K, Kita Y, Kokubo Y, Tsugane S.. Intake of fish and n3 fatty acids and risk of coronary heart disease among Japanese: the Japan Public Health Center-Based (JPHC). Study Cohort I. Circulation. 2006;113:195–202. [DOI] [PubMed] [Google Scholar]
- 19. Albert CM, Campos H, Stampfer MJ, Ridker PM, Manson JE, Willett WC, Ma J.. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N Engl J Med. 2002;346:1113–8. [DOI] [PubMed] [Google Scholar]
- 20. Lemaitre RN, King IB, Mozaffarian D, Kuller LH, Tracy RP, Siscovick DS.. n-3 Polyunsaturated fatty acids, fatal ischemic heart disease, and nonfatal myocardial infarction in older adults: the Cardiovascular Health Study. Am J Clin Nutr. 2003;77:319–25. [DOI] [PubMed] [Google Scholar]
- 21. Siscovick DS, Raghunathan TE, King I, Weinmann S, Wicklund KG, Albright J, Bovbjerg V, Arbogast P, Smith H, et al. . Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. JAMA. 1995;274:1363–7. [DOI] [PubMed] [Google Scholar]
- 22. Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, Elwood PC, Deadman NM.. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet. 1989;2:757–61. [DOI] [PubMed] [Google Scholar]
- 23. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico [Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial.] Lancet. 1999;354:447–55. [PubMed] [Google Scholar]
- 24. Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, et al. . Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–8. [DOI] [PubMed] [Google Scholar]
- 25. GISSI-HF Investigators Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–30. [DOI] [PubMed] [Google Scholar]
- 26. McLennan PL.. Myocardial membrane fatty acids and the antiarrhythmic actions of dietary fish oil in animal models. Lipids. 2001;36:S111–4. [DOI] [PubMed] [Google Scholar]
- 27. McLennan PL.. Omega-3 polyunsaturated fatty acid prevention of cardiac arrhythmia and sudden death: Cellular or circulating? Curr Top Nutraceut Res. 2004;2:101–12. [Google Scholar]
- 28. Mozaffarian D, Rimm EB.. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006;296:1885–99. [DOI] [PubMed] [Google Scholar]
- 29. Harris WS, Kris-Etherton PM, Harris KA.. Intakes of long-chain omega-3 fatty acid associated with reduced risk for death from coronary heart disease in healthy adults. Curr Atheroscler Rep. 2008;10:503–9. [DOI] [PubMed] [Google Scholar]
- 30. Dolecek TA.. Epidemiologic evidence of relationships between dietary polyunsaturated fatty acids and mortality in the multiple risk factor intervention trial. Proc Soc Exp Biol Med. 1992;200:177–82. [DOI] [PubMed] [Google Scholar]
- 31. Ascherio A, Rimm EB, Stampfer MJ, Giovannucci EL, Willett WC.. Dietary intake of marine n-3 fatty acids, fish intake, and the risk of coronary disease among men. N Engl J Med. 1995;332:977–82. [DOI] [PubMed] [Google Scholar]
- 32. Burr ML, Ashfield-Watt PA, Dunstan FD, Fehily AM, Breay P, Ashton T, Zotos PC, Haboubi NA, Elwood PC.. Lack of benefit of dietary advice to men with angina: results of a controlled trial. Eur J Clin Nutr. 2003;57:193–200. [DOI] [PubMed] [Google Scholar]
- 33. Leaf A, Albert CM, Josephson M, Steinhaus D, Kluger J, Kang JX, Cox B, Zhang H, Schoenfeld D.. Prevention of fatal arrhythmias in high-risk subjects by fish oil n-3 fatty acid intake. Circulation. 2005;112:2762–8. [DOI] [PubMed] [Google Scholar]
- 34. Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, et al. . Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–78. [DOI] [PubMed] [Google Scholar]
- 35. Thavendiranathan P, Bagai A, Brookhart MA, Choudhry NK.. Primary prevention of cardiovascular diseases with statin therapy: a meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166:2307–13. [DOI] [PubMed] [Google Scholar]
- 36. Willett WC.. Nutritional epidemiology. 2nd ed New York: Oxford University Press; 1998. [Google Scholar]
- 37. Erkkilä AT, Lichtenstein AH, Mozaffarian D, Herrington DM.. Fish intake is associated with a reduced progression of coronary artery atherosclerosis in postmenopausal women with coronary artery disease. Am J Clin Nutr. 2004;80:626–32. [DOI] [PubMed] [Google Scholar]
- 38. Sacks FM, Stone PH, Gibson CM, Silverman DI, Rosner B, Pasternak RC.. Controlled trial of fish oil for regression of human coronary atherosclerosis. HARP Research Group. J Am Coll Cardiol. 1995;25:1492–8. [DOI] [PubMed] [Google Scholar]
- 39. von Schacky C, Angerer P, Kothny W, Theisen K, Mudra H.. The effect of dietary omega-3 fatty acids on coronary atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1999;130:554–62. [DOI] [PubMed] [Google Scholar]
- 40. Angerer P, Kothny W, Stork S, von Schacky C.. Effect of dietary supplementation with omega-3 fatty acids on progression of atherosclerosis in carotid arteries. Cardiovasc Res. 2002;54:183–90. [DOI] [PubMed] [Google Scholar]
- 41. Block RC, Harris WS, Reid KJ, Sands SA, Spertus JA.. EPA and DHA in blood cell membranes from acute coronary syndrome patients and controls. Atherosclerosis. 2008;197:821–8. [DOI] [PubMed] [Google Scholar]
- 42. Sun Q, Ma J, Campos H, Rexrode KM, Albert CM, Mozaffarian D, Hu FB.. Blood concentrations of individual long-chain n-3 fatty acids and risk of nonfatal myocardial infarction. Am J Clin Nutr. 2008;88:216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. He K, Song Y, Daviglus ML, Liu K, Van Horn L, Dyer AR, Goldbourt U, Greenland P.. Fish consumption and incidence of stroke: a meta-analysis of cohort studies. Stroke. 2004;35:1538–42. [DOI] [PubMed] [Google Scholar]
- 44. Hooper L, Thompson RL, Harrison RA, Summerbell CD, Ness AR, Moore HJ, Worthington HV, Durrington PN, Higgins JPT, et al. . Risks and benefits of omega 3 fats for mortality, cardiovascular disease, and cancer: systematic review. BMJ. 2006;332:752–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tanaka K, Ishikawa Y, Yokoyama M, Origasa H, Matsuzaki M, Saito Y, Matsuzawa Y, Sasaki J, Oikawa S, et al. . Reduction in the recurrence of stroke by eicosapentaenoic acid for hypercholesterolemic patients: subanalysis of the JELIS trial. Stroke. 2008;39:2052–8. [DOI] [PubMed] [Google Scholar]
- 46. Geleijnse JM, Giltay EJ, Grobbee DE, Donders AR, Kok FJ.. Blood pressure response to fish oil supplementation: metaregression analysis of randomized trials. J Hypertens. 2002;20:1493–9. [DOI] [PubMed] [Google Scholar]
