Key Points
Question
Is nivolumab plus ipilimumab a cost-effective first-line treatment of metastatic renal cell carcinoma from the US payer perspective?
Findings
In this cost-effectiveness analysis of data from the CheckMate 214 randomized clinical trial, the incremental quality-adjusted life-years (QALYs) gained in the base case by using nivolumab plus ipilimumab was 0.96 years compared with using sunitinib, at a cost of $108 363 per QALY.
Meaning
In this model, nivolumab plus ipilimumab was estimated to be cost-effective compared with sunitinib for intermediate- and poor-risk patients with mRCC at a willingness-to-pay threshold of $100 000 to $150 000 per QALY.
This cost-effectiveness analysis compares nivolumab plus ipilimumab vs sunitinib to examine whether the drug combination is cost-effective from a US payer perspective as a first-line treatment for intermediate- and poor-risk patients with metastatic renal cell carcinoma.
Abstract
Importance
Recently, new drugs have been approved for the first-line treatment of metastatic renal cell carcinoma (mRCC). Nivolumab plus ipilimumab significantly increases overall survival for intermediate- and poor-risk patients with mRCC. However, considering the high cost of nivolumab plus ipilimumab, there is a need to assess its value by considering both efficacy and cost.
Objective
To evaluate the cost-effectiveness of nivolumab plus ipilimumab vs sunitinib in the first-line setting for intermediate- and poor-risk patients with mRCC from the US payer perspective.
Design, Setting, and Participants
A Markov model was developed to compare the lifetime cost and effectiveness of nivolumab plus ipilimumab vs sunitinib in the first-line treatment of mRCC using outcomes data from the CheckMate 214 phase 3 randomized clinical trial, which included 1096 patients with mRCC (median age, 62 years) and compared nivolumab plus ipilimumab vs sunitinib as first-line treatment of mRCC. In the analysis, patients were modeled to receive sunitinib or nivolumab plus ipilimumab for 4 doses followed by nivolumab monotherapy.
Main Outcomes and Measures
Life-years, quality-adjusted life-years (QALYs), and lifetime costs were estimated, at a willingness-to-pay threshold of $100 000 to $150 000 per QALY. Univariable, 2-way, and probabilistic sensitivity analyses were performed to evaluate the model uncertainty. Additional subgroup analyses were performed.
Results
Nivolumab plus ipilimumab provided an additional 0.96 QALYs, at a cost of $108 363 per QALY. Sensitivity analyses found the results to be most sensitive to overall survival hazard ratio (0.63; 95% CI, 0.44-0.89) and mean patient weight (70 kg, range, 40-200 kg). Other variables, such as the cost of nivolumab plus ipilimumab (mean, $32 213.44; range, $25 770.75-$38 656.13), utility values for nivolumab plus ipilimumab (mean, 0.82; range, 0.65-0.98), and proportion receiving nivolumab in sunitinib arm (mean, 0.27; range, 0.22-0.32), had a moderate or minor influence on model results. Subgroup analyses demonstrated that nivolumab plus ipilimumab was most cost-effective for patients with programmed cell death 1 ligand 1 expression of at least 1% ($86 390 per QALY).
Conclusions and Relevance
In this model, nivolumab plus ipilimumab was estimated to be cost-effective compared with sunitinib for intermediate- and poor-risk patients with mRCC at a willingness-to-pay threshold from $100 000 to $150 000 per QALY.
