This randomized clinical trial of patients with stage I gastric cancer examines whether long-term survival among patients undergoing laparoscopic distal gastrectomy is noninferior to that among patients undergoing open distal gastrectomy.
Key Points
Question
Is the long-term survival among patients with stage I gastric cancer undergoing laparoscopic distal gastrectomy noninferior to that among patients undergoing open distal gastrectomy?
Findings
In this randomized clinical trial of 1416 patients, 5-year overall survival rates were 94.2% in the laparoscopic gastrectomy group and 93.3% in the open gastrectomy group. The 5-year cancer-specific survival rates were also similar between the 2 groups: 97.1% in the laparoscopic gastrectomy group and 97.2% in the open gastrectomy group.
Meaning
Laparoscopic distal gastrectomy is an oncologically safe alternative to open surgery for stage I gastric cancer.
Abstract
Importance
Laparoscopic distal gastrectomy is gaining popularity over open distal gastrectomy for gastric cancer because of better early postoperative outcomes. However, to our knowledge, no studies have proved whether laparoscopic distal gastrectomy is oncologically equivalent to open distal gastrectomy.
Objective
To examine whether the long-term survival among patients with stage I gastric cancer undergoing laparoscopic distal gastrectomy is noninferior to that among patients undergoing open distal gastrectomy.
Design
The Korean Laparoendoscopic Gastrointestinal Surgery Study (KLASS) group, which includes 15 surgeons from 13 institutes, conducted a phase 3, multicenter, open-label, noninferiority, prospective randomized clinical trial (KLASS-01) of patients with histologically proven, preoperative clinical stage I gastric adenocarcinoma from January 5, 2006, to August 23, 2010. Survival and recurrence status of the patients was determined in December 2016.
Interventions
Patients were randomly assigned (1:1) to laparoscopic distal gastrectomy (n = 705) or open distal gastrectomy (n = 711). Of these patients, 85 received a surgical approach opposite the one to which they were randomized (63 randomized to the open surgery group and 22 to the laparoscopic group).
Main Outcomes and Measures
Difference in 5-year overall survival between the laparoscopic and open distal gastrectomy groups. The noninferiority margin was prespecified as −5% (corresponding hazard ratio of 1.54), with an assumed survival of 90% after 5 years in the open surgery group.
Results
Among the 1416 patients (mean [SD] age, 57.3 [11.1] years; 940 [66.4%] male) included in the study, the 5-year overall survival rates were 94.2% in the laparoscopic group and 93.3% in the open surgery group (log-rank P = .64). Intention-to-treat analysis confirmed the noninferiority of the laparoscopic approach compared with the open approach (difference, 0.9 percentage points; 1-sided 97.5% CI, −1.6 to infinity). The 5-year cancer-specific survival rates were similar between the 2 groups (97.1% in the laparoscopic group and 97.2% in the open surgery group, log-rank P = .91; difference, −0.03 percentage points; 1-sided 97.5% CI, −1.8 to infinity). Per-protocol analysis results were consistent with the intention-to-treat results for overall and cancer-specific survival rates.
Conclusions and Relevance
The KLASS-01 trial revealed similar overall and cancer-specific survival rates between patients receiving laparoscopic and open distal gastrectomy. Laparoscopic distal gastrectomy is an oncologically safe alternative to open surgery for stage I gastric cancer.
Trial Registration
ClinicalTrials.gov identifier: NCT00452751
Introduction
Laparoscopic gastrectomy has been applied in treatment of gastric cancer, especially early-stage gastric cancer.1,2,3 Compared with open procedures, laparoscopic gastrectomy for gastric cancer offers better short-term postoperative outcomes.4,5,6 Its oncologic safety, however, has remained controversial because of a lack of evidence from well-designed randomized clinical trials.
Most randomized clinical trials testing laparoscopic vs open surgery for early gastric cancer have reported early results on the procedural safety and short-term benefits of the laparoscopic approach.7,8,9,10 The Korean Laparoendoscopic Gastrointestinal Surgery Study (KLASS) group has also conducted a multicenter, phase 3 randomized clinical trial (KLASS-01) to provide definitive evidence on the surgical and oncologic safety of laparoscopic distal gastrectomy (LDG) compared with open distal gastrectomy (ODG).11
The KLASS group previously reported the short-term benefits of LDG for clinical stage I gastric cancer, demonstrating shorter hospital stay, less blood loss, and fewer wound complications compared with ODG.12,13 The KLASS group has since concluded that LDG for clinical stage I gastric cancer is a surgically safe procedure. In this article, we present the long-term outcomes of our prospective randomized clinical trial comparing LDG and ODG for clinical stage I gastric cancer.
