Abstract
Importance
Diabetic retinopathy is the leading cause of blindness in working-age adults. Studies have suggested that statins may reduce the risk of developing diabetic retinopathy.
Objective
To investigate the association between statin therapy and the development of diabetic retinopathy in patients with diabetes and dyslipidemia.
Design, Setting, and Participants
This population-based cohort study, conducted among 37 894 Taiwanese patients between January 1, 1998, and December 31, 2013, used the National Health Insurance Research Database to identify patients with type 2 diabetes and dyslipidemia. Outcomes were compared between those taking statins and those not taking statins. Statistical analysis was performed from May 1 to 31, 2018.
Exposure
Statin therapy with a medication possession rate of 80% or more with no other lipid-lowering medications.
Main Outcomes and Measures
Any stage of diabetic retinopathy and treatments for vision-threatening diabetic retinopathy.
Results
Of 1 648 305 patients with type 2 diabetes, 219 359 were eligible for analysis over the study period, including 199 760 patients taking statins and 19 599 patients not taking statins. After propensity score matching, there were 18 947 patients in the statin group (10 436 women and 8511 men; mean [SD] age, 61.5 [10.8] years) and 18 947 patients in the nonstatin group (10 430 women and 8517 men; mean [SD] age, 61.0 [11.0] years), with a mean follow-up of 7.6 years for the statin group and 7.3 years for the nonstatin group. During the study period, 2004 patients in the statin group (10.6%) and 2269 patients in the nonstatin group (12.0%) developed diabetic retinopathy. Patients in the statin group had a significantly lower rate of diabetic retinopathy (hazard ratio [HR], 0.86; 95% CI, 0.81-0.91), nonproliferative diabetic retinopathy (HR, 0.92; 95% CI, 0.86-0.99), proliferative diabetic retinopathy (HR, 0.64; 95% CI, 0.58-0.70), vitreous hemorrhage (HR, 0.62; 95% CI, 0.54-0.71), tractional retinal detachment (HR, 0.61; 95% CI, 0.47-0.79), and macular edema (HR, 0.60; 95% CI, 0.46-0.79) than the nonstatin group, as well as lower rates of interventions such as retinal laser treatment (HR, 0.71; 95% CI, 0.65-0.77), intravitreal injection (HR, 0.74; 95% CI, 0.61-0.89), and vitrectomy (HR, 0.58; 95% CI, 0.48-0.69), along with a smaller number of the interventions (retinal lasers: rate ratio, 0.61; 95% CI, 0.59-0.64; intravitreal injections: rate ratio, 0.68; 95% CI, 0.61-0.76; and vitrectomies: rate ratio, 0.54; 95% CI, 0.46-0.63). Statin therapy was also associated with lower risks of major adverse cardiovascular events (HR, 0.81; 95% CI, 0.77-0.85), new-onset diabetic neuropathy (HR, 0.85; 95% CI, 0.82-0.89), and new-onset diabetic foot ulcers (HR, 0.73; 95% CI, 0.68-0.78).
Conclusions and Relevance
Statin therapy was associated with a decreased risk of diabetic retinopathy and need for treatments for vision-threatening diabetic retinopathy in Taiwanese patients with type 2 diabetes and dyslipidemia.
This population-based cohort study uses a national database to investigate the association between statin therapy and development of diabetic retinopathy in Taiwanese patients with diabetes and dyslipidemia.
Key Points
Question
What is the association of statin therapy with development of diabetic retinopathy in patients with type 2 diabetes and dyslipidemia?
Findings
In this cohort study conducted among 37 894 Taiwanese patients with type 2 diabetes and dyslipidemia, those taking statins had a lower rate of diabetic retinopathy and need for treatments for vision-threatening diabetic retinopathy than those not taking statins. When treatment was required, patients taking statins needed a smaller number of interventions than patients not taking statins.
Meaning
These results suggest that statins are associated with a decreased prevalence of diabetic retinopathy and may decrease the progression of diabetic retinopathy in patients with type 2 diabetes and dyslipidemia.
Introduction
The increasing prevalence of diabetes has been a global health issue for decades.1 According to the latest investigation in the Diabetes Atlas, there are 425 million adults with diabetes worldwide.2 Although newer therapies have increased the life expectancy of patients with diabetes, macrovascular and microvascular complications still cause substantial morbidity.3 In 2012, the total estimated medical cost for treating patients with diabetes in the United States was $176 billion, and diabetes was especially costly if associated with any complications.4
Diabetic retinopathy, a leading microvascular complication of diabetes, is the primary cause of blindness in working-age adults.5 It has been estimated that the number of patients with diabetic retinopathy will reach 191 million globally by 2030.6 Treatment is required for vision-threatening diabetic retinopathy, which includes severe nonproliferative diabetic retinopathy, proliferative diabetic retinopathy, and diabetic macular edema.7 Although there are many interventions for vision-threatening diabetic retinopathy, including laser, intravitreal anti–vascular endothelial growth factor (anti-VEGF) injections, and vitrectomy, surgical interventions can only stop or decrease the progression of advanced diabetic retinopathy, and the interventions are associated with complications, such as peripheral vision loss with laser treatment8 and infection with surgery and anti-VEGF injections.9 Medical treatment to prevent diabetic retinopathy or slow its progression would be a preferable choice to these interventions.
Control of glucose levels, blood pressure, lipid levels, and body weight have been reported to prevent the development of or delay the progression of diabetic retinopathy.7 The Diabetes Control and Complications Trial10 and the United Kingdom Prospective Diabetes Study11 have shown the benefits of intensive control of blood glucose levels for preventing diabetic retinopathy. Therefore, better medical management could not only reduce the complications from diabetes but also decrease the additional cost of subsequent surgical intervention.