- 47. Mozaffarian D, Geelen A, Brouwer IA, Geleijnse JM, Zock PL, Katan MB.. Effect of fish oil on heart rate in humans: A meta-analysis of randomized controlled trials. Circulation. 2005;112:1945–52. [DOI] [PubMed] [Google Scholar]
- 48. Mozaffarian D.. Fish, n-3 fatty acids, and cardiovascular haemodynamics. J Cardiovasc Electrophysiol. 2007;8:S23–6. [DOI] [PubMed] [Google Scholar]
- 49. Mozaffarian D, Stein PK, Prineas RJ, Siscovick DS.. Dietary fish and omega-3 fatty acid consumption and heart rate variability in US adults. Circulation. 2008;117:1130–7. [DOI] [PubMed] [Google Scholar]
- 50. Mozaffarian D, Prineas RJ, Stein PK, Siscovick DS.. Dietary fish and n-3 fatty acid intake and cardiac electrocardiographic parameters in humans. J Am Coll Cardiol. 2006;48:478–84. [DOI] [PubMed] [Google Scholar]
- 51. Mozaffarian D, Gottdiener JS, Siscovick DS.. Intake of tuna or other broiled or baked fish versus fried fish and cardiac structure, function, and hemodynamics. Am J Cardiol. 2006;97:216–22. [DOI] [PubMed] [Google Scholar]
- 52. Balk EM, Lichtenstein AH, Chung M, Kupelnick B, Chew P, Lau J.. Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: a systematic review. Atherosclerosis. 2006;189:19–30. [DOI] [PubMed] [Google Scholar]
- 53. Raitt MH, Connor WE, Morris C, Kron J, Halperin B, Chugh SS, McClelland J, Cook J, MacMurdy K, et al. . Fish oil supplementation and risk of ventricular tachycardia and ventricular fibrillation in patients with implantable defibrillators: a randomized controlled trial. JAMA. 2005;293:2884–91. [DOI] [PubMed] [Google Scholar]
- 54. Brouwer IA, Zock PL, Camm AJ, Böcker D, Hauer RN, Wever EF, Dullemeijer C, Ronden JE, Katan MB, et al. ; SOFA Study Group . Effect of fish oil on ventricular tachyarrhythmia and death in patients with implantable cardioverter defibrillators: the Study on Omega-3 Fatty Acids and Ventricular Arrhythmia (SOFA) randomized trial. JAMA. 2006;295:2613–9. [DOI] [PubMed] [Google Scholar]
- 55. Harris WS.. The omega-3 index as a risk factor for coronary heart disease. Am J Clin Nutr. 2008;87:1997S–2002S. [DOI] [PubMed] [Google Scholar]
- 56. USDA National Nutrient Database for Standard Reference, Release 21 (SR 21) [database on the Internet] Washington, DC: USDA, Agricultural Research Service; ©2008. [cited 2008 September 29]. Available from: http://www.ars.usda.gov/Services/docs.htm?docid=8964. [Google Scholar]
- 57. Hirafuji M, Machida T, Hamaue N, Minami M.. Cardiovascular protective effects of n-3 polyunsaturated fatty acids with special emphasis on docosahexaenoic acid. J Pharmacol Sci. 2003;92:308–16. [DOI] [PubMed] [Google Scholar]
- 58. Mozaffarian D.. Does alpha-linolenic acid intake reduce the risk of coronary heart disease? A review of the evidence [review]. Altern Ther Health Med. 2005;11:24–30; quiz 31, 79. [PubMed] [Google Scholar]
- 59. Albert CM, Oh K, Whang W, Manson JE, Chae CU, Stampfer MJ, Willett WC, Hu FB.. Dietary alpha-linolenic acid intake and risk of sudden cardiac death and coronary heart disease. Circulation. 2005;112:3232–8. [DOI] [PubMed] [Google Scholar]
- 60. Brouwer IA, Katan MB, Zock PL.. Dietary alpha-linolenic acid is associated with reduced risk of fatal coronary heart disease, but increased prostate cancer risk: a meta-analysis. J Nutr. 2004;134:919–22. [DOI] [PubMed] [Google Scholar]
- 61. Harris WS.. Alpha linolenic acid—A gift from the and? Circulation. 2005;111:2872–4. [DOI] [PubMed] [Google Scholar]
- 62. O'Brien JS, Fillerup DL, Mead JF.. Quantification and fatty acid and fatty aldehyde composition of ethanolamine, choline, and serine glycerophosphatides in human cerebral grey and white matter. J Lipid Res. 1964;5:329–42. [PubMed] [Google Scholar]
- 63. Anderson RE, Maude MB, Zimmerman W.. Lipids of ocular tissues–X. Lipid composition of subcellular fractions of bovine retina. Vision Res. 1975;15:1087–90. [DOI] [PubMed] [Google Scholar]
- 64. Dutta-Roy AK.. Transport mechanisms for long-chain polyunsaturated fatty acids in the human placenta. Am J Clin Nutr. 2000;71:315S–22S. [DOI] [PubMed] [Google Scholar]
- 65. Ruyle M, Connor WE, Anderson GJ, Lowensohn RJ.. Placental transfer of essential fatty acids in humans: venous-arterial difference for docosahexaenoic acid in fetal umbilical erythrocytes. Proc Natl Acad Sci USA. 1990;87:7902–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Putnam JC, Carlson SE, Devoe PW, Barness LA.. The effect of variations in dietary fatty acids on the fatty acid composition of erythrocyte phosphatidylcholine and phosphatidylethanolamine in human infants. Am J Clin Nutr. 1982;36:106–14. [DOI] [PubMed] [Google Scholar]
- 67. Sanders TA, Naismith DJ.. A comparison of the influence of breast-feeding and bottle-feeding on the fatty acid composition of the erythrocytes. Br J Nutr. 1979;41:619–23. [DOI] [PubMed] [Google Scholar]
- 68. Neuringer M, Connor WE, Van Petten C, Barstad L.. Dietary omega-3 fatty acid deficiency and visual loss in infant rhesus monkeys. J Clin Invest. 1984;73:272–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bourre JM, Francois M, Youyou A, Dumont O, Piciotti M, Pascal G, Durand G.. The effects of dietary alpha-linolenic acid on the composition of nerve membranes, enzymatic activity, amplitude of electrophysiological parameters, resistance to poisons and performance of learning tasks in rats. J Nutr. 1989;119:1880–2. [DOI] [PubMed] [Google Scholar]
- 70. Garcia-Calatayud S, Redondo C, Martin E, Ruiz JI, Garacia-Guentes M, Sanjurjo P.. Brain docosahexaenoic acid status and learning in young rats submitted to dietary long-chain polyunsaturated fatty acid deficiency and supplementation limited to lactation. Pediatr Res. 2005;57:719–23. [DOI] [PubMed] [Google Scholar]
- 71. Lim SY, Hoshiba J, Moriguchi T, Salem N Jr.. N-3 fatty acid deficiency induced by a modified artificial rearing method leads to poorer performance in spatial learning tasks. Pediatr Res. 2005;58:741–8. [DOI] [PubMed] [Google Scholar]
- 72. Reisbick S, Neuringer M, Gohl E, Wald R, Anderson GJ.. Visual attention in infant monkeys: effects of dietary fatty acids and age. Dev Psychol. 1997;33:387–95. [DOI] [PubMed] [Google Scholar]
