Abstract
Importance
There are limited data concerning the risk of metabolic and cardiovascular disorders among individuals with Tourette syndrome (TS) or chronic tic disorder (CTD).
Objective
To investigate the risk of metabolic and cardiovascular disorders among individuals with TS or CTD over a period of 40 years.
Design, Settings, and Participants
This longitudinal population-based cohort study included all individuals living in Sweden between January 1, 1973, and December 31, 2013. Families with clusters of full siblings discordant for TS or CTD were further identified. Data analyses were conducted from August 1, 2017, to October 11, 2018.
Exposures
Previously validated International Classification of Diseases diagnoses of TS or CTD in the Swedish National Patient Register.
Main Outcomes and Measures
Registered diagnoses of obesity, dyslipidemia, hypertension, type 2 diabetes, and cardiovascular diseases (including ischemic heart diseases, arrhythmia, cerebrovascular diseases and transient ischemic attack, and arteriosclerosis).
Results
Of the 14 045 026 individuals in the cohort, 7804 individuals (5964 males [76.4%]; median age at first diagnosis, 13.3 years [interquartile range, 9.9-21.3 years]) had a registered diagnosis of TS or CTD in specialist care. Of 2 675 482 families with at least 2 singleton full siblings, 5141 families included siblings who were discordant for these disorders. Individuals with TS or CTD had a higher risk of any metabolic or cardiovascular disorders compared with the general population (hazard ratio adjusted by sex and birth year [aHR], 1.99; 95% CI, 1.90-2.09) and sibling controls (aHR for any disorder, 1.37; 95% CI, 1.24-1.51). Specifically, individuals with TS or CTD had higher risks for obesity (aHR, 2.76; 95% CI, 2.47-3.09), type 2 diabetes (aHR, 1.67; 95% CI, 1.42-1.96), and circulatory system diseases (aHR, 1.76; 95% CI, 1.67-1.86). The risk of any cardiometabolic disorder was significantly greater in males than in females (aHR, 2.13; 95% CI, 2.01-2.26 vs aHR, 1.79; 95% CI, 1.64-1.96), as was the risk of obesity (aHR, 3.24; 95% CI, 2.83-3.70 vs aHR, 1.97; 95% CI, 1.59-2.44). The risks were already evident from childhood (the groups were significantly different by age 8 years) and were significantly reduced with the exclusion of individuals with comorbid attention-deficit/hyperactivity disorder (aHR, 1.52; 95% CI, 1.42-1.62), while excluding other comorbidities did not significantly affect the results. Compared with patients with TS or CTD who were not taking antipsychotics, patients with a longer duration of antipsychotic treatment (>1 year) had significantly lower risks of metabolic and cardiovascular disorders.
Conclusions and Relevance
The findings of this study suggest that TS and CTD are associated with a substantial risk of metabolic and cardiovascular disorders. The results highlight the importance of carefully monitoring cardiometabolic health in patients with TS or CTD across the lifespan, particularly in those with comorbid attention-deficit/hyperactivity disorder.
This longitudinal population-based cohort study investigates the risk of metabolic and cardiovascular disorders among individuals with Tourette syndrome or chronic tic disorder over a period of 40 years.
Key Points
Question
Do patients with Tourette syndrome or chronic tic disorder have an increased risk of metabolic and cardiovascular disorders?
Findings
This longitudinal population-based cohort study included 14 045 026 individuals living in Sweden between 1973 and 2013, of whom 7804 individuals received a diagnosis of Tourette syndrome or chronic tic disorder. Individuals with Tourette syndrome or chronic tic disorder had an increased risk of metabolic and cardiovascular disorders compared with the general population and sibling controls; exclusion of individuals with comorbid attention-deficit/hyperactivity disorder attenuated but did not eliminate the risks.
Meaning
Tourette syndrome and chronic tic disorder are associated with a substantial risk of cardiometabolic disorders; these risks should be carefully monitored in these patients, particularly in those with comorbid attention-deficit/hyperactivity disorder.
Introduction
Tourette syndrome (TS) and chronic tic disorder (CTD) are neurodevelopmental movement disorders characterized by multiple motor and phonic tics in TS and multiple motor or phonic tics in CTD, lasting more than 1 year in both disorders.1 Combined, TS and CTD affect approximately 1% of the population,2 and they are more common in males.3 Tic disorders are associated with multiple adversities, including an increased risk of premature death.4 However, very little is known about the factors that contribute to these adverse outcomes.
Previous research has shown that individuals with neuropsychiatric disorders are more prone to develop health complications,5,6,7,8,9,10,11,12 which in turn are known to increase the risk of mortality.13,14,15 Among these complications, metabolic syndrome, a group of risk factors that generally includes abdominal obesity, increased triglyceride concentrations, reduced high-density lipoprotein cholesterol concentrations, hypertension, and hyperglycemia,16 together with cardiovascular problems, have received the most attention.
In TS and CTD, cardiometabolic disorders have been explored mainly as potential adverse effects of pharmacologic treatment, particularly antipsychotics.17,18,19,20 Although these studies were valuable, they were small, had short follow-up periods, and lacked control groups of unexposed individuals, thus limiting the generalizability of the results. In addition, previous studies could not examine the role of other potential variables, such as psychiatric comorbidity or familial factors, that may influence both the disorder and the outcomes, such as shared environment or genetic factors.21
In this longitudinal population-based cohort study, we aimed to study the risk of cardiometabolic disorders in almost 8000 individuals with TS or CTD identified in the Swedish national registers over 4 decades. We also aimed to explore whether common psychiatric comorbidities contribute to the risk of cardiometabolic disorders. To rule out alternative explanations of the association between TS and CTD and metabolic and cardiovascular disorders (eg, genetic or shared environmental factors), we used a full sibling comparison design that controlled for possible shared familial confounders. In additional exploratory analyses, we examined whether pharmacologic treatment with antipsychotics was associated with the risk of cardiometabolic disorders in patients who received a diagnosis of TS or CTD.
