Abstract
Psychological stress reactivity is associated with atherogenesis in youth. The novel hypothesis is that stress promotes atherogenic behaviors including snacking on energy dense foods and reduced physical activity; and it increases adiposity. Stress also increases systolic blood pressure cardiovascular reactivity, which may also be atherogenic. Exercise dampens stress reactivity and may be one mechanism by which it protects against the development of cardiovascular diseases.
Keywords: Children, adolescents, psychological stress, intima media thickness, exercise, eating, coping
INTRODUCTION
A stressor is an aversive physical, mental, or emotional event that threatens the well-being of the organism. An individual’s response to a stressor is formulated in the prefrontal and limbic areas of the brain where ongoing events are given cognitive evaluation and emotions are formed. The brain interprets how threatening the event is and establishes the behavioral and physiological responses to the event (27). This information is sent to the brainstem and hypothalamus which relay information regarding behavioral, endocrine, and autonomic responses appropriate for the emotional state and the associated behaviors to lower parts of the nervous system and to peripheral target tissues (27).
Stress is the reaction to the stressor; it is the total response to the internal and external environmental demands or pressures that are perceived as threatening to the individual. The stress response often takes both physiological and behavioral forms. Physiological responses to stress include activation of the nervous system and two neuroendocrine axes, and altered immune system function. The most immediate stress responses are driven by the autonomic nervous system (ANS), which is subdivided into the sympathetic (SNS) and parasympathetic nervous systems (PNS). The ANS is activated by centers located in the hypothalamus, brains stem, and spinal cord. The cerebral (limbic) cortex also transmits signals to these lower centers. Activation of the ANS produces the ‘fight or flight’ response via control of most of the visceral functions of the body and powerful effects on cardiovascular functions such as heart rate, myocardial contractility, vasodilation, and blood pressure. The ANS can double the heart rate (HR) within 3 to 5 seconds and arterial pressure within 10 to 15 seconds. The ANS produces it parasympathetic and sympathetic effects on effector organs via the secretion of acetylcholine and norepinephrine or epinephrine, which bind to specific receptors on the effector cells. The sympathetic adrenomedullary (SAM) axis is formed by innervation of the adrenal medullae by sympathetic nerves, which stimulate epinephrine and norepinephrine secretion. Circulating catecholamines produce nearly identical effects as those caused by direct sympathetic stimulation, but last 5- to 10-fold longer. Together, the shorter-term physiological responses promoted by the SAM axis activate the fight- or flight-type response and promote active coping (4).
The hypothalamic-pituitary-adrenal (HPA) axis includes the secretagogues and secretory products of corticotrophin-releasing hormone, adrenocorticotrophic hormone and cortisol and drives longer-term stress responses (4). Cortisol is often considered the stress hormone due to its effects on physiology (e.g., gluconeogenesis, lipolysis, proteolytic effects, inflammatory responses) and behavior (e.g., arousal, vigilance and cognition) that promote coping and a return to homeostasis (7, 20, 24, 26). Behavioral coping associated with HPA axis activation includes eating and distracting sedentary behaviors.
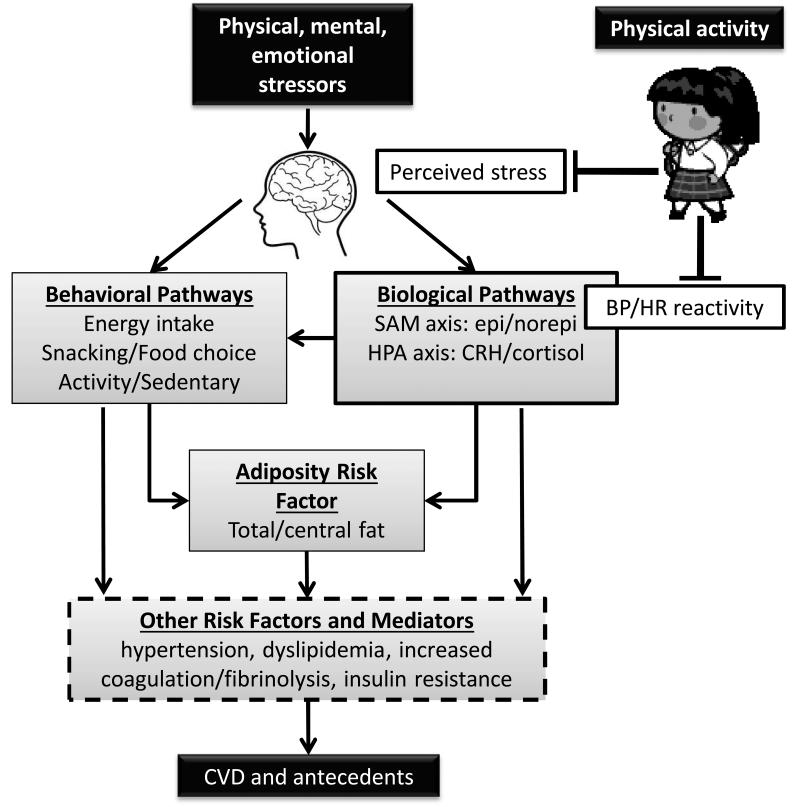
The purpose of this review is to : 1) highlight the great number of daily psychological stressors that can be experienced by children and adolescents; 2) describe the experimental and field trials demonstrating an association between cardiovascular reactivity to psychological stressors and atherogenesis in youth; 3) present new insights into the interacting behavioral and physiological pathways by which stress may increase risk of cardiovascular diseases (CVD) risk; 4) discuss how and why a short bout of vigorous or moderate physical activity may reduce stress reactivity and; 5) and develop a rationale for why a dampening psychological stress reactivity may be one way that exercise produces a cardio-protective effect. The central hypothesis of our work is that recurrent physiological responses and coping behaviors activated by stress reactivity increase risk factors for CVD in youth (Figure 1).
