Key Points
Question
What are the rates of local regrowth, pelvic control, and survival when using a watch-and-wait approach for patients with rectal cancer after a clinical complete response to neoadjuvant therapy?
Findings
A watchful waiting strategy for 113 patients with rectal cancer achieving a clinical complete response after neoadjuvant therapy resulted in excellent rectal preservation (82%) and pelvic tumor control (91%) in this case series study. However, worse survival was observed compared with 136 patients undergoing total mesorectal excision who had a pathologic complete response; a higher incidence of distant progression was also noted among patients managed by the watch-and-wait strategy who developed local regrowth vs those who did not develop local regrowth.
Meaning
A watch-and-wait strategy may be safe for most patients, but better risk stratification is needed for more precise patient selection to identify those at high risk of local regrowth who are not optimal candidates.
This case series of patients with rectal cancer compares outcomes between those who had a clinical complete response to neoadjuvant therapy and agreed to a watch-and-wait strategy and those who underwent total mesorectal excision and had a pathologic complete response at resection.
Abstract
Importance
The watch-and-wait (WW) strategy aims to spare patients with rectal cancer unnecessary resection.
Objective
To analyze the outcomes of WW among patients with rectal cancer who had a clinical complete response to neoadjuvant therapy.
Design, Setting, and Participants
This retrospective case series analysis conducted at a comprehensive cancer center in New York included patients who received a diagnosis of rectal adenocarcinoma between January 1, 2006, and January 31, 2015. The median follow-up was 43 months. Data analyses were conducted from June 1, 2016, to October 1, 2018.
Exposures
Patients had a clinical complete response after completing neoadjuvant therapy and agreed to a WW strategy of active surveillance and possible salvage surgery (n = 113), or patients underwent total mesorectal excision and were found to have a pathologic complete response (pCR) at resection (n = 136).
Main Outcomes and Measures
Kaplan-Meier estimates were used for analyses of local regrowth and 5-year rates of overall survival, disease-free survival, and disease-specific survival.
Results
Compared with the 136 patients in the pCR group, the 113 patients in the WW group were older (median [range], 67.2 [32.1-90.9] vs 57.3 [25.0-87.9] years, P < .001) with cancers closer to the anal verge (median [range] height from anal verge, 5.5 [0.0-15.0] vs 7.0 [0.0-13.0] cm). All 22 local regrowths in the WW group were detected on routine surveillance and treated by salvage surgery (20 total mesorectal excisions plus 2 transanal excisions). Pelvic control after salvage surgery was maintained in 20 of 22 patients (91%). No pelvic recurrences occurred in the pCR group. Rectal preservation was achieved in 93 of 113 patients (82%) in the WW group (91 patients with no local regrowths plus 2 patients with local regrowths salvaged with transanal excision). At 5 years, overall survival was 73% (95% CI, 60%-89%) in the WW group and 94% (95% CI, 90%-99%) in the pCR group; disease-free survival was 75% (95% CI, 62%-90%) in the WW group and 92% (95% CI, 87%-98%) in the pCR group; and disease-specific survival was 90% (95% CI, 81%-99%) in the WW group and 98% (95% CI, 95%-100%) in the pCR group. A higher rate of distant metastasis was observed among patients in the WW group who had local regrowth vs those who did not have local regrowth (36% vs 1%, P < .001).
Conclusions and Relevance
A WW strategy for select rectal cancer patients who had a clinical complete response after neoadjuvant therapy resulted in excellent rectal preservation and pelvic tumor control; however, in the WW group, worse survival was noted along with a higher incidence of distant progression in patients with local regrowth vs those without local regrowth.
Introduction
Rectal cancer treatment remains challenging as we work to reduce the risk of distant metastases,1 preserve quality of life,2 and tailor treatment to individual patients by identifying responders to neoadjuvant therapy (NAT).3 By definition, patients with pathologic complete response (pCR) in the total mesorectal excision (TME) specimen have maximal response to NAT. Pathologic complete response is associated with significantly better outcomes,4,5 but ascertainment requires radical resection. A watch-and-wait (WW) approach for patients with rectal cancer following a clinical complete response (cCR) to NAT is a nonstandard approach, but it has become more widely practiced with the advent of total neoadjuvant therapy6 and with increasing demand by patients in the context of a cCR.7
Pioneering work and updated case series studies by Dr Angelita Habr-Gama’s group in 2004 demonstrated that WW was safe and efficacious after cCR.8,9 In The Netherlands, a “wait-and-see” approach was examined in patients achieving cCR after NAT, and a high rate of organ preservation and equivalence of oncologic outcomes was noted.10,11 These data have also been bolstered by recent findings from the United Kingdom12 and in a recent meta-analysis13 and a large international multicenter registry.14 Some of these reports are reflected in recent National Comprehensive Cancer Network guidelines mentioning WW,15 although the National Comprehensive Cancer Network cannot formally endorse this approach because of the need for more rigorous study of the strategy in the context of clinical trials.