Methods
This study largely follows the methods and reports on the same outcomes examined in a previous study in which the exposure was obsessive-compulsive disorder.12 The study was approved by the Regional Ethical Review Board in Stockholm (reference number 2013/862-31/5). The requirement for informed consent was waived because the study is register based and the individuals are not identifiable at any time.
Data Sources
Data were obtained by linking several Swedish nationwide administrative registers through the unique personal national identification numbers assigned to Swedish citizens.22 Registers included (1) the Swedish Total Population Register, containing data on all Swedish inhabitants since 1968, from which demographic data were obtained; (2) the Migration Register, which contains a record on all migration flows in and out of Sweden; (3) the Multi-Generation Register, which connects every person born in Sweden since 1933 and ever registered as living in the country after 1960 with their parents, allowing for the identification of siblings and other relatives23; (4) the Cause of Death Register, which contains information on all deaths since 1952; (5) the National Patient Register (NPR), which includes data on all diagnoses given in both inpatient (since 1969, with good coverage for psychiatric disorders since 1973)24 and outpatient specialist services (since 2001) based on the International Classification of Diseases (ICD) in its eighth (ICD-8; 1969-1986), ninth (ICD-9; 1987-1996), and tenth (ICD-10; 1997-2013) revisions; and (6) the Prescribed Drug Register, which includes, since July 1, 2005, a record of all dispensed medications in Sweden, registered using the codes from the Anatomical Therapeutic Chemical Classification System.25
Study Cohort and Exposure Variables
The study cohort included all individuals living in Sweden at any given time between January 1, 1973, and December 31, 2013. Individuals were followed up from birth or 1973, whichever came last, until the date of the outcomes of interest (ie, metabolic or cardiovascular disorders), emigration, death, or December 31, 2013 (end of the study period), whichever came first.
Individuals with a lifetime diagnosis of TS or CTD between January 1, 1973, and December 31, 2013, were identified from the NPR (ICD-8 code 306.2, ICD-9 code 307C, and ICD-10 codes F95.0 [transient tic disorder], F95.1 [chronic motor or vocal tic disorder], F95.2 [TS], F95.8 [other tic disorders], or F95.9 [unspecified tic disorder]). The Swedish TS and CTD codes have been previously validated.26 As in previous works,27,28,29,30 we used an algorithm to minimize the inclusion of individuals with only transient tics.
Lifetime comorbid psychiatric diagnoses were obtained from the NPR and included (1) organic disorders (organic brain disorder and epilepsy), (2) obsessive-compulsive disorder, (3) attention-deficit/hyperactivity disorder (ADHD), (4) conduct disorders, (5) pervasive developmental disorders, (6) psychotic and bipolar disorders, (7) anxiety disorders, and (8) depressive disorders. All disorders were defined as at least 1 registered diagnosis in the NPR during follow-up (eTable 1 in the Supplement).
Antipsychotic drugs (Anatomical Therapeutic Chemical Classification System code N05A) were identified from the Prescribed Drug Register. Individuals were classified as receiving antipsychotics if they had been dispensed between July 1, 2005, and December 31, 2013. For each individual with TS or CTD, follow-up time was divided into treatment periods (when the patients were taking antipsychotics) and nontreatment periods. The duration of an individual’s treatment period was estimated as the length of time between the first and the final dispensation. As the Swedish pharmaceutical benefits allow for a maximum 3-month medication supply per prescription, the duration of each treatment period was extended by 91 days to ensure capturing the full length of treatment.31 Treatment periods consisted of single dispensations or 2 or more consecutive dispensations within a 6-month period. If there were gaps extending beyond 6 months, the next dispensation was considered as the initiation of a new period of treatment. Thus, each person could have more than 1 individual period of treatment of different duration during the follow-up. For each individual, the cumulative number of days of all treatment periods was summed and categorized into no antipsychotic medication, antipsychotic medication up to 1 year, and antipsychotic medication for more than 1 year.
Metabolic and Cardiovascular Disorders
The diagnosis of metabolic syndrome as such generally requires laboratory tests and medical examinations16 that are not recorded in the population health registers. As a proxy and in accordance with previous work,12 we selected several medical conditions known to be associated with the diagnostic criteria of metabolic syndrome (eTable 2 in the Supplement). In addition, because type 2 diabetes and cardiovascular diseases are strongly associated with metabolic syndrome,16 these diagnoses were also included as outcome variables. The study outcomes were defined by means of 2 different methods. First, diagnoses of obesity, hypertension, type 2 diabetes, and cardiovascular diseases (which included ischemic heart diseases, arrhythmia, cerebrovascular diseases and transient ischemic attack, and arteriosclerosis) were retrieved from the NPR (ICD codes are listed in eTable 2 in the Supplement). Individuals with a diagnosis of type 2 diabetes who also had a diagnosis of type 1 diabetes (ICD-10 code E10) were excluded to avoid these cases being confounded with a disorder that, etiologically, would be distinct. Second, in an attempt to improve the coverage of the outcomes, individuals who were dispensed drugs generally prescribed to treat dyslipidemia, type 2 diabetes, hypertension, or cardiovascular disorders (eTable 2 in the Supplement shows the specific drugs and corresponding Anatomical Therapeutic Chemical Classification System codes) were also considered as having these outcomes. Because the drugs prescribed to treat hypertension and cardiovascular problems usually overlap (eg, diuretics are prescribed for both conditions), the 2 categories were merged into a broader category called circulatory system diseases, as classified in ICD-10. For individuals having both an ICD diagnosis and 1 of the relevant drug prescriptions, the date of the first event was used as the censoring date for the survival analysis.