Methods
Study Design and Patients
Our investigator-initiated trial sought to compare short-term clinical outcomes and long-term oncologic results of LDG with those of ODG for clinical stage I gastric cancer (KLASS-01). KLASS-01 was designed as a phase 3, multicenter, open-label, noninferiority, prospective randomized clinical trial conducted by 15 surgeons from 13 tertiary hospitals in Korea. The study design and methods have been described in detail previously.11 All patients provided written informed consent before enrollment. The study protocol was approved by the institutional review boards of all participating hospitals (Seoul National University Bundang Hospital, Ajou University Hospital, Dong-A University Hospital, Yeouido St. Mary's Hospital, Seoul National University Hospital, Keimyung University Dongsan Medical Center, Soonchunhyang University Bucheon Hospital, Chonbuk National University Hospital, Seoul St. Mary's Hospital, Chungnam National University Hospital, Chonnam National University Hwasun Hospital, Ewha Womans University Mokdong Hospital, and Severance Hospital). The trial protocol is included in Supplement 1.
From January 5, 2006, to August 23, 2010, we enrolled patients with gastric cancer suitable for distal gastrectomy. Patients were between 20 and 80 years of age. All patients had histologically proven, preoperative clinical stage I gastric adenocarcinoma (T1N0M0, T1N1M0, or T2aN0M0). We excluded patients with the following criteria: American Society of Anesthesiologist score greater than 3, history or presence of other malignant tumors, previous chemotherapy or radiotherapy, and need for combined resection because of other benign conditions except cholecystectomy.
Objectives and End Points
Our primary objective was to demonstrate noninferior oncologic outcomes for LDG vs ODG for clinical stage I gastric cancer. The primary end point was 5-year overall survival calculated from the date of surgery to the date of death from any cause. Secondary end points were gastric cancer–specific survival, morbidity and mortality, quality of life, and cost-effectiveness. Cancer-specific survival was calculated as the number of months from gastrectomy to the date of death from gastric cancer. In the analysis of cancer-specific survival, we censored patients who died of causes other than gastric cancer.
Randomization and Data Management
Patients were enrolled and randomized to undergo LDG or ODG according to a computer-generated randomization list at a 1:1 ratio. Randomization was controlled by a centralized independent data center balancing the treatment arms using 60 codes for each investigator and institution. The patients were allocated in order of the day of enrollment. A trial steering committee was responsible for overseeing the trial. All data were collected by research coordinators at each hospital. To reduce the likelihood of incorrect data, data entry was conducted using a dual data entry procedure by 2 different data entry persons at the data center independent of the participating institutions. The 2 entries were compared, and any discrepancies were corrected. Patient death information was obtained from the Korea Statistics Promotion Institute database, allowing the exact dates of death for all enrolled patients to be collected. Survival and recurrence status was determined in December 2016.
Interventions, Quality Control, and Follow-up
For both approaches, standard radical distal gastrectomy with D1+β or D2 lymphadenectomy according to the Japanese classification was performed.14 Dissection of lymph node station 14v was optional. For all patients, a partial omentectomy was performed. Decisions on reconstruction methods depended on each surgeon's preference. During LDG, reconstruction was performed extracorporeally through a minilaparotomy of less than 5 cm in the upper abdomen. Adjuvant chemotherapy was recommended to patients with pathologic stage II or more advanced cancer.
To participate in the trial, surgeons must have performed at least 100 gastrectomies for gastric cancer by laparoscopic and open surgery (50 each), and hospitals had to have an annual volume of at least 80 gastrectomies. Before initiation of the trial, we established a standardized protocol for each surgical procedure. All candidate surgeons’ operations were assessed by 2 experienced surgeons (H.-H.K., W.K., S.-U.H., M.-C.K., G.S.C., and W.J.H.) during on-site visits for quality control. All participating surgeons thoroughly reviewed each other’s unedited videos to build consensus on procedural standardization. During the trial, video recordings of all LDG and photographic documentation of the operative field after lymphadenectomy by ODG were mandated. We evaluated morbidity and mortality between the 2 interventions by a planned interim analysis before continuing the study to ensure patient safety. We previously reported the results of this planned interim analysis, demonstrating no difference between the 2 approaches.12
All patients were followed up regularly, and follow-up data, including recurrence and death, were registered. The same follow-up protocol was used for both groups. Follow-up was conducted every 3 months for the first 2 years after surgery, every 6 months for the next 3 years, and then annually.
Statistical Analysis
The primary and secondary end points were analyzed in 2 different populations. The intention-to-treat population was defined as all eligible patients who were randomized except those excluded after randomization. The per-protocol population was defined as the patients who underwent the assigned approaches only. The patients who required conversion from ODG to LDG were not excluded from the per-protocol population. We planned to perform all analyses on an intention-to-treat population basis.
We calculated the effective sample size using a 5-year overall survival of 90% based on review of literature after open gastrectomy for clinical stage I gastric cancer.15,16,17 We tested the hypothesis that the Kaplan-Meier estimate of 5-year overall survival of LDG would be noninferior to that of ODG within a 5% margin (corresponding hazard ratio [HR] of 1.54). To prove the noninferiority of LDG, the lower limit of the 1-sided 97.5% CI of the difference in 5-year overall survival between the 2 groups should be greater than −5% (LDG minus ODG). Although the original analysis plan for the noninferiority results was using a 1-sided 95% CI, this was changed to a 1-sided 97.5% CI to correspond to recent, more conservative statistical practice. We assumed that the patient registration period would be 4.5 years and the follow-up period would be 5 years. The sample size was estimated using the noninferiority log-rank test. Finally, we calculated that 1400 patients (700 in each group, with at least 633 eligible patients in each intention-to-treat population) were needed to provide 80% power for a 1-sided type I error level of 0.05 and to allow for a dropout rate of 10% after randomization.