Since the association between hard exudates and serum cholesterol in patients with diabetic retinopathy was first investigated in 1991,12 several studies have been conducted to clarify the role of lipid-lowering therapy in controlling diabetic retinopathy. The 2005 Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study first identified the benefit of fenofibrate for decreasing the need for laser treatment in diabetic retinopathy.13 Then, the Action to Control Cardiovascular Risk in Diabetes-Eye (ACCORD-EYE) studies showed that fenofibrate plus simvastatin, a 3-hydroxy-methylglutaryl coenzyme-A reductase inhibitor, could decrease the progression of diabetic retinopathy compared with simvastatin alone, which, by itself, showed no improvement in this trial vs placebo.14,15 In addition, statin therapy alone was associated with reduced risk of development of diabetic macular edema and progression of diabetic retinopathy.16 Subsequently, a population-based study in Denmark showed that use of statins before receiving a diagnosis of diabetes decreased the cumulative incidence of diabetic retinopathy after diagnosis.17 However, the effectiveness of regular statin therapy as the primary prevention of the development of retinopathy in patients with diabetes remains uncertain. We conducted this population-based study to explore the association of statin therapy with the prevention of diabetic retinopathy in patients with type 2 diabetes and dyslipidemia.
Methods
Data Source
This population-based study used the National Health Insurance Research Database (NHIRD), which contains longitudinal claims data from medical practices receiving payment from the single-payer Taiwan National Health Insurance.18 All data from the NHIRD are encrypted and deidentified. This study adhered to the tenets of the Declaration of Helsinki19 and was approved by the Ethics Institutional Review Board of Chang Gung Memorial Hospital (201800199B1), which waived the requirement to obtain informed consent because the data were deidentified.
Patient Enrollment and Exclusion Criteria
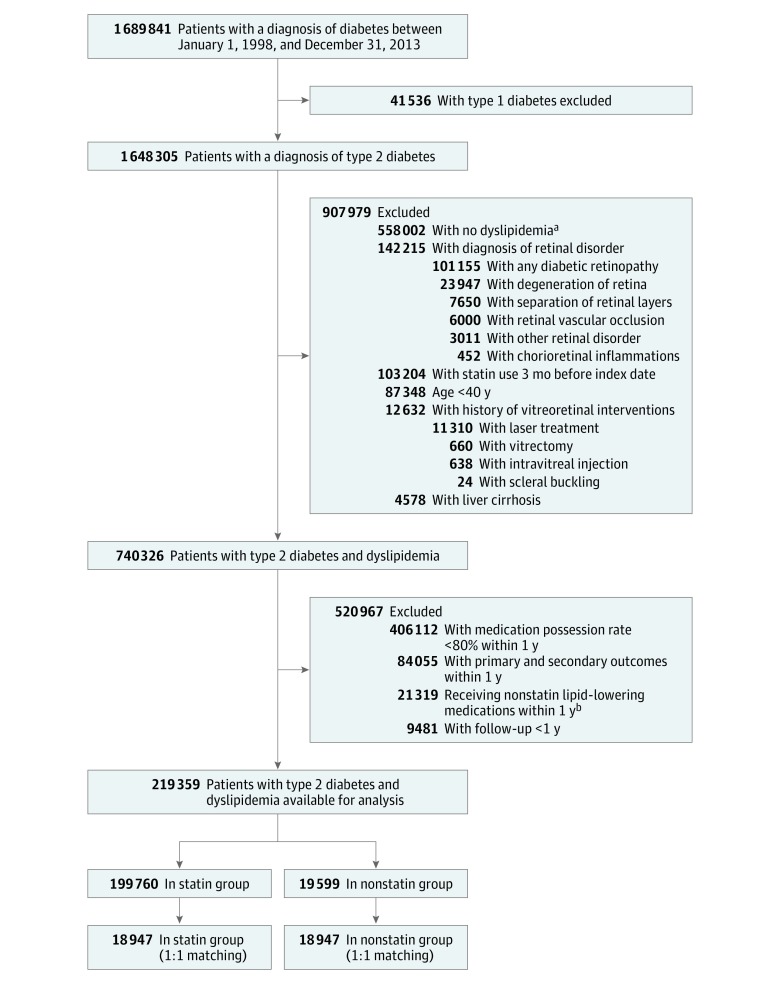
From January 1, 1998, to December 31, 2013, we identified patients with a diagnosis of any type of diabetes using International Classification of Diseases, Ninth Revision, Clinical Modification code 250 combined with a prescription for glucose-lowering agents (Figure 1). The accuracy of a diagnosis of diabetes has been validated in previous NHIRD studies.20 Eligible patients were those 40 years of age or older with a diagnosis of type 2 diabetes. We divided patients into those taking statins and those not taking statins. The index date for the statin group was the day of the first statin prescription. To minimize immortal time bias, the index date for the nonstatin group was assigned as the date on which prescription medication was prescribed for the randomly matched statin group.21 Exclusion criteria (before the index date) were (1) a serum low-density lipoprotein cholesterol (LDL-C) level of less than 100 mg/dL (to convert to millimoles per liter, multiply by 0.0259) or a serum total cholesterol level of less than 160 mg/dL (to convert to millimoles per liter, multiply by 0.0259), (2) any statin use 3 months before the index date, (3) previously documented retinal disorder (including diabetic retinopathy, retinal vascular occlusion, separation of retinal layers, retinal degeneration, and chorioretinal inflammations) or receipt of vitreoretinal interventions (including laser treatment, intravitreal injection, scleral buckling, and vitrectomy), and (4) liver cirrhosis, which may cause safety concerns that could preclude use of statins.