Our group previously noted that a WW approach was safe and efficacious after cCR; however, the intervals of follow-up were short and the numbers of patients were small.16,17 To address these shortcomings, we aimed to evaluate long-term outcomes of 113 rectal cancer patients managed by a WW strategy after achieving a cCR to NAT to determine rates of rectal preservation, salvage after local regrowth detection, and pelvic tumor control. In addition, we sought to determine the relevant oncologic outcomes of survival and metastasis.
Methods
Study Design and Participants
This retrospective study evaluated oncologic outcomes of patients with rectal cancer treated via a WW strategy off-protocol. We also examined outcomes of patients who underwent a TME with subsequent pCR as an aspirational criterion standard symbolic of achieving maximal response to NAT. These 2 groups were not directly compared given the retrospective design of the study and given that there was no randomization to TME or WW. Agreement among surgeon, patient, radiation oncologist, and medical oncologist was made to proceed with WW. With institutional approval, all patients with a diagnosis of rectal adenocarcinoma seen from January 1, 2006, to January 31, 2015, were identified and reviewed. Patients with localized, biopsy-proven adenocarcinoma of the rectum who received neoadjuvant therapy were included in this study. Exclusion criteria included metastatic disease, metastases during NAT, and transanal excision (TAE) before NAT initiation. Patients were included in the WW group if they had a cCR prior to January 31, 2015, without subsequent TME. Patients were included in the pCR group if they underwent a TME prior to January 31, 2015, and had no residual disease on pathology (ypT0N0). Data were extracted through medical record review, including inpatient and outpatient visit records, as well as records from outside health care professionals. Patient characteristics collected included age at diagnosis, sex, tumor height from the anal verge, clinical tumor and nodal staging, neoadjuvant regimen, surgical procedures performed, and associated pathology. The present study was approved by the Institutional Review Board at Memorial Sloan Kettering Cancer Center (MSK), New York, New York, which also waived the need for obtaining informed patient consent for the conduct of this case series analysis. Each patient and physician were in agreement to pursue a WW approach knowing the risks, benefits, and alternatives.
Neoadjuvant Therapy
Long-course chemoradiation was the most commonly used NAT. Radiation doses ranged from 45 to 54 Gy given across 25 to 28 fractions, with administration of concurrent, continuous infusion of fluorouracil or oral capecitabine. The second regimen used was induction chemotherapy,6 consisting of 8 cycles of FOLFOX (folinic acid, fluorouracil, and oxaliplatin) administration followed by long-course chemoradiation as described above. The third regimen was long-course chemoradiation followed by consolidation chemotherapy consisting of 8 cycles of FOLFOX. The fourth and least common regimen was chemotherapy only, with 8 cycles of FOLFOX treatment with or without administration of bevacizumab.
Clinical Complete Response
The final assessment of cCR was based on the clinical judgment and decision of the attending surgeon for each case. Patients in the initial 5 to 6 years of this study were considered cCRs based on digital rectal examination and endoscopy alone. Endoscopic findings consistent with a cCR included a flat white scar with or without telangiectasias and lack of ulceration or nodularity.18 Our current method of detecting cCR18 includes magnetic resonance imaging (MRI) of the pelvis, but this could not be applied in this study because of its retrospective nature and inconsistent use of MRI prior to 2013.
Local Regrowth
Once cCR was confirmed, and the patient was managed with a WW strategy, a surveillance examination and endoscopy were performed per the clinician with a goal of every 3 months for the first year, every 4 months for the second year, and then every 6 months for a total of 5 years of follow-up. Surveillance MRI was not used widely at MSK prior to 2013. Local regrowth was defined as any sign of tumor recurrence in the rectal wall on digital rectal examination or endoscopy (endoluminal) or concerning imaging findings, such as new rectal wall thickening or an enlarging mass in the mesorectum (extraluminal). Local regrowth was an indication for salvage surgery via TME and was confirmed at resection in all cases. At detection, patients underwent computed tomography of the chest, abdomen, and pelvis to detect metastases.
Organ Preservation
Patients were considered to have achieved organ preservation if they were treated with NAT and did not subsequently undergo TME. Local procedures, such as transanal excision, were considered consistent with organ preservation.
Outcomes
We evaluated 5-year overall survival (OS), disease-free survival (DFS), and disease-specific survival (DSS). In addition, we analyzed the rates of local regrowth (WW group), successful salvage surgery, pelvic control, organ preservation, and distant metastasis. Results from patients with pCR8,10,16 noted after radical resection were examined and then referenced as a criterion standard, aspirational comparison group achieving the best possible oncologic outcomes.
Statistical Analysis
Patient characteristics were summarized using median and range for continuous covariates and frequency and percentage for categorical covariates and were compared between groups using the Wilcoxon rank sum test and Fisher exact test, respectively. Survival was measured from the end of NAT for the WW and pCR cohorts. Disease-free survival events included locoregional recurrence, distant recurrence, and death from any cause but excluded local regrowth. Overall survival and DFS times were censored at the last follow-up visit. Local regrowth in the WW group was defined as time from the end of NAT until local regrowth and was censored at the time of death or the last follow-up visit. Organ preservation in the WW group was defined as time from the end of NAT to rectal resection. Overall survival, DSS, DFS, local regrowth, and organ preservation were estimated using Kaplan-Meier methods. All statistical analyses were performed from June 1, 2016, to October 1, 2018, using R, version 3.1.1 (R Foundation), and a 2-sided P < .05 was considered statistically significant.