We used R statistics, version 3.1.1 (R Foundation for Statistical Computing) for data analysis. The χ2 or Fisher exact tests for categorical variables and t tests or Mann-Whitney tests for continuous variables were used for statistical analyses. Kaplan-Meier curves were used to estimate overall survival and cancer-specific survival. The HRs and 1-sided 97.5% CIs were estimated with a Cox proportional hazards regression model after confirmation of the proportional hazard assumption. One-sided 97.5% CIs are reported for survival differences and HRs; other CIs are 2-sided 95% CIs unless otherwise specified. We used 2-sided values for the calculation of the P values of the HR for death in the post hoc analysis.
Results
Patients
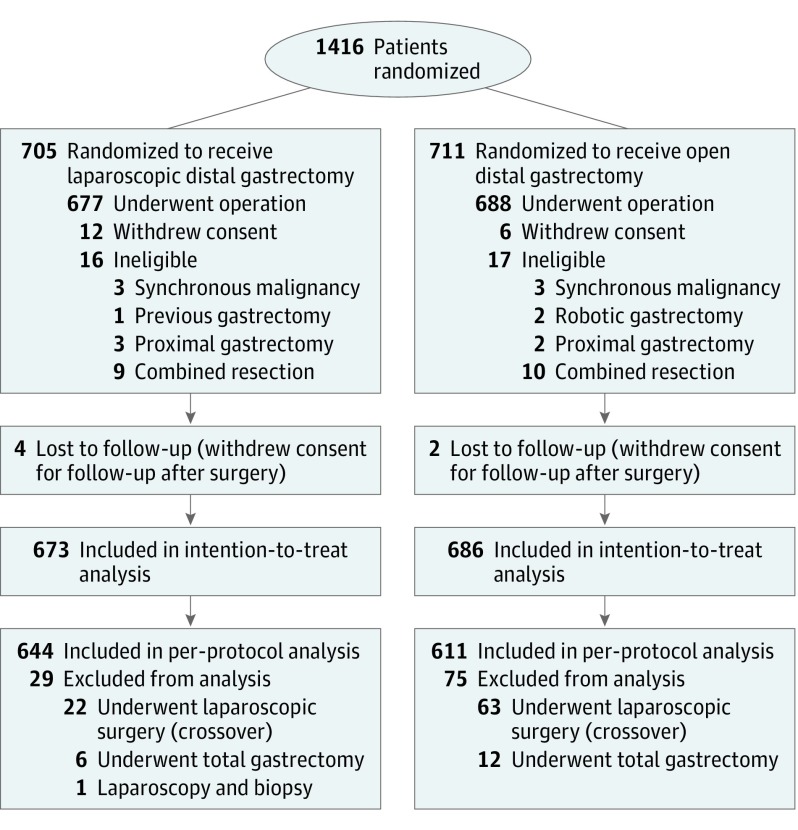
A total of 1416 patients (mean [SD] age, 57.3 [11.1] years; 940 [66.4%] male and 476 [33.6%] female) with clinical stage I gastric adenocarcinoma underwent randomization to LDG or ODG: 705 were randomized to the LDG group and 711 to the ODG group. After randomization and during follow-up, we excluded 57 patients for various reasons. Finally, we included 1359 patients in the intention-to-treat population: 673 in the LDG group and 686 in the ODG group. We excluded another 104 patients from the per-protocol population. Among them, 85 underwent surgery with an approach opposite the one to which they were randomized (63 randomized to the ODG group and 22 to the LDG group). Thus, the per-protocol population included 1255 patients: 644 in the LDG group and 611 in the ODG group (Figure 1). The 2 study groups were balanced regarding baseline clinical characteristics, surgical procedures, and pathologic features and staging (Table 1).
Figure 1. Recruitment and Inclusion of Patients in the Korean Laparoendoscopic Gastrointestinal Surgery Study 01 Randomized Clinical Trial.
The data on the number of individuals assessed for eligibility in the study were not collected.