Figure 1. Selection of Study Patients.
aA serum low-density lipoprotein cholesterol level of less than 100 mg/dL or a serum total cholesterol level of 160 mg/dL (to convert to millimoles per liter, multiply by 0.0259).
bIncluding fibrates, nicotinic acid and derivatives, bile acid sequestrants, and cholesterol absorption inhibitors.
Guaranteed Statin and No Statin Exposure Time
We defined the first year after the index date as the exposure period to measure the medication possession rate for the statin. A medication possession rate of 80% or higher was required for the statin group, and a medication possession rate of 0% was required for the nonstatin groups. Participants were not eligible if they used a nonstatin lipid-lowering medication during the statin exposure period (Figure 1). To ensure sufficient statin exposure, patients who either died or experienced primary or secondary outcomes during the statin and nonstatin exposure periods were excluded. Statin frequency and dosage were obtained, and the intensity for each statin was calculated as low, moderate, or high intensity according to the 2013 American College of Cardiology/American Heart Association guideline.22 Rosuvastatin calcium and pravastatin sodium were classified as hydrophilic statins, and atorvastatin calcium, simvastatin, fluvastatin sodium, lovastatin, and pitavastatin calcium were classified as lipophilic statins.
Outcome Definitions
The outcomes and complications were defined as an outpatient diagnosis listed on 3 or more visits or a 1-time inpatient diagnosis after the exposure period. Nonproliferative and proliferative diabetic retinopathy and diabetic macular edema were identified. We classified complications of diabetic retinopathy, including vitreous hemorrhage and tractional retinal detachment. In addition, we identified the rate and number of interventions for vision-threatening diabetic retinopathy, including retinal laser treatment, intravitreal injection, and vitrectomy. Safety outcomes between the study cohorts included major adverse cardiovascular events (ie, myocardial infarction, heart failure, and ischemic stroke), de novo hemodialysis, and diabetic neuropathy and diabetic foot ulcers. Established major adverse cardiovascular events required a principal diagnosis in an emergency department or during inpatient visits, and its validation in the NHIRD was performed in previous studies.20,23,24,25
Covariate Assessment
We ascertained comorbidities using the same method as for the outcomes. Study comorbidities included hypertension, heart diseases, ischemic stroke, chronic kidney disease, hemodialysis, and peripheral arterial disease. Diabetic severity was assessed using duration of diabetes, diabetic neuropathy, diabetic foot ulcers, and amputation, as well as use of glucose-lowering medications. Duration of diabetes was measured based on the first diagnosis of diabetes in the NHIRD that could be tracked to March 1995. Taking medication for more than 90 days within 1 year before and after the index date was required for confirmation of drug use. The Charlson Comorbidity Index score was recorded.26 eTable 1 in the Supplement provides details on the codes regarding the disease, and eTable 2 in the Supplement provides details on medications.
Statistical Analysis
Statistical analysis was performed from May 1 to 31, 2018. To compare the statin and nonstatin groups, we performed propensity score matching. The propensity score was the predicted probability of being in the statin group given the values of covariates. These covariates included all variables listed in Table 1 and the index date (eFigure 2 in the Supplement). Each patient in the statin group was matched with a counterpart in the nonstatin group to achieve minimal bias.27 We adopted an algorithm of greedy nearest neighbor matching and set the caliper width to 0.2 times the logit of the SD of the propensity score. An absolute standardized mean difference of less than 0.1 between the 2 groups after propensity score matching was considered well balanced. The cumulative incidence of follow-up outcomes was generated; a comparison between the 2 groups was made using a subdistribution hazard model in which death was considered a competing risk. The definition of death in the NHIRD was described previously.28 The mean number of surgical interventions during follow-up between the 2 groups was compared using a Poisson model in which the logarithm of the follow-up duration was treated as an offset variable. In a subgroup analysis of statin users who were prescribed different statin intensities, the linear trend of the risk of diabetic retinopathy was tested across intensity groups (low, moderate, and high) using a subdistribution hazard model. Likewise, the dose-dependent outcome across statin groups for adherence (medication possession rate, 0%, <80%, and ≥80%) on the risk of diabetic retinopathy was tested using a linear trend test. All P values were from 2-sided tests, and results were deemed statistically significant at P < .05; the 95% CI did not include 1. Statistical analysis including propensity score matching was conducted using SAS, version 9.4 (SAS Institute Inc).
Table 1. Demographic Information of the Study Population After Propensity Score Matching.
