Abstract
Importance
Effective treatment options are limited for patients with advanced, metastatic esophageal cancer progressing after 2 or more lines of systemic therapy.
Objective
To evaluate the efficacy and safety of pembrolizumab for patients with advanced, metastatic esophageal squamous cell carcinoma (ESCC) or advanced, metastatic adenocarcinoma of the esophagus and gastroesophageal junction that progressed after 2 or more lines of systemic therapy.
Design, Setting, and Participants
This phase 2, open-label, interventional, single-arm study, KEYNOTE-180, enrolled 121 patients from January 12, 2016, to March 21, 2017, from 57 sites in 10 countries. Patients had advanced, metastatic esophageal cancer that progressed after 2 or more lines of therapy and had evaluable tumor samples for biomarkers.
Interventions
Pembrolizumab, 200 mg, was administered intravenously every 3 weeks until disease progression, unacceptable toxic effects, or study withdrawal, for up to 2 years.
Main Outcomes and Measures
Primary end point was objective response rate per the Response Evaluation Criteria in Solid Tumors by central imaging review for all patients.
Results
As of September 18, 2017, of 121 enrolled patients (100 men and 21 women; median age, 65 years [range, 33-87 years]), 18 (14.9%) had undergone 3 or more prior therapies, 63 (52.1%) had ESCC, and 58 (47.9%) had tumors positive for programmed death ligand-1 (PD-L1), defined as a combined positive score of 10 or higher assessed by immunohistochemistry. Median duration of follow-up was 5.8 months (range, 0.2-18.3 months). Objective response rate was 9.9% (95% CI, 5.2%-16.7%) among all patients (12 of 121), and median duration of response was not reached (range, 1.9-14.4 months). Objective response rate was 14.3% (95% CI, 6.7%-25.4%) among patients with ESCC (9 of 63), 5.2% (95% CI, 1.1%-14.4%) among patients with adenocarcinoma (3 of 58), 13.8% (95% CI, 6.1%-25.4%) among patients with PD-L1–positive tumors (8 of 58), and 6.3% (95% CI, 1.8%-15.5%) among patients with PD-L1–negative tumors (4 of 63). Overall, 15 patients (12.4%) had treatment-related grade 3 to 5 adverse events. Only 5 patients (4.1%) discontinued treatment because of adverse events. There was 1 treatment-related death from pneumonitis.
Conclusions and Relevance
Where effective treatment options are an unmet need, pembrolizumab provided durable antitumor activity with manageable safety in patients with heavily pretreated esophageal cancer. Phase 3 studies evaluating pembrolizumab vs standard therapy for patients with esophageal cancer progressing after first-line therapy or in combination with chemotherapy as first-line therapy for patients with locally advanced unresectable or metastatic esophageal cancer are ongoing.
Trial Registration
ClinicalTrials.gov identifier: NCT02559687
This phase 2, open-label, interventional study examined the safety and efficacy of pembrolizumab for patients with advanced, metastatic esophageal carcinoma that has progressed after 2 or more lines of systemic therapy.
Key Points
Question
Is pembrolizumab effective and safe for patients with advanced, metastatic esophageal cancer that has progressed after 2 or more lines of systemic therapy?
Findings
Among 121 heavily pretreated patients with advanced, metastatic esophageal cancer enrolled in the phase 2 KEYNOTE-180 study, patients treated with pembrolizumab had an objective response rate of 9.9%, with partial responses observed in 12 patients per the Response Evaluation Criteria in Solid Tumors, version 1.1, by central imaging review. The safety profile was manageable and similar to that seen previously with pembrolizumab.
Meaning
These data suggest that pembrolizumab has modest activity in patients with heavily pretreated, metastatic esophageal cancer.
Introduction
Metastatic esophageal cancer is a fatal disease with a fatality to case ratio of 0.92 and a median overall survival ranging from 10 to 12 months.1,2 Squamous cell carcinoma accounts for 90% of cases of metastatic esophageal cancer in Asia, Africa, and France, while adenocarcinoma represents 62% of cases in the United States.3,4 In the first- and second-line setting, conventional chemotherapy is largely palliative, with limited evidence of durable benefit.5,6,7,8 Few patients whose disease progresses after 2 or more lines of therapy (<15% of patients who received first-line therapy) receive treatment, and there is a lack of clinical data in this setting.9 Given the absence of recommended treatment options for patients whose disease progresses after 2 or more lines of therapy, supportive care or participation in a clinical study is recommended.