Table 1. Patient Characteristics and Pathologic Outcomesa.
| Variable | Intention-to-Treat Population | Per-Protocol Population | |||
|---|---|---|---|---|---|
| LDG (n = 673) | ODG (n = 686) | LDG (n = 644) | ODG (n = 611) | ||
| Age, mean (SD), y | 56.9 (10.9) | 57.5 (11.3) | 56.8 (10.8) | 57.8 (11.2) | |
| Sex | |||||
| Male | 448 (66.6) | 458 (66.8) | 426 (66.1) | 412 (67.4) | |
| Female | 225 (33.4) | 228 (33.2) | 218 (33.9) | 199 (32.6) | |
| BMI | 23.8 (2.9) | 23.8 (3.0) | 23.8 (2.9) | 23.8 (3.0) | |
| Clinical TNM stageb | |||||
| cT1N0M0 | 530 (78.8) | 531 (77.4) | 511 (79.3) | 465 (76.1) | |
| cT1N1M0 | 15 (2.2) | 19 (2.8) | 14 (2.2) | 19 (3.1) | |
| cT2N0M0 | 128 (19.0) | 136 (19.8) | 119 (18.5) | 127 (20.8) | |
| Extent of resectionc | |||||
| Distal gastrectomy | 664 (98.7) | 673 (98.1) | 644 (100) | 611 (100) | |
| Total gastrectomy | 8 (1.2) | 13 (1.9) | 0 | 0 | |
| Extent of lymphadenectomyc | |||||
| D1+ | 293 (43.5) | 245 (35.7) | 282 (43.8) | 215 (35.2) | |
| D2 | 379 (56.3) | 441 (64.3) | 362 (56.2) | 396 (64.8) | |
| No. of retrieved lymph nodes, mean (SD) | 40.5 (15.4) | 43.2 (15.9) | 40.5 (15.5) | 43.6 (15.8) | |
| ≤15 | 13 (1.9) | 10 (1.5) | 10 (1.6) | 8 (1.3) | |
| ≥16 | 660 (98.1) | 676 (98.5) | 634 (98.4) | 603 (98.7) | |
| Tumor size, mm | 26.7 (17.8) | 27.4 (17.1) | 25.8 (15.1) | 27.0 (16.4) | |
| Pathologic T classificationc | |||||
| T1 | 548 (81.4) | 549 (80.0) | 531 (82.5) | 485 (79.4) | |
| ≥T2 | 124 (18.4) | 137 (20.0) | 113 (17.5) | 126 (20.6) | |
| Pathologic N classificationc | |||||
| N0 | 571 (84.8) | 571 (83.2) | 553 (85.9) | 508 (83.1) | |
| N+ | 101 (15.0) | 115 (16.8) | 91 (14.1) | 103 (16.9) | |
| Pathologic stage | |||||
| I | 583 (86.6) | 579 (84.4) | 567 (88.0) | 516 (84.5) | |
| II | 60 (8.9) | 75 (10.9) | 52 (8.1) | 66 (10.8) | |
| III | 29 (4.3) | 32 (4.7) | 25 (3.9) | 29 (4.7) | |
| IV | 1 (0.1) | 0 | 0 | 0 | |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); LDG, laparoscopic distal gastrectomy, ODG, open distal gastrectomy.
Data are presented as number (percentage) of patients unless otherwise indicated.
The TNM stage was determined according to the seventh edition of the American Joint Committee on Cancer/Union for International Cancer Control Cancer Staging Manual.
One patient who did not undergo gastrectomy because of carcinomatosis was excluded from the LDG group.
Surgical and Pathologic Outcomes
The KLASS group previously reported early postoperative outcomes in detail,13 although minor differences were found between the patients included in this long-term survival analysis and those in the early postoperative outcomes analysis. For 6 patients (0.9%), LDG was converted to ODG. Regarding postoperative mortality, 4 in-hospital deaths (0.6%) were recorded in the LDG group and 2 (0.3%) in the ODG group.
All patients underwent radical gastrectomy with systemic lymphadenectomy (D1+ or D2) except for 1 patient with peritoneal carcinomatosis in the LDG group. Mean numbers of retrieved lymph nodes and patients with fewer than 16 retrieved lymph nodes were similar between the 2 groups. There was no margin involvement of the tumor in any patient. The proportions of histologic types and pathologic TNM stages were similar between the 2 groups (Table 1). As for diagnostic accuracy of invasion depth, 72 patients (5.3%) had pathologic T3 tumors and 41 (3.0%) had T4 tumors in the intention-to-treat population. For clinical T1 tumors (n = 1095), 960 (87.7%) were pathologic T1, whereas 84 (7.7%) were pathologic T2, 34 (3.1%) were pathologic T3, and 17 (1.6%) were pathologic T4. For clinical T2 tumors (n = 264), 64 (24.2%) were pathologic T2, whereas 137 (51.9%) were pathologic T1, 38 (14.4%) were pathologic T3, and 24 (9.1%) were pathologic T4.
In total, 126 patients (59 in the LDG group and 67 in the ODG group) received adjuvant chemotherapy. Fifty-one patients (86.4%) in the LDG group and 61 patients (91.0%) in the ODG group received chemotherapy within 6 weeks after surgery (P = .41). The mean (SD) durations from surgery to adjuvant chemotherapy did not differ between the 2 groups (28.7 [13.9] days in the LDG group vs 29.4 [12.3] days in the ODG group; P = .75).