Figure 1.
Schematic of proposed behavioral and physiological pathways by which stress may promote CVD risk factors in youth. Environmental events that can act as physical, mental, or emotional stressors are interpreted and evaluated in the brain and assessed as to whether it is perceived as a stressor. Stressful events can initiate a host of behavioral, endocrine, and autonomic responses and coping mechanisms. Behavioral coping responses include greater energy intake through increased eating and a shift in food choice toward more energy dense comfort foods, especially in those children who restrain their dietary intake. Stress also increases youth's coping by engaging in distracting sedentary behaviors such as watching television and reduces willingness to engage in exercise. These stress coping behaviors increase the risk of excessive weight gain. Stress activates the sympatho-adrenal-meduallary (SAM) axis resulting in increased heart rate, myocardial contractility, vasodialtion/constriction and increased diastolic and systolic blood pressure. Stress also activates the hypothalamic-pituitary-adrenal (HPA) axis, which stimulates cortisol secretion, which shifts food choice towards comfort foods, promotes the deposition of body fat, especially abdominal fat. The effects of unhealthy stress coping behaviors and of stress-induced activation of the SAM and HPA axes on CVD risk factors are mediated through increases in adiposity and then through obesity-related comorbidities or mediated more directly through stress effects on blood pressure, blood lipid concentrations, coagulation/fibrinolysis states, etc. Physical activity, such as interval exercise that mimics children's natural pattern of play or a self-paced 1 mile walk that models a walk to school dampen perceived stress or the assessment of whether the stressor is as threatening, and blood pressure reactivity when children encounter a cognitive or interpersonal stressor. Dampening perceived stress reactivity and resultant blood pressure reactivity to a stressor may be one way that exercise produces a cardio-protective effect.
Childhood and Adolescence as Stressful Developmental Periods
Children and adolescents can experience many stressors each day including academic loads, expectations of academic excellence from parents and teachers, interpersonal conflicts including those with parents and friends, fitting-in, and teasing by peers. This stress is experienced in young people who do not yet have fully developed cognitive maturity, nor have gained the life experiences of adults, so that environmental events are more likely to be perceived as stressful and produce an intensified stress reaction. Adolescence, a period of especially heightened “storm and stress,” is considered a more difficult life period than others. During adolescence, most youth become very self-conscious and sensitive, this increases their stress reactivity. At the same time, they are pulling-away from parents as they endeavor towards greater independence and to establish their own identity. This reduces parents’ ability to provide support to help their adolescent reframe the thoughts or experiences that are causing them stress and to help their adolescent cope with stress in healthy ways (29).
PSYCHOSOCIAL STRESS AND ATHEROGENEISIS
Greater stress may have serious effects on children’s and adolescents’ physical and mental health, including psychological and emotional disturbances and the promotion of CVD. CVD is a leading cause of mortality and is a foremost health concern in developed nations. CVD develops slowly over decades, with the antecedents of atherosclerosis and CVD emerging during childhood and adolescence (39). There is much public health importance in understanding the early development of CVD risk factors, and there is growing evidence that stress is an important component of this process.
Traditional CVD risk factors such as family history, hypercholesterolemia, hypertension, smoking, and diabetes do not explain all future CVD, motivating the search for other risk factors. There is growing evidence that psychological stress (7), including stress experienced during childhood and adolescence (35), is an important risk factor for later CVD. Psychosocial stress imparts a meaningful relative risk for myocardial infarction and other types of CVD. Prolonged exposure to psychological stress is associated with elevated concentrations of intermediate markers of CVD pathophysiology such as blood inflammation markers (21) and development of overt atherosclerosis (13). Chronic stress is associated with a >2.1 fold increased risk for incident myocardial infarction and other CVDs (38). This is very similar to the classic risk factors investigated in the Framingham study, which found relative risks for developing CVD of >1.3 for hypercholesterolemia, >1.8 for diabetes, and >1.7 for hypertension and smoking (43). The INTERHEART study found that psychosocial stress accounted for 30% of the population attributable risk (PAR) of acute myocardial infarction (44). Only smoking (36% PAR) and lipid concentrations (49% PAR) accounted for a greater PAR, while hypertension (18%), diabetes (10%), abdominal obesity (20%) and physical activity (12%) accounted for lower PAR.
In the laboratory, stress is induced when participants engage in tasks that have a challenging or aversive quality (e.g., mild discomfort or ego threats). Common stressors include giving an interpersonal speech, mental arithmetic, mirror star tracing, reaction time tasks, and cold pressor tasks. Perceived stress reactivity and cardiovascular reactivity (e.g., heart rate (HR, blood pressure, total peripheral resistance) are measured as outcome responses from being exposed to a stressor. Reactivity is usually calculated as the stress-induced increase above the resting value. Perceived stress reactivity and cardiovascular reactivity are considered individual difference characteristics in that there can be large between-participant variability in reactivity to a particular stressor. Having participants complete a carefully selected set of varied stress tasks allows for the calculation of an aggregated stress reactivity score and provides a more reliable reflection of ‘trait’ cardiovascular reactivity (14).