Statistical Analysis
Analyses were performed from August 1, 2017, to October 11, 2018, using SAS, version 9.4 for Windows (SAS Institute Inc). We used Cox proportional hazards regression analyses to estimate hazard ratios (HRs) and corresponding 95% CIs for metabolic and cardiovascular disorders in exposed individuals (those with a lifetime diagnosis of TS or CTD) compared with unexposed individuals (those without a diagnosis of TS or CTD), taking into account the time at risk, with days since the start of follow-up as the underlying time scale. Analyses were adjusted for birth year (used as a continuous variable) and sex. The main analyses were also stratified by sex.
As a sensitivity analysis, the same models were repeated in the subgroup of individuals who entered the cohort at birth to ensure complete follow-up time, thus avoiding issues with left truncation (ie, exposure, outcome, or death occurring before the register started, which may lead to missing observations or individuals). In addition, the expected cumulative incidence of cardiometabolic disorders for exposed and unexposed individuals was calculated using Kaplan-Meier survival estimates in the subgroup of individuals with data available from birth.
In a further step, we implemented a fixed-effects model in the subsample of full siblings included in the cohort, also using stratified Cox proportional hazards regression models. This analysis compares all full siblings within a family with each other in a way that exposed siblings (those who have received a diagnosis of TS or CTD) have their unexposed full siblings as comparison. These models control for potential familial confounders that are shared by full siblings, including unmeasured shared environmental factors, such as parental socioeconomic status, and about 50% of the genetic factors.32 To determine how the presence of other psychiatric disorders was associated with the risk of cardiometabolic disorders in the cohort, the main analyses were repeated excluding different groups of comorbid psychiatric disorders, one at a time.
An exploratory analysis aimed to study the association of antipsychotic drugs with cardiometabolic disorders in individuals with TS or CTD. We excluded all individuals who had developed any cardiometabolic disorder before July 1, 2005 (start of the follow-up period in the Prescribed Drug Register). Then we performed Cox proportional hazards regression analyses (adjusted for birth year and sex) comparing individuals with a registered TS or CTD diagnosis who were not receiving antipsychotic drugs (reference group) with those receiving antipsychotic drugs as a cumulative time-varying exposure using the above-described duration categories.
Results
Descriptive Statistics
We identified 14 045 026 individuals who lived in Sweden during the period 1973-2013. The mean (SD) length of the follow-up was 22.2 (13.7) years (median, 22.8 years; range, 0-41 years). A total of 7804 individuals had a diagnosis of TS or CTD in the NPR (1840 females and 5964 males; median age at first diagnosis, 13.3 years [interquartile range, 9.9-21.3 years]), corresponding to an estimated cumulative incidence of 0.27% (95% CI, 0.26%-0.27%) by age 41 years.
Risk of Cardiometabolic Disorders
Individuals with TS or CTD had nearly double the risk of any cardiometabolic disorder compared with unaffected individuals from the general population (HR adjusted for sex and year of birth [aHR], 1.99; 95% CI, 1.90-2.09) (Table 1). Similarly, the risk for each specific disorder was also significantly higher in those with TS or CTD except for dyslipidemia (obesity: aHR, 2.76; 95% CI, 2.47-3.09; type 2 diabetes: aHR, 1.67; 95% CI, 1.42-1.96; circulatory system diseases: aHR, 1.76; 95% CI, 1.67-1.86; and dyslipidemia: aHR, 0.87; 95% CI, 0.75-1.00). The risk of any cardiometabolic disorder was significantly greater in males than in females (aHR, 2.13; 95% CI, 2.01-2.26 vs aHR, 1.79; 95% CI, 1.64-1.96), as was the risk of obesity (aHR, 3.24; 95% CI, 2.83-3.70 vs aHR, 1.97; 95% CI, 1.59-2.44) (Table 1).
Table 1. Risk of Metabolic and Cardiovascular Disorders Among Individuals With Tourette Syndrome or Chronic Tic Disorder, Compared With Unaffected Individuals From the General Population.
| Metabolic and Cardiovascular Disorders | Individuals, No. (%)a | HR (95% CI), Adjusted for Sex and Birth Yearb | |
|---|---|---|---|
| Tourette Syndrome or Chronic Tic Disorder Cohort (n = 7804) | Unaffected General Population (n = 14 037 222) | ||
| Any disorder, all | 1572 (20.1) | 4 933 345 (35.1) | 1.99 (1.90-2.09) |
| Male | 1067 (17.9) | 2 344 448 (33.2) | 2.13 (2.01-2.26) |
| Female | 505 (27.5) | 2 588 897 (37.2) | 1.79 (1.64-1.96) |
| Obesity, all | 301 (3.9) | 196 158 (1.4) | 2.76 (2.47-3.09) |
| Male | 217 (3.6) | 66 883 (1.0) | 3.24 (2.83-3.70) |
| Female | 84 (4.6) | 129 275 (1.9) | 1.97 (1.59-2.44) |
| Dyslipidemia, all | 180 (2.3) | 1 122 174 (8.0) | 0.87 (0.75-1.00) |
| Male | 111 (1.9) | 592 355 (8.4) | 0.79 (0.65-0.95) |
| Female | 69 (3.8) | 529 819 (7.6) | 1.04 (0.82-1.31) |
| Type 2 diabetes, all | 147 (1.9) | 426 448 (3.0) | 1.67 (1.42-1.96) |
| Male | 98 (1.6) | 228 344 (3.2) | 1.63 (1.34-1.99) |
| Female | 49 (2.7) | 198 104 (2.8) | 1.83 (1.38-2.42) |
| Circulatory system diseases, all | 1291 (16.5) | 4 790 365 (34.1) | 1.76 (1.67-1.86) |
| Male | 846 (14.2) | 2 288 283 (32.4) | 1.81 (1.69-1.94) |
| Female | 445 (24.2) | 2 502 082 (35.9) | 1.70 (1.55-1.87) |
Abbreviation: HR, hazard ratio.