Pembrolizumab is a high-affinity, humanized monoclonal antibody against programmed cell death protein 1 that blocks interaction between programmed cell death protein 1 and programmed death-ligand 1 (PD-L1) or programmed death-ligand 2.10 In the phase 1b KEYNOTE-028 study, durable responses to pembrolizumab were observed in patients with PD-L1–positive advanced and metastatic esophageal cancer.11 In the KEYNOTE-180 study, we evaluated the antitumor activity of pembrolizumab in patients with previously treated, advanced and metastatic adenocarcinoma or squamous cell carcinoma of the esophagus.
Methods
Study Design, Treatment, and Participants
The KEYNOTE-180 study is a global, open-label, phase 2 study of pembrolizumab for patients with histologically confirmed advanced and metastatic esophageal adenocarcinoma, advanced and metastatic esophageal squamous cell carcinoma (ESCC), or advanced and metastatic Siewert type 1 adenocarcinoma of the gastroesophageal junction that progressed after 2 or more lines of therapy, conducted at 57 sites in 10 countries. Patients received pembrolizumab, 200 mg, every 3 weeks for up to 2 years, until progression of disease, unacceptable toxic effects occurred, or withdrawal of consent. The protocol was approved by all participating institutions (trial protocol in Supplement 1). The study was conducted in accordance with the Declaration of Helsinki12 and International Good Clinical Practice Guidelines. All patients provided written informed consent.
The primary end point was the objective response rate (ORR) among all patients. Secondary end points included duration of response (DOR), progression-free survival (PFS), and overall survival (OS). Tumor response was assessed per the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1, by central review every 9 weeks. Adverse events (AEs) were monitored throughout the study.
Biomarker Analysis
Expression of PD-L1 was evaluated in pretreatment tissue samples by the PD-L1 IHC 22C3 pharmDx assay (Agilent Technologies). Tumors positive for PD-L1 had a combined positive score of 10 or more. The combined positive score is defined as the number of PD-L1–positive cells (tumor cells, macrophages, and lymphocytes) divided by the total number of tumor cells, multiplied by 100.
Statistical Analysis
All patients who received 1 or more doses of pembrolizumab were included in efficacy and safety analyses. For the ORR, point estimates and 2-sided 95% CIs were provided using the exact binomial Clopper Pearson method. Kaplan-Meier estimates were provided for DOR, PFS, and OS. Descriptive statistics were provided for safety analyses. The numbers and percentages of AEs were provided. Additional details of all the study methods can be found in the eAppendix in Supplement 2.
Results
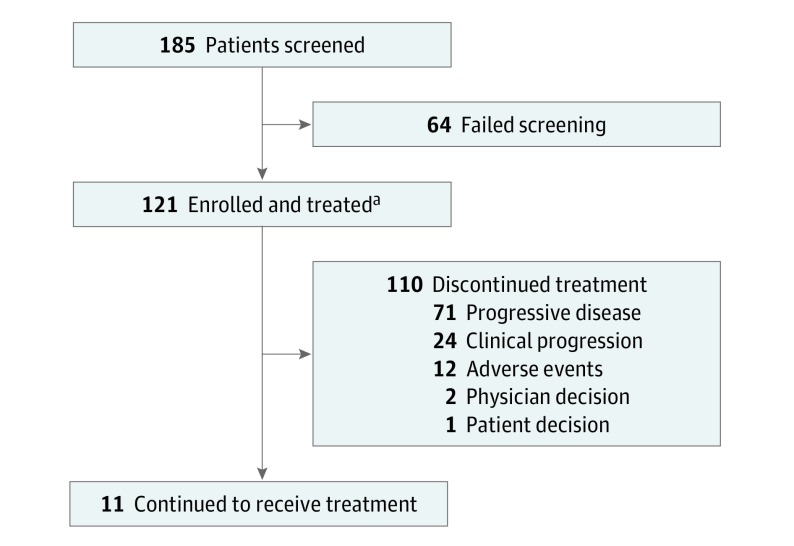
Between January 12, 2016, and March 21, 2017, a total of 121 patients were treated. The median age was 65 years (range, 33-87 years), 100 patients (82.6%) were male, 15 patients (12.3%) had undergone 3 or more prior therapies, 63 patients (52.1%) had ESCC, and 58 patients (47.9%) had PD-L1–positive tumors (eTable 1 in Supplement 2). As of September 18, 2017, the median duration of follow-up was 5.8 months (range, 0.2-18.3 months), and 11 patients (9.1%) continued to receive pembrolizumab (Figure).