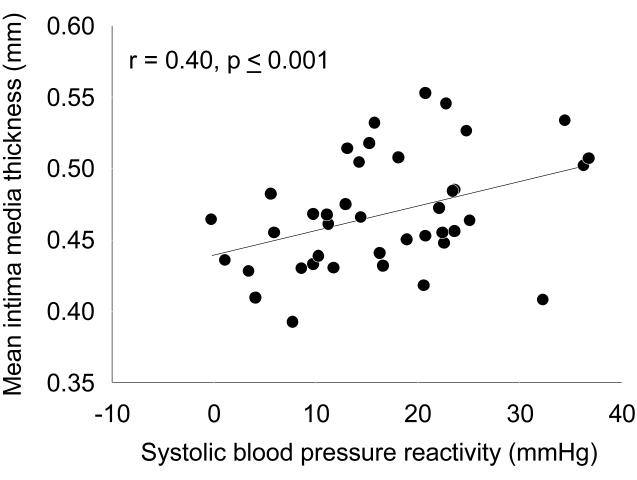
The reactivity hypothesis suggests that those individuals with the greatest cardiovascular reactivity to acute psychological stress are at the most risk for developing CVD. Cardiovascular reactivity to laboratory stress tasks in youth is associated with several CVD risk factors including essential hypertension (25), central adiposity (36), and greater left ventricular mass (1). Cardiovascular reactivity is also associated with subclinical atherosclerosis. Carotid artery intima-media thickness (IMT), a valid index of diffuse subclinical atherosclerosis has been shown in univariate fashion (Figure 2, ) to be positively correlated with systolic blood pressure (SBP) reactivity to an interpersonal speech stressor in children. Moreover, we demonstrated that SBP reactivity to a speech stressor increased (R2inc=0.10, p<0.05) the prediction of IMT after accounting for demographic variables and physical characteristics that could have explained the observed relationship (35). When we used a median split of the SBP reactivity to categorize children as high or low for SBP cardiovascular reactivity, the high stress reactive group (IMT = 0.48 ± 0.01 mm) had a 0.03 mm, or 6.2%, greater IMT than the low stress reactive group (IMT = 0.45 ± 0.01 mm). In comparison, youth with primary hypertension have a similar 0.04 mm (5.9%) greater IMT than normotensive control subjects (19). Moreover, 6 to 12 months of diet therapy or exercise training is necessary to reduce IMT by 0.02-0.04 mm (4.2% to 11.3%) (28). We extended this finding by demonstrating that a composite SBP reactivity score of reactivity from reaction-time, star-tracing, and speech tasks increased (R2inc=0.11, p<0.02,) the prediction of IMT (31). We recently found that SBP reactivity was also independently associated with IMT in an older sample of adolescents (17).
Figure 2.
Relationship between systolic blood pressure reactivity to an interpersonal speech stressor and mean common carotid intima media thickness in 8 to 12 year old boys and girls. (Reprinted from (35). Copyright © 2009 Wiley-Blackwell. Used with permission.)
While cardiovascular reactivity is associated with subclincal atherosclerosis in youth, it is only the initial part of the stress response. The greater the total stress load the more likely that stress will produce negative effects on cardiovascular health. Indeed, the diathesis-stress model suggests that individuals experiencing the greatest cumulative stress during daily life are most at risk for developing CVD. Thus, those youth who have the greatest cardiovascular reactivity to a stressor, who are reactive to a greater variety of different types of stressors, who have a greater duration of responding to each presentation of a stressor, those who habituate more slowly to a stressor (continue to respond after repeated presentations of the same stressor), and those that incur a lot of stressors during their daily lives may be the most at risk for CVD risk factors. The interactive relationships of individual differences in these aspects of the total stress load on CVD risk factors in children have not been widely studied.
MECHANISMS OF PSYCHOSOCIAL STRESS AND ATHEROGENEISIS
The mechanisms by which repeated stress reactivity contributes to an elevated risk for CVD are not yet completely understood. Psychological stress is formulated in the prefrontal and limbic areas of the brain where ongoing events are evaluated and emotions are formed, respectively. The brain interprets whether the events are threatening or nonthreatening and establishes the behavioral and physiological responses to the event. As shown in Figure 1, we propose that there are interacting behavioral and physiological pathways by which stress may increase CVD risk. We have been studying both pathways.
Behavioral Pathways between Stress and CVD Risk
Behaviorally, stress influences the cardiovascular health of youth by altering weight control behaviors (32, 37), which promotes greater total and central adiposity (9, 36). While most of the work regarding behavioral changes associated with stress-induced weight gain has been performed in adults, stress-induced alterations in weight control behaviors likely have their origins in childhood.
Stress could promote CVD risk by shifting food choices toward energy dense foods, resulting in greater energy intake, and/or by reducing the willingness to be physically active. Both types of coping behavior could promote excess weight gain. Of the two, stress-induced alterations in eating or snacking as coping behaviors have been more widely studied, mostly in adults. In a seminal review of the literature, Greeno and Wing (10) used the Individual Differences Model to understand how stress-induced eating responses can vary across individuals. They recognized that, in response to stress, not all obese adults increase their energy intake, and not all normal weight adults decrease their energy intake. Rather, other individual differences, such as the individual's gender, magnitude of stress reactivity, and level of dietary restraint, moderated the relationship between stress reactivity and amount of stress-induced eating. Dietary restraint occurs when an individual intentionally tries to control their body weight by restricting energy intake. Individuals high in dietary restraint are more aware of the amounts and types of foods that they consume and rely on cognitive control to eat less food than they really want. Psychological stressors, especially interpersonal stressors that require much cognitive appraisal and promote cognitive rumination, appear to be powerful disruptors of cognitive control of eating. Indeed, adults, especially women, who are reactive to the stressor and have a high level of dietary restraint increase their eating behavior when stressed (10).
