Abstract
This study assesses the outcomes of single-patient use of the US Food and Drug Administration Expanded Access program.
The US Food and Drug Administration (FDA) Expanded Access (compassionate use) program permits patient access to investigational products outside clinical trials. Little is known about outcomes of this program.1,2,3,4 We sought to assess the use, safety, and efficacy associated with single-patient use (SPU) at our institution.
Methods
All cancer product SPUs approved by the Memorial Sloan Kettering Institutional Review Board and the FDA between January 1, 2012, and January 1, 2018, were included. This study was retrospectively approved by the Memorial Sloan Kettering Institutional Review Board. The need for informed consent (written or verbal) was waived by the Memorial Sloan Kettering Institutional Review Board. Data were collected and then deidentified after collection.
Medical records and regulatory documents were reviewed. Response was physician assessed at 6 weeks or more into treatment. Patients who clinically deteriorated before formal assessment were considered nonresponders. Serious adverse event data were collected. Overall response rates were estimated with binomial exact CIs. Progression-free survival and overall survival were estimated by Kaplan-Meier methods from the date of first dosing. For patients with multiple SPUs (n = 6), progression-free survival was assessed for the first instance of first progression, and overall survival was defined from the first dose of the last SPU before death.
Results
In total, 208 SPUs were approved, and the product was administered in 185 instances to 179 patients (mean [SD] age, 39 [25] years; 112 [55.4%] male; 137 [65.9%] adults). Treated patients had 43 cancer types (117 [57.9%] had solid tumors and 85 [42.1%] had hematologic cancer). Solid tumors included neuroblastoma (31 [15.3%]), lung (16 [7.9%]), primary brain (16 [7.9%]), breast (12 [5.9%]), and other (42 [20.8%]). Patients received 66 unique investigational products, including kinase inhibitors (60 [28.8%]), naked antibodies (26 [12.5%]), and allogeneic cell therapy (25 [12.0%]). Patients were heavily pretreated (median number of prior treatments, 4). At the time of SPU approval, most agents were in phase 2 (75 [36.1%]) or phase 3 (82 [39.4%]) studies, although 39 (18.8%) were still in phase 1 studies. Prior supporting clinical experience was cited in 128 applications (61.5%) and preclinical evidence in 64 (30.8%). Genomic data were additionally cited in 79 cases (38.0%). Median time from initial SPU request to treatment was 19 days (interquartile range, 11-35 days).
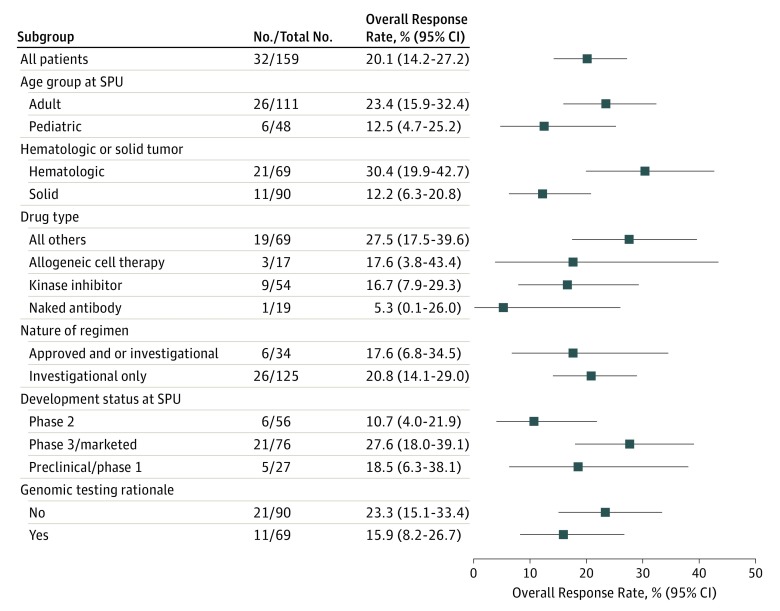
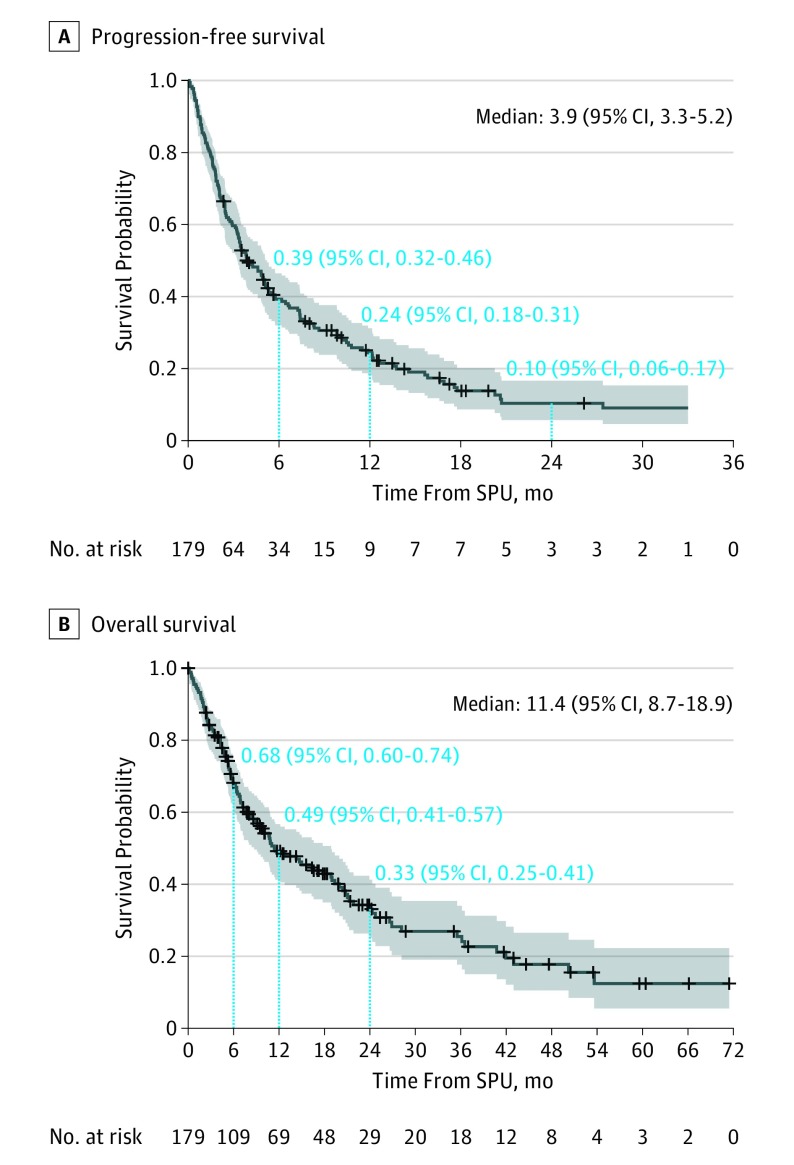
Twenty-six patients received therapy in a minimal residual disease state or remission and were thus not assessed for response. Of the remaining 159 patients with evaluable data, the overall response rate was 20.1% (95% CI, 14.2%-27.2%). Best response varied by patient and product characteristics (Figure 1). Responses occurred with a variety of agent types, most commonly kinase inhibitors (9 [16.7%]), allogeneic cell therapy (3 [17.6%]), and antibody drug conjugates (1 [5.3%]). Estimated progression-free survival rates were 38.9% (95% CI, 31.6%-46.1%) at 6 months and 24.5% (95% CI, 18.2%-31.4%) at 1 year (Figure 2A). Median overall survival was 11.4 months (95% CI, 8.7-18.9 months) (Figure 2B). Median follow-up for survivors was 16 months (range, 0-71 months).