Figure. Enrollment and Disposition of the KEYNOTE-180 Study.
Eligible patients were 18 years or older; had histologically confirmed advanced and metastatic adenocarcinoma, squamous cell carcinoma of the esophagus, or advanced and metastatic Siewert type 1 adenocarcinoma of the gastroesophageal junction; and had disease progression after at least 2 prior lines of therapy.
aA total of 31 patients in the study are being followed up.
Overall, the ORR was 9.9% (95% CI, 5.2%-16.7%) among all patients (12 of 121), with 12 patients having a partial response; 7 of the 12 responses were ongoing at analysis (Table 1). Among the 12 responders, the median DOR was not reached (range, 1.9-14.4 months), and an estimated 4 patients had a DOR of 6 months or more (2 patients with a DOR of ≥12 months). Of 106 patients with 1 or more postbaseline tumor assessments, 43 (40.6%) had a reduction from baseline in target lesion size (RECIST, version 1.1, central review) (eFigure 1 in Supplement 2). The median PFS (RECIST, version 1.1, central review) was 2.0 months (95% CI, 1.9-2.1 months), with a 6-month PFS rate of 16% (95% CI, 10%-23%) and a 9-month rate PFS of 9% (95% CI, 5%-16%) (eFigure 2A in Supplement 2). The median OS was 5.8 months (95% CI, 4.5-7.2 months), with a 6-month OS rate of 49% (95% CI, 40%-57%) and a 12-month OS rate of 28% (95% CI, 20%-37%) (eFigure 2B in Supplement 2).
Table 1. Antitumor Activity With Pembrolizumab in All Patients.
| Best Overall Response | No. (%) [95% CI] (N = 121) |
|---|---|
| Objective response rate (CR+PR) | 12 (9.9) [5.2-16.7]a |
| Disease control rate (CR+PR+SD) | 37 (30.6) [22.5-39.6] |
| CR | 0 [0-3] |
| PR | 12 (9.9) [5.2-16.7] |
| SD | 25 (20.7) [13.8-29.0] |
| No assessmentb | 13 (10.7) [5.8-17.7] |
| Progressive disease | 71 (58.7) [49.4-67.6] |
| Time to response, median (range), mo | 4.1 (2.0-6.3) |
| Duration of follow-up, median (range), moc | 13.3 (6.6-18.3) |
| Duration of response, median (range), mo | Not reached (1.9-14.4) |
Abbreviations: CR, complete response; PR, partial response; SD, stable disease.
A total of 7 of 12 responses were ongoing at analysis.
No assessment includes patients who did not have a postbaseline tumor assessment as of the data cutoff date because the scans were missing or because the patient discontinued the study or died before the first postbaseline scan.
Median duration of follow-up in responders.
The ORR was 14.3% (95% CI, 6.7%-25.4%) among patients with ESCC (9 of 63) and 5.2% (95% CI, 1.1%-14.4%) among patients with adenocarcinoma (3 of 58) (eTable 2 in Supplement 2). The median DOR was not reached in either group (patients with ESCC: range, 1.9-14.4 months; patients with adenocarcinoma: range, 2.1-5.4 months). Among patients with PD-L1–positive tumors, the ORR was 13.8% (95% CI, 6.1%-25.4%) (8 of 58), and among patients with PD-L1–negative tumors, the ORR was 6.3% (95% CI, 1.8%-15.5%) (4 of 63) (eTable 3 in Supplement 2). The median DOR was not reached for patients with PD-L1–positive tumors (range, 1.9-14.4 months); the median DOR for patients with PD-L1–negative tumors was 4.4 months (range, 2.1-5.3 months). Progression-free survival was similar across subgroups, while OS was higher among patients with ESCC (eTable 2 in Supplement 2) and PD-L1–positive tumors (eTable 3 in Supplement 2). In a retrospective analysis of 98 evaluable patients, 1 patient had high microsatellite instability and did not respond.
The median duration of pembrolizumab treatment was 2.0 months (range, 0.03-17.0 months), with a median of 4 treatment sessions (range, 1-26 treatment sessions). Overall, 70 patients (57.9%) experienced 1 or more treatment-related AEs, most commonly fatigue (13 [10.7%]), rash (9 [7.4%]), pruritus (8 [6.6%]), hypothyroidism (7 [5.8%]), and diarrhea (6 [5.0%]) (Table 2). Only 5 patients (4.1%) discontinued treatment with pembrolizumab because of AEs. Grade 3 to 5 treatment-related AEs occurred in 15 patients (12.4%) (eTable 4 in Supplement 2). One death from pneumonitis was attributed to treatment (Table 2). Twenty-five patients (20.7%) had 1 or more immune-mediated AEs, most commonly hypothyroidism (9 [7.4%]) and pneumonitis (9 [7.4%]).