Results
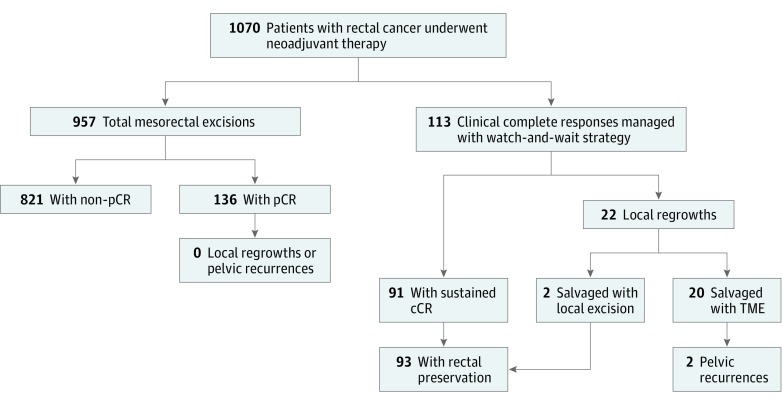
Between January 1, 2006, and January 31, 2015, 1070 patients with resectable nonmetastatic rectal adenocarcinoma underwent NAT at MSK. We identified 113 patients (11%) with post-NAT cCR who were subsequently followed up using the WW strategy. During the same period, 957 patients underwent TME, and 136 of those patients (13%) had a pCR (Figure 1). In 2006, the first year of the study, 3 patients were treated with the WW strategy compared with 37 patients in 2014.
Figure 1. Selection of Patients Included in the Watch-and-Wait and Pathologic Complete Response (pCR) Groups.
Patients were included in the watch-and-wait arm if they had a clinical complete response (cCR) prior to January 31, 2015 (n = 113). Patients were included in the pCR arm if they underwent total mesorectal excision (TME) and had a pCR (n = 136).
The cohorts were significantly different with respect to age, tumor height from the anal verge, and neoadjuvant regimen received (all P < .01; Table 1). Patients in the WW cohort were a decade older on average (median [range], 67.2 [32.1-90.9] vs 57.3 [25.0-87.9] years; P < .001) and had lower tumors (median [range] height from the anal verge, 5.5 [0.0-15.0] vs 7.0 [0.0-13.0] cm; P = .003). Patients in the pCR group were more likely to have received chemoradiation therapy only vs those in the WW cohort (83 patients [61%)] vs 31 patients [27%]), in which the predominant regimens were induction chemotherapy (47 patients [42%]) and consolidation chemotherapy (33 patients [29%]). There were no significant differences between groups with regard to sex, clinical tumor stage, or nodal stage (Table 1). From the end of NAT, the WW group had a median follow-up of 33 months, and the pCR group had a median follow-up of 55 months. We noted that 22 of the 113 patients in the WW group (20%) developed local regrowth, corresponding to a 5-year actuarial rate of 21% (95% CI, 12%-30%) (Figure 2). The 5-year rate of rectal preservation in the cCR group was 79% (95% CI, 71%-88%). To determine if any clinical characteristics were associated with cCR, we evaluated clinical T2/T3 stage, clinical nodal stage, neoadjuvant regimen, tumor size, age, and height of tumor from the anal verge. We found no significant associations with local regrowth in a univariate analysis (P > .05 for all).
Table 1. Clinical and Demographic Characteristics by Patient Cohort.
| Characteristic | Patients, No. (%)a | P Value | |
|---|---|---|---|
| Watch-and-Wait (n = 113) | pCR (n = 136) | ||
| Age, median (range), y | 67.2 (32.1-90.9) | 57.3 (25.0-87.9) | <.001b |
| Sex | |||
| Men | 67 (59) | 79 (58) | .90c |
| Women | 46 (41) | 57 (42) | |
| Height from anal verge, median (range), cm | 5.5 (0.0-15.0) | 7.0 (0.0-13.0) | .003b |
| Clinical tumor (T) classificationd | |||
| cT2 | 23 (20) | 27 (20) | .13c |
| cT3 | 90 (80) | 104 (76) | |
| cT4 | 0 | 5 (4) | |
| Clinical nodal (N) classificationd | |||
| cN0 | 39 (35) | 43 (32) | .63c |
| cN1 and N2 | 74 (66) | 93 (68) | |
| Neoadjuvant regimen | |||
| CRT only | 31 (27) | 83 (61) | <.001c |
| Induction chemotherapy | 47 (42) | 31 (23) | |
| Consolidation chemotherapy | 33 (29) | 4 (3) | |
| Chemotherapy only | 2 (2) | 18 (13) | |
Abbreviations: CRT, chemoradiation therapy; pCR, pathologic complete response.
Some totals do not add up to 100% due to rounding.
The Kruskal-Wallis test.
The χ2 test or Fisher exact test where applicable.