- 73. Wainwright PE, Huang YS, Coscina DV, Levesque S, McCutcheon D.. Brain and behavioral effects of dietary n-3 deficiency in mice: a three generational study. Dev Psychobiol. 1994;27:467–87. [DOI] [PubMed] [Google Scholar]
- 74. Yamamoto N, Saitoh M, Moriuchi A, Nomura M, Okuyama H.. Effect of dietary alpha-linolenate/linoleate balance on brain lipid compositions and learning ability of rats. J Lipid Res. 1987;28:144–51. [PubMed] [Google Scholar]
- 75. Yamamoto N, Hashimoto A, Takemoto Y, Okuyama H, Nomura M, Kitajima R, Togashi T, Tamai Y.. Effect of the dietary alpha-linolenate/linoleate balance on lipid compositions and learning ability of rats. II. Discrimination process, extinction process, and glycolipid compositions. J Lipid Res. 1988;29:1013–21. [PubMed] [Google Scholar]
- 76. Makrides M, Summer K, Goggin M, Gibson RA.. Erythrocyte docosahexaenoic acid correlates with the visual response of healthy, term infants. Pediatr Res. 1993;33:425–7. [DOI] [PubMed] [Google Scholar]
- 77. Birch EE, Birch DG, Hoffman DR, Uauy RD.. Dietary essential fatty acid supply and visual acuity development. Invest Ophthalmol Vis Sci. 1992;33:3242–53. [PubMed] [Google Scholar]
- 78. Carlson SE, Werkman SH, Rhodes PG, Tolley EA.. Visual-acuity development in healthy preterm infants: effect of marine-oil supplementation. Am J Clin Nutr. 1993;58:35–42. [DOI] [PubMed] [Google Scholar]
- 79. Innis SM.. Dietary (n-3) fatty acids and brain development. J Nutr. 2007;137:855–9. [DOI] [PubMed] [Google Scholar]
- 80. Koletzko B, Cetin I, Brenna JT; Perinatal Lipid Intake Working Group, Child Health Foundation; Diabetic Pregnancy Study Group; European Association of Perinatal Medicine; European Association of Perinatal Medicine; European Society for Clinical Nutrition and Metabolism; European Society for Paediatric Gastroenterology, Hepatology and Nutrition, Committee on Nutrition; International Federation of Placenta Associations; International Society for the Study of Fatty Acids and Lipids . Dietary fat intakes for pregnant and lactating women. Br J Nutr. 2007;98:873–7. [DOI] [PubMed] [Google Scholar]
- 81. SanGiovanni JP, Parra-Cabrera S, Colditz GA, Berkey CS, Dwyer JT.. Meta-analysis of dietary essential fatty acids and long-chain polyunsaturated fatty acids as they relate to visual resolution acuity in healthy preterm infants. Pediatrics. 2000;105:1292–8. [DOI] [PubMed] [Google Scholar]
- 82. SanGiovanni JP, Berkey CS, Dwyer JT, Colditz GA.. Dietary essential fatty acids, long-chain polyunsaturated fatty acids, and visual resolution acuity in healthy fullterm infants: a systematic review. Early Hum Dev. 2000;57:165–88. [DOI] [PubMed] [Google Scholar]
- 83. Makrides M, Gibson RA, McPhee AJ, Collins CT, Davis PG, Doyle LW, Simmer K, Colditz PB, Morris S, Smithers LG, Willson K, Ryan P.. Neurodevelopmental outcomes of preterm infants fed high-dose docosahexaenoic acid: a randomized controlled trial. JAMA. 2009;301:175–82. [DOI] [PubMed] [Google Scholar]
- 84. Carlson SE.. Lipid requirements of very-low-birth-weight infants for optimal growth and development. In: Dobbing J. editor. Lipids, learning, and the brain: fats in infant formulas. Report of the 103rd Ross Conference on Pediatric Research Columbus, OH: Ross Laboratories; 1993. p. 188. [Google Scholar]
- 85. Werkman SH, Carlson SE.. A randomized trial of visual attention of preterm infants fed docosahexaenoic acid until nine months. Lipids. 1996;31:91–7. [DOI] [PubMed] [Google Scholar]
- 86. Willatts P, Forsyth JS, DiModugno MK, Varma S, Colvin M.. Influence of long-chain polyunsaturated fatty acids on infant cognitive function. Lipids. 1998;33:973–80. [DOI] [PubMed] [Google Scholar]
- 87. Carlson SE, Werkman SH.. A randomized trial of visual attention of preterm infants fed docosahexaenoic acid until two months. Lipids. 1996;31:85–90. [DOI] [PubMed] [Google Scholar]
- 88. Colombo J, Kannass KN, Shaddy DJ, Knudurthi S, Maikranz JM, Anderson CJ, Blaga OM, Carlson SE.. Maternal DHA and the development of attention in infancy and toddlerhood. Child Dev. 2004;75:1254–67. [DOI] [PubMed] [Google Scholar]
- 89. O'Connor DL, Hall R, Adamkin D, Auestad N, Castillo M, Connor WE, Connor SL, Fitzgerald K, Groh-Wargo S, et al. . Growth and development in preterm infants fed long-chain polyunsaturated fatty acids: a prospective, randomized controlled trial. Pediatrics. 2001;108:359–71. [DOI] [PubMed] [Google Scholar]
- 90. Auestad N, Halter R, Hall RT, Blatter M, Bogle ML, Burks W, Erickson JR, Fitzgerald KM, Dobson V, et al. . Growth and development in term infants fed long-chain polyunsaturated fatty acids: a double-masked, randomized, parallel, prospective, multivariate study. Pediatrics. 2001;108:372–81. [DOI] [PubMed] [Google Scholar]
- 91. Birch EE, Hoffman DR, Castaneda YS, Fawsett SL, Birch DG, Uauy RD.. A randomized controlled trial of long-chain polyunsaturated fatty acid supplementation of formula in term infants after weaning at 6 wk of age. Am J Clin Nutr. 2002;75:570–80. [DOI] [PubMed] [Google Scholar]
- 92. Helland IB, Saugstad CD, Smith L, Saarem K, Solvoll K, Ganes T, Drevon CA.. Similar effects on infants of n-3 and n-6 fatty acids supplementation to pregnant and lactating women. Pediatrics. 2001;108:E82. [DOI] [PubMed] [Google Scholar]
- 93. Innis SM, Gilley J, Werker J.. Are human milk long-chain polyunsaturated fatty acids related to visual and neural development in breast-fed term infants? J Pediatr. 2001;139:532–8. [DOI] [PubMed] [Google Scholar]
- 94. Ryan AS, Nelson EB.. Assessing the effect of docosahexaenoic acid on cognitive functions in healthy, preschool children: a randomized, placebo-controlled, double-blind study. Clin Pediatr. 2008;47:355–62. [DOI] [PubMed] [Google Scholar]
- 95. Lauritzen L, Jorgensen MH, Mikkelsen TP, Skorvgaard M, Straarup EM, Olsen SF, Hoy CE, Michaelsen KF.. Maternal fish oil supplementation in lactation: effect on visual acuity and n-3 fatty acid content of infant erythrocytes. Lipids. 2004;39:195–206. [DOI] [PubMed] [Google Scholar]
