Abstract
Trauma exposure leads to various psychiatric disorders including depression, anxiety, bipolar disorders, personality disorders, psychotic disorders, and trauma related disorders, especially posttraumatic stress disorder (PTSD). There are some overlapping symptoms of both PTSD and psychosis that make diagnosis challenging. Despite this overlap, the evidence of PTSD with comorbid psychosis as a distinct entity lies in the research showing biologic, genetic and treatment management differences between psychotic PTSD, non-psychotic PTSD, psychotic disorders and healthy controls. There is emerging evidence that PTSD with secondary psychotic features (PTSD-SP) might be a discrete entity of PTSD with known risk factors that increase its prevalence. This review has presented evidence for individuals with PTSD-SP being distinct in genetics and neurobiological factors. Individuals with PTSD and comorbid psychosis can benefit from evidence based psychotherapy (EBT). There is not enough evidence to recommend second generation antipsychotics (SGA) for PTSD-SP given that risperidone and quetiapine are the only SGAs studied in randomized controlled trials. Hence, developing an operational diagnostic criteria and treatment framework for clinical and research use is critical.
Keywords: PTSD, psychosis, phenomenology, genetics, antipsychotics
1. Introduction
Trauma exposure leads to various psychiatric disorders including depression, anxiety, bipolar disorders, personality disorders, psychotic disorders, and trauma related disorders, especially posttraumatic stress disorder (PTSD) (Schäfer and Fisher, 2011a). Posttraumatic stress disorder (PTSD) is a nosological diagnosis characterized by the presence of trauma exposure with a minimal of one month of persistent symptoms, at least one symptom from the four clusters: intrusion, avoidance, negative mood and cognitive alterations, as well as arousal and reactivity (Gayle and Raskin, 2017). The lifetime prevalence of trauma exposure found in the US community is 40–80% with an average of 3.5 different events (Kessler, 1995; Read, 2005). However, the lifetime prevalence of PTSD is approximately 7% (Kessler, 1995; Read, 2005). Since the addition of PTSD into the Diagnostic and statistical manual of mental disorders-3rd edition (DSM-III), there has been emerging evidence for a variant of PTSD involving the presence of psychosis when full PTSD criteria are met (Association, 1980; Braakman, 2009; Hamner, M B, Frueh, Christopher, Ulmer, Helen, Huber, Michael, Twomey, Timothy, Tyson, Clare, Arana, 2000; Hamner, 1997; Hamner et al., 1999; Ivezic et al., 2000; Seedat S, Stein MB, Oosthuizen PP, Emsley RA, 2003). There are some overlapping symptoms of both PTSD and psychosis that make diagnosis challenging. Despite this overlap, the evidence of PTSD with comorbid psychosis as a distinct entity lies in the research showing biologic, genetic and treatment management differences between psychotic PTSD, non-psychotic PTSD and psychotic disorders. Based on research, there are two types of PTSD with psychosis—post psychotic PTSD (PP-PTSD) and PTSD with secondary psychotic features1 (PTSD-SP). However, this review will focus on the latter. Thus, it is important to note that there are risk factors that predispose individuals to develop PTSD-SP such as trauma types, cultural differences and comorbid diagnoses. In addition, proper diagnosis and treatment of PTSD-SP is of great importance. Individuals with PTSD and comorbid psychosis can benefit from evidence based psychotherapy2 (EBT). There is not enough evidence to recommend second generation antipsychotics3 (SGA) for PTSD-SP given that risperidone and quetiapine are the only SGAs studied in randomized controlled trials. However, there is still conflicting information about the relationship between trauma burden, PTSD symptom severity, chronicity, and treatment resistance with PTSD-SP. It is important to address the methodological issues found in studies of PTSD-SP. Given emerging evidence for PTSD-SP as a distinct entity, this review paper aims to not only summarize the extant data on PTSD-SP, but also proposes a symptom criterion, and create diagnostic and treatment recommendations.
2. Methodology
Studies published between 1997 and 2017 were reviewed using multiple databases—PubMed and Google Scholar—with full-text terms: “posttraumatic stress disorder”, “PTSD”, “trauma”, “traumatic life events”, “combat”, “combat-trauma”, “childhood trauma”, “PTSD-secondary features”, “PTSD-SP”, with varied combinations: “psychosis”, “neurobiological”, “genetics”, “minority”, “refugees”, “immigrants”, “treatment”, “psychotherapy”, “therapy”, “second generation antipsychotic”. Studies were screened using the title and abstracts and eventually selected based on topic relevance and exceeding the evidence level for case studies. To find other additional and relevant studies, reference lists were examined for any appropriate articles.
104 publications were found which discussed topics on PTSD-SP, excluding case reports, opinion, and correspondence reports. While childhood trauma will be extensively mentioned throughout, this review focuses on individuals with PTSD-SP, age 18 and above. For the PTSD-SP management, occasionally, numerous publications with less rigorous standards will be cited to illustrate both the paucity of information available and barriers to formulating comprehensive conclusion with the available information. Only statistically significant findings will be discussed with p<0.05, unless authors utilize a different p value threshold, which will be explicitly stated. If the authors identify that p<0.05 is statistically significant, then any findings with p value ≥0.05 will be considered as insignificant, even if the authors did not exclusively comment on that finding. There are common methodological limitations with the majority, if not all studies, discussed; these limitations will be discussed where relevant.
3.1. General Overview: PTSD with secondary psychotic features (PTSD-SP)
Evaluation of psychotic symptoms in patients with post-traumatic symptoms or disorders is important. There is an increased likelihood of psychotic symptoms with lifetime PTSD diagnoses in the community (Shevlin et al., 2011). Thus, many authors are proposing PTSD-SP as a discrete diagnostic entity (Braakman, 2009; Coentre, 2011; Hamner, 2011; Kilcommons and Morrison, 2005; Kroll, 2007; Morrison et al., 2003; Seedat S, Stein MB, Oosthuizen PP, Emsley RA, 2003; Shevlin et al., 2011). However, Gaudiano and Zimmerman (2010) make the opposite case that PTSD-SP should not be a discrete diagnostic entity based on their data, which showed decrease PTSD-SP prevalence after psychiatric comorbidities were excluded (Gaudiano and Zimmerman, 2010). But, the authors’ careful examination of their data revealed statistically significant lifetime prevalence of PTSD-SP without major depressive disorder with an odds ratio of 3.48 (Gaudiano and Zimmerman, 2010). Meanwhile, after the exclusion of psychiatric comorbidities, the lifetime prevalence of PTSD-SP decreased from 24% to 2.4%; however, this was not statistically significant (Gaudiano and Zimmerman, 2010). The case of psychiatric comorbidities contributing to PTSD-SP prevalence has its merits (see risk factors below). Gaudiano and Zimmerman (2010)’s case was based on limited data with low study sample and high p-value (Gaudiano and Zimmerman, 2010). Thus, there is some evidence that PTSD-SP should be a distinct diagnostic psychopathology, even with the presence of psychiatric comorbidities, such as depression and substance use disorders.
Mueser et al. (2002) proposed an adaptation of the stress-vulnerability model for shared mechanism between trauma, PTSD and severe mental illness (Mueser et al., 2002). This model explored trauma as a mediator and PTSD and psychosis as disorders that interact with each other and exacerbate each other’s symptoms (Morrison et al., 2003; Mueser et al., 2002). Based on a modified Morrison et al. (2003)’s model, there are different modes for the direction causality between trauma, PTSD and psychosis: psychosis causing PTSD, called PP-PTSD; and trauma causing psychosis; and trauma causing both psychosis and PTSD (Morrison et al., 2003). The remainder of this section will focus PTSD-SP then will discuss how trauma along with other risk factors can cause both psychosis and PTSD.