| Variable | Patients, No. (%) | ASMD | |
|---|---|---|---|
| Statin Group (n = 18 947) | Nonstatin Group (n = 18 947) | ||
| Sex | |||
| Male | 8511 (44.9) | 8517 (45.0) | 0.001 |
| Female | 10 436 (55.1) | 10 430 (55.0) | 0.001 |
| Outpatient visits in the previous year, mean (SD), No. | 16.4 (11.7) | 15.6 (14.3) | 0.060 |
| Age, mean (SD), y | 61.5 (10.8) | 61.0 (11.0) | 0.044 |
| Age ≥65 y | 7347 (38.8) | 7006 (37.0) | 0.037 |
| Comorbidity | |||
| Hypertension | 8476 (44.7) | 8149 (43.0) | 0.035 |
| Ischemic heart disease | 2721 (14.4) | 2432 (12.8) | 0.045 |
| Heart failure | 674 (3.6) | 612 (3.2) | 0.018 |
| Ischemic stroke | 1797 (9.5) | 1703 (9.0) | 0.017 |
| Chronic kidney disease | 1213 (6.4) | 1140 (6.0) | 0.016 |
| Hemodialysis | 54 (0.3) | 51 (0.3) | 0.003 |
| Peripheral arterial disease | 310 (1.6) | 274 (1.4) | 0.015 |
| Severity of type 2 diabetes | |||
| Duration, mean (SD), y | 2.8 (3.4) | 2.8 (3.3) | 0.015 |
| Diabetic neuropathy | 943 (5.0) | 906 (4.8) | 0.009 |
| Diabetic foot ulcers | 302 (1.6) | 319 (1.7) | 0.007 |
| Amputation | 83 (0.4) | 72 (0.4) | 0.009 |
| Glycemic-lowering medication | |||
| Metformin | 14 193 (74.9) | 14 214 (75.0) | 0.003 |
| Sulfonylurea | 15 253 (80.5) | 15 119 (79.8) | 0.018 |
| Thiazolidinediones | 2065 (10.9) | 2064 (10.9) | 0.000 |
| DPP4 inhibitor | 586 (3.1) | 536 (2.8) | 0.016 |
| α-Glucosidase inhibitors | 2160 (11.4) | 2097 (11.1) | 0.011 |
| Meglitinides | 1742 (9.2) | 1688 (8.9) | 0.010 |
| Insulin | 2335 (12.3) | 2245 (11.8) | 0.015 |
| Hypertension medication | |||
| Calcium channel blocker | 9171 (48.4) | 8728 (46.1) | 0.047 |
| β Blocker | 8431 (44.5) | 7974 (42.1) | 0.049 |
| ACE inhibitor | 7423 (39.2) | 7076 (37.3) | 0.038 |
| ARB | 5295 (27.9) | 5072 (26.8) | 0.026 |
| Thiazide | 2613 (13.8) | 2419 (12.8) | 0.030 |
| α Blocker | 1532 (8.1) | 1434 (7.6) | 0.019 |
| CCI score, mean (SD) | 1.6 (1.4) | 1.6 (1.5) | 0.023 |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; ASMD, absolute standardized mean difference; CCI, Charlson Comorbidity Index; DPP4, dipeptidyl peptidase 4.
Results
Participants
From January 1, 1998, to December 31, 2013, a total of 1 689 841 patients received a diagnosis of any type of diabetes (1 648 305 with type 2 diabetes) (Figure 1). After applying the exclusion criteria, we selected a total of 219 359 patients, of whom 199 760 were in the statin group and 19 599 were in the nonstatin group. Finally, after propensity score matching, 18 947 patients were analyzed in each group.
Characteristics of the Study Cohort
Table 1 shows the demographics and clinical characteristics of the study participants after propensity score matching. The mean follow-up time after matching was 7.6 years in the statin group and 7.3 years in the nonstatin group. After matching, 17 028 of the patients were men and 20 866 were women, with a mean (SD) age of 61.3 (10.9) years. A total of 2335 patients in the statin group (12.3%) and 2245 patients in the nonstatin group (11.8%) were administered insulin therapy for diabetes. The leading comorbidity was hypertension (statin group, 8476 [44.7%] and nonstatin group, 8149 [43.0%]). The mean (SD) Charlson Comorbidity Index score was 1.6 (1.4) in the statin group and 1.6 (1.5) in the nonstatin group. All standardized mean difference values were less than 0.1, indicating a well-balanced study population between the 2 groups regarding characteristics. The characteristics before propensity score matching are provided in eTable 3 in the Supplement.
Study Outcomes
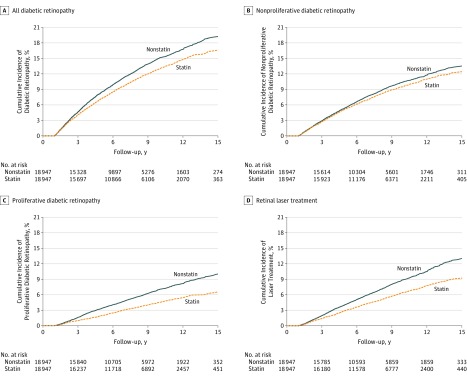
The outcomes analyzed in our study are listed in Table 2, and the cumulative incidence of diabetic retinopathy and retinal laser treatment are presented in Figure 2. A total of 2004 patients in the statin group (10.6%) and 2269 patients in the nonstatin group (12.0%) had diabetic retinopathy. The statin group had a significantly lower rate of diabetic retinopathy (hazard ratio [HR], 0.86; 95% CI, 0.81-0.91), nonproliferative diabetic retinopathy (HR, 0.92; 95% CI, 0.86-0.99), proliferative diabetic retinopathy (HR, 0.64; 95% CI, 0.58-0.70), vitreous hemorrhage (HR, 0.62; 95% CI, 0.54-0.71), tractional retinal detachment (HR, 0.61; 95% CI, 0.47-0.79), and macular edema (HR, 0.60; 95% CI, 0.46-0.79) than the nonstatin group. As for interventions for vision-threatening diabetic retinopathy, the statin group had not only significantly lower rates of retinal laser treatment (HR, 0.71; 95% CI, 0.65-0.77), intravitreal injection (HR, 0.74; 95% CI, 0.61-0.89), and vitrectomy (HR, 0.58; 95% CI, 0.48-0.69) but also a significantly lower number of retinal laser treatments (rate ratio, 0.61; 95% CI, 0.59-0.64), intravitreal injections (rate ratio, 0.68; 95% CI, 0.61-0.76), and vitrectomies (rate ratio, 0.54; 95% CI, 0.46-0.63) than the nonstatin group. Moreover, the statin group had a significantly lower rate of major adverse cardiovascular events (HR, 0.81; 95% CI, 0.77-0.85), new-onset diabetic neuropathy (HR, 0.85; 95% CI, 0.82-0.89), and new-onset diabetic foot ulcers (HR, 0.73; 95% CI, 0.68-0.78).