Survival Outcomes
Overall Survival
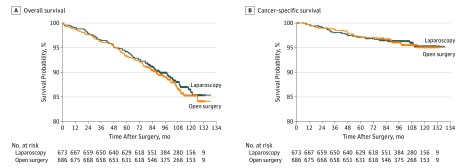
After a median follow-up of 99.8 months, 79 patients (11.7%) in the LDG group died compared with 85 patients (12.4%) in the ODG group by the last follow-up date. Overall causes of death were similar between the 2 groups (Table 2). The 5-year overall survival rates were 94.2% (95% CI, 92.4%-96.0%) for the LDG group and 93.3% (95% CI, 91.4%-95.2%) for the ODG group (log-rank P = .64) (Figure 2A). The difference in 5-year overall survival was 0.9 percentage points; the difference in the lower limit of the 97.5% CI was −1.6 percentage points, which was greater than the noninferiority margin of −5% (1-sided 97.5% CI, −1.6 to infinity; HR, 0.93, 1-sided 97.5% CI, −infinity to 1.26). The absence of differences in causes of death (eTable in the Supplement 2), HRs for death, and 5-year overall survival also persisted in the per-protocol population (eFigure 1A in the Supplement 2).
Table 2. Causes of Death and Recurrence Patterns in the Intention-to-Treat Population.
| Variable | No. (%) of Patients | P Value | |
|---|---|---|---|
| LDG Population (n = 673) | ODG Population (n = 686) | ||
| Total deaths | |||
| Gastric cancer related | 28 (4.1) | 28 (4.1) | .49 |
| Other malignant tumor related | 11 (1.6) | 8 (1.1) | |
| Other | 32 (4.7) | 34 (5.0) | |
| Unknown | 8 (1.2) | 15 (2.1) | |
| Total recurrence | |||
| Locoregional | 9 (1.3) | 5 (0.7) | .60 |
| Hematogenous | 13 (1.9) | 13 (1.9) | |
| Peritoneal | 8 (1.2) | 7 (1.0) | |
| Distant lymph node | 1 (0.1) | 3 (0.4) | |
| Mixed | 7 (1.0) | 5 (0.7) | |
Abbreviations: LDG, laparoscopic distal gastrectomy, ODG, open distal gastrectomy.
Figure 2. Kaplan-Meier Survival Curves for Patients Included in the Final Analysis in the Intention-to-Treat Population.
A, Overall survival between the laparoscopic group and the open surgery group was similar (94.2% [95% CI, 92.4%-96.0%] in the laparoscopic group and 93.3% [95% CI, 91.4%-95.2%] in the open surgery group; log-rank P = .64; difference, 0.9 percentage points; 1-sided 97.5% CI, −1.6 to infinity). B, Cancer-specific survival between the laparoscopic group and the open surgery group was also similar (97.1% [95% CI, 95.9%-98.4%] in the laparoscopic group and 97.2% [95.9%-98.4%] in the open surgery group, log-rank P = .91; difference, −0.03 percentage points; 1-sided 97.5% CI, −1.8 to infinity). The median follow-up of patients was 99.8 months in all groups.
Cancer-Specific Survival
By the cutoff date, 28 patients (4.2%) in the LDG group had died of gastric cancer compared with 28 patients (4.1%) in the ODG group. The 5-year cancer-specific survival rates were similar between the 2 groups (97.1% in the LDG group and 97.2% in the ODG group, log-rank P = .91; difference of −0.03 percentage points; 1-sided 97.5% CI, −1.8 to infinity; HR, 0.97; 1-sided 97.5% CI, −infinity to 1.64) (Figure 2B). In the per-protocol population, cancer-specific survival was similar to that in the intention-to-treat population (eFigure 1B in Supplement 2).
Recurrence was recorded in 38 patients (5.6%) in the LDG group and 33 patients (4.8%) in the ODG group; the difference was not statistically significant (P = .49). Recurrence patterns for these 2 groups were also similar (Table 2). Including patients with mixed recurrence, 13 (1.9%) in the LDG group and 7 (1.0%) in the ODG group experienced locoregional recurrence. In the per-protocol population, we observed similar recurrence rates and patterns as those in the intention-to-treat population (eTable in Supplement 2).
Post Hoc Subgroup Analyses
Post hoc subgroup analysis of overall survival revealed no significant interaction between treatment effects and any baseline clinical findings in the intention-to-treat population (eFigure 2 in Supplement 2). Patients with advanced tumor (T2 or deeper) or node-positive disease had no survival difference. The HRs for death in the LDG group were 0.71 (95% CI, 0.44-1.16) for 261 patients with T2 or deeper tumor and 0.85 (95% CI, 0.49-1.46) for 216 patients with node-positive disease compared with the ODG group. We observed similar survival between the 2 approaches in patients with clinically advanced cancer and with pathologically advanced tumor. No significant interaction was found between patients in the LDG and ODG groups among different body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) groups (<20.0, 20.0-24.9, or ≥25.0).