- 96. Auestad N, Scott DT, Janowsky JS, Jacobsen C, Carroll RE, Montalto MB, Halter R, Qiu W, Jacobs JR, et al. . Visual, cognitive, and language assessments at 39 months: a follow-up study of children fed formulas containing long-chain polyunsaturated fatty acids to 1 year of age. Pediatrics. 2003;112:e177–83. [DOI] [PubMed] [Google Scholar]
- 97. Agostoni C, Trojan S, Bellù R, Riva E, Giovannini M.. Neurodevelopmental quotient of healthy term infants at 4 months and feeding practice: the role of long-chain polyunsaturated fatty acids. Pediatr Res. 1995;38:262–6. [DOI] [PubMed] [Google Scholar]
- 98. Birch EE, Garfield S, Hoffman DR, Uauy R, Birch DG.. A randomized controlled trial of early dietary supply of long-chain polyunsaturated fatty acids and mental development in term infants. Dev Med Child Neurol. 2000;42:174–81. [DOI] [PubMed] [Google Scholar]
- 99. Clandinin MT, Van Aerde LE, Merkel KL, Harris CL, Springer MA, Hansen JW, Diersen-Schade DA.. Growth and development of preterm infants fed infant formulas containing docosahexaenoic acid and arachidonic acid. J Pediatr. 2005;146:461–8. [DOI] [PubMed] [Google Scholar]
- 100. Agostoni C, Trojan S, Bellu R, Riva E, Bruzzese MG, Giovanni M.. Developmental quotient at 24 months and fatty acid composition of diet in early infancy: a follow up study. Arch Dis Child. 1997;76:421–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lucas A, Stafford M, Morley R, Abbott R, Stephenson T, MacFadyen U, Elias-Jones A, Clements H.. Efficacy and safety of long-chain polyunsaturated fatty acid supplementation of infant-formula milk: a randomised trial. Lancet. 1999;354:1948–54. [DOI] [PubMed] [Google Scholar]
- 102. Makrides M, Neumann MA, Simmer K, Gibson RA.. A critical appraisal of the role of dietary long-chain polyunsaturated fatty acids on neural indices of term infants: a randomized, controlled trial. Pediatrics. 2000;105:32–8. [DOI] [PubMed] [Google Scholar]
- 103. McNamara RK, Carlson SE.. Role of omega-3 fatty acids in brain development and function: potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot Essent Fatty Acids. 2006;75:329–49. [DOI] [PubMed] [Google Scholar]
- 104. Colombo J, Carlson SE, Cheatham CL, Kannass KN, Gustafson KM, Schmeidler T.. Dietary DHA supplementation in infancy lowers heart rate and increases sustained attention. Kansas City, MO: International Society for the Study of Fats and Lipids (ISSFAL); 2008. [Google Scholar]
- 105. Birch EE, Garfield S, Castaneda Y, Hughbanks-Wheaton D, Uauy R, Hoffman D.. Visual acuity and cognitive outcomes at 4 years of age in a double-blind, randomized trial of long-chain polyunsaturated fatty acid-supplemented infant formula. Early Hum Dev. 2007;83:279–84. [DOI] [PubMed] [Google Scholar]
- 106. Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, Bennett DA.. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002;17:179–93. [PubMed] [Google Scholar]
- 107. Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, Barnes LL, Fox JH, Bach J, et al. . Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. [DOI] [PubMed] [Google Scholar]
- 108. Evans DA, Funkenstein HH, Albert MS, Scherr PA, Cook NR, Chown MJ, Hebert LE, Hennekens CH, Taylor JO.. Prevalence of Alzheimer's disease in a community population of older persons. Higher than previously reported. JAMA. 1989;262:2551–6. [PubMed] [Google Scholar]
- 109. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM.. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–44. [DOI] [PubMed] [Google Scholar]
- 110. Christen Y.. Oxidative stress and Alzheimer disease. Am J Clin Nutr. 2000;71:621S–9S. [DOI] [PubMed] [Google Scholar]
- 111. Bennett DA, Part I.. Epidemiology and public health impact of Alzheimer's disease. Dis Mon. 2000;46:657–65. [DOI] [PubMed] [Google Scholar]
- 112. Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA.. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology. 2004;62:1148–55. [DOI] [PubMed] [Google Scholar]
- 113. Neuringer M, Anderson GJ, Connor WE.. The essentiality of n-3 fatty acids for the development and function of the retina and brain. Annu Rev Nutr. 1988;8:517–41. [DOI] [PubMed] [Google Scholar]
- 114. Carrie I, Clement M, de Javel D, Frances H, Bourre JM.. Specific phospholipid fatty acid composition of brain regions in mice. Effects of n-3 polyunsaturated fatty acid deficiency and phospholipid supplementation. J Lipid Res. 2000;41:465–72. [PubMed] [Google Scholar]
- 115. McGahon BM, Martin DS, Horrobin DF, Lynch MA.. Age-related changes in synaptic function: analysis of the effect of dietary supplementation with omega-3 fatty acids. Neuroscience. 1999;94:305–14. [DOI] [PubMed] [Google Scholar]
- 116. Kawakita E, Hashimoto M, Shido O.. Docosahexaenoic acid promotes neurogenesis in vitro and in vivo. Neuroscience. 2006;139:991–7. [DOI] [PubMed] [Google Scholar]
- 117. Wu A, Ying Z, Gomez-Pinilla F.. Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience. 2008;155:751–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Hashimoto M, Tanabe Y, Fujii Y, Kikuta T, Shibata H, Shido O.. Chronic administration of docosahexaenoic acid ameliorates the impairment of spatial cognition learning ability in amyloid beta-infused rats. J Nutr. 2005;135:549–55. [DOI] [PubMed] [Google Scholar]
- 119. Cao D, Yang B, Hou L, Xu J, Xue R, Sun L, Zhou C, Liu Z.. Chronic daily administration of ethyl docosahexaenoate protects against gerbil brain ischemic damage through reduction of arachidonic acid liberation and accumulation. J Nutr Biochem. 2007;18:297–304. [DOI] [PubMed] [Google Scholar]
- 120. Cole GM, Lim GP, Yang F, Teter B, Begum A, Ma Q, Harris-White ME, Frautschy SA.. Prevention of Alzheimer's disease: Omega-3 fatty acid and phenolic anti-oxidant interventions. Neurobiol Aging. 2005;26:S133–6. [DOI] [PubMed] [Google Scholar]
- 121. Lim GP, Calon F, Morihara T, Yang F, Teter B, Ubeda O, Salem N Jr, Frautschy SA, Cole GM.. A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. J Neurosci. 2005;25:3032–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Kalmijn S, Launer LJ, Ott A, Witteman JC, Hofman A, Breteler MM.. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann Neurol. 1997;42:776–82. [DOI] [PubMed] [Google Scholar]