Table 2. Primary Outcomes and Number of Interventions Between the Study Cohorts.
| Outcome | Events, No. (%) | Statin Group vs Nonstatin Group | ||
|---|---|---|---|---|
| Statin Group (n = 18 947) | Nonstatin Group (n = 18 947) | HR (95% CI)a | P Value | |
| Primary outcomes | ||||
| Diabetic retinopathy | 2004 (10.6) | 2269 (12.0) | 0.86 (0.81-0.91) | <.001 |
| Nonproliferative | 1478 (7.8) | 1568 (8.3) | 0.92 (0.86-0.99) | .03 |
| Proliferative | 691 (3.6) | 1047 (5.5) | 0.64 (0.58-0.70) | <.001 |
| Vitreous hemorrhage | 348 (1.8) | 546 (2.9) | 0.62 (0.54-0.71) | <.001 |
| Tractional RD | 90 (0.5) | 145 (0.8) | 0.61 (0.47-0.79) | <.001 |
| Macular edema | 84 (0.4) | 136 (0.7) | 0.60 (0.46-0.79) | <.001 |
| VTDR with surgical intervention | ||||
| Retinal laser | 985 (5.2) | 1339 (7.1) | 0.71 (0.65-0.77) | <.001 |
| Intravitreal injection | 189 (1.0) | 249 (1.3) | 0.74 (0.61-0.89) | .001 |
| Vitrectomy | 185 (1.0) | 310 (1.6) | 0.58 (0.48-0.69) | <.001 |
| Safety outcomes | ||||
| Major adverse cardiovascular eventsb | 3199 (16.9) | 3767 (19.9) | 0.81 (0.77-0.85) | <.001 |
| De novo hemodialysis | 706 (3.7) | 754 (4.0) | 0.91 (0.82-1.002) | .06 |
| Diabetic neuropathy | 4410 (23.3) | 5003 (26.4) | 0.85 (0.82-0.89) | <.001 |
| Diabetic foot ulcers | 1690 (8.9) | 2232 (11.8) | 0.73 (0.68-0.78) | <.001 |
| No. of interventions per 10 y, mean (SD) | RR (95% CI)c | |||
| Retinal laser | 3.30 (19.73) | 5.11 (24.69) | 0.61 (0.59-0.64) | <.001 |
| Intravitreal injection | 0.35 (5.72) | 0.50 (6.67) | 0.68 (0.61-0.76) | <.001 |
| Vitrectomy | 0.17 (1.99) | 0.33 (2.98) | 0.54 (0.46-0.63) | <.001 |
Abbreviations: HR, hazard ratio; RD, retinal detachment; RR, rate ratio; VTDR, vision-threatening diabetic retinopathy.
Estimated from a subdistribution hazard model that considered death as a competing risk.
Any one of myocardial infarction, heart failure, or ischemic stroke.
Estimated under a Poisson model in which the logarithm of follow-up duration was treated as an offset variable.
Figure 2. Cumulative Incidence of Outcomes.
A, All diabetic retinopathy in the statin and nonstatin groups (hazard ratio [HR], 0.86; 95% CI, 0.81-0.91; P < .001). B, Nonproliferative diabetic retinopathy (HR, 0.92; 95% CI, 0.86-0.99; P = .03). C, Proliferative diabetic retinopathy (HR, 0.64; 95% CI, 0.58-0.70; P < .001). D, Undergoing retinal laser treatment (HR, 0.71; 95% CI, 0.65-0.77; P < .001).
Statin Intensity
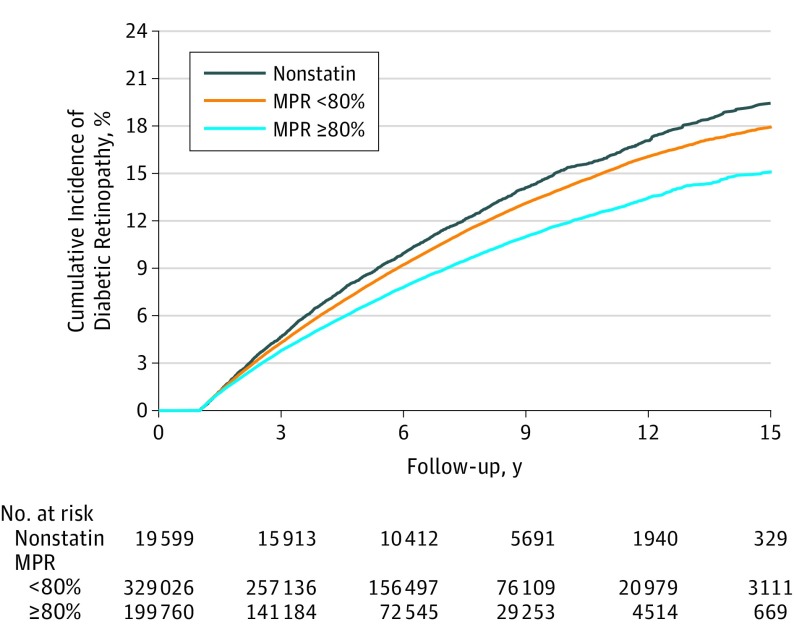
A subgroup analysis of the association of statin intensity with diabetic retinopathy was performed using the cohort before propensity score matching (n = 199 760) (eTable 4 in the Supplement), as was a subgroup analysis of the association of statin intensity with the cumulative incidence of diabetic retinopathy (eFigure 1 in the Supplement). A graded correlation was found between intensity of statin therapy and diabetic retinopathy, with an overall rate of diabetic retinopathy of 13.4% for patients administered low-intensity statin therapy, 6.0% for patients administered moderate-intensity statin therapy, and 3.4% for patients administered high-intensity statin therapy (P < .001 for linear trend). The dose-dependent outcome for adherence across statin groups is shown in Figure 3; higher statin adherence was associated with a lower cumulative risk of diabetic retinopathy (unadjusted event rate: nonstatin group, 12.3%; those with a medication possession rate <80%, 10.1%; and those with a medication possession rate ≥80%, 7.1%; P < .001 for trend for the competing risk model).