First, it is important to examine common developmental and symptomatological processes of PTSD and psychosis. One is dissociation. On the one hand, trauma-induced dissociation and dissociative detachment render people vulnerable to psychotic symptoms (Morrison et al., 2003). For individuals with PTSD, hearing voices was correlated with dissociative experiences in Brewin and Patel (2010)’s study (Brewin and Patel, 2010). In addition, Morrison et al 2003 alluded to dissociative symptoms affecting reality-testing difficulties (Morrison et al., 2003) while Brewin and Patel (2010) found in their sample that those with dissociative symptoms retained normal thought processes (Brewin and Patel, 2010; Morrison et al., 2003; Seedat S, Stein MB, Oosthuizen PP, Emsley RA, 2003). There were studies refuting that PTSD-SP is associated with dissociation (Braakman, 2009; David et al., 1999). Hence, there are some indications that dissociation in PTSD might be associated with psychotic symptoms.
Another shared commonality in developmental and symptomatological processes of PTSD and psychosis are positive symptoms of hallucinations and delusions. These symptoms can be conceptualized as intrusions and flashbacks, which might be interpreted as psychotic symptoms based on cultural unacceptability (Morrison et al., 2003; Seedat S, Stein MB, Oosthuizen PP, Emsley RA, 2003). It is important to note that these positive psychotic symptoms should be delineated from flashbacks (Hamner, M B, Frueh, Christopher, Ulmer, Helen, Huber, Michael, Twomey, Timothy, Tyson, Clare, Arana, 2000; Hamner, 2011) and intrusions. Although, if present, hallucinations and delusions in PTSD patients tend to be more paranoid and persecutory in nature compared with those found in primary psychotic disorders which are more bizarre (Seedat S, Stein MB, Oosthuizen PP, Emsley RA, 2003). More studies are revealing that psychotic symptoms in PTSD do not necessarily need to be trauma-related (Hamner, 2011; Seedat S, Stein MB, Oosthuizen PP, Emsley RA, 2003). Hence, Hamner (2011) argued that lack of complex hallucinations and delusions is one major differentiating factor between those with PTSD-SP and a primary thought disorder (Hamner, 2011). Positive psychotic symptoms must be delineated from PTSD intrusions and flashbacks. If patients retain their cognitive thought processes and preserved reality testing, then these positive psychotic symptoms may be attributed to PTSD-SP.
Some negative psychotic symptoms such as withdrawal and lack of motivation can be interpreted as avoidance or emotional numbing in patients with PTSD (Morrison et al., 2003; Seedat S, Stein MB, Oosthuizen PP, Emsley RA, 2003). They enumerated many shared features between PTSD and psychosis: paranoia, arousal, hypervigilance, interrupted sleep along with emotional numbing, affective constriction, detachment, estrangement from others, and derealization (Morrison et al., 2003; Seedat S, Stein MB, Oosthuizen PP, Emsley RA, 2003). Despite these shared symptoms between PTSD and psychosis, the nature and complexity of psychosis along with thought process must be considered to help differentiate PTSD-SP from psychotic disorders. Negative psychotic symptoms are harder to differentiate from some PTSD symptoms. Nonetheless, patients should have preserved reality testing before the negative psychotic symptoms can be attributed to PTSD-SP.
Hence, there is emerging evidence that PTSD-SP might be a discrete entity with some proposing an operational diagnostic criteria.
3.2. Proposed diagnostic criterion for PTSD with secondary psychotic features (PTSD-SP)
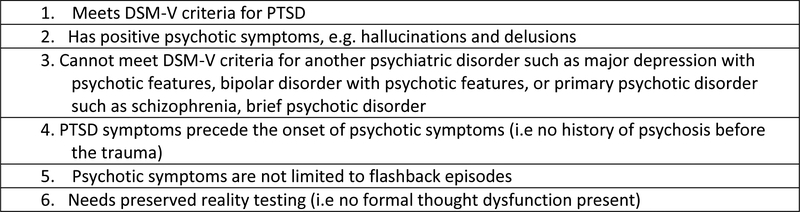
Hamner et al. (1999) and Braakman et al. (2008) suggested operational criteria for PTSD-SP (Braakman et al., 2008; Hamner et al., 1999). The combined criteria which was summarized in table 2 in Hamner et al. (2011) was an effort to establish some structure to the definition and characterization of PSTD-SP (Hamner, 2011). In order, to meet criteria for PTSD-SP, an individual cannot have any history of psychotic symptoms before a traumatic event; thus, PTSD precedes psychotic symptoms. Moreover, the psychotic symptoms cannot meet criteria for a primary psychotic disorder diagnosis. In other words, the clinical interview should not reveal a formal thought disorder pattern or brief psychotic disorder, i.e. preserved reality testing (Brewin and Patel, 2010; David et al., 1999; Hamner, 2011; Kastelan et al., 2007). To help differentiate PTSD symptoms from psychosis, the psychotic symptoms cannot “occur exclusively in the context of flashbacks” (Hamner, 2011; Kastelan et al., 2007; Kozaric-Kovacic and Borovecki, 2005). Hamner et al. (1999) and Braakman et al. (2008) suggested that the positive symptoms of delusions and hallucinations should be present (Braakman et al., 2008; Hamner et al., 1999). For research, individuals should score a minimal “moderate or higher on at least one positive items” on the Positive and Negative Syndrome Scale (PANSS) (Hamner et al., 1999). This proposed criterion will be expanded on and included in the later section of diagnostic and treatment framework.
Given the importance of establishing a criterion, a modified and comprehensive framework will be proposed for PTSD-SP upon conclusion of the paper. Thus, risk factors, neurobiological factors and management of PTSD-SP will be included in the proposed diagnostic and treatment diagram.
Modified from (Hamner, 2011)
4.1. Risk factors for PTSD-SP
There are prominent risk factors that increase an individual’s likelihood to endorse PTSD-SP. Certain trauma types, such as childhood and war traumas, ethnicity, culture and comorbid diagnoses have higher prevalence of PTSD-SP (Braakman, 2009; Hamner et al., 1999; Murphy et al., 2014; Schäfer and Fisher, 2011a, 2011b; van Nierop et al., 2013).
Childhood trauma is commonly associated with the development of psychotic disorders (Schäfer and Fisher, 2011a, 2011b; van Nierop et al., 2013), PTSD only and PTSD-SP (Bendall et al., 2012; Bendall Sarah, 2013; Jackson et al., 2004; Kilcommons and Morrison, 2005; Shaw et al., 2002). Childhood trauma has been consistently shown to increase the risk of having PTSD-SP (Read, 2005; Schäfer and Fisher, 2011b; van Nierop et al., 2013). Read (2005) summarized findings that childhood abuse had a strong relationship with positive psychotic symptom of hallucinations and there was a weak relationship between childhood abuse and negative psychotic symptoms (Read, 2005). In addition, childhood physical abuse was reportedly the only significant predictor of psychosis according to US National Comorbidity survey (Kroll, 2007). Different studies found that delusions and hallucinations are associated with a history of bullying and sexual trauma (Morgan and Fisher, 2007; Murphy et al., 2014). In general, Kilcommon and Morrison (2005) found that lifetime physical and sexual assault is associated with psychosis (Kilcommons and Morrison, 2005).
However, Calhoun et al. (2007) did not find an association between childhood and adult sexual traumas and PTSD in men (Calhoun et al., 2007). Thus, there are inconsistencies across studies about what types of childhood and adult traumas are associated with psychosis. Despite controlling for depression, a common PTSD comorbidity, there is still a significant association between childhood abuse and psychosis as found in US National Comorbidity Survey (Shevlin et al., 2011). However, cumulatively, there is a trend that childhood and adult physical and sexual traumas increase an individual’s risk of PTSD-SP.