- 123. Barberger-Gateau P, Letenneur L, Deschamps V, Peres K, Dartigues JF, Renaud S.. Fish, meat, and risk of dementia: cohort study. BMJ. 2002;325:932–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Huang TL, Zandi PP, Tucker KL, Fitzpatrick AL, Kuller LH, Fried LP, Burke GL, Carlson MC.. Benefits of fatty fish on dementia risk are stronger for those without APOE epsilon4. Neurology. 2005;65:1409–14. [DOI] [PubMed] [Google Scholar]
- 125. Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Wilson RS, Aggawal N, Schneider J.. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch Neurol. 2003;60:940–6. [DOI] [PubMed] [Google Scholar]
- 126. Engelhart MJ, Geerlings MI, Ruitenberg A, van Swieten JC, Hofmann A, Witteman JC, Breteler NM.. Diet and risk of dementia: Does fat matter? Neurology. 2002;59:1915–21. [DOI] [PubMed] [Google Scholar]
- 127. Plourde M, Fortier M, Vandal M, Tremblay-Mercier J, Freemantle E, Begin M, Pifferi F, Cunnane SC.. Unresolved issues in the link between docosahexaenoic acid and Alzheimer's disease. Prostaglandins Leukot Essent Fatty Acid. 2007;77:301–8. [DOI] [PubMed] [Google Scholar]
- 128. Schaefer EJ, Bongard V, Beiser AS, Lamon-Fava S, Robins SJ, Au R, Tucker L, Kyle DJ.. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: the Framingham Heart Study. Arch Neurol. 2006;63:1545–50. [DOI] [PubMed] [Google Scholar]
- 129. Laurin D, Verreault R, Lindsay J, Dewailly E, Holub BJ.. Omega-3 fatty acids and risk of cognitive impairment and dementia. J Alzheimers Dis. 2003;5:315–22. [DOI] [PubMed] [Google Scholar]
- 130. Heude B, Ducimetiere P, Berr C.. Cognitive decline and fatty acid composition of erythrocyte membranes—The EVA Study. Am J Clin Nutr. 2003;77:803–8. [DOI] [PubMed] [Google Scholar]
- 131. Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS.. Fish consumption and cognitive decline with age in a large community study. Arch Neurol. 2005;62:1849–53. [DOI] [PubMed] [Google Scholar]
- 132. van Gelder BM, Tijhuis M, Kalmijn S, Kromhout D.. Fish consumption, n-3 fatty acids, and subsequent 5-y cognitive decline in elderly men: the Zutphen Elderly Study. Am J Clin Nutr. 2007;85:1142–7. [DOI] [PubMed] [Google Scholar]
- 133. Beydoun MA, Kaufman JS, Sloane PD, Heiss G, Ibrahim J.. n-3 Fatty acids, hypertension and risk of cognitive decline among older adults in the Atherosclerosis Risk in Communities (ARIC) study. Public Health Nutr. 2008;11:17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Freund-Levi Y, Eriksdotter-Jonhagen M, Cederholm T, Basun H, Faxen-Irving G, Garlind A, Grut M, Vedin I.. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch Neurol. 2006;63:1402–8. [DOI] [PubMed] [Google Scholar]
- 135. MacLean CH, Newberry SJ, Mojica WA, Issa A, Khanna P, Lim YW, Morton SC, Suttorp M, Tu W, et al. . Effects of omega-3 fatty acids on cancer. Evidence report/technology assessment No. 113. Prepared by the Southern California Evidence-based Practice Center, under Contract No. 290–02–0003. AHRQ Publication No. 05–E010–2 Rockville, MD: Agency for Healthcare Research and Quality; February 2005. [Google Scholar]
- 136. Geelen A, Schouten JM, Kamphuis C, Stam BE, Burema J, Renkema JM, Bakker EJ, van't Veer P, Kampman E.. Fish consumption, n-3 fatty acids, and colorectal cancer: a meta-analysis of prospective cohort studies. Am J Epidemiol. 2007;166:1116–25. [DOI] [PubMed] [Google Scholar]
- 137. Engeset D, Alsaker E, Lund E, Welch A, Khaw KT, Clavel-Chapelon F, Thiebaut A, Chajes V, Key TJ.. Fish consumption and breast cancer risk. The European Prospective Investigation into Cancer and Nutrition (EPIC). Int J Cancer. 2006;119:175–82. [DOI] [PubMed] [Google Scholar]
- 138. Engeset D, Andersen V, Hjartaker A, Lund E.. Consumption of fish and risk of colon cancer in the Norwegian Women and Cancer (NOWAC) study. Br J Nutr. 2007;98:576–82. [DOI] [PubMed] [Google Scholar]
- 139. Terry PD, Rohan TE, Wolk A.. Intakes of fish and marine fatty acids and the risks of cancers of the breast and prostate and of other hormone-related cancers: a review of the epidemiologic evidence. Am J Clin Nutr. 2003;77:532–43. [DOI] [PubMed] [Google Scholar]
- 140. Leitzmann MF, Stampfer MJ, Michaud DS, Augustsson K, Colditz GC, Willett WC, Giovannucci EL.. Dietary intake of n-3 and n-6 fatty acids and the risk of prostate cancer. Am J Clin Nutr. 2004;80:204–16. [DOI] [PubMed] [Google Scholar]
- 141. Wallstrom P, Bjartell A, Gullberg B, Olsson H, Wirfalt E.. A prospective study on dietary fat and incidence of prostate cancer (Malmo, Sweden). Cancer Causes Control. 2007;18:1107–21. [DOI] [PubMed] [Google Scholar]
- 142. Augustsson K, Michaud DS, Rimm EB, Leitzmann MF, Stampfer MJ, Willett WC, Giovannucci E.. A prospective study of intake of fish and marine fatty acids and prostate cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:64–7. [PubMed] [Google Scholar]
- 143. Terry P, Lichtenstein P, Feychting M, Ahlbom A, Wolk A.. Fatty fish consumption and risk of prostate cancer. Lancet. 2001;357:1764–6. [DOI] [PubMed] [Google Scholar]
- 144. Crowe FL, Allen NE, Appleby PN, Overvad K, Aardestrup IV, Johnsen NF, Tjonneland A, Linseisen J, Kaaks R, et al. . Fatty acid composition of plasma phospholipids and risk of prostate cancer in a case-control analysis nested within the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2008;88:1353–63. [DOI] [PubMed] [Google Scholar]
- 145. Astorg P.. Dietary N-6 and N-3 polyunsaturated fatty acids and prostate cancer risk: a review of epidemiological and experimental evidence. Cancer Causes Control. 2004;15:367–86. [DOI] [PubMed] [Google Scholar]
- 146. Giovannucci E, Liu Y, Platz EA, Stampfer MJ, Willett WC.. Risk factors for prostate cancer incidence and progression in the health professionals follow-up study. Int J Cancer. 2007;121:1571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Koralek DO, Peters U, Andriole G, Reding D, Kirsh V, Subar A, Schatzki A, Hayes R, Leitzmann MF.. A prospective study of dietary alpha-linolenic acid and the risk of prostate cancer (United States). Cancer Causes Control. 2006;17:783–91. [DOI] [PubMed] [Google Scholar]
- 148. Fritsche K.. Important differences exist in the dose-response relationship between diet and immune cell fatty acids in humans and rodents. Lipids. 2007;42:961–79. [DOI] [PubMed] [Google Scholar]