Figure 3. Cumulative Incidence of All Diabetic Retinopathy.
All diabetic retinopathy in the nonstatin group and in the statin group with a medication possession rate (MPR) of lower than 80% and in the statin group with an MPR of 80% or higher. P < .001 for trend of competing risk model.
Discussion
This study investigated the association of statins with the prevention of diabetic retinopathy in a Taiwanese patient population with type 2 diabetes and dyslipidemia. We found that statin use in patients with diabetes was associated with a decreased prevalence of diabetic retinopathy and a lower need for invasive treatments for vision-threatening diabetic retinopathy. The benefit increased as the statin intensity and patient adherence increased.
Since the Wisconsin Epidemiologic Study of Diabetic Retinopathy12 found a correlation between serum cholesterol and retinal exudates in diabetic retinopathy, several studies have investigated the association of lipid-lowering therapy with diabetic retinopathy. In the FIELD13 and ACCORD-EYE14 trials, fibrates slowed the progression of diabetic retinopathy. As for statins, the ACCORD-EYE study showed that the combination of fenofibrate and simvastatin also slowed the progression of diabetic retinopathy compared with simvastatin alone.14,15 A nationwide study of 62 716 individuals in Denmark found that statin use prior to receiving a diagnosis of diabetes decreased the risk of developing diabetic retinopathy by approximately 40%.17 However, a small, nested case-control study of 684 individuals by Zhang and McGwin29 did not support the association between statins and the development of diabetic retinopathy without considering baseline dyslipidemia. Unlike fibrates, the use of statin therapy as a strategy to prevent diabetic retinopathy remains controversial.
In our study, we found that statin use was associated with a lower risk of diabetic retinopathy in patients with type 2 diabetes and dyslipidemia. Statin therapy could delay and avert the development of diabetic retinopathy compared with patients not administered statin therapy, as statin users had a lower incidence of nonproliferative diabetic retinopathy. In vision-threatening diabetic retinopathy, statin users had not only a lower incidence of proliferative diabetic retinopathy but also a lower rate of complications associated with diabetic retinopathy and fewer interventions, including retinal laser treatments, intravitreal injections, and vitrectomies. Furthermore, the association seen between statins and a lower incidence of diabetic retinopathy was more significant with higher-intensity statin therapy and better efficacy of LDL-C reduction.30 We found that statins could delay all stages of diabetic retinopathy and decrease the number of invasive procedures needed; this finding was associated with the intensity and duration of statin use.
According to the ACCORD-EYE studies, the addition of fenofibrate to simvastatin was associated with a slower progression of diabetic retinopathy compared with simvastatin alone.14,15 In our study, we found that statin therapy alone was associated with a reduced risk of diabetic retinopathy compared with the nonstatin group (10.6% vs 12.0%). Our finding could expand the conclusion from the ACCORD-EYE study14,15 that the group receiving statins and fenofibrate had the lowest risk of diabetic retinopathy (6.5% in the ACCORD-EYE study), followed by the group receiving statins alone (10.6% in our study and 10.2% in the ACCORD-EYE study) and the nonstatin group (12.0% in our study). Compared with the ACCORD-EYE studies, our study used data from the clinical practice setting with all types of statins. Our study and the ACCORD-EYE study could offer medical strategies to reduce the risk of diabetic retinopathy.
Statin therapy was reported to reduce all-cause mortality and composite cardiovascular disease end points that were attributed to a reduction in total cholesterol and LDL-C.31 Statins have pleiotropic effects, including improving endothelial function, and anti-inflammatory, antioxidation, and antithrombotic effects along with lipid-lowering abilities.32 Moreover, Tuuminen et al33 found lower intravitreal levels of proangiogenic factors, angiopoietin 2, VEGF, fibrotic factors, matrix metalloproteinase 9, and transforming growth factor β1 in patients with diabetic retinopathy who were treated with simvastatin compared with controls who did not receive a statin. The aforementioned effects of statins seem to prevent the development of diabetic retinopathy, including improving endothelial function and inhibiting fibrotic proliferation, inflammation, oxidative stress, and VEGF-associated angiogenesis.34 Those mechanisms could provide reasons for the efficacy of statin therapy for the prevention of diabetic retinopathy in our study.