Introduction
Renal cell carcinoma (RCC) is the most common type of kidney cancer, accounting for more than 330 000 cases diagnosed and 140 000 deaths worldwide each year.1 Up to 30% of patients have advanced disease at the time of diagnosis, with a 5-year survival rate of 11%.2
Recently, several new drugs have been approved for the treatment of metastatic RCC (mRCC). Approved treatments in the first-line setting include vascular epithelial growth factor inhibitors (axitinib, sunitinib, pazopanib, cabozantinib, bevacizumab) and mammalian target of rapamycin inhibitors (temsirolimus). Sunitinib is approved for this setting because of superior progression-free survival benefit over interferon alfa.3 Although sunitinib is the current standard of care for this setting, no overall survival (OS) benefit is observed.3,4
Nivolumab is a fully human immunoglobulin G4 monoclonal antibody that binds to the programmed death 1 receptor and restores T-cell immune activity. Ipilimumab is a fully human monoclonal antibody that binds to the inhibitory cytotoxic T-lymphocyte antigen 4. The CheckMate 214 randomized clinical trial5 compared nivolumab plus ipilimumab with sunitinib for previously untreated mRCC. The trial demonstrated a significant improvement in OS for International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) intermediate- and poor-risk patients receiving nivolumab plus ipilimumab when compared with sunitinib (hazard ratio [HR], 0.63; 95% CI, 0.44-0.89). Nivolumab plus ipilimumab was associated with a lower incidence of grade 3 or 4 adverse events than sunitinib (46% vs 63%). Nivolumab plus ipilimumab was subsequently approved for intermediate- or poor-risk mRCC by the US Food and Drug Administration (FDA) in April 2018 and has become a new first-line standard of care.4,5,6
The objective of this study was to evaluate the cost-effectiveness of nivolumab plus ipilimumab vs sunitinib in the first-line setting for intermediate- and poor-risk patients with mRCC from the US payer perspective.
Methods
A Markov model was developed to estimate the costs and effectiveness of treatment of mRCC (eFigure 1 in the Supplement). This model-based economic evaluation of exempt from institutional review board approval; patients were simulated using a mathematical model. Two first-line treatment options were evaluated in the model: (1) nivolumab plus ipilimumab for 4 doses followed by nivolumab monotherapy and (2) sunitinib. We assumed that the first-line treatments continued until disease progression or unacceptable toxic effects. Upon progression of the disease or unacceptable toxic effects, both groups could receive subsequent treatment until death. As observed in the CheckMate 214 trial,5 39% of patients (217 of 550) in the nivolumab plus ipilimumab arm and 54% (295 of 546) in the sunitinib arm received subsequent therapy; sunitinib, pazopanib and axitinib were the most common therapies (eTable 1 in the Supplement).
We considered only direct medical care costs. Each model cycle represents 6 weeks. The time horizon was lifetime. Half-cycle correction was applied in the model. We adopted a 3% discount rate per year for both costs and outcomes. The primary outputs of the models included the total cost, life-years, quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratios (ICERs). The Markov model was implemented using TreeAge Pro 2018 software (TreeAge, Williamstown, Massachusetts), and statistical analyses were performed using R software (version 3.3.1, http://www.r-project.org).
Model Survival and Progression Risk Estimates
The overall probability of death in any state was estimated as the probability of death from mRCC and background mortality rate from other causes. The probability of death from mRCC for each treatment strategy was estimated based on the OS curves of the CheckMate 214 trial.5 GetData Graph Digitizer software package (version 2.25; http://www.getdata-graph-digitizer.com/index.php) was used to extract the OS probabilities from the published OS curve in the CheckMate 214 trial. Pseudo-individual patient data were generated using the algorithm derived by Hoyle et al,7 which improves estimates accuracy of mean survival time.8 A Weibull distribution was fitted to the pseudo-individual patient data as it provided the best fit among the exponential, log-logistic, and log-normal distributions according to Akaike information criterion.