War trauma is a potential risk factor given it is associated with a high prevalence of PTSD-SP. For Western countries, 15–64% of combat-exposed veterans have psychosis associated with PTSD (David et al., 1999; Hamner, 1997; Kaštelan et al., 2007; Kozarić-Kovačić and Borovečki, 2005; Sautter and Brailey K, Uddo MM, Hamilton MF, Beard MG, 1999). It is unclear if this could be related to veterans having a more complex and chronic course of PTSD or if psychosis is an indicator of a severe PTSD type (Calhoun et al., 2007; Hamner, 1997; Hamner et al., 1999; Mueser et al., 2002; Pivac and Kozaric-Kovacic, 2006; Sautter and Brailey K, Uddo MM, Hamilton MF, Beard MG, 1999). Of note, veterans had been reported to endorse higher symptomology on various psychiatric scales for various reasons (Frueh et al., 2000). Similar to civilians, combat veterans report similar psychotic symptoms such as auditory hallucinations and delusions, especially paranoia (David et al., 1999; Kozarić-Kovačić and Borovečki, 2005; Sautter and Brailey K, Uddo MM, Hamilton MF, Beard MG, 1999). Common auditory hallucinations usually consist of combat related themes, such as, the dead soldiers screaming or telling the veterans that “they did not deserve to survive” (Kozarić-Kovačić and Borovečki, 2005). As previous discussed elsewhere, the psychotic symptoms in combat- exposed veterans were different from dissociative symptoms or flashbacks (Kaštelan et al., 2007; Kozarić-Kovačić and Borovečki, 2005). Veterans with combat exposure have been shown to have higher prevalence of psychosis associated with their PTSD.
Meanwhile, for non-Western countries, individuals from war torn countries have a higher prevalence of PTSD with estimates of 10–30% compared to the US prevalence of PTSD of 7% (Ayazi et al., 2016; Kessler, 1995; Nygaard et al., 2017). There is a similar or higher prevalence of PTSD-SP amongst individuals from war torn nations than those in Western nations (Kroll et al., 2011; Soosay et al., 2012). Some studies focus on non-Western immigrants and refugees in Western nations with the latter group being at high risk of developing severe trauma related psychopathology (Nygaard et al., 2017). First and second generation immigrants are at increase risk of psychosis (Kroll, 2007). However, as many of the studies admitted, there is usually little to no information to confirm that trauma preceded the psychotic symptoms as mostly patients’ and family/friends’ collaterals are the only available sources (Kroll et al., 2011; Soosay et al., 2012). In addition, in some cases, researchers omitted some trauma type items (such as rape) due to recommendations from leaders in the local community of interest (Soosay et al., 2012). Another source of limitation for immigrant and refugee populations is the underreporting or overreporting of trauma and psychotic symptoms due to language barriers (i.e. use of interpreters), cultural barriers, normative responses of mistrust from trauma, reluctance to acknowledge trauma-related symptoms, or possible implication in visa/deportation status in Western nations (Adeponle et al., 2012; Buhmann, 2014; Kirmayer, 2003; Kroll et al., 2011; Soosay et al., 2012).
Adeponle et al. (2012) suggested using either the DSM cultural formulation interviews or Cultural Consultation Service (CCS), which consists of psychologist or psychiatrist along with a language interpreter, or cultural broker (Adeponle et al., 2012). CCS had direct impact in diagnoses at least 23% and treatment by 70% and in some cases, facilitated with therapeutic alliance of the providers who placed the consult (Adeponle et al., 2012; Kirmayer, 2003). CCS is still in its infancy with needs for more development and addition into clinical practice (Adeponle et al., 2012; Kirmayer, 2003). CCS seems promising with its impact on mental health care.
Despite these limitations, hallucinations, especially auditory hallucinations, and delusions are the most common psychotic symptoms in refugees with PTSD (Nygaard et al., 2017; Soosay et al., 2012). At times, it is unclear if a belief is culturally acceptable, culturally aberrant or a different manifestation of PTSD in non-Western cultures (Ayazi et al., 2016; Kroll et al., 2011; Soosay et al., 2012). In other words, psychiatric diagnoses depend on deviation from what is culturally acceptable. Thus, during psychosis assessment, evaluators must make sure that the beliefs and symptoms are abnormal for the patient’s culture. Also, evaluators must ensure that the symptoms being attributed as psychosis are not a different manifestations of PTSD as Kroll and colleagues elaborated on (Kroll, 2007). Kroll et al 2007 points out that PTSD symptoms manifest differently between Westernized populations and non-Westernized populations (Kroll, 2007). Kroll and colleagues illustrates this using the symptoms of intrusion and numbing which they identify as Westernized categories (Kroll, 2007). They contrast this to the somatic and economic complaints which they identify as non-Westernized manifestations of PTSD symptoms (Kroll, 2007). With immigrant and refugee populations, cultural and ethnicity differences play significant roles along with the stress related to transitioning to a new cultural environmental and at times with lack of social support (Nygaard et al., 2017). Some evidence exists that immigrant and refugee populations have higher rates of psychosis with their trauma history and PTSD. Certainly, more prospective studies with consistency of scales are needed to further explore relationship of trauma, PTSD and psychosis for immigrants and refugees, since this research is in its infancy.
Nonetheless, in most cases, refugees and immigrants are considered minorities in Western countries. Hence, research findings are confounded by their minority status as there is evidence that minorities (even citizen-born, i.e. African Americans and Hispanics) have higher prevalence of psychosis in PTSD (David et al., 1999; Frueh et al., 2002; Soosay et al., 2012) and psychosis in general (Kaštelan et al., 2007). Minorities, such as African Americans, underutilize mental and medical services (Frueh et al., 2002). Given the scarcity of research in this field, it is still unclear if the higher prevalence is related to: severe disease course, delayed diagnoses, access to care, avoidance of mental health services, patients’ communication with providers or combinations of these factors (Adeponle et al., 2012; David et al., 1999; Frueh et al., 2002). There are reports that oftentimes African Americans are misdiagnosed or underdiagnosed as summarized by Frueh et al. 2002 (Frueh et al., 2002). In a prior Frueh et al. (2000) study and David et al. (1999), African Americans in the Veteran Affairs outpatient PTSD clinic endorsed more positive psychotic symptoms than their Caucasians counterparts, but no racial differences on PTSD diagnosis (David et al., 1999; Frueh et al., 2000; Monnier et al., 2002). Although David et al. (1999) did find racial differences with comorbid depression, i.e current comorbid depression was more likely amongst African Americans and Hispanics than Caucasian counterparts (David et al., 1999). Also Frueh et al. (2000) did not find any racial differences in anxiety, depression, or other psychiatric comorbidities (Frueh et al., 2000). Hence, clinician awareness of cultural differences is pertinent especially in mental health to avoid under or over diagnosis, both of which can lead to inadequate care of patients with PTSD-SP. For these reasons, African Americans and other minority groups are at higher likelihood to endorse PTSD-SP.
Currently, the prognosis of PTSD with psychosis compared to non-psychotic PTSD is unclear given multiple uncertainties pertaining to PTSD severity, psychosis severity and co-morbidities such as depression and substance use (Braakman et al., 2009; Hamner et al., 2000; Hamner and Gold, 1998; Seedat Soraya, 2003). In many if not all the studies, participants with substance use or dependence were excluded (Ronconi et al., 2014); although, the parameters for these exclusion criteria differ with the majority of the studies using 30 days (Hamner et al., 2000; Hamner and Gold, 1998; Sautter et al., 2003) or 60 days (Cerbone et al., 2003; Pivac et al., 2006) since last use. In one combat exposed veteran study, alcohol dependence was the most prevalent comorbidity with PTSD (Kozarić-Kovačić and Borovečki, 2005).