Discussion
In this KLASS-01 trial, we confirmed the noninferiority of 5-year overall survival outcomes for LDG for gastric cancer and better short-term clinical outcomes compared with ODG. We found overall survival, cancer-specific survival, and recurrence patterns after LDG to be similar to those after ODG. Although LDG exhibited longer operation times in our trial, it allowed for less estimated blood loss, fewer postoperative complications, and a shorter hospital stay, as described in a previous report13 on short-term outcomes of the KLASS-01 trial. Together with these better, or at least similar, early postoperative outcomes and the minimal invasiveness of laparoscopic surgery over open surgery, these long-term oncologic outcomes of LDG support the adoption of this procedure as a standard treatment for clinical stage I gastric cancer.
The oncologic safety of laparoscopic surgery for gastric cancer was doubted because of a potentially increased risk of locoregional recurrence caused by inadequate lymphadenectomy.18 However, we previously observed equivalent surgical and pathologic oncologic efficacy with LDG compared with ODG, achieving both an adequate number of harvested lymph nodes and a safe resection margin with LDG. We thus anticipated comparable long-term oncologic outcomes for overall and cancer-specific survival because these early outcomes indicated the oncologic safety of the laparoscopic procedure. In this trial, the difference in locoregional recurrence between LDG and ODG did not reach statistical significance, although the total number of recurrences was small and the recurrence rate was 0.8% higher with LDG than with ODG. Supporting our results, several large-scale retrospective studies19,20 have reported similar long-term oncologic results between laparoscopic and open gastrectomy. Although the survival rates in our trial are somewhat lower than those in the JCOG0703 (Japan Clinical Oncology Group 0703) study, this finding might be explained by a comparatively lower proportion of patients with pathologic stage IA disease in our study.21 Moreover, 13% of patients in our study had pathologic stage II or more advanced disease, and 15% of patients had lymph node metastasis. Along with minimally invasive advantages of laparoscopic gastrectomy, our trial found decreased morbidity and mortality for LDG compared with ODG within the same study cohort. We also found similar survival in the LDG group compared with that in the ODG group regardless of BMI, although the mean BMI of the patients was less than that of Western patients. Thus, our results on the noninferiority of LDG in regard to survival compared with ODG suggest potential benefits in obese or even morbidly obese patients, even if lymphadenectomy may be more difficult by laparoscopy in obese patients.
We strived to maintain the quality of the trial by using hospitals’ and surgeons’ case volumes as eligibility criteria as well as evaluation of surgeons’ technical proficiency. Our study revealed satisfactory surgical outcomes for both approaches. Moreover, the mean number of patients enrolled in the study was 108.9 patients per hospital and 23.3 patients per hospital each year. These numbers are greater than those registered in any other surgical trial on this topic to our knowledge.
Limitations
Our trial has several limitations. First, we only included patients with clinical stage I cancer suitable for distal subtotal gastrectomy. Applying laparoscopic surgery for more advanced cancers and different operations, such as total gastrectomy, needs to be verified through other clinical trials. Accordingly, the KLASS-02 trial is being conducted to compare LDG with ODG for locally advanced gastric cancer.22,23 The KLASS-06 trial is in the planning stages to compare laparoscopic total gastrectomy with open surgery for advanced gastric cancer located in the upper body of the stomach.
Second, after randomization, the surgical approaches crossed over from ODG to LDG in 63 patients and from LDG to ODG in 22 patients. Increasing interest in laparoscopic surgery among patients on initiation of the KLASS-01 trial may have influenced these patients’ decisions to switch surgical approaches after randomization. However, analysis of the per-protocol population after excluding these crossover patients revealed the same long-term oncologic results.
Third, it would be difficult to verify the generalizability of our findings to surgeons and centers with less experience. Along with an accumulation of cases, proper education and training, such as participation in a laparoscopic gastrectomy team of experts and proctoring program, would help surgeons and centers with less experience perform laparoscopic gastrectomy safely and reduce the learning curve period.24,25,26 In addition, studies27,28,29 have found that improvements in survival after gastrectomy, as well as reduction of operative morbidity and mortality, can be obtained in the West by centralization. Thus, centralization should be advocated for better surgical and oncologic outcomes after gastrectomy for gastric cancer by open distal gastrectomy and laparoscopy.
Fourth, reconstruction performed extracorporeally in LDG is another limitation. When we designed and started the study in 2004, the intracorporeal anastomosis technique was not popular. Thus, we performed extracorporeal anastomosis in LDG, although intracorporeal anastomosis has now become the standard technique. Moreover, advantages of the laparoscopic approach are lessened by performing extracorporeal anastomosis because an easier and faster reconstruction can be achieved intracorporeally in laparoscopic procedures, especially in patients with a high BMI.
Fifth, the 5% noninferiority margin of the 5-year overall survival rate was large compared with the observed 5-year overall mortality of approximately 6.7% in the ODG group. However, the difference in the 5-year overall survival rate was 0.9 percentage points, which was within the lower limit of the 97.5% CI of −1.6%. Thus, we can rule out a larger decrease in 5-year overall survival.