Table 2. Adverse Events With Pembrolizumab in All Patients-as-Treated.
| Adverse Events | No. (%) (N = 121) |
|---|---|
| Treatment-related | 70 (57.9) |
| Grades 3-5 | 15 (12.4) |
| Led to discontinuation | 5 (4.1) |
| Led to death | 1 (0.8)a |
| Treatment-related AEs with incidence ≥5% | |
| Grades 1-2 | |
| Fatigue | 13 (10.7) |
| Rash | 9 (7.4) |
| Pruritus | 8 (6.6) |
| Hypothyroidism | 7 (5.8) |
| Diarrhea | 6 (4.9) |
| Pneumonitis | 6 (4.9) |
| Grades 3-5 | |
| Fatigue | 0 |
| Rash | 0 |
| Pruritus | 0 |
| Hypothyroidism | 0 |
| Diarrhea | 1 (0.8) |
| Pneumonitis | 3 (2.4) |
| Immune-mediated AEs | 25 (20.7) |
| Grades 3-5 | 7 (5.8) |
| Led to discontinuation | 5 (4.1) |
| Led to death | 1 (0.8)a |
| Incidence ≥5% | |
| Grades 1-2 | |
| Hypothyroidism | 9 (7.4) |
| Pneumonitis | 6 (4.9) |
| Grades 3-5 | |
| Hypothyroidism | 0 |
| Pneumonitis | 3 (2.4) |
Abbreviation: AE, adverse event.
There was 1 grade 5 treatment-related AE of pneumonitis as determined by investigator.
Discussion
In the KEYNOTE-180 study, pembrolizumab provided durable antitumor activity and manageable safety for heavily pretreated patients with advanced and metastatic esophageal cancer whose disease progressed after 2 or more lines of therapy (including 15 of 121 patients [12.4%] who received fourth-line or higher treatment). In this patient population notable for limited treatment options, pembrolizumab provided a clinically meaningful tumor response, with an ORR of 9.9% by central review. At median follow-up of 13.3 months among responders, the median DOR was not reached, suggesting the durability of the response. This finding compares favorably with an ORR of 3%, with a response duration ranging from 1.17 to 7.33 months, previously observed in the third-line setting.13 In addition, an estimated 4 patients achieved a DOR of 6 months or more; 7 of 12 responses were ongoing at analysis. Clinically meaningful antitumor activity was observed regardless of histologic characteristics, with a higher ORR among patients with ESCC (14.3%) compared with patients with adenocarcinoma (5.2%). This finding is similar to the ORR (17%) observed in a study of nivolumab among patients with advanced ESCC.14 In addition, although responses were enriched in patients with PD-L1–positive tumors, antitumor activity was observed regardless of PD-L1 status. Conclusive comparisons of subgroups are limited by small sample sizes, and apparent differences in antitumor activity will be confirmed in the larger, ongoing, phase 3 KEYNOTE-181 (NCT02564263) and KEYNOTE-590 (NCT03189719) clinical studies.
Survival outcomes were encouraging, with a median OS of 5.8 months, a 6-month OS rate of 49% and a 12-month OS rate of 28%. This finding compares favorably with the historically observed median OS of less than 4 months and a 12-month OS rate of less than 13% in the second-line setting,13 and it supports the durability of clinical benefit among these patients. The estimated 6-month PFS rate of 16% also compared favorably with the 6-month PFS rate of 12% previously observed.13 However, the PFS rate plateaued after 6 months, suggesting prolonged survival of patients who were alive and whose disease was not progressing after 6 months. There was 1 treatment-related death due to pneumonitis. No new safety signals were observed.10,11,15,16
Limitations
This study has some limitations. It shows the antitumor activity of pembrolizumab in patients with metastatic esophageal cancer in a single-arm, nonrandomized study. Randomized phase studies evaluating pembrolizumab in patients with metastatic esophageal cancer in the first- and second-line setting are ongoing.
Conclusions
These data support pembrolizumab as a valuable treatment option with durable benefit for heavily pretreated patients with advanced, metastatic esophageal cancer whose disease progressed after 2 or more lines of therapy.