Figure 1. Overall Response Rate by Patient and Single-Patient Use (SPU) Characteristics.
Overall response rate is shown according to age, tumor type, drug type, nature of regimen, development status, and genomic rationale. Error bars provide 95% CIs.
Figure 2. Progression-Free Survival (PFS) and Overall Survival (OS).
Survival from time of single-patient use (SPU) initiation. Numbers above dotted lines provide point estimate of progression-free and overall survival rate at time point indicated. Blue shading indicates 95% CI; crosses, censored data.
A total of 55 of 185 patients (29.7%) experienced 1 or more treatment-related serious adverse event (12 [19.1%] in pediatric patients, 43 [35.3%] in adults). There were no treatment-related deaths.
Discussion
The FDA Expanded Access program provided access to investigational products at all stages of development and to patients of all ages with a variety of cancer types. Despite being heavily pretreated, a small but meaningful proportion of patients appeared to benefit. Children represented 34.1% of the cohort despite being only approximately 2% of the patients seen at the center in 2017, suggesting SPUs may provide an important means of pediatric drug access.5
Our study has some important limitations. It was conducted at a single academic cancer center, and the retrospective design precluded the collection of certain data elements. Moreover, although the federal Right-to-Try law promises to further expand prescribing of unapproved products, differences in the precise requirements and implementation of this new access mechanism may make aspects of our current SPU analysis only partially applicable.3,4,6
In summary, our data provide an initial evidence basis to evaluate the FDA Expanded Access mechanism. We find its use is broad, involving a wide variety of patients and products, and clinical benefit was observed. Routine prospective collection of key safety and efficacy metrics should be considered moving forward.
References
- 1.Jarow JP, Lemery S, Bugin K, Khozin S, Moscicki R. Expanded access of investigational drugs: the experience of the Center of Drug Evaluation and Research over a 10-year period. Ther Innov Regul Sci. 2016;50(6):705-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKee AE, Markon AO, Chan-Tack KM, Lurie P. How often are drugs made available under the Food and Drug Administration’s Expanded Access process approved? J Clin Pharmacol. 2017;57(suppl 10):S136-S142. doi: 10.1002/jcph.960 [DOI] [PubMed] [Google Scholar]
- 3.Holbein ME, Berglund JP, Weatherwax K, Gerber DE, Adamo JE. Access to investigational drugs: FDA Expanded Access programs or “Right-to-Try” legislation? Clin Transl Sci. 2015;8(5):526-532. doi: 10.1111/cts.12255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoerger M. Right-to-try laws and individual patient “compassionate use” of experimental oncology medications: a call for improved provider-patient communication. Death Stud. 2016;40(2):113-120. doi: 10.1080/07481187.2015.1077356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.RACE for Children Act of 2017-2018, S 456, 115th Cong (2017-2018).
- 6.Traynor K. Federal right-to-try law aims to broaden access to investigational drugs. Am J Health Syst Pharm. 2018;75(15):1085-1086. doi: 10.2146/news180048 [DOI] [PubMed] [Google Scholar]