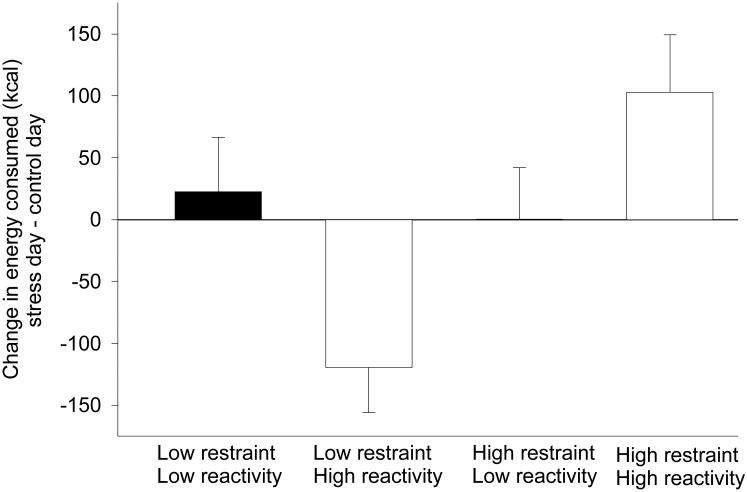
We have completed a series of controlled laboratory experiments studying the influence of interpersonal stress on weight control behaviors. In our initial investigation of stress reactivity, dietary restraint and eating behavior, we found that children responded similarly as adults (37). As shown in Figure 3, exposure to a psychological stressor increased children’s energy intake when they were provided a snack following the stressor. The increase in energy intake was greatest in children who were highly stress reactive and who had greater dietary restraint (37).
Figure 3.
Change (mean ± SE) in energy of snack foods consumed (stress day minus control day) of high and low dietary restraint and stress reactivity groups. A total of 40 children age 8 to 11 years were studied on a interpersonal speech stress day and reading control day. Subjects groups were assigned to low-restraint/low-reactive, low-restraint/high-reactive, high-restraint/low-reactive, and high-restraint/high-reactive groups based on median splits of dietary restraint and stress reactivity. The low-restrained/high-reactive group consumed fewer calories of snacks and high-restrained/high-reactive children consumed more calories of snacks in the stress compared with the control condition. Created from data presented in (37).
Two hypotheses have been proposed to explain stress-induced eating. Self-regulatory resources theory proposes that self-regulatory resources are finite (41). Maintaining dietary restraint requires great amounts of self-regulation because constant vigilance is necessary to keep from eating in our current obesigenic environment where large amounts of palatable food are readily accessible. The additional cognitive load of coping with a stressor overwhelms the self-regulatory resources and the child can no longer maintain their dietary restraint (41). A second hypothesis is that eating could be an effective means of distracting the attention from the stressor (12).
As shown in Figure 3, not all children were reactive to the interpersonal speech stressor used in this study. These children did not need to cope with an ongoing stressor and their eating did not change between the control and stress days (37). For other children who were reactive to the stress but low in dietary restraint, stress resulted in a reduction in energy intake (37). For these youth, eating may not be very engaging and therefore not distractive enough to shift attention away from the stress. These youth may have learned to use other types of coping behaviors to reduce their stress.
Indeed, the choice of coping behavior likely depends on access to the behavior and a number of individual difference factors. Youth spend a large portion of their time watching television and engaging in other sedentary behaviors. Watching television and listening to music are the two coping mechanisms adolescents most frequently list to deal with stress. It would not be surprising that children who regularly watch television turn to television as a distractor when stressed.
A limitation of research on stress and coping behaviors is that it has studied coping options in isolation. Recently, we tested the effects of stress on the coping preferences of children in a free choice setting by presenting children with equal access to eating a highly-palatable snack food, watching television, and exercise. Thus, we were able to assess which behavioral alternative youth would most frequently choose to cope with stress (2). We also assessed whether individual difference factors such as dietary restraint or usual engagement in sedentary or physically active behaviors moderated which behaviors children chose to cope with stress. These results more accurately modeled the choices youth are presented with in daily life where multiple coping behaviors are available after experiencing stress. As expected, children chose to spend more time watching television than eating or exercising after being stressed. We also found that children who watched more television in their daily lives were more likely to increase their television time after being stressed. As predicted from previous research, children high in dietary restraint increased their energy intake when stressed. This work also extended previous research by demonstrating that stress still increased energy intake in dietary-restrained individuals in an environment where there is easy access to other coping choices. In toto, these results are well aligned with the individual differences model (10) in that between-participant differences in behavioral or psychological factors moderated the effect of stress reactivity on choice of both sedentary and eating coping behaviors.
In addition to increasing energy intake, stress can also change food choices. In an independent sample of children, we replicated the above results to show that stress reactive dietary restrained youth consumed more energy after stress. Additionally, we demonstrated that stress reactive children with high dietary restraint shifted their food choices toward consumption of highly palatable energy dense foods (34). The choice of sweeter, higher-fat foods when stressed could be a function of the greater palatability of these foods and their ability to improve mood and reduce stress. Emotional state and food choice are linked as early as infancy. The ingestion of sweet or fatty foods calms infants and alleviates their crying and pain (40). Sucrose also produces an analgesic effect in prepubertal children (30).
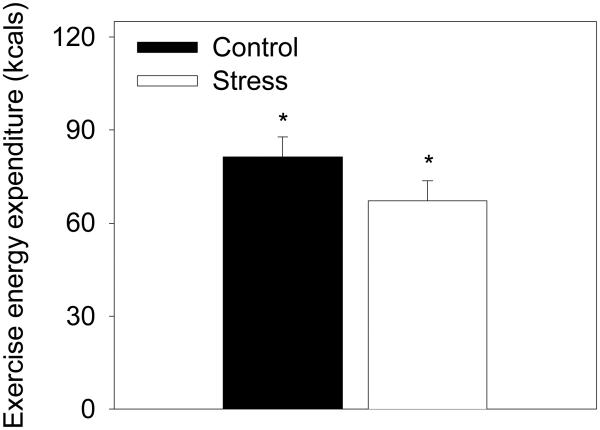
In another study (32), we used a behavioral choice paradigm where children were monetarily reinforced for exercising or being sedentary and found that children were less willing to exercise and more willing to watch television after being stressed. After giving a videotaped speech, children engaged in 20% less exercise when stressed compared to a control condition (Figure 4). Though exercise may be an effective and healthy stress coping mechanism, children are less willing to engage in exercise after being stressed.