Percentages for male and female individuals are based on the total numbers by sex in each group (Tourette syndrome or chronic tic disorder: 5964 male individuals and 1840 female individuals; unaffected general population: 7 070 359 male individuals and 6 966 863 female individuals).
Male and female HRs adjust only for birth year.
A total of 6245 individuals with TS or CTD and 4 244 718 individuals from the general population cohort had full data available from birth. When the analysis was limited to this cohort to ensure complete follow-up time, the risks for cardiometabolic disorders remained overall unchanged for any disorder (aHR, 2.06; 95% CI, 1.93-2.19) and for all the specific diagnoses (obesity: aHR, 2.88; 95% CI, 2.55-3.25; type 2 diabetes: aHR, 1.85; 95% CI, 1.50-2.29; and circulatory system diseases: aHR, 1.95; 95% CI, 1.81-2.10) except for dyslipidemia, which became statistically significant (aHR, 1.58; 95% CI, 1.06-2.36) (eTable 3 in the Supplement).
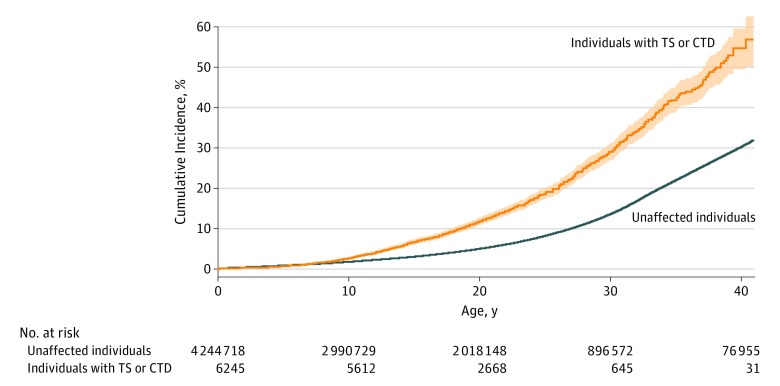
The cumulative incidences of any cardiometabolic disorder at the end of follow-up (under the assumption of no competing risks estimated as 1 – the Kaplan-Meier estimate of survival function, with the oldest individual being 41 years) were 52.5% (95% CI, 47.7%-57.4%) for those with TS or CTD and 29.5% (95% CI, 29.3%-29.6%) for the general population cohort (Figure). The differences between the 2 cohorts for any cardiometabolic disorder were already significant (nonoverlapping 95% CIs) from childhood (age, 8 years).
Figure. Cumulative Incidence of Any Cardiometabolic Disorder.
Cumulative incidence under the assumption of no competing risks estimated as 1 – the Kaplan-Meier estimate of survival function (with 95% CIs) for any metabolic or cardiovascular disorder among individuals with Tourette syndrome (TS) or chronic tic disorder (CTD) and unaffected individuals from the general population using the subgroup of individuals who were followed up from birth (N = 4 250 963).
Sibling Comparison
Of the 2 675 482 families who had at least 2 singleton children, 5141 families included clusters of full siblings who were discordant for TS or CTD. Those with TS or CTD had a higher risk of any cardiometabolic disorder compared with their unaffected full siblings without TS or CTD (aHR, 1.37; 95% CI, 1.24-1.51) (Table 2). The risks of obesity (aHR, 1.57; 95% CI, 1.25-1.98) and circulatory system diseases (aHR, 1.39; 95% CI, 1.25-1.56) were also significantly increased among individuals with TS or CTD compared with their full siblings (Table 2) but with lower risk estimates compared with the general population.
Table 2. Sibling Comparison for the Risk of Metabolic and Cardiovascular Disorders.
| Metabolic and Cardiovascular Disorders | No. (%) | HR (95% CI), Adjusted for Sex and Birth Year | |
|---|---|---|---|
| Individuals With TS or CTD With Unaffected Full Siblings (n = 5193) | Unaffected Full Siblings of Individuals With TS or CTD (n = 8116) | ||
| Any disorder | 974 (18.8) | 1266 (15.6) | 1.37 (1.24-1.51) |
| Obesity | 182 (3.5) | 219 (2.7) | 1.57 (1.25-1.98) |
| Dyslipidemia | 111 (2.1) | 191 (2.4) | 1.27 (0.97-1.66) |
| Type 2 diabetes | 84 (1.6) | 120 (1.5) | 1.17 (0.86-1.60) |
| Circulatory system diseases | 802 (15.4) | 1040 (12.8) | 1.39 (1.25-1.56) |
Abbreviations: CTD, chronic tic disorder; HR, hazard ratio; TS, Tourette syndrome.
Effect of Comorbidities
Frequencies of comorbid conditions in the cohort with TS or CTD and in the general population are displayed in eTable 4 in the Supplement. When different groups of psychiatric comorbidities were excluded from the analyses, the risk estimates for metabolic and cardiovascular disorders remained largely similar. The only exception was ADHD; the risks were reduced, but remained substantial, when individuals with this disorder were excluded (aHR, 1.52; 95% CI, 1.42-1.62) (Table 3).