Substance associated psychosis with use of cannabis and khat use might not remit after use (Kroll, 2007). Khat is a plant substance with stimulant psychoactivity due to its chemical content of cathinone, (S (−) alpha-aminopropiophenone) (Bhui and Warfa, 2010; Odenwald et al., 2009). Since Khat is culturally sanctioned in certain parts of the world, mainly East Africa and the Arabian peninsula (Bhui and Warfa, 2010), many researchers have to be cognizant of its use and effects on self-report (Bhui and Warfa, 2010; Odenwald et al., 2009). With psychotic symptoms (such as paranoia), it is unclear if Khat is an etiology (Widmann Marina, 2014) or mediating variable for trauma and psychosis (Bhui and Warfa, 2010; Odenwald et al., 2009); although, overall, Khat reportedly has weak association with causality of mental illness (Hamner et al., 2000; Kroll et al., 2011). Clearly, there needs to be more research on Khat and its role in trauma, PTSD and psychosis.
Meanwhile, like Khat, the research is unclear about cannabis and its association with psychosis amidst legalization in several states (Walsh et al., 2017; Wilkinson et al., 2016). As Walsh et al. (2017) and Wilkerson et al. (2016)’s reviews pointed out that there was limited research on cannabis and PTSD; also, cannabis had been documented to worsen preexisting psychosis (Walsh et al., 2017; Wilkinson et al., 2016). Individuals with PTSD and comorbid substance use are known to have worsened symptom severity and illness course (Grella, 2003). In Grella (2003)’s study of 400 men and women in drug treatment rehab, she found that psychosis was significant in individuals with PTSD and comorbid substance use (Grella, 2003). Some researchers did not find any association between substance use and PTSD-SP (Braakman, 2009; David et al., 1999; Hamner et al., 2000). Researchers and clinicians need to be aware that substance use increases the risk of PTSD-SP.
As Braakman et al. (2009) and Hamner (1999) suggested, a portion of individuals with PTSD-SP have comorbid depression (Braakman et al., 2009; Hamner et al., 1999). Sautter et al. (1999) found an increase prevalence of depression in PTSD-SP than in non-psychotic PTSD with the least prevalence of depression in the psychosis-only group in a sample of combat veterans (Sautter et al., 1999). Meanwhile, Braakman et al. (2009) cited that 68% of individuals have comorbid depression with PTSD and that those who suffer from depression with psychosis were four times more likely to suffer from PTSD (Braakman et al., 2009). However, some researchers did not find an association between PTSD-SP and depression (Brewin and Patel, 2010; Hamner et al., 1999; Kaštelan et al., 2007). Frueh et al. (2002) and David et al. (1999) illustrated the inconsistent findings of PTSD-SP in combat related veterans and depression (David et al., 1999; Frueh et al., 2002). Both studies found an increase in psychotic symptoms in minority populations (David et al., 1999; Frueh et al., 2002). However, David et al. (1999) did find any racial differences with comorbid depression while Frueh et al. (2002) did not (David et al., 1999; Frueh et al., 2002). Although David et al. (1999) distinguished between current and lifetime history of major depression (David et al., 1999). Despite ethnicity being a risk factor, the David et al. (1999) study found that psychotic symptoms were associated with current major depression but not with lifetime major depression. Thus, highlighting that there might be a different association of PTSD-SP and depression temporally.
For instance, in a Somali refugee population in Minnesota, the comorbidity of depression and PTSD were age and gender dependent in the cross-sectional study (Kroll et al., 2011; Kroll, 2007). Somali females age 18 to 50 had a higher rate of PTSD with depression than Somali males; however, Somali males had higher rates of psychosis than Somali females in the same age group (Kroll et al., 2011; Kroll, 2007).
When Somali group was compared to non-Somali groups for PTSD/depression and psychosis, the results were complicated as Somali males from age 18 to 30 had more psychosis than non-Somali counterparts; however, Somali males from that age group had low and “almost identical” PTSD/depression rates to non-Somali males (Kroll et al., 2011). Somali females age 18 and above had higher PTSD/depression compared to non-Somali females age 18 and above. Whereas for psychosis, Somali females only age 18 to 30 had higher psychosis rate than non-Somali females of same age group. However, Somali females age 50 and above had less psychosis rate than non-Somali females of same age group (Kroll et al., 2011).
Within the Somali group and between the group (Somali versus Non-Somali) comparisons for PTSD and psychosis showed unclear association between comorbid depression, psychosis, and PTSD.
Given current evidence, the relationship between PTSD-SP and the comorbid depression is not clear. Although, some studies excluding comorbid depression and substance use along with other DSM-IV Axis I disorder comorbidities still found individuals with PTSD-SP (Mustapic, 2007; Pivac et al., 2006).
Gaudiano and Zimmerman (2010) made an important claim on the effects of psychiatric comorbidities on PTSD-SP prevalence (Gaudiano and Zimmerman, 2010). The magnitude and temporality of these comorbidities effect on PTSD-SP is not well understood at this time.
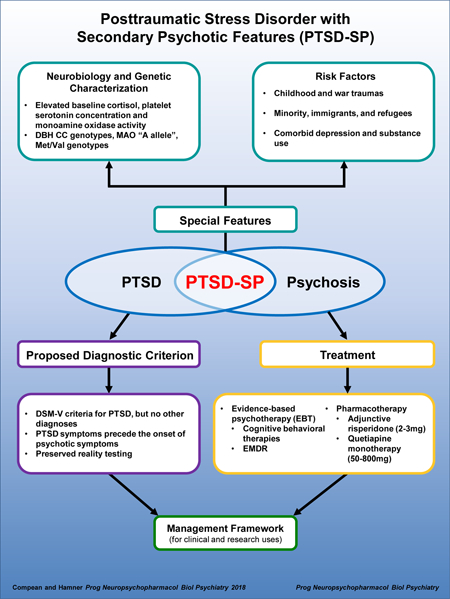
In conclusion, there are prominent risk factors that predispose individuals to PTSD-SP. These factors— trauma types, ethnicity, culture, and comorbid mood disorders, and substance use—need to be considered by clinicians during assessments and in treatment plans.
4.2. Genetics and Neurobiology of PTSD-SP
A lack of family history of psychosis is found in patients with PTSD-SP (Sautter et al., 2002). With this in mind, there are a paucity of genetic and neurobiological studies pertaining to PTSD and psychosis (Braakman et al., 2008; Hamner and Gold, 1998).
Over the last few years, there have been studies that primarily focus on genetic differences of individuals with PTSD-SP and non-psychotic PTSD focusing on genes coding for three proteins: Dopamine βeta hydroxylase (DBH), Brain-derived neurotrophic factor (BNDF) and Monoamine oxidase B (MAO-B) (Hamner and Gold, 1998; Mustapić et al., 2007; Pivac et al., 2012, 2007). DBH gene contains a −1021 C/T (rs161115) single nucleotide polymorphism in the 5’ flanking region (Mustapic, 2007). In a fairly homogeneous sample of Croatian Caucasian veterans of 167, Mustapic et al. (2007) demonstrated that only DBH CC genotype was associated with decreased DBH activity in individuals with PTSD, and there was no change in DBH activity with CT and TT genotype (Mustapic, 2007). DBH is involved in converting dopamine to norepinephrine; both are neurotransmitters implicated in PTSD (Hamner and Gold, 1998).