Figure 4.
Effect of stress on amount of time children chose to be physically active rather than watch television. Data are mean ± SE from 25 children studied across an interpersonal speech stress day and reading control day. Children were reinforced with small amounts of money to engage in either behavior. Children were less willing to engage in physical activity when stressed compared to a control condition. A * indicates significant difference (p < 0.05) across conditions. (Reprinted from (32). Copyright © 2003 Nature Publishing Group. Used with permission.)
One important concern is that of coping with stress by eating energy dense foods, engaging in sedentary behaviors, and decreasing physical activity may promote positive energy balance and fat deposition. Indeed, we have found that children who are the most reactive to psychological stress have the greatest total and abdominal body fat (36) and that obese children are more likely to shift their food choice toward consumption of highly palatable energy dense foods (34). Learned coping behaviors may provide relief from stress, but can lead to greater total and central adiposity as early as childhood, which can contribute to hypertension, insulin resistance, and CVD risk later in life (Figure 1).
Biological Pathways between Stress and CVD Risk
In addition to psychological stress activating behavioral pathways, as shown in Figure 1, it also stimulates the afore-mentioned SNS, which may play a key role in a physiologic pathway of CVD risk. Stress reactivity begins in the brain, which interprets events as threatening. Perception of a threat results in increased SNS tone and reduced vagal tone. This increases HR, myocardial contractility, and vascular resistance, resulting in greater arterial pressure and turbulent blood flow that may produce mechanical injury to the endothelial lining. At the same time, psychological stress-induced SNS activation also promotes general platelet aggregation, endothelial dysfunction, release of proinflammatory cytokines and a procoagulant state. The endothelium serves the important role of preventing cellular adhesion and platelet activation and aggregation to the lumen wall. Local damage to the endothelium initiates an inflammatory response. Monocytes adhere to the lumen, migrate into the intimal layer, develop into foam cells, and secrete proinflammatory cytokines, which promotes further adhesion and macrophage formation (24). These sympathetically-driven promotions of a procoagulant state result in occlusive effects within the micro-circulation (42).
Another hypothesis proposed to explain the link between stress reactivity and CVD risk is that the cardiovascular responses during psychological stress are greater than what would be expected based on the lower metabolic demand (i.e., rate of oxygen consumption) (6). Indeed, studies of adults found that the HR and cardiac output responses to psychological stress are greater than expected based on the metabolic demand of the task (6). However, these studies did not report the associations between blood pressure reactivity to psychological stress and metabolic demand. This seemed important for our work given that SBP reactivity to psychological stress was a consistent predictor of atherogenesis and CVD risk in youth (17, 31, 35). We demonstrated for the first time that blood pressure responses during psychological stress were greater than expected based on the metabolic demand in adolescents (16). Notably, the excess SBP based on metabolic demand (β=0.30, p=0.02, R2 increase=0.08) was associated with greater IMT (17).
Regular exercise has numerous health benefits, including protection against CVD. However, exercise may also be a stressor. In fact, we have reported that greater exercise SBP reactivity was associated with greater IMT in adolescents (18), similar to the associations between SBP reactivity to psychological stress and IMT. The anticipation or ‘threat’ of exercise could also be a stressor and studies in adults have demonstrated increases in cardiovascular reactivity prior to physical exertion (14). Common with other types of laboratory stressors, greater pre-exercise anticipatory cardiovascular responses are associated with an increased risk for CVD (14).
Frequent stress reactivity may result in increased arterial stiffness and sustained increases in vascular resistance. These changes act to maintain arterial pressure at a high level, even in the absence of stress resulting in hypertension and the development of atherosclerosis and increasing the potential for life threatening cardiac events. Some stress-induced cardiovascular alterations appear to be more atherogenic than others. We have shown (Figure 2) that SBP reactivity to a speech task is associated with IMT in youth (17, 31, 35). Cross-sectional studies in adults have confirmed an association of SBP reactivity with IMT and atherosclerosis (13). Stress-induced SBP reactivity is also prospectively associated with the progression of IMT in adults (13). Stress-induced chronotropic effects on the heart do not appear to be atherogenic as HR reactivity is not associated with CVD risk cross-sectionally or prospectively in adults or children (17, 22, 31, 35). Given that the antecedents of atherosclerosis appear during childhood (39), it is important to understand the links between psychological stress-induced alterations in biological pathways that may promote the development of CVD risk factors early in life in order to prevent or delay the onset of CVD later in life.
EXERCISE DAMPENS STRESS REACTIVITY
If psychological stress is indeed atherogenic, and if the effects are mediated in part by SNS activation-driven cardiovascular reactivity, then reducing the SNS response to stress may be protective against CVD risk factors. Exercise may be one approach to reduce the magnitude of cardiovascular reactivity to psychological stress and thus protect against the detrimental effects of heightened stress responses on cardiovascular health. A number of studies in adults have reported reductions in blood pressure reactivity to psychological stress after an acute bout of aerobic exercise (11). To our knowledge, we are the only group to investigate whether acute exercise dampens increases in SNS activity in response to stress in children, as marked by a reduction in cardiovascular stress reactivity. We found that 20 min of interval exercise before giving a speech reduced (p<0.05) SBP, DBP and HR reactivity (33). The choice to use interval exercise in this study was based on our research that children find interval exercise more reinforcing and are more motivated to engage in interval exercise than constant load exercise (3). The interval exercise more closely matched the usual intensity and duration of physical activity during spontaneous activity in children.