In addition to statin intensity, adherence to statins was associated with the prevention of diabetic retinopathy. Lower adherence to statins was reported to be associated with increased risks of cardiovascular diseases.35 In our study, lower adherence to statins was associated with an increased risk of diabetic retinopathy. As for safety outcomes, a meta-analysis of clinical studies demonstrated the beneficial outcomes of statins on reducing cardiovascular events.31 Our study also found that those in the statin group experienced additional benefits regarding the prevention of major adverse cardiovascular events, de novo hemodialysis, and the development of diabetic neuropathy and diabetic foot ulcers.
Limitations
There were some limitations to this study. First, data on the participants’ serum lipid profiles were not available in the NHIRD, so we were unable to ensure that lipid levels had in fact been lowered. However, the Taiwanese National Health Insurance program has regulations regarding reimbursement for lipid-lowering therapy. Reimbursement for lipid-lowering agents is granted only to patients with diabetes and a serum LDL-C level of 100 mg/dL or more or a serum total cholesterol level of 160 mg/dL or more. This regulation could make the inclusion of dyslipidemia robust. Second, we were unable to obtain biomarker values regarding blood pressure and serum glucose levels. Nevertheless, medications for hypertension and glycemic control were matched in the 2 groups. Theoretically, the blood pressure values and serum glucose levels in the 2 groups were similar. Third, the NHIRD lacks information on eye laterality, so it is unclear if 1 eye or both eyes were similarly affected or treated. Diabetic retinopathy is a systemic vascular complication of diabetes that would generally involve both eyes simultaneously.34 Identification of diabetic retinopathy, rather than localization of a diseased eye, was the main concern in this study. Fourth, the study involved an Asian population, so these data may or may not be applicable to other populations, although statin use for the prevention of cardiovascular events is strongly recommended for many different populations.31 Last, we used strict inclusion and exclusion criteria and propensity score-matched the 2 groups, but some bias may not be eliminated entirely because of the study’s retrospective nature. However, our study could provide meaningful results regarding the association between statin use and diabetic retinopathy in an Asian population.
Conclusions
For patients with type 2 diabetes and dyslipidemia, statin therapy was associated with a decreased risk of diabetic retinopathy. Statin therapy could also retard the progression of vision-threatening diabetic retinopathy and reduce the number of subsequent retinal laser treatments, intravitreal injections, and vitrectomies.
eTable 1. ICD-9-CM Codes of Diagnoses Used for Defining the Population, Comorbidities, and Outcomes
eTable 2. Anatomical Therapeutic Chemical (ATC) Codes of the Medications Used in This Study
eTable 3. Demographic Information of the Study Population before Propensity Score Matching
eTable 4. The Effect of Statin on Diabetic Retinopathy in Patients with Type 2 Diabetes Mellitus by Intensity
eFigure 1. Cumulative Incidence of All Diabetic Retinopathy With Low-, Moderate-, and High-Intensity Statin Therapy
eFigure 2. Distribution of the Index Date in the Statin and Nonstatin Groups After Propensity Score Matching
References
- 1.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387(10027):1513-1530. doi: 10.1016/S0140-6736(16)00618-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Diabetes Federation IDF Diabetes Atlas—8th edition. http://www.diabetesatlas.org/. Accessed January 13, 2018. [PubMed]
- 3.Beckman JA, Creager MA. Vascular complications of diabetes. Circ Res. 2016;118(11):1771-1785. doi: 10.1161/CIRCRESAHA.115.306884 [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association Economic costs of diabetes in the US in 2012. Diabetes Care. 2013;36(4):1033-1046. doi: 10.2337/dc12-2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein BE. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. 2007;14(4):179-183. doi: 10.1080/09286580701396720 [DOI] [PubMed] [Google Scholar]
- 6.Zheng Y, He M, Congdon N. The worldwide epidemic of diabetic retinopathy. Indian J Ophthalmol. 2012;60(5):428-431. doi: 10.4103/0301-4738.100542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ting DS, Cheung GC, Wong TY. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Ophthalmol. 2016;44(4):260-277. doi: 10.1111/ceo.12696 [DOI] [PubMed] [Google Scholar]
- 8.Reddy SV, Husain D. Panretinal photocoagulation: a review of complications. Semin Ophthalmol. 2018;33(1):83-88. doi: 10.1080/08820538.2017.1353820 [DOI] [PubMed] [Google Scholar]
- 9.Gupta A, Sun JK, Silva PS. Complications of intravitreous injections in patients with diabetes. Semin Ophthalmol. 2018;33(1):42-50. doi: 10.1080/08820538.2017.1353811 [DOI] [PubMed] [Google Scholar]
- 10.Nathan DM, Genuth S, Lachin J, et al. ; Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977-986. doi: 10.1056/NEJM199309303291401 [DOI] [PubMed] [Google Scholar]
- 11.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837-853. doi: 10.1016/S0140-6736(98)07019-6 [DOI] [PubMed] [Google Scholar]
- 12.Klein BE, Moss SE, Klein R, Surawicz TS. The Wisconsin Epidemiologic Study of Diabetic Retinopathy, XIII: relationship of serum cholesterol to retinopathy and hard exudate. Ophthalmology. 1991;98(8):1261-1265. doi: 10.1016/S0161-6420(91)32145-6 [DOI] [PubMed] [Google Scholar]