Trial Protocol
eAppendix. Methods
eTable 1. Baseline Characteristics
eTable 2. Antitumor Activity in Patients with Squamous Cell Carcinoma or Adenocarcinoma
eTable 3. Antitumor Activity in Patients With PD-L1-Positive or PD-L1-Negative Tumors
eTable 4. Grade 3-5 Treatment-Related Adverse Events
eFigure 1. Best Percentage Change From Baseline in Target Lesion Size (RECIST v1.1, Central Review)
eFigure 2. Survival Outcomes in All Patients
Data Sharing Statement
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30. doi: 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 2.Enzinger PC, Burtness BA, Niedzwiecki D, et al. CALGB 80403 (Alliance)/E1206: a randomized phase II study of three chemotherapy regimens plus cetuximab in metastatic esophageal and gastroesophageal junction cancers. J Clin Oncol. 2016;34(23):2736-2742. doi: 10.1200/JCO.2015.65.5092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87-108. doi: 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 4.Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381(9864):400-412. doi: 10.1016/S0140-6736(12)60643-6 [DOI] [PubMed] [Google Scholar]
- 5.Bang YJ, Van Cutsem E, Feyereislova A, et al. ; ToGA Trial Investigators . Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687-697. doi: 10.1016/S0140-6736(10)61121-X [DOI] [PubMed] [Google Scholar]
- 6.Fuchs CS, Tomasek J, Yong CJ, et al. ; REGARD Trial Investigators . Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383(9911):31-39. doi: 10.1016/S0140-6736(13)61719-5 [DOI] [PubMed] [Google Scholar]
- 7.Shah MA. Update on metastatic gastric and esophageal cancers. J Clin Oncol. 2015;33(16):1760-1769. doi: 10.1200/JCO.2014.60.1799 [DOI] [PubMed] [Google Scholar]
- 8.Smyth EC, Lagergren J, Fitzgerald RC, et al. Oesophageal cancer. Nat Rev Dis Primers. 2017;3:17048. doi: 10.1038/nrdp.2017.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cafferkey C, Davidson M, Goode E, et al. Survival in advanced oesophagogastric adenocarcinoma (OGA) improves with the use of multiple lines of therapy: results from an analysis of over 500 patients (pts). Ann Oncol. 2017;28(suppl_5):mdx369.026. doi: 10.1093/annonc/mdx369.026 [DOI] [PubMed] [Google Scholar]
- 10.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti–PD-1) in melanoma. N Engl J Med. 2013;369(2):134-144. doi: 10.1056/NEJMoa1305133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doi T, Piha-Paul SA, Jalal SI, et al. Safety and antitumor activity of the anti–programmed death-1 antibody pembrolizumab in patients with advanced esophageal carcinoma. J Clin Oncol. 2018;36(1):61-67. doi: 10.1200/JCO.2017.74.9846 [DOI] [PubMed] [Google Scholar]
- 12.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 13.Dutton SJ, Ferry DR, Blazeby JM, et al. Gefitinib for oesophageal cancer progressing after chemotherapy (COG): a phase 3, multicentre, double-blind, placebo-controlled randomised trial. Lancet Oncol. 2014;15(8):894-904. doi: 10.1016/S1470-2045(14)70024-5 [DOI] [PubMed] [Google Scholar]
- 14.Kudo T, Hamamoto Y, Kato K, et al. Nivolumab treatment for oesophageal squamous-cell carcinoma: an open-label, multicentre, phase 2 trial. Lancet Oncol. 2017;18(5):631-639. doi: 10.1016/S1470-2045(17)30181-X [DOI] [PubMed] [Google Scholar]
- 15.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1–positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540-1550. doi: 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 16.Zinzani PL, Ribrag V, Moskowitz CH, et al. Safety and tolerability of pembrolizumab in patients with relapsed/refractory primary mediastinal large B-cell lymphoma. Blood. 2017;130(3):267-270. doi: 10.1182/blood-2016-12-758383 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix. Methods
eTable 1. Baseline Characteristics
eTable 2. Antitumor Activity in Patients with Squamous Cell Carcinoma or Adenocarcinoma
eTable 3. Antitumor Activity in Patients With PD-L1-Positive or PD-L1-Negative Tumors
eTable 4. Grade 3-5 Treatment-Related Adverse Events
eFigure 1. Best Percentage Change From Baseline in Target Lesion Size (RECIST v1.1, Central Review)
eFigure 2. Survival Outcomes in All Patients
Data Sharing Statement