We estimated the progression risk using the same approach, with an exception that a log-logistic distribution was used to fit the pseudo-individual patient data, as it provided the best fit based on Akaike information criterion. The background mortality rate for each age group was estimated based on US life tables (eTable 2 in the Supplement).9
Utility Estimates
QALYs were calculated by combining survival time and health-related quality of life (QOL) that is often referred to as utility (a health status value from 0 for death to 1 for perfect health). We used previously published utilities of 0.7310 and 0.6611 for patients in the first-line sunitinib and all patients in the second-line therapy, respectively. In the CheckMate 214 trial,5 the Functional Assessment of Cancer Therapy–Kidney Symptom Index (FKSI-19) questionnaire was used to assess QOL. The trial results showed that the nivolumab plus ipilimumab group had significantly higher FKSI-19 scores than the sunitinib group. To account for the QOL improvement in the nivolumab plus ipilimumab group compared with the sunitinib group, the utility increment was estimated by dividing the FSKI-19 score by 76 (the maximum value of FKSI-19 score), similar to the analyses by Sarfaty et al.12,13 At baseline, the mean FKSI-19 QOL score was 60.1 in the nivolumab plus ipilimumab group (utility value, 0.79) and 59.1 in the sunitinib group (utility value, 0.77) and was assessed every 4 weeks. After 104 weeks of follow-up, the nivolumab plus ipilimumab group had a mean FKSI-19 QOL score of 64 (utility value, 0.84), and the sunitinib group had a mean score of 52 (utility value, 0.68). The average utility for the nivolumab plus ipilimumab group and sunitinib groups was 0.81 and 0.72, respectively, and the utility increment was 0.09. The utility increment (0.09) was added to the utility value for sunitinib (0.73) to estimate that for nivolumab plus ipilimumab (0.82).
Cost Estimates
Direct medical costs included drug, administration, and management of adverse effect costs. The mean US patient weight was 74.7 kg, and we used a body weight of 70.0 kg, accounting for weight loss effects of the metastatic disease.14 Grade 3 to 4 adverse effects were included in the model, which had significantly different rates between treatments in the CheckMate 214 trial5 and included fatigue, hypertension, thrombocytopenia, and palmar–plantar erythrodysesthesia.5
We used the 2018 average sale price from the Centers for Medicare & Medicaid Services to estimate the unit price of nivolumab and ipilimumab.15 The unit price of other drugs was derived from previously published studies.16,17 Adverse effect costs were derived from previously published studies.18,19,20
Following the approach used by Goldstein et al,21 administration costs were estimated according to the Medicare physician fee schedule for 2018.22 We adjusted costs for inflation to reflect 2018 US dollars using the US consumer price index. All information regarding the drug dose and the costs are listed in eTables 1 and 3 in the Supplement.
Sensitivity Analysis
A series of sensitivity analyses were performed to explore how results varied across plausible ranges. In univariable sensitivity analysis, all variables varied over a plausible range, as shown in eTable 1 in the Supplement, which were obtained from credible intervals or by assuming a variance of 20% from base-case values. We performed 10 000 Monte Carlo simulations to conduct probabilistic sensitivity analyses, with the variables simultaneously varied with a specific pattern of distribution (eTable 1 in the Supplement).
We performed a 2-way sensitivity analysis to test the interplay between the utility for nivolumab plus ipilimumab and for sunitinib. We also considered all the patient subgroups presented in the forest plot of the CheckMate 214 trial.5 For these subgroups, in the absence of sufficient data, we assumed the same data as for all subgroups in the trial except for the OS HRs. The subgroup-specific OS HRs are listed in eTable 4 in the Supplement.
Results
Base Case Results
The model projected that the life expectancy of patients receiving nivolumab plus ipilimumab was 3.99 life-years, which was 1.27 life-years more than patients receiving sunitinib (Table). Accounting for QOL, patients receiving nivolumab plus ipilimumab gained 2.84 QALYs; this value was 0.96 QALYs more than for patients receiving sunitinib. The use of nivolumab plus ipilimumab cost an additional $104 072, resulting in an ICER of $82 035 per life-year, or $108 363 per QALY compared with sunitinib.
Table. Base Case Results.
| Results | Nivolumab Plus Ipilimumab | Sunitinib | ICER |
|---|---|---|---|
| Life-years | 3.994 | 2.725 | 1.27 |
| QALYs | 2.842 | 1.882 | 0.96 |
| Total cost of regimen, $ | 350 646 | 246 573 | 104 072 |
| ICER, $ | |||
| Per life-year | 82 035 (38 665 to 234 641)a | ||
| Per QALY | 108 363 (47 452 to 286 275)a |
Abbreviations: ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year.
Values in the parentheses represent 95% credible intervals derived from the results of probabilistic sensitivity analyses.