Thus DBH activity is relevant to assess in PTSD (Hamner and Gold, 1998). There was reportedly a 35 to 52% variance in DBH structural locus, which controls plasma DBH activity in this sample population (Mustapic et al., 2007). Furthermore, Mustapic et al. (2007) showed lower DBH activity in war veterans with PTSD than in veterans without PTSD (Mustapic et al., 2007). This genotype-independent trend on plasma DBH had been reported in patients with psychotic disorders such as paranoid schizophrenia, as well as unipolar psychotic depression (Mustapic et al., 2007). However, these studies were inconsistent with Hamner and Gold (1998) (Hamner and Gold, 1998). Hamner and Gold (1998) showed that PTSD-SP is associated with increase in plasma DBH activity (Hamner and Gold, 1998). Mustapic et al. (2007) argued that Hamner and Gold (1998)’s smaller sample size and no correlation with DBH genotype were confounders (Hamner and Gold, 1998; Mustapic et al., 2007). Another confound for DBH studies includes the potential alteration from previous medication exposure, especially from neuroleptics (Mustapic et al., 2007), although the authors mitigated this confound with medication-free participants (Mustapic et al., 2007). However, it is relevant to note that prior medication exposure for Mustapic et al. (2007)’s participants were not presented (Mustapic et al., 2007). In summary, these are initial indications that plasma DBH activity and the DBH gene might be implicated in PTSD-SP.
The second gene study is about MAO-B intron 13 polymorphism. Pivac et al. (2007) studied differences in platelet Monoamine oxidase-B (MAO-B) activity and MAO-B gene A/G substitution in intron 13 polymorphism in 4 groups of male war veterans: PTSD-SP, non-psychotic PTSD, combat exposed without PTSD, and healthy control (Pivac et al., 2007). When smoking status was controlled, PTSD-SP had a higher platelet MAO-B activity than PTSD in other groups (Pivac et al., 2007). In addition, A allele carriers in a group of PTSD-SP veterans had higher platelet MAO-B activity when compared to A allele carriers from the other groups of veterans (Pivac et al., 2007). While these findings need to be replicated in a larger prospective trials, the potential differences in platelet MAO-B activity and MAO-B intron 13 polymorphism in PTSD-SP group compared to the other groups strengthen the authors’ argument that PTSD-SP might be distinct.
The third gene study in PTSD-SP is about BDNF single nucleotide polymorphism (SNP), Val66Met (Pivac et al., 2012). Brain-derived neurotrophic factor (BDNF) is one of the most frequently studied genes with Val66Met, rs6265, being of interest to PTSD-SP (Pivac et al., 2012). Val66Met is a base pair substitution in which G is replaced by A leading to Met (methionine) replacing Val (valine) at the 66th position in pro-BDNF sequence (Pivac et al., 2012). This functional polymorphism is a good study candidate for PTSD and psychotic PTSD as Met carriers have been shown to have decreased activation of ventromedial prefrontal cortex which can be associated with impaired fear extinction (Pivac et al., 2012). In addition, Met carriers are shown to be overrepresented in psychotic disorders (Pivac et al., 2012). Thus, Pivac et al. (2012) examined the BDNF Val66Met polymorphism in individuals with psychotic PTSD, specifically PTSD-SP in a homogenous unrelated combat exposed Croatian Caucasian veteran population of 576 males (Pivac et al., 2012). There were three groups of participants determined by Structural Clinical Interview for DSM-IV (SCID), Clinician Administered PTSD Scales (CAPS) and PANSS: no PTSD (n= 206); PTSD only (n=294); and PTSD-SP (n=76) (Pivac et al., 2012). This study replicated that there was no association between BDNF Val66Met and PTSD (Pivac et al., 2012; Zhang et al., 2006). However, when subdivided into the presence or absence of psychotic features, there was significant difference in the frequencies of BDNF Val66Met variants between the three groups (Pivac et al., 2012). There were no differences between PTSD symptom severity and PTSD comorbidities of MDD and anxious depressive disorder. Patients with different BDNF Val66Met genotypes had the same CAPS and PANSS total and subscale scores, despite PTSD-SP having statistically significant higher CAPS and PANSS scores than non-psychotic PTSD (Pivac et al., 2012). While Pivac et al. (2012) detected a higher frequency of Met/Val genotype and Met carriers for PTSD-SP than the two other groups, there were multiple limitations hindering the generalization of these findings (Pivac et al., 2012). As the authors recognized, the small sample size increases the risk of a false negative finding (Pivac et al., 2012). Moreover, these findings cannot be applied outside the Caucasian male veteran population. Along with the above mentioned DBH and MAO-B genes’ polymorphisms with respective associated plasma activities, BDNF Val66Met polymorphism presents emerging evidence that PTSD-SP might be genetically distinct from non- psychotic PTSD individuals and healthy controls.
In addition to the genetic findings, other neurobiological factors have been shown to be different between individuals with PTSD-SP compared to non-psychotic PTSD, healthy controls and/or individuals with schizophrenia. Braakman et al. (2008) summarized these findings for baseline cortisol, cerebrospinal fluid (CSF) corticotropin-releasing factor (CRF), plasma DBH, platelet 5-HT concentration, and smooth pursuit eye movement (SPEM) in chapter 27 of the Progress in Brain research textbook (Braakman et al., 2008). Individuals with PTSD-SP had higher baseline cortisol, CSF CRH, and plasma DBH activity when compared to individuals with nonpsychotic PTSD (Braakman et al., 2008). When compared to those with depressive disorder with psychotic features, individuals with PTSD-SP had cortisol hyper-suppression subsequent to dexamethasone (Braakman et al., 2008). There have been studies showing no association between a neurobiological factor and PTSD-SP, even when researchers found a significant association between PTSD psychosis and a neurobiological factor. For instance, Sautter et al. (2003)’s findings of CSF CRF differences between PTSD-SP and non-psychotic PTSD individuals was included in the Braakman et al 2008 review (Braakman et al., 2008; Sautter et al., 2003). Sautter et al. (2003) also did not find any differences amongst the three groups— non-psychotic PTSD, PTSD-SP and controls—for CSF somatotropin-release- inhibiting hormone (SRIF) (Sautter et al., 2003). It is imperative to highlight that the study found no significant associations as well with SRIF. Sautter et al. (2003) did not find a statistically significant difference for CSF CRF between non-psychotic PTSD individuals and controls (Sautter et al., 2003). Nonetheless, cortisol and associated pathways might be contributing to differences between non-psychotic PTSD, PTSD-SP and healthy controls.
Meanwhile, Pivac et al. (2006) found significant elevations in peripheral platelet serotonin (5-HT) concentrations in individuals with psychotic PTSD when compared to 3 groups consisting of non- psychotic PTSD, trauma exposed only, and healthy control individuals. This elevation in platelet 5-HT concentration was limited to PANSS-positive scale, especially delusion subscale (Pivac et al., 2006). The authors concluded that (1) platelet 5-HT concentration could be related to trait markers and (2) platelet 5-HT is not associated with PTSD diagnosis, since platelet 5-HT concentration was not elevated in non-psychotic PTSD but it was in psychotic (Pivac et al., 2006). The latter conclusion was a replication of van Praag (2004); although, Pivac et al 2006 pointed out that platelet 5-HT has been associated with depressive symptoms in PTSD (Pivac et al., 2006; van Praag, 2004). Given the study design, individuals with depression and/or other Axis I comorbid disorders were excluded (Pivac et al., 2006). Moreover, the study design controlled for other confounds of platelet 5-HT variations such as: sex (all male sample), age (no difference with age and 5-HT), and season (all sampled in the same season). Thereby, Pivac et al. (2006) were able to make a convincing conclusion that the elevation in platelet 5-HT concentration is unique to PTSD-SP individuals (Pivac et al., 2006). Overall, this data further suggests that PTSD-SP has a distinctive neurobiological profile from non-psychotic PTSD and healthy individuals.