Table 3. Risk of Metabolic and Cardiovascular Disorders Among Individuals With Tourette Syndrome or Chronic Tic Disorder, Compared With Unaffected Individuals From the General Population.
| Metabolic and Cardiovascular Disorders | HR (95% CI), Adjusted for Sex and Birth Year | |||||||
|---|---|---|---|---|---|---|---|---|
| Excluding Organic Disordersa | Excluding OCD | Excluding ADHD | Excluding Conduct Disorders | Excluding Pervasive Developmental Disorders | Excluding Psychotic and Bipolar Disorders | Excluding Anxiety Disorders | Excluding Depressive Disorders | |
| Any disorder | 2.01 (1.90-2.11) | 1.94 (1.84-2.05) | 1.52 (1.42-1.62) | 1.92 (1.82-2.02) | 1.83 (1.73-1.94) | 2.03 (1.92-2.15) | 1.95 (1.83-2.08) | 2.05 (1.93-2.18) |
| Obesity | 2.86 (2.55-3.22) | 2.82 (2.49-3.20) | 2.09 (1.76-2.50) | 2.69 (2.39-3.03) | 2.39 (2.08-2.76) | 2.78 (2.46-3.14) | 2.97 (2.59-3.40) | 3.00 (2.64-3.42) |
| Dyslipidemia | 0.88 (0.75-1.03) | 0.88 (0.75-1.03) | 0.92 (0.79-1.09) | 0.87 (0.75-1.01) | 0.92 (0.79-1.07) | 0.86 (0.73-1.02) | 0.94 (0.79-1.12) | 0.88 (0.73-1.05) |
| Type 2 diabetes | 1.77 (1.50-2.09) | 1.58 (1.31-1.90) | 1.39 (1.13-1.71) | 1.59 (1.34-1.88) | 1.52 (1.26-1.83) | 1.54 (1.27-1.87) | 1.72 (1.40-2.10) | 1.75 (1.44-2.13) |
| Circulatory system diseases | 1.76 (1.67-1.87) | 1.71 (1.61-1.82) | 1.42 (1.32-1.52) | 1.71 (1.62-1.81) | 1.67 (1.57-1.77) | 1.77 (1.66-1.88) | 1.67 (1.56-1.79) | 1.77 (1.65-1.89) |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; HR, hazard ratio; OCD, obsessive-compulsive disorder.
Organic brain disorder or epilepsy.
Effect of Antipsychotic Use
A total of 6324 individuals in the cohort with TS or CTD with data in the Prescribed Drug Register were used in these exploratory analyses. Use of antipsychotic medication for up to 1 year was not associated with a significantly increased risk of metabolic and cardiovascular disorders (aHR, 0.83; 95% CI, 0.56-1.24). However, use of antipsychotic medication for more than 1 year was associated with a significantly decreased risk of these disorders (aHR, 0.27; 95% CI, 0.17-0.43).
Discussion
To our knowledge, this is the first study to report on the risk of cardiometabolic disorders among individuals with TS or CTD at the population level. The results expand our understanding of the broader health consequences of TS and CTD and have direct clinical implications across health services, including neurology, pediatrics, psychiatry, and primary care.
The main finding was that individuals who received a diagnosis of TS or CTD were nearly twice as likely to develop at least 1 cardiometabolic disorder, compared with individuals from the general population without TS or CTD. In particular, the risks of obesity, circulatory system diseases, and type 2 diabetes were significantly elevated. The risks of any cardiometabolic disorder and obesity were significantly greater in males than in females. By the end of the follow-up (age, 41 years), 29.5% of the general population cohort were expected to have developed at least 1 cardiometabolic disorder, well in line with the literature.33,34 The corresponding figure in those with TS or CTD was 52.5%. The risk for any disorder was already significantly higher from childhood.
Our risk estimates remained unchanged overall in sensitivity and subgroup analyses. Exclusion of different groups of comorbidities did not significantly affect the estimates. An exception to this finding was the exclusion of individuals with ADHD from the cohort, which resulted in attenuated risk estimates, although individuals with TS or CTD without comorbid ADHD still had a 52% higher risk of developing cardiometabolic problems. This finding may indicate that a diagnosis of ADHD significantly adds to the observed risk, in line with previous research showing adverse health risk outcomes in ADHD.8 However, ADHD was the most prevalent comorbid condition within those with TS or CTD, with nearly half of the individuals with TS or CTD also receiving a diagnosis of ADHD; therefore, exclusion of such individuals results in a less representative cohort of individuals with TS or CTD.