Sensitivity Analysis
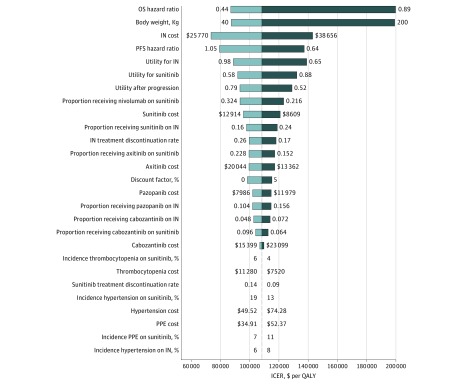
Figure 1 presents the results of univariable sensitivity analysis. The variables with greatest influence on the ICER was OS HR and patient weight. The ICER was higher than a willingness-to-pay threshold of $150 000 per QALY when the OS HR (0.63; 95% CI, 0.44-0.89) increased to the upper limit of the 95% CI (0.89) or the patient’s weight increased to 200 kg. Other variables, such as the drug cost, utility values, and proportions of receiving subsequent therapy, had a moderate or minor influence on the ICER (Figure 1).
Figure 1. The Results of Univariable Sensitivity Analysis.
This diagram shows incremental cost-effectiveness ratio (ICER) of nivolumab plus ipilimumab (IN) vs sunitinib for different model input parameters. The dotted line intersecting the light and dark blue bars represents the ICER of $108 363 per quality-adjusted life-year (QALY) from the base case results. OS indicates overall survival; PFS, progression-free survival; PPE, palmar-plantar erythrodysesthesia.
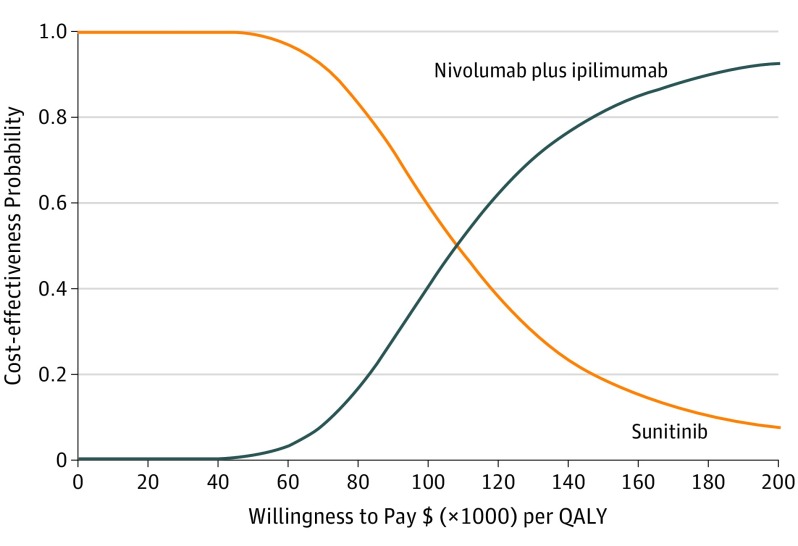
The results of probabilistic sensitivity analyses suggested that the probability of nivolumab plus ipilimumab being cost-effective compared with sunitinib is 42.5% and 80.2% at a willingness-to-pay threshold of $100 000 and $150 000 per QALY, respectively (Figure 2). The results of the 2-way sensitivity analyses demonstrated that the ICERs were lower than $150 000 per QALY across the majority of utility combinations (eFigure 2 in the Supplement).
Figure 2. The Cost-effectiveness Acceptability Curve.
Results of the probabilistic sensitivity analyses based on 10 000 Monte Carlo simulations, which involves sampling model variable values from distributions imposed on variables to indicate uncertainty about whether nivolumab plus ipilimumab are cost-effective at different willingness-to-pay thresholds. For example, nivolumab plus ipilimumab was found to be cost-effective in 80.2% at a willingness-to-pay threshold of $150 000 per QALY. QALY indicates quality-adjusted life-year.