Interestingly, individuals with PTSD-SP were reported to have different abnormalities in SPEM from those with schizophrenia. Those with schizophrenia had deficits in smooth pursuit initiation of a visual stimulus in contrast to those with PTSD-SP who had SPEM deficit at lower velocity (Cerbone et al., 2003). While at higher velocity, PTSD-SP individuals demonstrated impaired SPEM performance when compared to healthy controls and individuals with schizophrenia (Braakman et al., 2008).
As discussed above, while it is exciting to report significant findings, negative findings should be highlighted for two important reasons. The first reason is related to a common limitation for these studies—small, non-representative sample size—as this increases the likelihood of either false positive or false negative results. Secondly, the negative findings might be a true positive for that particular neurobiological factor. Of course, with no replications of these studies currently, any definite conclusion is tentative.
Thus, this review has presented evidence for individuals with PTSD-SP being distinct in genetics—DBH −1021 C/T, MAO-B intron 13, and BDNF Val66Met polymorphisms—and neurobiological factors—plasma DBH activity, platelet MAO-B activity, baseline cortisol, CSF CRH, platelet 5-HT and SPEM. While there are major limitations to these studies and a need for replication, the presence of differences between PTSD-SP and other groups, such as non-psychotic PTSD, healthy controls and individuals with schizophrenia, suggests that PTSD-SP is most likely a distinct entity that warrant more clinical recognition and research.
5.0. Management for PTSD-SP
Many guidelines recommend evidence based psychotherapy (EBT) for individuals with PTSD (Benedek et al., 2009; Courtois et al., 2017; Katzman et al., 2014). The two commonly recommended psychotherapies are cognitive behaviorally based therapies (such as trauma focused, prolonged exposure, cognitive processing) and eye movement desensitization and reprocessing therapy (EMDR) (Benedek et al., 2009; Courtois et al., 2017; Katzman et al., 2014). Goodnight et al. (2018) summarized evidence based treatment guidelines for PTSD including some adaptions ((Goodnight et al., 2018) this issue). In addition Goodnight et al. (2018) explored barriers for patients in completing EBT for PTSD ((Goodnight et al., 2018) this issue). However, for individuals with PTSD and comorbid psychosis, many clinicians are wary of recommending or performing these EBTs due to multiple fears: (1) exacerbating PTSD or psychotic symptoms, (2) dubious of credibility of these therapies in this clinical population, and lastly, (3) burden on the individuals (Boden et al., 2016; Frueh et al., 2006; van den Berg et al., 2016). Various studies with individuals with PTSD and comorbid psychosis had shown that psychotherapies for PTSD do not exacerbate symptoms with all studies recommending these treatments (Bernard et al., 2006; de Bont et al., 2013; Mueser et al., 2015). Ronconi et al. (2014) meta-analysis addressed the clinician’s credibility fear through examining exclusion criteria in multiple PTSD studies (Ronconi et al., 2014). They found that while many studies did exclude individuals with comorbidities such as psychosis and substance use, these studies were more inclusive than clinicians were when offering EBT for PTSD to individuals with PTSD and comorbid psychosis (Ronconi et al., 2014). In other words, the clinicians were “more conservative” than the studies (Ronconi et al., 2014). Frueh et al. (2006) and van den Berg et al. (2016) pioneered clinician focused intervention, through groups, to help overcome the resistance associated with offering EBTs to individuals with PTSD-SP (Frueh et al., 2006; van den Berg et al., 2016). Many researchers are working on combating the clinician’s resistance to offering/treating PTSD patients with psychosis with EBT for (Frueh et al., 2006; van den Berg et al., 2016). Psychotherapy has been known to be beneficial to individuals with PTSD and psychosis. However, there needs to be more research and collaboration in encouraging clinicians to treat these individuals with EBTs.
In addition to psychotherapy, many clinical guidelines recommend pharmacotherapy for individuals with PTSD (Benedek et al., 2009; Courtois et al., 2017; Katzman et al., 2014). In the 2004 American Psychiatric Association (APA) guideline for PTSD and 2009 Guideline Watch, second generation antipsychotics were recommended if first line PTSD treatment (ie SSRI) were ineffective or if psychotic symptoms were present (Benedek et al., 2009; Courtois et al., 2017; Katzman et al., 2014). Both APA and Canadian Psychiatric Association acknowledged the need for robust studies with PTSD and SGAs (Benedek et al., 2009; Courtois et al., 2017). For non-psychotic PTSD, the first line treatment of PTSD is selective serotonin reuptake inhibitors (SSRIs) which have an efficacy of about 60% (Benedek et al., 2009; Coentre, 2011; Courtois et al., 2017), but less with PTSD-SP, given more severe pathology with comorbid psychosis (Kozaric-Kovacic and Pivac, 2007; Pae et al., 2008; Pivac et al., 2004; Sokolski, 2003; Stein et al., 2002). Thus, there is a need to examine current studies on PTSD-SP with SGAs.
However, there is a scarcity of double blinded placebo control trials (RCTs) SGAs (Bartzokis et al., 2005; Hamner et al., 2003; Rothbaum et al., 2008; Villarreal et al., 2016). Many studies have been open label trials (Kozaric-Kovacic and Pivac, 2007; Petty et al., 2001; Pivac et al., 2004; Sokolski, 2003; Stein et al., 2002). Even for non-psychotic PTSD, antipsychotics are not first line due to their side effect profiles and risks (Butterfield, 2001). In addition, most RCTs of pharmacologic interventions for PTSD-SP are on risperidone as an adjunctive medication; thus the participants were receiving other medications, mostly antidepressants (Bartzokis et al., 2005; Hamner et al., 2003). Table 1 summarizes key points of each study.
Table 1.