According to the 7th edition of the American Joint Committee on Cancer TNM staging system. The induction chemotherapy regimen consists of a course of neoadjuvant chemotherapy followed by long-course chemoradiation. The consolidation chemotherapy regimen consists of long-course chemoradiation followed by chemotherapy.
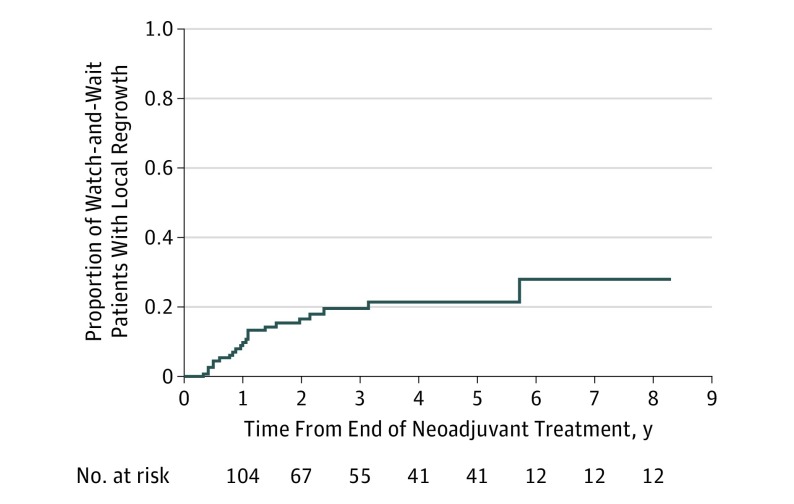
Figure 2. Local Regrowth and Rectal Preservation in the Watch-and-Wait Cohort.
At 5 years, the rate of local regrowth was 21%. After a median follow-up of 33 months from the end of neoadjuvant therapy, 22 of the 113 patients (19.5%) included in the watch-and-wait group developed a local regrowth, which corresponds to a 5-year actuarial rate of 21.4% (95% CI, 12%-30%) and thus an organ preservation rate of 79% (95% CI, 70%-88%).
Regarding salvage after detection of local regrowth, in the WW cohort, we found that the median time to local regrowth for these 22 patients was 11.2 months (range, 3.5-74.4 months) from the date of cCR and that the majority of patients had a local regrowth in the first 12 months (eFigure 1 in the Supplement). Nineteen of these 22 regrowths (86%) had an endoluminal component detectable on digital rectal examination and endoscopy in the absence of symptoms (Table 2). In 3 of those 19 regrowths, clinical examination was complemented by imaging, leading to increased suspicion, confirmation of regrowth, and consideration for salvage TME. Three extraluminal regrowths were found by surveillance imaging alone.
Table 2. Relevant Clinical Characteristics of Patients With Local Regrowth and Subsequent Salvage Surgery.
| Patient | Pattern of Regrowth | Salvage Operation | Height From AV, cm | Initial Clinical Staginga | Surgical Pathology Staginga | CRM | Pelvic Recurrence | Distant Metastases | Disease Status |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Extraluminal | LAR | 6.0 | cT3N2 | ypT3N0 | Negative | No | No | NED |
| 2 | Endoluminal | LAR | 6.5 | cT2N0 | ypT2N0 | Negative | No | No | NED |
| 3 | Endoluminal | LAR | 6.0 | cT3N1 | ypT2N0 | Negative | No | No | NED |
| 4 | Endoluminal | LAR | 4.0 | cT2N0 | ypT3N0 | Negative | No | Yes (lung) | AWD |
| 5 | Endoluminal | LAR | 6.5 | cT3N0 | ypT1N0 | Negative | No | No | NED |
| 6 | Endoluminal | TAE | 10.0 | cT3N1 | NAb | NA | No | Yes (liver, SBRT) | DOC |
| 7 | Endoluminal | LAR | 12.0 | cT3N1 | ypT3N0 | Negative | No | Yes (liver) | DOD |
| 8 | Endoluminal | PRc | 5.0 | cT3N0 | ypT2N0 | Negative | No | Yes (lung/liver) | DOD |
| 9 | Endoluminal | APR | 7.5 | cT2N1 | ypT2N0 | Negative | No | No | NED |
| 10 | Endoluminal | APR | 5.5 | cT3N1 | ypT2N0 | Negative | No | No | NED |
| 11 | Extraluminal | APR | 4.0 | cT3N1 | ypT3N0 | Negative | No | No | NED |
| 12 | Extraluminal | LAR | 7.0 | cT3N1 | ypT3N1 | Negative | No | No | NED |
| 13 | Endoluminal | APR | 7.0 | cT3N0 | ypT2N0 | Negative | No | Yes (lung)d | NED |
| 14 | Endoluminal | APR | 8.0 | cT3N0 | ypT3N1 | Negative | Yes | Yes (lung/liver) | DOD |
| 15 | Endoluminal | APR | 0.5 | cT2N0 | ypT3N0 | Negative | No | Yes (lung) | DOD |
| 16 | Endoluminal | LAR | 10.0 | cT3N1 | ypT3N0 | Negative | No | No | NED |
| 17 | Endoluminal | APRc | 3.0 | cT2N0 | ypT2N2 | Positive | Yes | Yes (lung) | DOD |
| 18 | Endoluminal | APR | 5.0 | cT3N0 | ypT3N0 | Negative | No | No | NED |
| 19 | Endoluminal | LAR | 5.5 | cT3N1 | ypT2N0 | Negative | No | No | NED |
| 20 | Endoluminal | TAE | 5.0 | cT3N0 | ypT1Nx | NA | No | No | NED |
| 21 | Endoluminal | APR | 4.0 | cT3N1 | ypT2N0 | Negative | No | No | NED |
| 22 | Endoluminal | APR | 5.0 | cT3N0 | ypT3N0 | Negative | No | No | NED |
Abbreviations: APR, abdominoperineal resection; AV, anal verge; AWD, alive with disease; CRM, circumferential resection margin; DOC, died of other causes; DOD, died of disease; LAR, low anterior resection; NA, not applicable; NED, no evidence of disease; PR, perineal resection; SBRT, stereotactic body radiation therapy; TAE, transanal excision.