The risk estimates were reduced, but not eliminated, in the sibling comparison analysis (from 1.99 to 1.37 for any cardiometabolic disorder). This finding is unlike that for obsessive-compulsive disorder, a closely related condition, in which the risk of metabolic and cardiovascular disorders remains intact in similar sibling analyses.12 There are several possible explanations for these findings. One possibility is that there are genetic and/or environmental risk factors that may partially influence both the disorder and the cardiovascular outcomes (eg, pleiotropic genetic effects). Another possible interpretation is that, while siblings of affected individuals did not have a diagnosis of TS or CTD, they may have had undiagnosed tic disorders or other neuropsychiatric disorders that are, in turn, known to be associated with cardiometabolic disorders.8,12 Nonetheless, the risk was still 37% higher for individuals with TS or CTD even after controlling for familial factors, which suggests that at least part of the observed health complications might be attributable to the tic disorders themselves. Individuals with TS or CTD experience a substantial number of stressors in their daily lives,35 have poorer educational outcomes,29,36 and, like individuals with other neuropsychiatric disorders, might have unhealthier lifestyles (eg, lack of physical activity and poor diet),37 which have also been shown to be associated with the development of metabolic and cardiovascular diseases.38,39,40
In exploratory analyses, patients taking long-term (>1 year) antipsychotics had a lower risk of metabolic and cardiovascular diseases, compared with those who were not using antipsychotic medications. A seemingly protective effect of medication for the risk of adverse outcomes, such as cardiometabolic disorders and mortality, has also been found in other neuropsychiatric disorders, such as obsessive-compulsive disorder12 and schizophrenia.41 However, previous clinical studies, although smaller and with shorter follow-ups, found an association between use of antipsychotics and increased risk of metabolic disorders.17,18,19,20 Because this is an observational study, we are careful not to ascribe the reduction of the risk to the medication itself. Patients taking medication may represent an inherently different group than those who are not taking medication. Furthermore, patients seen in specialist services are more likely to have frequent follow-ups and receive closer monitoring of their general health. Thus, the findings might mostly reflect the indirect effects of greater medical vigilance. Our results should not be taken as evidence that antipsychotics are free from cardiometabolic adverse effects, and they should continue to be used with caution in this patient group.
Limitations
This study has some limitations. Although the Swedish registers have national coverage, the study cohort does not represent the totality of all Swedish patients with TS or CTD because many individuals with mild tics do not seek help, the register had incomplete coverage until 2001 (prior to this date, only hospital admissions were registered), and patients diagnosed in primary care by general practitioners and other nonspecialists are not included. Thus, while the patients included in our cohort resemble other patients recruited from specialist tic disorder clinics around the world, it is possible that our results do not generalize to less complex patient samples or individuals with mild tics. Similarly, it is likely that coverage is incomplete for at least some of the outcomes that are more likely to be diagnosed in primary care settings than in specialist services, such as obesity. In addition, the Prescribed Drug Register started in 2005, resulting in a relatively short follow-up time in the medication analyses. Although many diagnostic codes in the NPR have been successfully validated,26,42,43,44,45 others (eg, conduct disorder and anxiety disorders) have yet to be validated. Despite having specific indications, some drugs used to identify specific outcomes may have been prescribed to treat more than 1 cardiovascular disorder, which means that the estimates for any cardiometabolic disorder may be more precise than those of individual disorders. Finally, the registers do not include information on other behavioral variables known to have an effect on the outcomes of interest (such as sedentary lifestyle, unhealthy eating habits, or smoking)16 or nonpharmacologic strategies to manage them (eg, behavioral interventions).
Conclusions
Tourette syndrome and CTD are associated with a substantial risk of cardiometabolic problems, even after taking into account a number of covariates and shared familial confounders and excluding relevant psychiatric comorbidities.
eTable 1. International Classification of Diseases (ICD) Eighth (ICD-8; 1969-1986), Ninth (ICD-9; 1987-1996), and Tenth (ICD-10; 1997-2013) Revisions Codes From the Swedish National Patient Register for all Comorbidities in the Study
eTable 2. Correspondence Between the Harmonized Definition of the Metabolic Syndrome and the Selected Metabolic and Cardiovascular Disorders and Drug Prescriptions for Such Disorders Used as Proxy in the Current Study
eTable 3. Sensitivity Analysis – Risk of Metabolic and Cardiovascular Disorders in Individuals with Tourette Syndrome or Chronic Tic Disorder, Compared With Unaffected Individuals From the General Population, in a Subcohort of Individuals Followed Up From Birth
eTable 4. Frequencies of Comorbidities in Individuals With Tourette Syndrome or Chronic Tic Disorder and in the Unaffected General Population Cohort
References
- 1.World Health Organization International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10). Geneva, Switzerland: World Health Organization; 1992. [Google Scholar]
- 2.Robertson MM. A personal 35 year perspective on Gilles de la Tourette syndrome: prevalence, phenomenology, comorbidities, and coexistent psychopathologies. Lancet Psychiatry. 2015;2(1):68-87. doi: 10.1016/S2215-0366(14)00132-1 [DOI] [PubMed] [Google Scholar]
- 3.Knight T, Steeves T, Day L, Lowerison M, Jette N, Pringsheim T. Prevalence of tic disorders: a systematic review and meta-analysis. Pediatr Neurol. 2012;47(2):77-90. doi: 10.1016/j.pediatrneurol.2012.05.002 [DOI] [PubMed] [Google Scholar]
- 4.Meier SM, Dalsgaard S, Mortensen PB, Leckman JF, Plessen KJ. Mortality risk in a nationwide cohort of individuals with tic disorders and with Tourette syndrome. Mov Disord. 2017;32(4):605-609. doi: 10.1002/mds.26939 [DOI] [PubMed] [Google Scholar]