PTSD-SP pharmacological intervention
| RCTs | ||||
|---|---|---|---|---|
| (Hamner et al., 2003) | (Bartzokis et al., 2005) | (Rothbaum et al., 2008) | (Villarreal et al., 2016) | |
| Drug | Risperidone | Risperidone | Sertraline—open label phase 1 Risperidone—RCT phase 2 | Quetiapine |
| Dosage | 2.5mg +/− 1.25 | 2–3mg | 2–3mg | Ave 258mg (50800mg) |
| Time | 6 weeks | 16 weeks | 8 weeks on risperidone (16 weeks total) | 12 weeks |
| Groups (completers) | Risperidone (n=19) Placebo (n=18) | Unclear Risperidone (n=22) Placebo (n=25) | Risperidone (n=9) Placebo (n=11) | Risperdone (n=29) Placebo (n=18) |
| PTSD | CAPS | CAPS | CAPS | CAPS |
| Psychosis | PANSS | PANSS-P | PANSS | PANSS |
| Depression | n/a | HAM-D | BDI | HAM-D HAM-A |
| Other clinical measures | n/a | Anxiety: HAM-A | Davidson Trauma Scale (DTS) Clinical Global Impressions- Improvement scale (CGI-I) | Clinical Global Impressions- Improvement scale (CGI-I) |
| Outcome | Improvement on PANSS general psychopathology and mean score (favoring risperidone) | CAPS total, CAPS-D (Category D—negative altercations in mood and cognition) and PANSS-P (favoring risperidone) | Risperdone: improvement in DTS. Other measures (PANSS, CGI- I) were nonsignificant | Improvement in PTSD for re- experiencing and hyperarousal (via CAPS) Improvement in DTS, CGI, HAM- D, HAM-A, PANSS global psychopathology and positive symptoms (favoring quetiapine). No significance for PANSS negative symptom subscale |
| Side effects (ex. EPS) | Akathisia (n=1). None (measured by AIMS, SAS, BAS, weight, heart rate, systolic and diastolic blood pressure) | No difference between two groups (measured BAS, Columbia Scale, AIMS, weight, heart rate, systolic and diastolic blood pressure) | AIMS, SAS, BAS Tachycardia, chest pain, “probable dystonic reaction”, elevated liver function test | Most common: dry mouth, somnolence, sedation. AIMS, SAS, BAS, Arizona Sexual Experiences Scale |
| Retention rates (and reasons) | n= 22/37 (60%) most common 2 reasons: consent withdrawals and transportation difficulties. None were side effect related | n=47/65 No significance between the two groups | n=20/25. No significance between the two groups of termination due to study related adverse events | n=47/80. No significance between the two groups of termination in general and also to medication-related adverse events |
CAPS: Clinician Administered PTSD Scale; PANSS: Positive and Negative Syndrome Scale; PANSS-P: PANSS: Positive and Negative Syndrome Scale-Positive subscale; HAM-D: Hamilton Depression Scale; BDI: Beck Depression Inventory; HAM-A: Hamilton Anxiety Scale; DTS: Davidson Trauma Scale; CGI-I: Clinician Global Impressions- Improvement; AIMS: Abnormal Involuntary Movement Scale; SAS: Simpson-Angus Scale BAS: Barnes Akathisia scale
Some RCTs were not exclusively studying PTSD-SP; however, they included data on PANSS. Most RCTs to date consist of male combat veterans (Bartzokis et al., 2005; Hamner et al., 2003). Nevertheless, risperidone had varying degree(s) of improvements across the studies. For instance, Hamner et al. (2003) showed that risperidone was more efficacious than placebo only on PANSS mean score and PANSS general psychopathology subscale (Hamner et al., 2003). Rothbaum et al. (2008) examined risperidone for SSRI resistant civilian PTSD with PANSS to evaluate for psychosis; in this study, the first phase was an open label sertraline trial with randomized placebo-controlled adjunctive risperidone if less than 70% response reduction was seen in the first phase (Rothbaum et al., 2008). While there was significant improvement with risperidone over placebo for the PTSD symptoms, measured by Davidson Trauma Scale (DTS), there were no statistically significant findings between the intervention and placebo groups for PANSS and Clinical Global Impression-Global Improvement (CGI-I) (Rothbaum et al., 2008). However, the risperidone dose was capped at 3mg (Rothbaum et al., 2008). In addition, those on adjunctive risperidone had lesser end target dose of sertraline (144mg) compared to 177mg of sertraline for the placebo (Rothbaum et al., 2008). Rothbaum et al. (2008) study was notable because it was a multicenter trial and recruited community participants as opposed to many studies with combat veteran participants (Bartzokis et al., 2005; Hamner et al., 2003; Rothbaum et al., 2008). While Bartzokis et al. (2005) adjunctive risperidone study was not exclusively on PTSD-SP patients, the individuals suffered from chronic combat-related PTSD, and psychosis items were assessed via PANSS-Positive subscale (PANSS-P) (Bartzokis et al., 2005). The study reported significant improvement with risperidone group over placebo group in all measures, especially CAPS total, CAPS-D (Category D—negative altercations in mood and cognition) and PANSS-P (Bartzokis et al., 2005).
Lastly, quetiapine was the other SGA examined for PTSD with data about psychosis (Villarreal et al., 2016). Villarreal et al. (2016) examined quetiapine monotherapy for chronic PTSD and included PANSS. This study included multicenter Veteran Affairs sites with majority subjects being male veterans(Villarreal et al., 2016); for demographics, quetiapine group had more education and CAP-B than the placebo (Villarreal et al., 2016). The quetiapine group improved in many measures: CAP-B, mean total CAPS score, DTS, HAM-D, HAM-A, CGI, PANSS global psychopathology (Villarreal et al., 2016). The two measures that did not separate the two groups were PANSS negative (PANSS-N) and Pittsburgh Sleep Quality Index (Villarreal et al., 2016). Villarreal et al. (2016) team noted dry mouth, somnolence and sedation as most common side effects (Villarreal et al., 2016). In fact, 40% of the participants dropped out after randomization due to mostly lack of efficacy and side effect; however, there were no statistically differences between the two groups. Surprisingly, weight gain was not statistically different between the groups; the team suggested that quetiapine might be safe for short team use (Villarreal et al., 2016). Although this quetiapine study was not exclusively on PTSD-SP, this RCT presented enough evidence that suggests that quetiapine might be beneficial for PTSD-SP.
Collectively, the studies used similar risperidone doses ranges (2–4mg) of risperidone with wide range of quetiapine 50–800mg and all reported minimal side effects (Bartzokis et al., 2005; Hamner et al., 2003; Rothbaum et al., 2008; Villarreal et al., 2016). Regarding risperidone and extrapyramidal symptoms (EPS), there were minimal EPS side effects. In the Hamner et al. (2003), there was 1 subject out of the 40 subjects who had akathisia; otherwise, the studies did not find any differences between risperidone and placebo when it comes to objective scales, such as Barnes Akathisia Scale (BAS) and Abnormal Involuntary Movement Scale (AIMS) (Bartzokis et al., 2005; Hamner et al., 2003; Rothbaum et al., 2008; Villarreal et al., 2016). Table 1 summarizes these RCT studies.
Braakman et al. (2008) chapter publication cited Hamner et al. (2003) as the only RCT on PTSD-SP (Braakman et al., 2008; Hamner et al., 2003). Since that chapter publication in 2008, there has been one additional RCT published focusing on psychotic PTSD (Villarreal et al., 2016) and two PTSD RCTs with psychosis rating scales included (Bartzokis et al., 2005; Rothbaum et al., 2008). This speaks to the relative paucity of PTSD-SP research and scarcity of well-designed studies to evaluate SGAs as potential PTSD-SP treatment speaks to the difficulty in conducting such studies for this research field. As noted, risperidone and quetiapine are the only SGAs studied with RCTs for PTSD with psychosis data. Thus, there is modest evidence to recommend (adjunctive) treatment for PTSD-SP with risperidone. While mean target dose of risperidone is between 2 and 3 mg, there is insufficient evidence to recommend a target dose titration for patients with PTSD-SP. Similarly, for quetiapine, more RCT studies for PTSD and psychosis are needed to help elucidate the dose titration. Clearly, more RCTs with larger and diverse sample populations are needed for both risperidone and other potential pharmacological interventions to better inform treatment of PTSD-SP.
6.0. Discussion
This review has described a discrete subtype of PTSD—PTSD-SP (i.e. psychotic symptoms after PTSD development). Individuals with PTSD can develop psychotic symptoms, oftentimes positive symptoms. These positive symptoms can be trauma or non-trauma related. Nevertheless, individuals with PTSD-SP should not have a formal thought disorder. There are factors that increase likelihood of having PTSD-SP, including but not limited to: ethnicity, trauma types and comorbid mood symptoms and/or substance use. There are neurobiological findings suggesting that PTSD-SP, is distinct from non-psychotic PTSD and healthy controls. The effective management of PTSD-SP points to potential beneficial use of SGAs such as risperidone. Of note, cultural consultation services (CCS) might be beneficial when treating minorities, refugees or immigrants. Based on evidence presented, the next revision of DSM-5 should consider including PTSD-SP subtype.
Common to the studies reviewed are two major limitations: small, non-representative sample populations and varied assessment scales. For better characterization of PTSD-SP, large, population- representative prospective cohort studies are indicated. Similarly, for management of PTSD-SP, large, multicenter randomized, placebo-controlled trials are recommended in order for researchers to be confident that any improvement noted is due to the intervention.