According to the 7th edition of the American Joint Committee on Cancer TNM staging system.
Patient underwent a salvage procedure at an outside hospital, and the pathology report was not available.
Patient initially underwent transanal excision and subsequently required radical resection for salvage.
Patient developed lung metastasis and underwent resection; now NED.
The median time from detection of local regrowth to salvage surgery was 5 weeks, and all 22 patients with local regrowth underwent salvage surgery. Eleven of the 22 patients (50%) with local regrowth had a ypT stage consistent with clinical staging, 8 patients (36%) had their diagnosis downstaged, and 2 patients (9%) had their diagnosis upstaged from T2 to T3. None had T4 disease on pathology. Table 2 summarizes relevant clinical and pathology findings in the patients with local regrowth. Nine patients (41%) underwent low anterior resection, 9 patients underwent abdominoperineal resection, and 2 patients underwent TAE for definitive salvage therapy. The remaining 2 patients initially underwent TAE in an attempt to avoid major surgery owing to multiple comorbidities, but later required radical resection for salvage. One of those patients underwent an abdominoperineal resection with a positive circumferential resection margin and subsequently developed a second local recurrence, whereas the other underwent perineal resection and diverting colostomy with negative margins and no further pelvic recurrence. In regard to sustained pelvic control after salvage, we noted that 20 of 22 patients (91%) in the salvage group were free of pelvic progression at the last follow-up visit. There were no cases of pelvic regrowth in the pCR group.
In total, 91 of 113 patients (81%) in the WW cohort remained free of disease in the rectum after NAT. Along with 2 patients salvaged by TAE, 93 of 113 patients (82%) had rectal preservation (Figure 1 and Table 2). At the end of follow-up, 11 patients (10%) in the WW group had a permanent stoma compared with 21 patients (15%) in the pCR group.
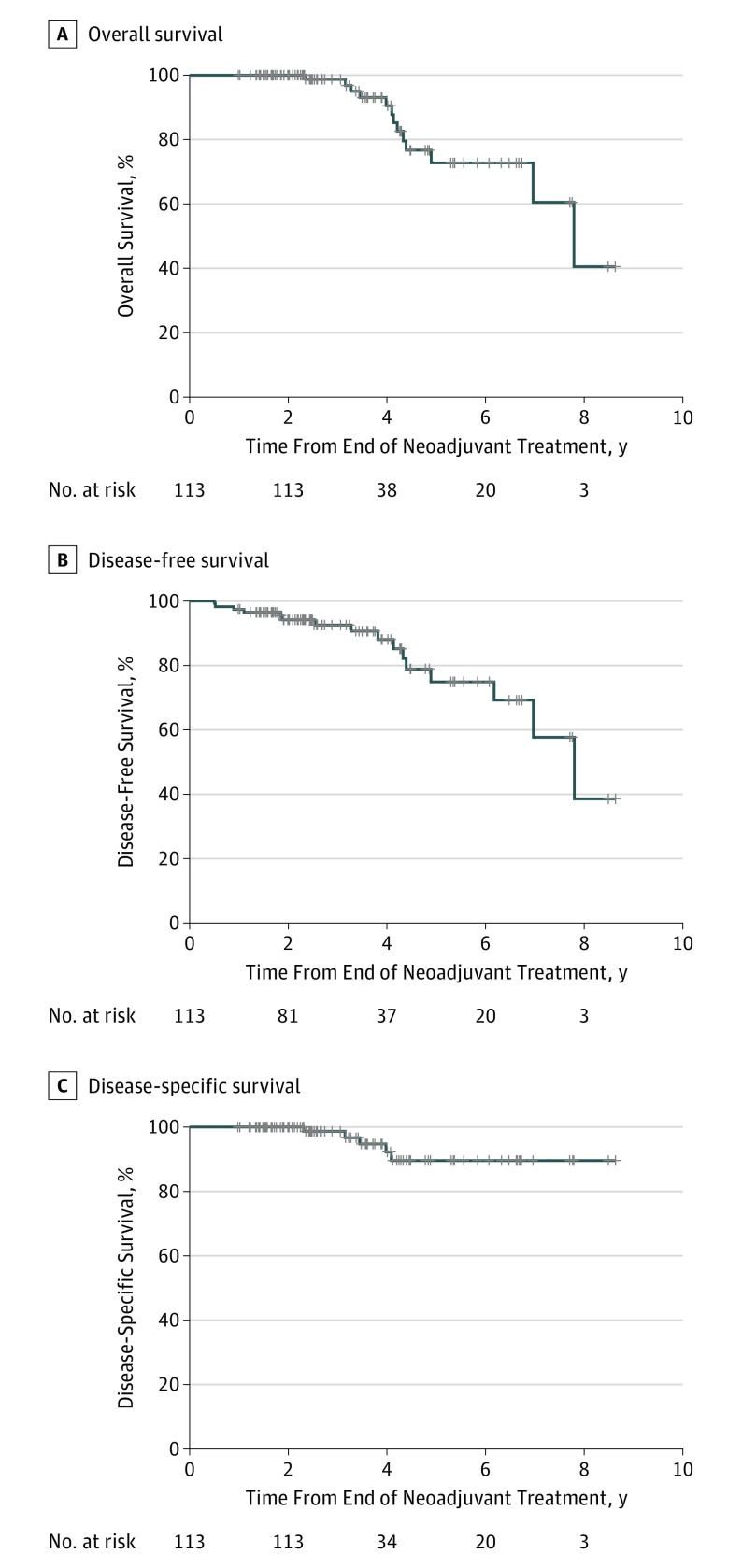
After a median follow-up of 43 months (interquartile range, 27-43 months) from the end of NAT, 19 (8%) of 249 patients (113 in the WW group and 136 in the pCR group) died. Five-year OS was 73% in the WW group (95% CI, 60%-89%) and 94% in the pCR group (95% CI, 90%-99%). Five-year DFS was 75% in the WW group (95% CI, 62%-90%) and 92% in the pCR group (95% CI, 87%-98%). Five-year DSS was 90% in the WW group (95% CI, 81%-99%) and 98% in the pCR group (95% CI, 95%-100%) (Figure 3 for WW; eFigure 2 in the Supplement for pCR). In the WW group, use of induction or consolidation chemotherapy was not associated with worse OS rates compared with use of chemoradiotherapy alone (eFigure 3 in the Supplement). In addition, OS (eFigure 4 in the Supplement) did not differ significantly among patients in the WW group staged with or without MRI.
Figure 3. Overall Survival, Disease-Free Survival, and Disease-Specific Survival at 5 Years in the Watch-and-Wait Cohort.