Sixth, the period of follow-up was relatively short compared with the median survival time for patients; therefore, we only observed the first part of the survival curve, leaving open the possibility that the observed noninferiority may not be maintained in the long term. Thus, a study with more long-term follow-up results is warranted.
Conclusions
We found that, when used for clinical stage I gastric cancer, LDG is associated with low morbidity and with survival comparable to those for ODG without compromising long-term oncologic outcomes. Our trial supports the use of LDG as a standard treatment option for clinical stage I distal gastric cancer when it can be performed by surgeons with sufficient experience.
Trial Protocol
eTable. Comparison of causes of death and recurrence patterns for patients included in the per-protocol population
eFigure 1. Kaplan-Meier survival curves for patients included in the final analysis in the per-protocol population
eFigure 2. Post hoc subgroup analysis for overall survival
Data Sharing Statement
References
- 1.Information Committee of Korean Gastric Cancer Association Korean gastric cancer association nationwide survey on gastric cancer in 2014. J Gastric Cancer. 2016;16(3):131-140. doi: 10.5230/jgc.2016.16.3.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Son SY, Kim HH. Minimally invasive surgery in gastric cancer. World J Gastroenterol. 2014;20(39):14132-14141. doi: 10.3748/wjg.v20.i39.14132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Son T, Hyung WJ. Laparoscopic gastric cancer surgery: current evidence and future perspectives. World J Gastroenterol. 2016;22(2):727-735. doi: 10.3748/wjg.v22.i2.727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deng Y, Zhang Y, Guo TK. Laparoscopy-assisted versus open distal gastrectomy for early gastric cancer: a meta-analysis based on seven randomized controlled trials. Surg Oncol. 2015;24(2):71-77. doi: 10.1016/j.suronc.2015.02.003 [DOI] [PubMed] [Google Scholar]
- 5.Kelly KJ, Selby L, Chou JF, et al. . Laparoscopic versus open gastrectomy for gastric adenocarcinoma in the West: a case-control study. Ann Surg Oncol. 2015;22(11):3590-3596. doi: 10.1245/s10434-015-4381-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee SI, Choi YS, Park DJ, Kim HH, Yang HK, Kim MC. Comparative study of laparoscopy-assisted distal gastrectomy and open distal gastrectomy. J Am Coll Surg. 2006;202(6):874-880. doi: 10.1016/j.jamcollsurg.2006.02.028 [DOI] [PubMed] [Google Scholar]
- 7.Katai H, Sasako M, Fukuda H, et al. ; JCOG Gastric Cancer Surgical Study Group . Safety and feasibility of laparoscopy-assisted distal gastrectomy with suprapancreatic nodal dissection for clinical stage I gastric cancer: a multicenter phase II trial (JCOG 0703). Gastric Cancer. 2010;13(4):238-244. doi: 10.1007/s10120-010-0565-0 [DOI] [PubMed] [Google Scholar]
- 8.Kim YW, Baik YH, Yun YH, et al. . Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg. 2008;248(5):721-727. doi: 10.1097/SLA.0b013e318185e62e [DOI] [PubMed] [Google Scholar]
- 9.Kitano S, Shiraishi N, Fujii K, Yasuda K, Inomata M, Adachi Y. A randomized controlled trial comparing open vs laparoscopy-assisted distal gastrectomy for the treatment of early gastric cancer: an interim report. Surgery. 2002;131(1)(suppl):S306-S311. doi: 10.1067/msy.2002.120115 [DOI] [PubMed] [Google Scholar]
- 10.Lee JH, Han HS, Lee JH. A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc. 2005;19(2):168-173. doi: 10.1007/s00464-004-8808-y [DOI] [PubMed] [Google Scholar]
- 11.Kim HH, Han SU, Kim MC, et al. ; Korean Laparoscopic Gastrointestinal Surgery Study (KLASS) Group . Prospective randomized controlled trial (phase III) to comparing laparoscopic distal gastrectomy with open distal gastrectomy for gastric adenocarcinoma (KLASS 01). J Korean Surg Soc. 2013;84(2):123-130. doi: 10.4174/jkss.2013.84.2.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim HH, Hyung WJ, Cho GS, et al. . Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report: a phase III multicenter, prospective, randomized trial (KLASS trial). Ann Surg. 2010;251(3):417-420. doi: 10.1097/SLA.0b013e3181cc8f6b [DOI] [PubMed] [Google Scholar]
- 13.Kim W, Kim HH, Han SU, et al. ; Korean Laparo-endoscopic Gastrointestinal Surgery Study (KLASS) Group . Decreased morbidity of laparoscopic distal gastrectomy compared with open distal gastrectomy for stage I gastric cancer: short-term outcomes from a multicenter randomized controlled trial (KLASS-01). Ann Surg. 2016;263(1):28-35. doi: 10.1097/SLA.0000000000001346 [DOI] [PubMed] [Google Scholar]