The results of subgroup analyses showed that nivolumab plus ipilimumab was most cost-effective for patients with 1% or greater programmed cell death 1 ligand 1 (PD-L1) expression, followed by patients younger than 65 years, female patients, and patients free of lymph-node metastases (eTable 4 in the Supplement).
Discussion
To our knowledge, this study is the first cost-effectiveness analysis of nivolumab plus ipilimumab vs sunitinib for intermediate- and poor-risk patients with previously untreated mRCC. Based on our model, nivolumab plus ipilimumab cost $108 363 per additional QALY gained compared with sunitinib. The probabilistic sensitivity analyses suggested a high likelihood that nivolumab plus ipilimumab would be considered cost-effective at a willingness-to-pay threshold of $100 000 to $150 000 per QALY, as recommended by Neumann et al.23
One factor influencing our model the most was OS HR, justifying subgroup analyses to identify potential subsets where nivolumab plus ipilimumab is more cost-effective. The results of subgroup analyses demonstrated that the probability of nivolumab plus ipilimumab being cost-effective for patients with at least 1% PD-L1 expression was as high as 97% at a willingness-to-pay threshold of $150 000 per QALY, suggesting nivolumab plus ipilimumab was most cost-effective for this subset. Pending the development of a reliable PD-L1 assay, this might be one way to use nivolumab plus ipilimumab more cost effectively. However, from a more far-sighted perspective, the development of a predictive biomarker for both sunitinib and nivolumab plus ipilimumab would allow for the utilization of the least costly regimen for the best responders of both regimens. The results of univariable sensitivity analyses demonstrated that nivolumab plus ipilimumab might not be cost-effective in patients weighing more than 160 kg at a willingness-to-pay threshold of $150 000/ per QALY. However, this brings into question ethical issues of charging obese patients more money for the same life-sustaining treatments.
It should be noted that, from the patient perspective, the high price of anticancer drugs might place individual patients with cancer at risk for serious financial toxicity—the distress caused by the financial burden of out-of-pocket expenses incurred for medical care that is not covered by health insurance.24 It has been demonstrated that financial toxicity leads to delay, abandonment, and discontinuation of treatment among patients with cancer.24 For health care systems, ensuring that patients have access to innovative treatment is as important as minimizing financial toxicity.25
Although treatments other than sunitinib (eg, pazopanib, cabozantinib, bevacizumab, temsirolimus) are also used in mRCC, we did not evaluate these therapies because of the lack of robust head-to-head trial data. Furthermore, the FDA approved cabozantinib for previously untreated patients with mRCC based on the results of the phase 2 CABOSUN trial.26 However, the progression-free survival of patients receiving sunitinib in the CABOSUN trial was much lower than expected based on previous reports, which might exaggerate the efficacy of cabozantinib.27 This suggests that future phase III randomized trials are warranted to confirm the efficacy of cabozantinib.
Limitations
As with any model, there are limitations to our analysis. First, we acknowledge that the CheckMate 214 trial5 is the only randomized phase III trial that compared nivolumab plus ipilimumab with sunitinib in mRCC. It is a large and well-designed trial, but our model is essentially reliant on the validity and generalizability of the trial and any biases within the trial will be reflected in our study. Second, the results of subgroup analyses in the present study should be interpreted with caution because of the model assumption, the exploratory nature of the subgroup analyses, and the small sample size (eTable 4 in the Supplement). Third, the utility values in the first-line nivolumab plus ipilimumab were estimated by dividing the FSKI-19 value by the maximum value owing to the lack of data mapping FSKI-19 scores to utility values. This estimation is not ideal, but we performed a series of sensitivity analyses in which wide variability in utility values was included. Fourth, Medicare reimbursement was used to estimate the cost of nivolumab plus ipilimumab in the model. It is possible that the alternative public and commercial reimbursement in the United States may be lower and higher than Medicare reimbursement, respectively. The lack of publicly available sources on commercial drug costs presents barriers to the application of commercial reimbursement to cost-effectiveness analyses.21,28 However, the ranges of the drug cost used in the sensitivity analyses account for the variation in the payer’s reimbursement rates. Finally, long-term efficacy of nivolumab plus ipilimumab in the model was extrapolated from the clinical data from the CheckMate 214 trial,5 which is inevitably subject to uncertainty. We have performed a series of sensitivity analyses and subgroup analyses to evaluate the uncertainty, however, long-term benefit of nivolumab plus ipilimumab remains an open question. Where more mature data are available, the model could be validated against the long-term survival data.