- 13.Keech A, Simes RJ, Barter P, et al. ; FIELD Study Investigators . Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366(9500):1849-1861. doi: 10.1016/S0140-6736(05)67667-2 [DOI] [PubMed] [Google Scholar]
- 14.Chew EY, Ambrosius WT, Davis MD, et al. ; ACCORD Study Group; ACCORD Eye Study Group . Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363(3):233-244. doi: 10.1056/NEJMoa1001288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chew EY, Ambrosius WT. Update of the ACCORD Eye Study. N Engl J Med. 2011;364(2):188-189. doi: 10.1056/NEJMc1011499 [DOI] [PubMed] [Google Scholar]
- 16.Chung YR, Park SW, Choi SY, et al. . Association of statin use and hypertriglyceridemia with diabetic macular edema in patients with type 2 diabetes and diabetic retinopathy. Cardiovasc Diabetol. 2017;16(1):4. doi: 10.1186/s12933-016-0486-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nielsen SF, Nordestgaard BG. Statin use before diabetes diagnosis and risk of microvascular disease: a nationwide nested matched study. Lancet Diabetes Endocrinol. 2014;2(11):894-900. doi: 10.1016/S2213-8587(14)70173-1 [DOI] [PubMed] [Google Scholar]
- 18.Hsing AW, Ioannidis JP. Nationwide population science: lessons from the Taiwan National Health Insurance Research Database. JAMA Intern Med. 2015;175(9):1527-1529. doi: 10.1001/jamainternmed.2015.3540 [DOI] [PubMed] [Google Scholar]
- 19.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 20.Lin CC, Lai MS, Syu CY, Chang SC, Tseng FY. Accuracy of diabetes diagnosis in health insurance claims data in Taiwan. J Formos Med Assoc. 2005;104(3):157-163. [PubMed] [Google Scholar]
- 21.Zhou Z, Rahme E, Abrahamowicz M, Pilote L. Survival bias associated with time-to-treatment initiation in drug effectiveness evaluation: a comparison of methods. Am J Epidemiol. 2005;162(10):1016-1023. doi: 10.1093/aje/kwi307 [DOI] [PubMed] [Google Scholar]
- 22.Stone NJ, Robinson JG, Lichtenstein AH, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25)(suppl 2):S1-S45. doi: 10.1161/01.cir.0000437738.63853.7a [DOI] [PubMed] [Google Scholar]
- 23.Cheng CL, Chien HC, Lee CH, Lin SJ, Yang YH. Validity of in-hospital mortality data among patients with acute myocardial infarction or stroke in National Health Insurance Research Database in Taiwan. Int J Cardiol. 2015;201:96-101. doi: 10.1016/j.ijcard.2015.07.075 [DOI] [PubMed] [Google Scholar]
- 24.Wu CS, Lai MS, Gau SS, Wang SC, Tsai HJ. Concordance between patient self-reports and claims data on clinical diagnoses, medication use, and health system utilization in Taiwan. PLoS One. 2014;9(12):e112257. doi: 10.1371/journal.pone.0112257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang CC, Chen PC, Hsu CW, Chang SL, Lee CC. Validity of the age-adjusted Charlson Comorbidity Index on clinical outcomes for patients with nasopharyngeal cancer post radiation treatment: a 5-year nationwide cohort study. PLoS One. 2015;10(1):e0117323. doi: 10.1371/journal.pone.0117323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 27.Austin PC. Statistical criteria for selecting the optimal number of untreated subjects matched to each treated subject when using many-to-one matching on the propensity score. Am J Epidemiol. 2010;172(9):1092-1097. doi: 10.1093/aje/kwq224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu CY, Chen YJ, Ho HJ, et al. . Association between nucleoside analogues and risk of hepatitis B virus–related hepatocellular carcinoma recurrence following liver resection. JAMA. 2012;308(18):1906-1914. doi: 10.1001/2012.jama.11975 [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, McGwin G Jr. Association of statin use with the risk of developing diabetic retinopathy. Arch Ophthalmol. 2007;125(8):1096-1099. doi: 10.1001/archopht.125.8.1096 [DOI] [PubMed] [Google Scholar]
- 30.Michigan Medicine, University of Michigan Screening and management of lipids. http://www.med.umich.edu/1info/FHP/practiceguides/lipids/lipidsupdate.pdf. Published May 2014. Accessed February 18, 2018.
- 31.Taylor F, Huffman MD, Macedo AF, et al. . Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;(1):CD004816. doi: 10.1002/14651858004816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89-118. doi: 10.1146/annurev.pharmtox.45.120403.095748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tuuminen R, Sahanne S, Loukovaara S. Low intravitreal angiopoietin-2 and VEGF levels in vitrectomized diabetic patients with simvastatin treatment. Acta Ophthalmol. 2014;92(7):675-681. doi: 10.1111/aos.12363 [DOI] [PubMed] [Google Scholar]
- 34.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124-136. doi: 10.1016/S0140-6736(09)62124-3 [DOI] [PubMed] [Google Scholar]
- 35.Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119(23):3028-3035. doi: 10.1161/CIRCULATIONAHA.108.768986 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. ICD-9-CM Codes of Diagnoses Used for Defining the Population, Comorbidities, and Outcomes
eTable 2. Anatomical Therapeutic Chemical (ATC) Codes of the Medications Used in This Study
eTable 3. Demographic Information of the Study Population before Propensity Score Matching
eTable 4. The Effect of Statin on Diabetic Retinopathy in Patients with Type 2 Diabetes Mellitus by Intensity
eFigure 1. Cumulative Incidence of All Diabetic Retinopathy With Low-, Moderate-, and High-Intensity Statin Therapy
eFigure 2. Distribution of the Index Date in the Statin and Nonstatin Groups After Propensity Score Matching