- 14.Japanese Gastric Cancer Association Japanese gastric cancer treatment guidelines 2010 (version 3). Gastric Cancer. 2011;14(2):113-123. doi: 10.1007/s10120-011-0042-4 [DOI] [PubMed] [Google Scholar]
- 15.Bando E, Kojima N, Kawamura T, Takahashi S, Fukushima N, Yonemura Y. Prognostic value of age and sex in early gastric cancer. Br J Surg. 2004;91(9):1197-1201. doi: 10.1002/bjs.4541 [DOI] [PubMed] [Google Scholar]
- 16.Karpeh MS, Leon L, Klimstra D, Brennan MF. Lymph node staging in gastric cancer: is location more important than number? an analysis of 1,038 patients. Ann Surg. 2000;232(3):362-371. doi: 10.1097/00000658-200009000-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nashimoto A, Akazawa K, Isobe Y, et al. . Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry. Gastric Cancer. 2013;16(1):1-27. doi: 10.1007/s10120-012-0163-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Memon MA, Khan S, Yunus RM, Barr R, Memon B. Meta-analysis of laparoscopic and open distal gastrectomy for gastric carcinoma. Surg Endosc. 2008;22(8):1781-1789. doi: 10.1007/s00464-008-9925-9 [DOI] [PubMed] [Google Scholar]
- 19.Kim HH, Han SU, Kim MC, et al. . Long-term results of laparoscopic gastrectomy for gastric cancer: a large-scale case-control and case-matched Korean multicenter study. J Clin Oncol. 2014;32(7):627-633. doi: 10.1200/JCO.2013.48.8551 [DOI] [PubMed] [Google Scholar]
- 20.Lee JH, Nam BH, Ryu KW, et al. . Comparison of outcomes after laparoscopy-assisted and open total gastrectomy for early gastric cancer. Br J Surg. 2015;102(12):1500-1505. doi: 10.1002/bjs.9902 [DOI] [PubMed] [Google Scholar]
- 21.Hiki N, Katai H, Mizusawa J, et al. ; Stomach Cancer Study Group of Japan Clinical Oncology Group . Long-term outcomes of laparoscopy-assisted distal gastrectomy with suprapancreatic nodal dissection for clinical stage I gastric cancer: a multicenter phase II trial (JCOG0703). Gastric Cancer. 2018;21(1):155-161. doi: 10.1007/s10120-016-0687-0 [DOI] [PubMed] [Google Scholar]
- 22.Hur H, Lee HY, Lee HJ, et al. . Efficacy of laparoscopic subtotal gastrectomy with D2 lymphadenectomy for locally advanced gastric cancer: the protocol of the KLASS-02 multicenter randomized controlled clinical trial. BMC Cancer. 2015;15:355. doi: 10.1186/s12885-015-1365-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim HI, Hur H, Kim YN, et al. . Standardization of D2 lymphadenectomy and surgical quality control (KLASS-02-QC): a prospective, observational, multicenter study [NCT01283893]. BMC Cancer. 2014;14:209. doi: 10.1186/1471-2407-14-209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim MG, Kim KC, Yook JH, Kim BS, Kim TH, Kim BS. A practical way to overcome the learning period of laparoscopic gastrectomy for gastric cancer. Surg Endosc. 2011;25(12):3838-3844. doi: 10.1007/s00464-011-1801-3 [DOI] [PubMed] [Google Scholar]
- 25.Yang SJ, Ahn EJ, Park SH, Kim JH, Park JM. The early experience of laparoscopy-assisted gastrectomy for gastric cancer at a low-volume center. J Gastric Cancer. 2010;10(4):241-246. doi: 10.5230/jgc.2010.10.4.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brenkman HJF, Ruurda JP, Verhoeven RHA, van Hillegersberg R. Safety and feasibility of minimally invasive gastrectomy during the early introduction in the Netherlands: short-term oncological outcomes comparable to open gastrectomy. Gastric Cancer. 2017;20(5):853-860. doi: 10.1007/s10120-017-0695-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith JK, McPhee JT, Hill JS, et al. . National outcomes after gastric resection for neoplasm. Arch Surg. 2007;142(4):387-393. doi: 10.1001/archsurg.142.4.387 [DOI] [PubMed] [Google Scholar]
- 28.Jensen LS, Nielsen H, Mortensen PB, Pilegaard HK, Johnsen SP. Enforcing centralization for gastric cancer in Denmark. Eur J Surg Oncol. 2010;36(suppl 1):S50-S54. doi: 10.1016/j.ejso.2010.06.025 [DOI] [PubMed] [Google Scholar]
- 29.Chan DS, Reid TD, White C, et al. . Influence of a regional centralised upper gastrointestinal cancer service model on patient safety, quality of care and survival. Clin Oncol (R Coll Radiol). 2013;25(12):719-725. doi: 10.1016/j.clon.2013.08.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable. Comparison of causes of death and recurrence patterns for patients included in the per-protocol population
eFigure 1. Kaplan-Meier survival curves for patients included in the final analysis in the per-protocol population
eFigure 2. Post hoc subgroup analysis for overall survival
Data Sharing Statement