Conclusions
From the perspective of US payers, nivolumab plus ipilimumab is estimated to be cost-effective vs sunitinib for intermediate- and poor-risk patients with previously untreated mRCC at a willingness-to-pay threshold of $100 000 to $150 000 per QALY.
eTable 1. Model parameters: baseline values, ranges, and distributions for sensitivity analysis
eTable 2. Background mortality rate
eTable 3. Drug dose and costs
eTable 4. Results for subgroup analyses
eFigure 1. Markov model simulating outcomes for the CheckMate 214 trial
eFigure 2. Results of two-way sensitivity analyses for utility values
References
- 1.Choueiri TK, Escudier B, Powles T, et al. ; METEOR Investigators . Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1814-1823. doi: 10.1056/NEJMoa1510016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher R, Gore M, Larkin J. Current and future systemic treatments for renal cell carcinoma. Semin Cancer Biol. 2013;23(1):38-45. doi: 10.1016/j.semcancer.2012.06.004 [DOI] [PubMed] [Google Scholar]
- 3.Motzer RJ, Hutson TE, Tomczak P, et al. . Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115-124. doi: 10.1056/NEJMoa065044 [DOI] [PubMed] [Google Scholar]
- 4.Powles T, Albiges L, Staehler M, et al. . Updated European Association of Urology Guidelines recommendations for the treatment of first-line metastatic clear cell renal cancer. Eur Urol. 2017;73(3):311-315. doi: 10.1016/j.eururo.2017.11.016 [DOI] [PubMed] [Google Scholar]
- 5.Motzer RJ, Tannir NM, McDermott DF, et al. ; CheckMate 214 Investigators . Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277-1290. doi: 10.1056/NEJMoa1712126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.FDA approves nivolumab plus ipilimumab combination for intermediate or poor-risk advanced renal cell carcinoma. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm604685.htm. Accessed 13 June, 2018.
- 7.Hoyle MW, Henley W. Improved curve fits to summary survival data: application to economic evaluation of health technologies. BMC Med Res Methodol. 2011;11:139. doi: 10.1186/1471-2288-11-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wan X, Peng L, Li Y. A review and comparison of methods for recreating individual patient data from published Kaplan-Meier survival curves for economic evaluations: a simulation study. PLoS One. 2015;10(3):e0121353. doi: 10.1371/journal.pone.0121353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arias E, Heron M, Xu J. United States Life Tables, 2014. Natl Vital Stat Rep. 2017;66(4):1-64. [PubMed] [Google Scholar]
- 10.Remák E, Charbonneau C, Négrier S, Kim ST, Motzer RJ. Economic evaluation of sunitinib malate for the first-line treatment of metastatic renal cell carcinoma. J Clin Oncol. 2008;26(24):3995-4000. doi: 10.1200/JCO.2007.13.2662 [DOI] [PubMed] [Google Scholar]
- 11.de Groot S, Redekop WK, Versteegh MM, et al. . Health-related quality of life and its determinants in patients with metastatic renal cell carcinoma. Qual Life Res. 2018;27(1):115-124. doi: 10.1007/s11136-017-1704-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarfaty M, Leshno M, Gordon N, et al. . Cost effectiveness of nivolumab in advanced renal cell carcinoma. Eur Urol. 2018;73(4):628-634. doi: 10.1016/j.eururo.2017.07.041 [DOI] [PubMed] [Google Scholar]
- 13.Sarfaty M, Hall PS, Chan KKW, et al. . Cost-effectiveness of pembrolizumab in second-line advanced bladder cancer. Eur Urol. 2018;74(1):57-62. doi: 10.1016/j.eururo.2018.03.006 [DOI] [PubMed] [Google Scholar]
- 14.Oh A, Tran DM, McDowell LC, et al. . Cost-effectiveness of nivolumab-ipilimumab combination therapy compared with monotherapy for first-line treatment of metastatic melanoma in the United States. J Manag Care Spec Pharm. 2017;23(6):653-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.2018 ASP Drug Pricing Files. 2018. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/2018ASPFiles.html. Accessed May 16, 2018.