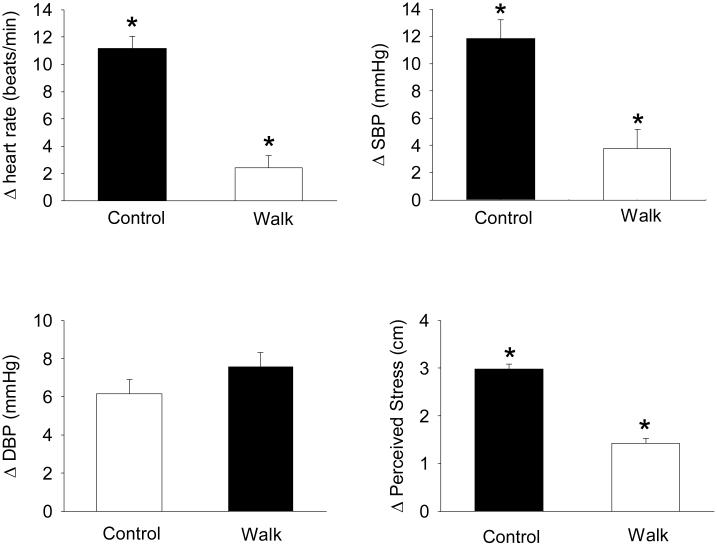
As shown in Figure 5, we also tested whether a moderate intensity walk to school would protect against increases in cardiovascular responses to a cognitive stressor, much like children experience at school (15). Walking to school is one way for youth to garner the health benefits of daily physical activity while at the same time dampening cardiovascular responses to up-coming school-related stressors. Children in the walk group performed a self-paced 1.6 km (1 mile) walk on a treadmill while images of a real walk to school through a pleasant neighborhood with a sidewalk, mature trees and shrubs were projected in front of them. Children in the control group sat in a chair and watched the same images to simulate being driven to school. The walk group had lower (p<0.001) SBP, HR and perceived stress reactivity than the control group to the cognitive stressor. Thus, a usual-intensity walk can protect against reactivity to a cognitive stressor typical to what children might experience during school. Time spent outdoors in natural or greener environments may also be restorative and protective against stress. We recently demonstrated that a greater percentage of park area in adolescent’s neighborhoods predicted lower (beta = −62.6, p<0.03) perceived stress (8).
Figure 5.
Protective effect of a self-paced 1.6 km (1 mile) walk on children's cardiovascular reactivity and perceived stress reactivity to a cognitive stressor. A total of 40 children ages 10 to 14 years completed either an active commute (walking) to school or a simulated sedentary drive to school. The walking group completed a self-paced 1.6 km walk on a treadmill while images from a real 1.6 km walk through a pleasant neighborhood that finished at a school were projected in front of them. The sedentary commute to school group watched the same slideshow of images of the neighborhood environment while sitting in a chair. The simulated active commute reduced children's heart rate, systolic blood pressure and perceived stress reactivity to a cognitive stressor that models stressors children may experience during a school day. [Adapted from (15). Copyright © 2010 Wolters Kluwer Health. Used with permission.]
Mechanisms for the Protective Effect of Exercise on Stress Reactivity
One way that exercise may lower cardiovascular stress reactivity and be protective for atherogenesis is by dampening the initial interpretation of events as stressful resulting in lower SNS activation in response to a stressor. Indeed, we have shown that a moderate intensity bout of exercise dampens children’s perceived stress reactivity (15). These data suggest that the preceding exercise reduced children’s interpretation of whether the stressor that they were encountering was in fact stressful. We have also found (unpublished data) that salivary □-amylase, a marker of SNS tone, is reduced during stress if children complete 20 minutes of exercise beforehand. Likewise, 25 minutes of exercise dampens stress-induced catecholamine secretion in adults (5). Thus, one way that regular exercise could produce its cardioprotective effect is by reducing autonomic stress reactivity for a portion of the day.
Another possible mechanism for the reduced blood pressure reactivity after exercise is a reduction in resting blood pressure after aerobic exercise. However, our research does not support post-exercise hypotension as a mechanism contributing to attenuated blood pressure responses to acute psychological stress after exercise in youth (15, 33). Our findings are consistent with studies in adults which found that post-exercise hypotension could not statistically account for the dampening effect of exercise on blood pressure reactivity (11).
In addition to acute exercise, usual physical activity may reduce stress reactivity (23). Given the effects of acute exercise on cardiovascular reactivity to stress, regular physical activity in youth may be beneficial for increasing the probability that children will be in the protective post-exercise window when encountering stressors in their daily lives. The effects of exercise training on perceived stress and stress reactivity are less clear. Exercise training could result in habituation to the repeated exercise sessions, or other effects of training, which would negate the stress-reducing effects of acute exercise on reactivity to psychological stress. The data for a training effect on alterations in stress reactivity is not clear for adults and not well studied in children.
CONCLUSION
The reviewed work represents initial evidence that psychological stress is associated with atherogenesis and CVD risk factors as early as childhood. A series of laboratory studies have provided plausible behavioral and physiological pathways or mechanisms by which stress may promote CVD risk in children. Physiologically, cardiovascular responses to stress, especially SBP reactivity are consistently associated with subclinical atherosclerosis as evidenced by greater carotid artery IMT. Behavioral stress coping responses in children include greater snacking and a shift in food choice toward more energy dense foods, especially in those children who report high levels of dietary restraint. Stress also increases children's willingness to engage in distracting sedentary behaviors (e.g, watching television) and reduces their willingness to engage in exercise. Psychological stress reactivity is also associated with greater adiposity in youth. Repeated activation of the SAM and HPA axes and the effects of unhealthy stress coping behaviors on CVD risk factors are likely partially mediated through increases in adiposity and alterations in blood pressure, blood lipid concentrations, and coagulation/fibrinolysis states. For children, a short bout of physical activity reduces the initial perception of threat due to an environmental event and reduces SBP reactivity. Dampening perceived stress reactivity and resultant blood pressure reactivity to a stressor may be one way that exercise produces a cardio-protective effect in youth.
Summary.
Stress increases snacking, sedentary behaviors, adiposity, and blood pressure all of which are atherogenic for children. Exercise dampens stress reactivity.