- 5.Cabral LS, Cherubini PA, de Oliveira MA, Bianchini L, Torres CM, Bianchin MM. Diagnostic yield and accuracy of different metabolic syndrome criteria in adult patients with epilepsy. Front Neurol. 2017;8:460. doi: 10.3389/fneur.2017.00460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lantz M, Sieurin J, Sjölander A, Waldenlind E, Sjöstrand C, Wirdefeldt K. Migraine and risk of stroke: a national population-based twin study. Brain. 2017;140(10):2653-2662. doi: 10.1093/brain/awx223 [DOI] [PubMed] [Google Scholar]
- 7.Zheng Z, Zhang L, Li S, et al. . Association among obesity, overweight and autism spectrum disorder: a systematic review and meta-analysis. Sci Rep. 2017;7(1):11697. doi: 10.1038/s41598-017-12003-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spencer TJ, Faraone SV, Tarko L, McDermott K, Biederman J. Attention-deficit/hyperactivity disorder and adverse health outcomes in adults. J Nerv Ment Dis. 2014;202(10):725-731. doi: 10.1097/NMD.0000000000000191 [DOI] [PubMed] [Google Scholar]
- 9.Mitchell AJ, Vancampfort D, Sweers K, van Winkel R, Yu W, De Hert M. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders—a systematic review and meta-analysis. Schizophr Bull. 2013;39(2):306-318. doi: 10.1093/schbul/sbr148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Czepielewski L, Daruy Filho L, Brietzke E, Grassi-Oliveira R. Bipolar disorder and metabolic syndrome: a systematic review. Braz J Psychiatr. 2013;35(1):88-93. doi: 10.1016/j.rbp.2012.07.004 [DOI] [PubMed] [Google Scholar]
- 11.Vancampfort D, Correll CU, Wampers M, et al. . Metabolic syndrome and metabolic abnormalities in patients with major depressive disorder: a meta-analysis of prevalences and moderating variables. Psychol Med. 2014;44(10):2017-2028. doi: 10.1017/S0033291713002778 [DOI] [PubMed] [Google Scholar]
- 12.Isomura K, Brander G, Chang Z, et al. . Metabolic and cardiovascular complications in obsessive-compulsive disorder: a total population, sibling comparison study with long-term follow-up. Biol Psychiatry. 2018;84(5):324-331. doi: 10.1016/j.biopsych.2017.12.003 [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Ruotsalainen S, Moilanen L, Lepistö P, Laakso M, Kuusisto J. The metabolic syndrome predicts cardiovascular mortality: a 13-year follow-up study in elderly non-diabetic Finns. Eur Heart J. 2007;28(7):857-864. doi: 10.1093/eurheartj/ehl524 [DOI] [PubMed] [Google Scholar]
- 14.Li Z, Yang X, Yang J, et al. . The cohort study on prediction of incidence of all-cause mortality by metabolic syndrome. PLoS One. 2016;11(5):e0154990. doi: 10.1371/journal.pone.0154990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Correll CU, Solmi M, Veronese N, et al. . Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry. 2017;16(2):163-180. doi: 10.1002/wps.20420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alberti KG, Eckel RH, Grundy SM, et al. ; International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity . Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640-1645. doi: 10.1161/CIRCULATIONAHA.109.192644 [DOI] [PubMed] [Google Scholar]
- 17.Gulisano M, Calì PV, Cavanna AE, Eddy C, Rickards H, Rizzo R. Cardiovascular safety of aripiprazole and pimozide in young patients with Tourette syndrome. Neurol Sci. 2011;32(6):1213-1217. doi: 10.1007/s10072-011-0678-1 [DOI] [PubMed] [Google Scholar]
- 18.Rizzo R, Eddy CM, Calí P, Gulisano M, Cavanna AE. Metabolic effects of aripiprazole and pimozide in children with Tourette syndrome. Pediatr Neurol. 2012;47(6):419-422. doi: 10.1016/j.pediatrneurol.2012.08.015 [DOI] [PubMed] [Google Scholar]
- 19.Pringsheim T, Pearce M. Complications of antipsychotic therapy in children with Tourette syndrome. Pediatr Neurol. 2010;43(1):17-20. doi: 10.1016/j.pediatrneurol.2010.02.012 [DOI] [PubMed] [Google Scholar]
- 20.Pringsheim T, Ho J, Sarna JR, Hammer T, Patten S. Feasibility and relevance of antipsychotic safety monitoring in children with Tourette syndrome: a prospective longitudinal study. J Clin Psychopharmacol. 2017;37(5):498-504. doi: 10.1097/JCP.0000000000000760 [DOI] [PubMed] [Google Scholar]
- 21.Kjøllesdal MKR, Ariansen I, Mortensen LH, Næss Ø. The importance of early life family factors in the association between cardiovascular risk factors and early cardiovascular mortality. Open Heart. 2017;4(2):e000608. doi: 10.1136/openhrt-2017-000608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24(11):659-667. doi: 10.1007/s10654-009-9350-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ekbom A. The Swedish Multi-generation Register. Methods Mol Biol. 2011;675:215-220. doi: 10.1007/978-1-59745-423-0_10 [DOI] [PubMed] [Google Scholar]
- 24.Ludvigsson JF, Andersson E, Ekbom A, et al. . External review and validation of the Swedish National Inpatient Register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO Collaborating Centre for Drug Statistics Methodology ATC/DDD index 2018. https://www.whocc.no/atc_ddd_index/. Accessed June 9, 2018.
- 26.Rück C, Larsson KJ, Lind K, et al. . Validity and reliability of chronic tic disorder and obsessive-compulsive disorder diagnoses in the Swedish National Patient Register. BMJ Open. 2015;5(6):e007520. doi: 10.1136/bmjopen-2014-007520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mataix-Cols D, Isomura K, Pérez-Vigil A, et al. . Familial risks of Tourette syndrome and chronic tic disorders: a population-based cohort study. JAMA Psychiatry. 2015;72(8):787-793. doi: 10.1001/jamapsychiatry.2015.0627 [DOI] [PubMed] [Google Scholar]
- 28.Fernández de la Cruz L, Rydell M, Runeson B, et al. . Suicide in Tourette’s and chronic tic disorders. Biol Psychiatry. 2017;82(2):111-118. doi: 10.1016/j.biopsych.2016.08.023 [DOI] [PubMed] [Google Scholar]
- 29.Pérez-Vigil A, Fernández de la Cruz L, Brander G, et al. . Association of Tourette syndrome and chronic tic disorders with objective indicators of educational attainment: a population-based sibling comparison study. JAMA Neurol. 2018;75(9):1098-1105. doi: 10.1001/jamaneurol.2018.1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brander G, Rydell M, Kuja-Halkola R, et al. . Perinatal risk factors in Tourette’s and chronic tic disorders: a total population sibling comparison study. Mol Psychiatry. 2018;23(5):1189-1197. doi: 10.1038/mp.2017.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sveriges Riksdag [Swedish Parliament]. Förordning (2002:687) om läkemedelsförmåner m.m. [Ordinance (2002:687) on Pharmaceutical Benefits, etc]. https://www.riksdagen.se/sv/dokument-lagar/dokument/svensk-forfattningssamling/forordning-2002687-om-lakemedelsformaner-mm_sfs-2002-687. Accessed November 29, 2018.