- 16.Swallow E, Messali A, Ghate S, McDonald E, Duchesneau E, Perez JR. The additional costs per month of progression-free survival and overall survival: an economic model comparing everolimus with cabozantinib, nivolumab, and axitinib for second-line treatment of metastatic renal cell carcinoma. J Manag Care Spec Pharm. 2018;24(4):335-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delea TE, Amdahl J, Diaz J, Nakhaipour HR, Hackshaw MD. Cost-effectiveness of pazopanib versus sunitinib for renal cancer in the United States. J Manag Care Spec Pharm. 2015;21(1):46-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein DA, Ahmad BB, Chen Q, et al. . Cost-effectiveness analysis of regorafenib for metastatic colorectal cancer. J Clin Oncol. 2015;33(32):3727-3732. doi: 10.1200/JCO.2015.61.9569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arondekar B, Curkendall S, Monberg M, et al. . Economic burden associated with adverse events in patients with metastatic melanoma. J Manag Care Spec Pharm. 2015;21(2):158-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perrin A, Sherman S, Pal S, et al. . Lifetime cost of everolimus vs axitinib in patients with advanced renal cell carcinoma who failed prior sunitinib therapy in the US. J Med Econ. 2015;18(3):200-209. doi: 10.3111/13696998.2014.985789 [DOI] [PubMed] [Google Scholar]
- 21.Goldstein DA, Chen Q, Ayer T, et al. . First- and second-line bevacizumab in addition to chemotherapy for metastatic colorectal cancer: a United States-based cost-effectiveness analysis. J Clin Oncol. 2015;33(10):1112-1118. doi: 10.1200/JCO.2014.58.4904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Medicare & Medicaid Services 2018. Medicare physician fee schedule. https://www.cms.gov/apps/physician-fee-schedule/license-agreement.aspx.
- 23.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796-797. doi: 10.1056/NEJMp1405158 [DOI] [PubMed] [Google Scholar]
- 24.Carrera PM, Kantarjian HM, Blinder VS. The financial burden and distress of patients with cancer: Understanding and stepping-up action on the financial toxicity of cancer treatment. CA Cancer J Clin. 2018;68(2):153-165. doi: 10.3322/caac.21443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Souza JA, Conti RM. Mitigating financial toxicity among US patients with cancer. JAMA Oncol. 2017;3(6):765-766. doi: 10.1001/jamaoncol.2016.4850 [DOI] [PubMed] [Google Scholar]
- 26.Choueiri TK, Halabi S, Sanford BL, et al. . Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: The Alliance A031203 CABOSUN Trial. J Clin Oncol. 2017;35(6):591-597. doi: 10.1200/JCO.2016.70.7398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buti S, Bersanelli M. Is cabozantinib really better than sunitinib as first-line treatment of metastatic renal cell carcinoma? J Clin Oncol. 2017;35(16):1858-1859. doi: 10.1200/JCO.2016.71.6506 [DOI] [PubMed] [Google Scholar]
- 28.McRae J, Vogenberg FR, Beaty SW, Mearns E, Varga S, Pizzi L. A review of US drug costs relevant to Medicare, Medicaid, and commercial insurers post-Affordable Care Act enactment, 2010-2016. Pharmacoeconomics. 2017;35(2):215-223. doi: 10.1007/s40273-016-0458-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Model parameters: baseline values, ranges, and distributions for sensitivity analysis
eTable 2. Background mortality rate
eTable 3. Drug dose and costs
eTable 4. Results for subgroup analyses
eFigure 1. Markov model simulating outcomes for the CheckMate 214 trial
eFigure 2. Results of two-way sensitivity analyses for utility values