- 149. Hulbert AJ, Rana T, Couture P.. The acyl composition of mammalian phospholipids: an allometric analysis. Comp Biochem Physiol B Biochem Mol Biol. 2002;132:515–27. [DOI] [PubMed] [Google Scholar]
- 150. Nagata C, Takatsuka N, Shimizu H.. Soy and fish oil intake and mortality in a Japanese community. Am J Epidemiol. 2002;156:824–31. [DOI] [PubMed] [Google Scholar]
- 151. Studer M, Briel M, Leimenstoll B, Glass TR, Bucher HC.. Effect of different antilipidemic agents and diets on mortality: a systematic review. Arch Intern Med. 2005;165:725–30. [DOI] [PubMed] [Google Scholar]
- 152. Aeberhard EE, Corbo L, Menkes JH.. Polyenoic acid metabolism in cultured human skin fibroblasts. Lipids. 1978;13:758–67. [DOI] [PubMed] [Google Scholar]
- 153. Blond JP, Lemarchal P, Spielmann D.. Comparative desaturation of linoleic and dihomo-gamma-linolenic acids by homogenates of human liver in vitro. C R Seances Acad Sci III. 1981;292:911–4. [PubMed] [Google Scholar]
- 154. Biagi PL, Hrelia S, Stefanini GF, Zunarelli P, Bordoni A.. Delta-6-desaturase activity of human liver microsomes from patients with different types of liver injury. Prostaglandins Leukot Essent Fatty Acids. 1990;39:39–42. [DOI] [PubMed] [Google Scholar]
- 155. de Gomez Dumm IN, Brenner RR.. Oxidative desaturation of α-linolenic, linoleic, and stearic acids by human liver microsomes. Lipids. 1975;10:315–7. [DOI] [PubMed] [Google Scholar]
- 156. Plourde M, Cunnane SC.. Extremely limited synthesis of long chain polyunsaturates in adults: implications for their dietary essentiality and use as supplements. Appl Physiol Nutr Metab. 2007;32:619–634. [DOI] [PubMed] [Google Scholar]
- 157. Burdge GC, Wootton SA.. Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br J Nutr. 2002;88:411–20. [DOI] [PubMed] [Google Scholar]
- 158. Hussein N, Ah-Sing E, Wilkinson P, Leach C, Griffin BA, Millward DJ.. Long-chain conversion of [13C]linoleic acid and alpha-linolenic acid in response to marked changes in their dietary intake in men. J Lipid Res. 2005;46:269–80. [DOI] [PubMed] [Google Scholar]
- 159. Harris WS, Lemke SL, Hansen SN, Goldstein DA, DiRienzo MA, Su H, Nemeth MA, Taylor ML, Ahmed G, George C.. Stearidonic acid-enriched soybean oil increased the omega-3 index, an emerging cardiovascular risk marker. Lipids. 2008;43:805–11. [DOI] [PubMed] [Google Scholar]
- 160. Liou YA, King DJ, Zibrik D, Innis SM.. Decreasing linoleic acid with constant alpha-linolenic acid in dietary fats increases (n-3) eicosapentaenoic acid in plasma phospholipids in healthy men. J Nutr. 2007;137:945–52. [DOI] [PubMed] [Google Scholar]
- 161. Cunnane SC.. Role of zinc in lipid and fatty acid metabolism and in membranes. Prog Food Nutr Sci. 1988;12:151–88. [PubMed] [Google Scholar]
- 162. Harris WS, Sands SA, Windsor SL, Ali HA, Stevens TL, Magalski A, Porter CB, Borkon AM.. Omega-3 fatty acids in cardiac biopsies from heart transplant patients: correlation with erythrocytes and response to supplementation. Circulation. 2004;110:1645–9. [DOI] [PubMed] [Google Scholar]
- 163. American Soybean Association Soy Stats 2008. [cited 2008 Jul 11]. Available from: http://www.soystats.com/2008/default.htm.
- 164. What We Eat in America National Health and Nutrition Examination Survey (NHANES) 2005–2006 [database on the Internet]. Beltsville, MD: USDA, Agricultural Research Service, Beltsville Human Nutrition Research Center, Food Surveys Research Group; (Beltsville, MD and Hyattsville, MD): U.S. Department of Health and Human Services, CDC, National Center for Health Statistics [updated 2008 July 31; cited 2008 July 15]. Available from: http://www.ars.usda.gov/Services/docs.htm?docid=15044. [Google Scholar]
- 165. NHANES 1999–2000 [database on the Internet] Hyattsville, MD: U.S. Department of Health and Human Services, CDC, National Center for Health Statistics; [reviewed 2008 September 8; cited 2008 July 15]. Available from: http://www.cdc.gov/nchs/about/major/nhanes/nhanes99_00.htm. [Google Scholar]
- 166. Food Availability Spreadsheets [database on the Internet] Washington, DC: USDA, Economic Research Service; [15 March 2008; cited 11 September 2008]. Available from: http://www.ers.usda.gov/Data/FoodConsumption/FoodAvailSpreadsheets.htm#mtfish. [Google Scholar]
- 167. USDA, Office of Communications Agriculture Fact Book, 2001–2002. Washington, DC: U.S. Government Printing Office; March 2003 [cited 29 September 2008]. Available from: http://www.usda.gov/factbook/2002factbook.pdf. [Google Scholar]
- 168. Howe P, Meyer B, Record S, Baghurst K.. Dietary intake of long-chain omega-3 polyunsaturated fatty acids: contribution of meat sources. Nutrition. 2006;22:47–53. [DOI] [PubMed] [Google Scholar]
- 169. National Food and Nutrient Analysis Program (NFNAP) [homepage on the Internet] Washington, DC: Agricultural Research Service, U.S. Department of Agriculture; [updated 2008. September 18; cited 2008 October 19]. Available from: http://www.ars.usda.gov/Research/docs.htm?docid=9446. [Google Scholar]
- 170. Eurodiet 2000. Eurodiet Core Report. [cited 2008 July 22] Available from: http://eurodiet.med.uoc.gr/eurodietcorereport.pdf.
- 171. Martin A. editor. Apports nutritionnels conseillés pour la population Française. 3rd ed Tech. & Doc Lavoisier: France, 2001. [Google Scholar]
- 172. United Kingdom Scientific Advisory Committee on Nutrition (SACN), Committee on Toxicity Advice on fish consumption: benefits and risks. Norwich, UK: The Stationery Office, 2004. [cited 2008 July 22] Available from: http://www.food.gov.uk/news/newsarchive/2004/jun/fishreport2004. [Google Scholar]
- 173. International Society for the Study of Fats and Lipids (ISSFAL) ISSFAL Policy Statement 3: Recommendations for intake of polyunsaturated fatty acids in healthy adults. 2004. [cited 2008 July 22]. Available from: http://www.issfal.org.uk/lipid-matters/issfal-policy-statements/issfal-policy-statement-3-2.html.
- 174. Australian Department of Health and Ageing, National Health and Medical Research Council Nutrient reference values for Australia and New Zealand including recommended dietary intakes. 2005. [cited 2008 July 22]. Available from: http://www.nhmrc.gov.au/publications/synopses/_files/n35.pdf.