For the watch-and-wait group, (A) overall survival is 73% (95% CI, 60%-89%), (B) disease-free survival is 75% (95% CI, 62%-90%), and (C) disease-specific survival is 90% (95% CI, 81%-99%). In the overall survival analysis, 70% of the watch-and-wait group died of other causes. Survival was measured from the end of neoadjuvant treatment. The disease-free survival events included locoregional recurrence, distant recurrence, and death from any cause, but specifically excluded local regrowth.
Nine patients (8%) from the WW group developed distant metastases. Five patients with pCR (4%) developed metastases. Eight of the 22 patients (36%) with local regrowth in the WW group developed distant metastases (Table 2). Only 1 of 91 patients (1%) without local regrowth developed distant metastasis. We noted a significant difference in the rates of distant metastasis among the patients in the WW group with local regrowth vs those patients without local regrowth (36% vs 1%, P < .001); the difference remained significant after 4 patients with less than 1 year of post-cCR follow-up were excluded.
Discussion
Our present experience with a WW strategy for 113 patients with rectal cancer achieving a cCR after NAT showed a high rate of rectal preservation, effective surgical salvage, and excellent pelvic tumor control. Thus, like other large WW case series, our data support both the benefit and overall safety of WW as a strategy for managing the primary rectal tumor in select patients. However, with longer follow-up and more patients in the present study compared with our prior work,16 we noted more distant metastases and worse survival among the patients in the WW group having local regrowth compared with those having a sustained cCR (36% vs 1% distant metastases). These findings suggest an oncologic risk of local regrowth that may not be mitigated by aggressive surveillance and surgical salvage. Whether this risk is due in part to deferral of surgery or is simply evidence that local regrowth identifies tumors at high risk of metastatic progression is unknown, although we do expect some degree of increased risk for the trade-off of rectal preservation when using a WW approach.
Our rate of rectal preservation (82%) is consistent with or higher than other reported rates from recent studies.9,11,12,14,19 It is remarkable that the rates of local regrowth across these modern case series and studies are consistent at approximately 20% to 30% despite the heterogeneity of patient cohorts and treatment regimens (eTable in the Supplement). In addition, the ability to salvage local regrowth was shown in our case series with a very high rate of local control (91%). We found a higher rate of distant metastases in the WW group than in the pCR group (8% vs 4%), but the event rate was low, and the numbers preclude meaningful statistical analysis. Nonetheless, our data strengthen the assertion that pathologically verified complete response correlates with reduced risk of both local and systemic failure. Other major WW studies report similar rates of distant metastases9,11,12,14,19 (eTable in the Supplement).