ACKNOWLEDGEMENT
The authors recognize the important work of other researchers which could not be cited due to the reference limitations.
Funding: This work was funded by a North American Association for the Study of Obesity Young Investigator Award and a University at Buffalo 2020 Interdisciplinary Research Development Fund to Dr. Roemmich.
Footnotes
James N. Roemmich, Ph.D. - no conflict of interest with companies or manufacturers who will benefit from the results of the present study
Maya J. Lambiase, Ph.D. - no conflict of interest with companies or manufacturers who will benefit from the results of the present study
Katherine N. Balantekin, M.S, R.D. - no conflict of interest with companies or manufacturers who will benefit from the results of the present study
Denise Feda, Ph.D. - no conflict of interest with companies or manufacturers who will benefit from the results of the present study
Joan Dorn, Ph.D. - no conflict of interest with companies or manufacturers who will benefit from the results of the present study
References
- 1.Allen, Matthews, Sherman Cardiovascular reactivity to stress and left ventricular mass in youth. Hypertension. 1997;30(4):782–7. doi: 10.1161/01.hyp.30.4.782. [DOI] [PubMed] [Google Scholar]
- 2.Balantekin, Roemmich Children's coping after psychological stress. Choices among food, physical activity, and television. Appetite. 2012;59(2):298–304. doi: 10.1016/j.appet.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 3.Barkley, Epstein, Roemmich Reinforcing value of interval and continuous physical activity in children. Physiol. Behav. 2009;98(1-2):31–6. doi: 10.1016/j.physbeh.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bitsika, Sharpley, Sweeney, McFarlane HPA and SAM axis responses as correlates of self- vs parental ratings of anxiety in boys with an Autistic Disorder. Physiol. Behav. 2014;127:1–7. doi: 10.1016/j.physbeh.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 5.Brownley, Hinderliter, West, Girdler, Sherwood, Light Sympathoadrenergic mechanisms in reduced hemodynamic stress responses after exercise. Med. Sci. Sports Exerc. 2003;35(6):978–86. doi: 10.1249/01.MSS.0000069335.12756.1B. [DOI] [PubMed] [Google Scholar]
- 6.Carroll, Phillips, Balanos Metabolically exaggerated cardiac reactions to acute psychological stress revisited. Psychophysiology. 2009;46(2):270–5. doi: 10.1111/j.1469-8986.2008.00762.x. [DOI] [PubMed] [Google Scholar]
- 7.Cohen, Janicki-Deverts, Miller Psychological stress and disease. JAMA. 2007;298(14):1685–7. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 8.Feda, Seelbinder, Baek, Raja, Yin, Roemmich Neighbourhood parks and reduction in stress among adolescents: results from Buffalo, New York. Indoor and Built Environment. 2014 1420326X14535791. [Google Scholar]
- 9.Francis, Granger, Susman Adrenocortical regulation, eating in the absence of hunger and BMI in young children. Appetite. 2013;64:32–8. doi: 10.1016/j.appet.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greeno, Wing Stress-induced eating. Psychol Bull. 1994;115(3):444–64. doi: 10.1037/0033-2909.115.3.444. [DOI] [PubMed] [Google Scholar]
- 11.Hamer, Taylor, Steptoe The effect of acute aerobic exercise on stress related blood pressure responses: a systematic review and meta-analysis. Biol Psychol. 2006;71(2):183–90. doi: 10.1016/j.biopsycho.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Herman, Polivy . Excess and restraint in bulimia. In: Pirke, Vandereycken, Ploog, editors. The Psychobiology of Bulemia. Springer Verlag; Munich: 1988. pp. 33–41. [Google Scholar]
- 13.Jennings, Kamarck, Everson-Rose, Kaplan, Manuck, Salonen Exaggerated blood pressure responses during mental stress are prospectively related to enhanced carotid atherosclerosis in middle-aged Finnish men. Circulation. 2004;110(15):2198–203. doi: 10.1161/01.CIR.0000143840.77061.E9. [DOI] [PubMed] [Google Scholar]
- 14.Kamarck, Lovallo Cardiovascular reactivity to psychological challenge: conceptual and measurement considerations. Psychosomat. Med. 2003;65(1):9–21. doi: 10.1097/01.psy.0000030390.34416.3e. [DOI] [PubMed] [Google Scholar]
- 15.Lambiase, Barry, Roemmich Effect of a simulated active commute to school on cardiovascular stress reactivity. Med. Sci. Sports Exerc. 2010;42(8):1609–16. doi: 10.1249/MSS.0b013e3181d0c77b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lambiase, Dorn, Chernega, McCarthy, Roemmich Excess heart rate and systolic blood pressure during psychological stress in relation to metabolic demand in adolescents. Biol Psychol. 2012;91(1):42–7. doi: 10.1016/j.biopsycho.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Lambiase, Dorn, Roemmich Metabolic and cardiovascular adjustments during psychological stress and carotid artery intima-media thickness in youth. Physiol. Behav. 2012;105(5):1140–7. doi: 10.1016/j.physbeh.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Lambiase, Dorn, Roemmich Systolic blood pressure reactivity during submaximal exercise and acute psychological stress in youth. Am. J. Hyperten. 2013;26(3):409–15. doi: 10.1093/ajh/hps036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lande, Carson, Roy, Meagher Effects of childhood primary hypertension on carotid intima media thickness: a matched controlled study. Hypertension. 2006;48(1):40–4. doi: 10.1161/01.HYP.0000227029.10536.e8. [DOI] [PubMed] [Google Scholar]