- 175. Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris-Etherton P, Harris WS, et al. . Diet and lifestyle recommendations revision 2006. A scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. [DOI] [PubMed] [Google Scholar]
- 176. Health Council of the Netherlands Guidelines for a healthy diet 2006. The Hague: Health Council of the Netherlands, 2006; publication no. 2006/21E. [cited 2008 July 22]. Available from: http://www.gr.nl/samenvatting.php?ID=1481. [Google Scholar]
- 177. Superior Health Council of Belgium Advisory report: Recommendations and claims made on omega-3 fatty acids (SCH 7945). 2004. [cited 2008 July 22]. Available from:https://portal.health.fgov.be/pls/portal/docs/page/internet_pg/homepage_menu/aboutus1_menu/institutionsapparentees1_menu/hogegezondheidsraad1_menu/adviezenenaanbevelngen1_menu/adviezenenaabevelingen1_docs/omega-3%20english.pdf.
- 178. Kris-Etherton PM, Innis S, American Dietetic Assocition, Dietitians of Canada . Position of the American Dietetic Association and Dietitians of Canada: dietary fatty acids. J Am Diet Assoc. 2007;107:1599–611. [PubMed] [Google Scholar]
- 179. Harris WS, von Schacky C.. The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med. 2004;39:212–20. [DOI] [PubMed] [Google Scholar]
- 180. Block RC, Harris WS, Pottala JV.. Determinants of blood cell omega-3 fatty acid content. Open Biomarkers J. 2008;1:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Cao J, Schwichtenberg KA, Hanson NQ, Tsai MY.. Incorporation and clearance of omega-3 fatty acids in erythrocyte membranes and plasma phospholipids. Clin Chem. 2006;52:2265–72. [DOI] [PubMed] [Google Scholar]
- 182. Leaf A, Jorgensen MB, Jacobs AK, Cote G, Schoenfeld DA, Scheer J, Weimer BH, Slack JD, Kellett MA, et al. . Do fish oils prevent restenosis after coronary angioplasty? Circulation. 1994;90:2248–57. [DOI] [PubMed] [Google Scholar]
- 183. Harris WS, Pottala JV, Sands SA, Jones PG.. Comparison of the effects of fish and fish-oil capsules on the n 3 fatty acid content of blood cells and plasma phospholipids. Am J Clin Nutr. 2007;86:1621–5. [DOI] [PubMed] [Google Scholar]
- 184. Harris WS.. Omega-3 Fatty Acids and Cardiovascular Disease: A Case for Omega-3 Index as a New Risk Factor. Pharmacol Res. 2007;55:217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Albert CM, Ma J, Rifai N, Stampfer MJ, Ridker PM.. Prospective study of C-reactive protein, homocysteine, and plasma lipid levels as predictors of sudden cardiac death. Circulation. 2002;105:2595–9. [DOI] [PubMed] [Google Scholar]
- 186. Zheng ZJ, Croft JB, Giles WH, Mensah GA.. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104:2158–63. [DOI] [PubMed] [Google Scholar]
- 187. Mori TA, Woodman RJ.. The independent effects of eicosapentaenoic acid and docosahexaenoic acid on cardiovascular risk factors in humans. Curr Opin Clin Nutr Metab Care. 2006;9:95–104. [DOI] [PubMed] [Google Scholar]
- 188. Harris WS.. Omega-3 fatty acids and bleeding: cause for concern? Am J Cardiol. 2007;99:44C–6C. [DOI] [PubMed] [Google Scholar]
- 189. Department of Health and Human Services, US Food and Drug Administration Substances Affirmed as Generally Recognized as Safe: Menhaden Oil. Fed Regist. 1997;62:30751–7. [cited 2009 February 17]. Available from: http://frwebgate6.access.gpo.gov/cgi-bin/TEXTgate.cgi?WAISdoclD=929075363553+1+1+0&WAISaction=retrieve. [Google Scholar]
- 190. Worm B, Barbier EB, Beaumont N, Duffy JE, Folke C, Halpern BS, Jackson JB, Lotze HK, Micheli F, et al. . Impacts of biodiversity loss on ocean ecosystem services. Science. 2006;314:787–90. [DOI] [PubMed] [Google Scholar]
- 191. Fisheries Statistics and Information [Internet] Rome: FAO; [updated 15 September 2006; cited 29 September 2008]. Available from: http://www.fao.org/fishery/topic/2017/en. [Google Scholar]
- 192. Doughman SD, Krupanidhi S, Sanjeevi CB.. Omega-3 fatty acids for nutrition and medicine: considering microalgae oil as a vegetarian source of EPA and DHA. Curr Diabetes Rev. 2007;3:198–203. [DOI] [PubMed] [Google Scholar]
- 193. Graham IA, Larson T, Napier JA.. Rational metabolic engineering of transgenic plants for biosynthesis of omega-3 polyunsaturates. Curr Opin Biotechnol. 2007;18:142–7. Epub 2007 Feb 9. [DOI] [PubMed] [Google Scholar]
- 194. Damude HG, Kinney AJ.. Engineering oilseeds to produce nutritional fatty acids. Physiol Plant. 2008;132:1–10. [DOI] [PubMed] [Google Scholar]
- 195. Murphy KJ, Meyer BJ, Mori TA, Burke V, Mansour J, Patch CS, Tapsell LC, Noakes M, Clifton PA, et al. . Impact of foods enriched with n-3 long-chain polyunsaturated fatty acids on erythrocyte n-3 levels and cardiovascular risk factors. Br J Nutr. 2007;97:749–57. [DOI] [PubMed] [Google Scholar]
- 196. Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, Di Mascio R, Franzosi MG, Geraci E, Levantesi G, et al. . Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002;105:1897–903. [DOI] [PubMed] [Google Scholar]
- 197. He K, Rimm EB, Merchant A, Rosner BA, Stampfer MJ, Willett WC, Ascherio A.. Fish consumption and risk of stroke in men. JAMA. 2002;288:3130–6. [DOI] [PubMed] [Google Scholar]
- 198. Bucher HC, Hengstler P, Schindler C, Meier C.. N-3 polyunsaturated fatty acids in coronary heart disease: a meta-analysis of randomized controlled trials. Am J Med. 2002;112:298–304. [DOI] [PubMed] [Google Scholar]
- 199. Nestel P, Shige H, Pomeroy S, Cehun M, Abbey M, Raederstorff D.. The n-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid increase systemic arterial compliance in humans. Am J Clin Nutr. 2002;76:326–30. [DOI] [PubMed] [Google Scholar]
- 200. Thies F, Garry JM, Yaqoob P, Rerkasem K, Williams J, Shearman CP, Gallagher PJ, Calder PC, Grimble RF.. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomised controlled trial. Lancet. 2003;361:477–85. [DOI] [PubMed] [Google Scholar]
- 201. Erkkilä AT, Lehto S, Pyörälä K, Uusitupa MI.. n-3 Fatty acids and 5-y risks of death and cardiovascular disease events in patients with coronary artery disease. Am J Clin Nutr. 2003;78:65–71. [DOI] [PubMed] [Google Scholar]