Despite geographic and patient heterogeneity, organ preservation rates and DFS rates are similar in the most mature data sets (eTable in the Supplement). Of note, 5-year OS in our WW cohort (73%) differed from those in the studies by Habr-Gama et al9 (91%), Martens et al11 (97%), Appelt et al19 (100%; 2-year OS), and Renehan et al12 (96%). The reasons for this are undoubtedly multifactorial, including selection bias, age differences, maturity of follow-up, and institutional differences. It does not appear that T or N stage alone is implicated in our review of these studies, although we do note fewer node-positive patients in the study by Habr-Gama et al.9 In the context of modern randomized trials examining multimodal treatment regimens for locally advanced rectal cancer, OS rates range from 75% to 80%,20,21 remarkably similar to the rate of survival in our WW cohort (73%).
Our data raises the question, does the deferral of surgery with the WW approach add to the risk of distant progression in the subset of patients with local regrowth? Could these metastases have arisen prior to neoadjuvant therapy and have occurred regardless of the timing of rectal resection? If so, this would suggest an underlying aggressive biology inherent in tumor cells that resists chemoradiation. Alternatively, these resistant cells could survive chemoradiation and populate the distant target organ during the surveillance period of a cCR. Given the complexity of tumor biology,22 local tumor response and metastatic capacity may be 2 disparate biological processes. Our data, however, show that development of local regrowth was associated with a higher risk of distant metastasis. Whether radical resection after NAT would have mitigated this risk or whether the metastases were formed by early disseminating cancer cells prior to consideration for resection is unknown. Attempting to understand the biology of metastases and local regrowth after NAT will require prospective translational studies with evaluation of clinical and molecular features of the primary, recurrent, and metastatic clones.
Strengths and Limitations
Our study was a large, single-institution experience of the WW strategy with sufficient cases and follow-up to assess recurrence patterns and the outcomes of surgical salvage. The weaknesses of the study included those intrinsic to retrospective studies, including selection bias and recall bias. In the WW group, patients were much older and likely sicker, although the comorbidity data available were too sparse for formal analysis. In addition, the tumors in the WW group were lower, representing a potentially different biology that could alter survival outcomes. Furthermore, the treatment regimens were heterogeneous; however, our analysis did not implicate induction or consolidation chemotherapy as associated with worse outcomes in the WW cohort. Final cCR assessment was based on clinical judgment and assessment of the attending surgeon at the time of data collection. Lastly, the staging and restaging modalities were not consistent throughout but did not appear to alter outcomes.
Conclusions
In conclusion, our study showed that 82% of patients with rectal cancer managed by a WW strategy achieved rectal preservation. All 22 local regrowths in our study were detected on routine surveillance visits, and 20 of 22 (91%) were successfully salvaged. Differing from prior and recent studies, survival outcomes were worse among patients in the WW group in our study, but this was likely due to selection bias and could be due to higher rates of distant metastases in patients with local regrowth. These data advise a measure of caution as we weigh the risks of WW for each patient with the benefits of organ preservation and quality of life. The data also suggest that although WW may be effective in most patients, better risk stratification is needed to inform more precise patient selection and to better understand which patients should be excluded from a WW strategy to minimize local failure and distant progression.
eFigure 1. Swimmer Plot of Local Regrowth Patients
eFigure 2. Overall Survival (OS), Disease-Free Survival (DFS) and Disease-Specific Survival (DSS) At 5 Years in the Pathologic Complete Response (pCR) Cohort
eFigure 3. Five-Year Survival in the WW Cohort in Relation to Treatment Received
eFigure 4. Five-Year Overall Survival in the WW Cohort in Relation to MRI Staging
eTable. Comparison With Previous Watch-And-Wait Studies
References
- 1.Cunningham D, Atkin W, Lenz H-J, et al. . Colorectal cancer. Lancet. 2010;375(9719):-. doi: 10.1016/S0140-6736(10)60353-4 [DOI] [PubMed] [Google Scholar]
- 2.Pucciarelli S, Del Bianco P, Efficace F, et al. . Patient-reported outcomes after neoadjuvant chemoradiotherapy for rectal cancer: a multicenter prospective observational study. Ann Surg. 2011;253(1):71-77. doi: 10.1097/SLA.0b013e3181fcb856 [DOI] [PubMed] [Google Scholar]
- 3.Chow OS, Smith JJ, Gollub MJ, Garcia-Aguilar J. Can we predict response and/or resistance to neoadjuvant chemoradiotherapy in patients with rectal cancer? Curr Colorectal Cancer Rep. 2014;10(2):164-172. doi: 10.1007/s11888-014-0210-0 [DOI] [Google Scholar]