- 20.Lovallo Cardiovascular reactivity: mechanisms and pathways to cardiovascular disease. Int. J. Psychophysiol. 2005;58(2-3):119–32. doi: 10.1016/j.ijpsycho.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Low, Matthews, Hall Elevated C-reactive protein in adolescents: roles of stress and coping. Psychosomat. Med. 2013;75(5):449–52. doi: 10.1097/PSY.0b013e31828d3f1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Low, Salomon, Matthews Chronic life stress, cardiovascular reactivity, and subclinical cardiovascular disease in adolescents. Psychosom Med. 2009;71(9):927–31. doi: 10.1097/PSY.0b013e3181ba18ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martikainen, Pesonen, Lahti, Heinonen, Feldt, Pyhala, et al. Higher levels of physical activity are associated with lower hypothalamic-pituitary-adrenocortical axis reactivity to psychosocial stress in children. J. Clin. Endocrinol. Metab. 2013;98(4):E619–27. doi: 10.1210/jc.2012-3745. [DOI] [PubMed] [Google Scholar]
- 24.Matthews Psychological perspectives on the development of coronary heart disease. Amer. Psychol. 2005:783–96. doi: 10.1037/0003-066X.60.8.783. [DOI] [PubMed] [Google Scholar]
- 25.Matthews, Salomon, Brady, Allen Cardiovascular reactivity to stress predicts future blood pressure in adolescence. Psychosomat. Med. 2003;65(3):410–5. doi: 10.1097/01.psy.0000057612.94797.5f. [DOI] [PubMed] [Google Scholar]
- 26.McEwen Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 2007;87(3):873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 27.McVicar, Ravalier, Greenwood Biology of Stress Revisited: Intracellular Mechanisms and the Conceptualization of Stress. Stress & Health. doi: 10.1002/smi.2508. DOI: 10.1002/smi.2508, 7/23/ 2013. [DOI] [PubMed] [Google Scholar]
- 28.Meyer, Kundt, Lenschow, Schuff-Werner, Kienast Improvement of early vascular changes and cardiovascular risk factors in obese children after a six-month exercise program. J. Amer. Coll. Cardiol. 2006;48(9):1865–70. doi: 10.1016/j.jacc.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 29.Nilsen, Karevold, Roysamb, Gustavson, Mathiesen Social skills and depressive symptoms across adolescence: social support as a mediator in girls versus boys. J. Adolesc. 2013;36(1):11–20. doi: 10.1016/j.adolescence.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Pepino, Mennella Sucrose-induced analgesia is related to sweet preferences in children but not adults. Pain. 2005;119(1-3):210–8. doi: 10.1016/j.pain.2005.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roemmich, Feda, Seelbinder, Lambiase, Kala, Dorn Stress-induced cardiovascular reactivity and atherogenesis in adolescents. Atherosclerosis. 2011;215(2):465–70. doi: 10.1016/j.atherosclerosis.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roemmich, Gurgol, Epstein Influence of an interpersonal laboratory stressor on youths' choice to be physically active. Obes. Res. 2003;11(9):1080–7. doi: 10.1038/oby.2003.148. [DOI] [PubMed] [Google Scholar]
- 33.Roemmich, Lambiase, Salvy, Horvath Protective effect of interval exercise on psychophysiological stress reactivity in children. Psychophysiology. 2009;46(4):852–61. doi: 10.1111/j.1469-8986.2009.00808.x. [DOI] [PubMed] [Google Scholar]
- 34.Roemmich, Lambiase, Lobarinas, Balantekin Interactive effects of dietary restraint and adiposity on stress-induced eating and the food choice of children. Eating Behav. 2011;12(4):309–12. doi: 10.1016/j.eatbeh.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Roemmich, Lobarinas, Joseph, Lambiase, Archer, Dorn Cardiovascular reactivity to psychological stress and carotid intima-media thickness in children. Psychophysiology. 2009;46(2):293–9. doi: 10.1111/j.1469-8986.2008.00776.x. [DOI] [PubMed] [Google Scholar]
- 36.Roemmich, Smith, Epstein, Lambiase Stress reactivity and adiposity of youth. Obesity. 2007;15(9):2303–10. doi: 10.1038/oby.2007.273. [DOI] [PubMed] [Google Scholar]
- 37.Roemmich, Wright, Epstein Dietary restraint and stress-induced snacking in youth. Obes. Res. 2002;10(11):1120–6. doi: 10.1038/oby.2002.152. [DOI] [PubMed] [Google Scholar]
- 38.Rosengren, Hawken, Ounpuu, Sliwa, Zubaid, Almahmeed, et al. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):953–62. doi: 10.1016/S0140-6736(04)17019-0. [DOI] [PubMed] [Google Scholar]
- 39.Skilton, Marks, Ayer, Garden, Garnett, Harmer, et al. Weight gain in infancy and vascular risk factors in later childhood. Pediatrics. 2013;131(6):e1821–8. doi: 10.1542/peds.2012-2789. [DOI] [PubMed] [Google Scholar]
- 40.Upadhyay, Aggarwal, Narayan, Joshi, Paul, Deorari Analgesic effect of expressed breast milk in procedural pain in term neonates: a randomized, placebo-controlled, double-blind trial. Acta Paediatr. 2004;93(4):518–22. doi: 10.1080/08035250410022792. [DOI] [PubMed] [Google Scholar]
- 41.Vohs, Heatherton Self-regulatory failure: A resource-depletion approach. Psychol. Sci. 2000;11:249–54. doi: 10.1111/1467-9280.00250. [DOI] [PubMed] [Google Scholar]
- 42.von Kanel, Mills, Ziegler, Dimsdale Effect of beta2-adrenergic receptor functioning and increased norepinephrine on the hypercoagulable state with mental stress. Am. Heart. J. 2002;144(1):68–72. doi: 10.1067/mhj.2002.123146. [DOI] [PubMed] [Google Scholar]
- 43.Wilson, D'Agostino, Levy, Belanger, Silbershatz, Kannel Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 44.Yusuf, Hawken, Ounpuu, Dans, Avezum, Lanas, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]