- 202. Dallongeville J, Yarnell J, Ducimetière P, Arveiler D, Ferrières J, Montaye M, Luc G, Evans A, Bingham A, et al. . Fish consumption is associated with lower heart rates. Circulation. 2003;108:820–5. [DOI] [PubMed] [Google Scholar]
- 203. Pischon T, Hankinson SE, Hotamisligil GS, Rifai N, Willett WC, Rimm EB.. Habitual dietary intake of n-3 and n-6 fatty acids in relation to inflammatory markers among US men and women. Circulation. 2003;108:155–60. [DOI] [PubMed] [Google Scholar]
- 204. Hino A, Adachi H, Toyomasu K, Yoshida N, Enomoto M, Hiratsuka A, Hirai Y, Satoh A, Imaizumi T.. Very long chain N-3 fatty acids intake and carotid atherosclerosis: an epidemiological study evaluated by ultrasonography. Atherosclerosis. 2004;176:145–9. [DOI] [PubMed] [Google Scholar]
- 205. Mozaffarian D, Psaty BM, Rimm EB, Lemaitre RN, Burke GL, Lyles MF, Lefkowitz D, Siscovick DS.. Fish intake and risk for incident atrial fibrillation. Circulation. 2004;110:368–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Schrepf R, Limmert T, Claus Weber P, Theisen K, Sellmayer A.. Immediate effects of n-3 fatty acid infusion on the induction of sustained ventricular tachycardia. Lancet. 2004;363:1441–2. [DOI] [PubMed] [Google Scholar]
- 207. Hooper L, Thompson RL, Harrison RA, Summerbell CD, Moore H, Worthington HV, Durrington PN, Ness AR, Capps NE, et al. . Omega-3 fatty acid and prevention and treatment of cardiovascular disease. The Cochrane Database of Systematic Reviews. New York: John Wiley & Sons, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Mozaffarian D, Longstreth WT Jr, Lemaitre RN, Manolio TA, Kuller LH, Burke GL, Siscovick DS.. Fish consumption and stroke risk in elderly individuals: the cardiovascular health study. Arch Intern Med. 2005;165:200–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Calò L, Bianconi L, Colivicchi F, Lamberti F, Loricchio ML, de Ruvo E, Meo A, Pandozi C, Staibano M, Santini M.. N-3 Fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: a randomized, controlled trial. J Am Coll Cardiol. 2005;45:1723–8. [DOI] [PubMed] [Google Scholar]
- 210. Wang C, Harris WS, Chung M, Lichtenstein AH, Balk EM, Kupelnick B, Jordan HS, Lau J.. n-3 Fatty acids from fish or fish-oil supplements, but not {alpha}-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review. Am J Clin Nutr. 2006;84:5–17. [DOI] [PubMed] [Google Scholar]
- 211. Radaelli A, Cazzaniga M, Viola A, Balestri G, Janetti MB, Signorini MG, Castiglioni P, Azzellino A, Mancia G, Ferrari AU.. Enhanced baroreceptor control of the cardiovascular system by polyunsaturated fatty acids in heart failure patients. J Am Coll Cardiol. 2006;48:1600–6. [DOI] [PubMed] [Google Scholar]
- 212. Hjerkinn EM, Abdelnoor M, Breivik L, Bergengen L, Ellingsen I, Seljeflot I, Aase O, Ole Klemsdal T, Hjermann I, Arnesen H.. Effect of diet or very long chain omega-3 fatty acids on progression of atherosclerosis, evaluated by carotid plaques, intima-media thickness and by pulse wave propagation in elderly men with hypercholesterolaemia. Eur J Cardiovasc Prev Rehabil. 2006;13:325–33. [DOI] [PubMed] [Google Scholar]
- 213. Harris WS, Gonzales M, Laney N, Sastre A, Borkon AM.. Effects of omega-3 fatty acids on heart rate in cardiac transplant recipients. Am J Cardiol. 2006;98:1393–5. [DOI] [PubMed] [Google Scholar]
- 214. Harris WS, Poston WC, Haddock CK.. Tissue n-3 and n-6 fatty acids and risk for coronary heart disease event. Atherosclerosis. 2007;193:1–10. [DOI] [PubMed] [Google Scholar]
- 215. Wennberg M, Bergdahl IA, Stegmayr B, Hallmans G, Lundh T, Skerfving S, Strömberg U, Vessby B, Jansson JH.. Fish intake, mercury, long-chain n-3 polyunsaturated fatty acids and risk of stroke in northern Sweden. Br J Nutr. 2007;98:1038–45. [DOI] [PubMed] [Google Scholar]
- 216. Mita T, Watada H, Ogihara T, Nomiyama T, Ogawa O, Kinoshita J, Shimizu T, Hirose T, Tanaka Y, Kawamori R.. Eicosapentaenoic acid reduces the progression of carotid intima-media thickness in patients with type 2 diabetes. Atherosclerosis. 2007;191:162–7. [DOI] [PubMed] [Google Scholar]
- 217. Sekikawa A, Curb JD, Ueshima H, El-Saed A, Kadowaki T, Abbott RD, Evans RW, Rodriguez BL, Okamura T, et al. . Marine-derived n-3 fatty acids and atherosclerosis in Japanese, Japanese-American, and white men: a cross-sectional study. J Am Coll Cardiol. 2008;52:417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Cohen BE, Garg SK, Ali S, Harris WS, Whooly MA.. Red blood cell docosahexaenoic acid and eicosapentaenoic acid concentrations are positively associated with socioeconomic status in patients with established coronary artery disease: data from the heart and soul study. J Nutr. 2008;138:1135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Aarsetøy H, Pönitz V, Nilsen OB, Grundt H, Harris WS, Nilsen DW.. Low levels of cellular omega-3 increase the risk of ventricular fibrillation during the acute ischaemic phase of a myocardial infarction. Resuscitation. 2008;78:258–64. [DOI] [PubMed] [Google Scholar]
- 220. Walser B, Stebbins CL.. Omega-3 fatty acid supplementation enhances stroke volume and cardiac output during dynamic exercise. Eur J Appl Physiol. 2008;104:455–61. [DOI] [PubMed] [Google Scholar]
- 221. Wilhelm M, Tobias R, Asskali F, Kraehner R, Kuly S, Klinghammer L, Boehles H, Daniel WG.. Red blood cell omega-3 fatty acids and the risk of ventricular arrhythmias in patients with heart failure. Am Heart J. 2008;155:971–7. [DOI] [PubMed] [Google Scholar]
- 222. Hall WL, Sanders KA, Sanders TA, Chowienczyk PJ.. A high-fat meal enriched with eicosapentaenoic acid reduces postprandial arterial stiffness measured by digital volume pulse analysis in healthy men. J Nutr. 2008;138:287–91. [DOI] [PubMed] [Google Scholar]
- 223. Yamagishi K, Iso H, Date C, Fukui M, Wakai K, Kikuchi S, Inaba Y, Tanabe N, Tamakoshi A, Japan Collaborative Cohort Study for Evaluation of Cancer Risk Study Group . Fish, omega-3 polyunsaturated fatty acids, and mortality from cardiovascular diseases in a nationwide community-based cohort of Japanese men and women, the JACC (Japan Collaborative Cohort Study for Evaluation of Cancer Risk) Study. J Am Coll Cardiol. 2008;52:988–96. [DOI] [PubMed] [Google Scholar]