- 32.Sjölander A, Lichtenstein P, Larsson H, Pawitan Y. Between-within models for survival analysis. Stat Med. 2013;32(18):3067-3076. doi: 10.1002/sim.5767 [DOI] [PubMed] [Google Scholar]
- 33.Scuteri A, Laurent S, Cucca F, et al. ; Metabolic Syndrome and Arteries Research (MARE) Consortium . Metabolic syndrome across Europe: different clusters of risk factors. Eur J Prev Cardiol. 2015;22(4):486-491. doi: 10.1177/2047487314525529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003-2012. JAMA. 2015;313(19):1973-1974. doi: 10.1001/jama.2015.4260 [DOI] [PubMed] [Google Scholar]
- 35.Eapen V, Cavanna AE, Robertson MM. Comorbidities, social impact, and quality of life in Tourette syndrome. Front Psychiatry. 2016;7:97. doi: 10.3389/fpsyt.2016.00097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang J, Hirsch L, Martino D, Jette N, Roberts J, Pringsheim T. The prevalence of diagnosed Tourette syndrome in Canada: a national population-based study. Mov Disord. 2016;31(11):1658-1663. doi: 10.1002/mds.26766 [DOI] [PubMed] [Google Scholar]
- 37.Henderson DC, Vincenzi B, Andrea NV, Ulloa M, Copeland PM. Pathophysiological mechanisms of increased cardiometabolic risk in people with schizophrenia and other severe mental illnesses. Lancet Psychiatry. 2015;2(5):452-464. doi: 10.1016/S2215-0366(15)00115-7 [DOI] [PubMed] [Google Scholar]
- 38.van Dijk G, Buwalda B. Neurobiology of the metabolic syndrome: an allostatic perspective. Eur J Pharmacol. 2008;585(1):137-146. doi: 10.1016/j.ejphar.2007.11.079 [DOI] [PubMed] [Google Scholar]
- 39.Ross CE, Wu C-L. The links between education and health. Am Sociol Rev. 1995;60(5):719-745. doi: 10.2307/2096319 [DOI] [Google Scholar]
- 40.Agardh E, Allebeck P, Hallqvist J, Moradi T, Sidorchuk A. Type 2 diabetes incidence and socio-economic position: a systematic review and meta-analysis. Int J Epidemiol. 2011;40(3):804-818. doi: 10.1093/ije/dyr029 [DOI] [PubMed] [Google Scholar]
- 41.Tiihonen J, Mittendorfer-Rutz E, Torniainen M, Alexanderson K, Tanskanen A. Mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines in patients with schizophrenia: an observational follow-up study. Am J Psychiatry. 2016;173(6):600-606. doi: 10.1176/appi.ajp.2015.15050618 [DOI] [PubMed] [Google Scholar]
- 42.Dalman Ch, Broms J, Cullberg J, Allebeck P. Young cases of schizophrenia identified in a national inpatient register—are the diagnoses valid? Soc Psychiatry Psychiatr Epidemiol. 2002;37(11):527-531. doi: 10.1007/s00127-002-0582-3 [DOI] [PubMed] [Google Scholar]
- 43.Ekholm B, Ekholm A, Adolfsson R, et al. . Evaluation of diagnostic procedures in Swedish patients with schizophrenia and related psychoses. Nord J Psychiatry. 2005;59(6):457-464. doi: 10.1080/08039480500360906 [DOI] [PubMed] [Google Scholar]
- 44.Sellgren C, Landén M, Lichtenstein P, Hultman CM, Långström N. Validity of bipolar disorder hospital discharge diagnoses: file review and multiple register linkage in Sweden. Acta Psychiatr Scand. 2011;124(6):447-453. doi: 10.1111/j.1600-0447.2011.01747.x [DOI] [PubMed] [Google Scholar]
- 45.Idring S, Rai D, Dal H, et al. . Autism spectrum disorders in the Stockholm Youth Cohort: design, prevalence and validity. PLoS One. 2012;7(7):e41280. doi: 10.1371/journal.pone.0041280 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. International Classification of Diseases (ICD) Eighth (ICD-8; 1969-1986), Ninth (ICD-9; 1987-1996), and Tenth (ICD-10; 1997-2013) Revisions Codes From the Swedish National Patient Register for all Comorbidities in the Study
eTable 2. Correspondence Between the Harmonized Definition of the Metabolic Syndrome and the Selected Metabolic and Cardiovascular Disorders and Drug Prescriptions for Such Disorders Used as Proxy in the Current Study
eTable 3. Sensitivity Analysis – Risk of Metabolic and Cardiovascular Disorders in Individuals with Tourette Syndrome or Chronic Tic Disorder, Compared With Unaffected Individuals From the General Population, in a Subcohort of Individuals Followed Up From Birth
eTable 4. Frequencies of Comorbidities in Individuals With Tourette Syndrome or Chronic Tic Disorder and in the Unaffected General Population Cohort