- 4.Maas M, Nelemans PJ, Valentini V, et al. . Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11(9):835-844. doi: 10.1016/S1470-2045(10)70172-8 [DOI] [PubMed] [Google Scholar]
- 5.Park IJ, You YN, Agarwal A, et al. . Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol. 2012;30(15):1770-1776. doi: 10.1200/JCO.2011.39.7901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cercek A, Goodman KA, Hajj C, et al. . Neoadjuvant chemotherapy first, followed by chemoradiation and then surgery, in the management of locally advanced rectal cancer. J Natl Compr Canc Netw. 2014;12(4):513-519. doi: 10.6004/jnccn.2014.0056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith JJ, Garcia-Aguilar J. Advances and challenges in treatment of locally advanced rectal cancer. J Clin Oncol. 2015;33(16):1797-1808. doi: 10.1200/JCO.2014.60.1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Habr-Gama A, Perez RO, Nadalin W, et al. . Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240(4):711-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Habr-Gama A, Gama-Rodrigues J, São Julião GP, et al. . Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: impact of salvage therapy on local disease control. Int J Radiat Oncol Biol Phys. 2014;88(4):822-828. doi: 10.1016/j.ijrobp.2013.12.012 [DOI] [PubMed] [Google Scholar]
- 10.Maas M, Beets-Tan RGH, Lambregts DMJ, et al. . Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol. 2011;29(35):4633-4640. doi: 10.1200/JCO.2011.37.7176 [DOI] [PubMed] [Google Scholar]
- 11.Martens MH, Maas M, Heijnen LA, et al. . Long-term outcome of an organ preservation program after neoadjuvant treatment for rectal cancer. J Natl Cancer Inst. 2016;108(12):djw171. doi: 10.1093/jnci/djw171 [DOI] [PubMed] [Google Scholar]
- 12.Renehan AG, Malcomson L, Emsley R, et al. . Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): a propensity-score matched cohort analysis. Lancet Oncol. 2016;17(2):174-183. doi: 10.1016/S1470-2045(15)00467-2 [DOI] [PubMed] [Google Scholar]
- 13.Dossa F, Chesney TR, Acuna SA, Baxter NN. A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017;2(7):501-513. doi: 10.1016/S2468-1253(17)30074-2 [DOI] [PubMed] [Google Scholar]
- 14.van der Valk MJM, Hilling DE, Bastiaannet E, et al. ; IWWD Consortium . Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet. 2018;391(10139):2537-2545. doi: 10.1016/S0140-6736(18)31078-X [DOI] [PubMed] [Google Scholar]
- 15.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN Guidelines): rectal cancer, version 3.2018. https://www.nccn.org/professionals/physician_gls/recently_updated.aspx. Published August 7, 2018. Accessed December 3, 2018.
- 16.Smith JD, Ruby JA, Goodman KA, et al. . Nonoperative management of rectal cancer with complete clinical response after neoadjuvant therapy. Ann Surg. 2012;256(6):965-972. doi: 10.1097/SLA.0b013e3182759f1c [DOI] [PubMed] [Google Scholar]
- 17.Smith JJ, Chow OS, Eaton A, et al. Organ preservation in rectal cancer patients with clinical complete response after neoadjuvant therapy [abstract 509]. Presented at the Gastrointestinal Cancers Symposium; January 17, 2015. Cold Spring Harbor, New York. [Google Scholar]
- 18.Smith JJ, Chow OS, Gollub MJ, et al. ; Rectal Cancer Consortium . Organ Preservation in Rectal Adenocarcinoma: a phase II randomized controlled trial evaluating 3-year disease-free survival in patients with locally advanced rectal cancer treated with chemoradiation plus induction or consolidation chemotherapy, and total mesorectal excision or nonoperative management. BMC Cancer. 2015;15:767. doi: 10.1186/s12885-015-1632-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Appelt AL, Pløen J, Harling H, et al. . High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study. Lancet Oncol. 2015;16(8):919-927. doi: 10.1016/S1470-2045(15)00120-5 [DOI] [PubMed] [Google Scholar]
- 20.Fernandez-Martos C, Garcia-Albeniz X, Pericay C, et al. . Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: long-term results of the Spanish GCR-3 phase II randomized trial. Ann Oncol. 2015;26(8):1722-1728. doi: 10.1093/annonc/mdv223 [DOI] [PubMed] [Google Scholar]
- 21.Rödel C, Graeven U, Fietkau R, et al. ; German Rectal Cancer Study Group . Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015;16(8):979-989. doi: 10.1016/S1470-2045(15)00159-X [DOI] [PubMed] [Google Scholar]
- 22.Bettoni F, Masotti C, Habr-Gama A, et al. . Intratumoral Genetic Heterogeneity in Rectal Cancer: Are Single Biopsies representative of the entirety of the tumor? Ann Surg. 2017;265(1):e4-e6. doi: 10.1097/SLA.0000000000001937 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Swimmer Plot of Local Regrowth Patients
eFigure 2. Overall Survival (OS), Disease-Free Survival (DFS) and Disease-Specific Survival (DSS) At 5 Years in the Pathologic Complete Response (pCR) Cohort
eFigure 3. Five-Year Survival in the WW Cohort in Relation to Treatment Received
eFigure 4. Five-Year Overall Survival in the WW Cohort in Relation to MRI Staging
eTable. Comparison With Previous Watch-And-Wait Studies