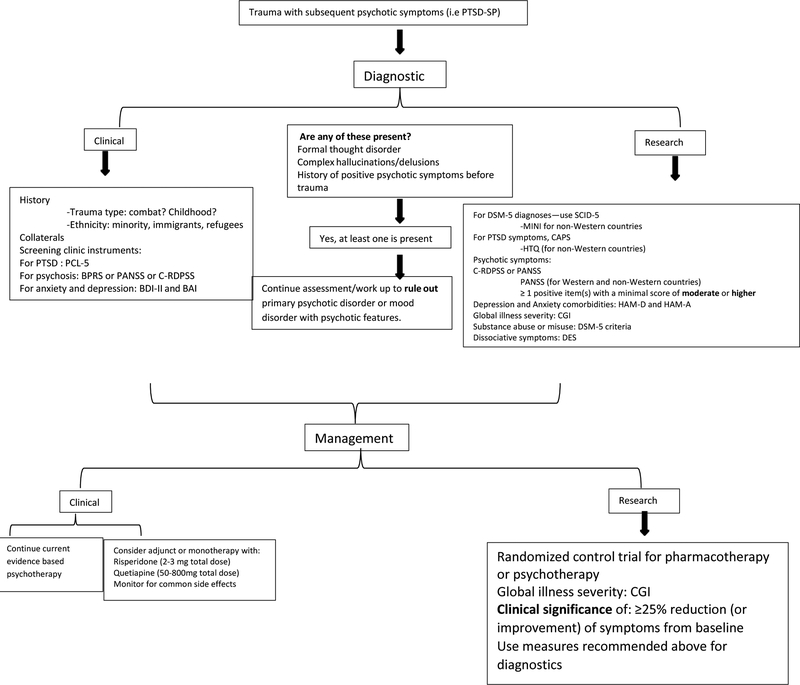
The second prominent limitation is the diverse scales utilized in all studies. There is a multitude of instrumental scales used for PTSD characterization in clinical studies, for instance, Clinician Administrated PTSD Scale (CAPS), Harvard Trauma Questionnaire (HTQ) and many others. Similar arrays of scales are found for psychosis as well. In order to eliminate bias and standardize this field of research, the authors would like to recommend the utilization of the Structured Clinical Interview for DSM-5 (SCID-5) for making DSM-5 diagnoses along with CAPS for PTSD assessments and Clinician-Rated Dimensions of Psychosis Symptom Severity (C-RDPSS) (Gayle and Raskin, 2017; Weathers et al., 2013). However, due to lack of reliability and validity measures of these instruments for PTSD-SP, the instruments utilized in research discussing PTSD-SP will also be recommended. Certainly, research in non-Western nations with limited resources might encounter difficulties and barriers to using these gold standard instruments such as CAPS. Thus, utilizing HTQ for PTSD assessment and PANSS for psychotic symptoms is recommended. For non-Western countries who cannot perform SCID-5, we recommend MINI (mini-international neuropsychiatric interview) as this has already been translated to certain languages (Ayazi et al., 2016). For depression and anxiety measures, researchers should consider utilizing the structured interview for Hamilton Depression Rating Scale (HAM-D) and Hamilton Anxiety Rating Scale (HAM-A) (Roffman et al., 2010). Now that dissociative symptoms have been added to PTSD criteria in DSM-5, research needs to uniformly monitor dissociation to further evaluate its role in PTSD-SP development (Gayle and Raskin, 2017), using Dissociative Experience Scale (DES). The authors acknowledge that diagnostic changes in the PTSD criteria from prior DSMs to DSM-V will affect the prevalence of not only PTSD-SP but also the associated comorbidities. For secondary measures, the Clinician Global Impressions (CGI) which includes subscales of Severity (CGI-S) and Improvement (CGI-I) should be implemented to standardize social functioning not only for research but also for clinical use (Busner and Targum, 2007). For clinical use, these scales are available: consider self-report PTSD Checklist for DSM-5 (PCL-5) for PTSD (Weathers et al., 2013); consider PANSS or Brief Psychiatric Rating Scales (BPRS) for psychosis; consider self-report Beck Depression Inventory version 2 (BDI-II) and Beck Anxiety Inventory (BAI) for depression and anxiety (Mortimer, 2007; Roffman et al., 2010). Hence, developing a clinical and research framework that summarizes the above information is critical (see attachment: Diagnostic and Treatment Framework).
7.0. Conclusion
Based on all the evidence, PTSD-SP is most likely a distinct entity that warrants clinical and research recognition. The research is in infancy with need for uniformity in instrumental scales and improvement in study designs for which the authors propose numerous ways of mitigating these methodological issues. Although the authors did not use formal flow chart such as Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), this literature review offers a quality assessment of PTSD-SP as well as diagnostic and management for PTSD-SP. There are key areas of research improvement on PTSD-SP such as: risk factors for PTSD-SP in prospective cohort studies; role of plasma DBH activity; effective dosages of risperidone and quetiapine; monotherapies for PTSD-SP when SSRI are ineffective.
Figure 1.
Proposed Diagnostic Criterion for PTSD with Secondary Psychotic Features (PTSD-SP)
Figure 2. Diagnostic and Management Framework for PTSD-SP.
To facilitate clinical decisions and research, the illustration provides guidance in identifying, diagnosing and treating PTSD-SP. PCL-5: PTSD Checklist for DSM-5; BPRS: Brief Psychiatric Rating Scales; PANSS: Positive and Negative Syndrome Scale; C-RDPSS: Clinician-Rated Dimensions of Psychosis Symptom Severity; BDI-II: Beck Depression Inventory version 2; BAI: Beck Anxiety Inventory; SCID-5: Structured Clinical Interview for DSM-5; MINI: Mini-International Neuropsychiatric Interview; CAPS: Clinician Administrated PTSD Scale; HTQ: Harvard Trauma Questionnaire; HAM-D: Hamilton Depression Rating Scale; HAM-A: Hamilton Anxiety Rating Scale; CGI: Clinician Global Impressions; DES: Dissociative Experience Scale
Acknowledgements
Thanks to the Drug Abuse Research Training (DART) program supported by the NIH-sponsored research track (R25 DA020537 by Drs. Brady and Back)
Special thanks to Michael Madson and the Center For Academic Excellence
Funding: This work was supported by the National Institutes of Health NIH R25 DA020537, Charleston SC
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
PTSD with secondary psychotic features (PTSD-SP)
Evidence based psychotherapy (EBT)
Second generation antipsychotics (SGAs)
References
- 1.Adeponle AB, Thombs BD, Groleau D, Jarvis E, Kirmayer LJ, 2012. Using the cultural formulation to resolve uncertainty in diagnoses of psychosis among ethnoculturally diverse patients. Psychiatr Serv 63, 147–153. [DOI] [PubMed] [Google Scholar]
- 2.Association AP, 1980. Diagnostic and statistical manual of mental disorder (3rd rev ed). Washington, DC. [Google Scholar]
- 3.Ayazi T, Swartz L, Eide AH, Lien L, Hauff E, 2016. Psychotic-like experiences in a conflict-affected population: a cross-sectional study in South Sudan. Soc Psychiatry Psychiatr Epidemiol 51, 971–979. [DOI] [PubMed] [Google Scholar]
- 4.Bartzokis G, Lu PH, Turner J, Mintz J, Saunders CS, 2005. Adjunctive risperidone in the treatment of chronic combat-related posttraumatic stress disorder. Biol Psychiatry 57, 474–479. [DOI] [PubMed] [Google Scholar]
- 5.Bendall S, Alvarez-Jimenez M, Hulbert CA, McGorry PD, Jackson HJ, 2012. Childhood trauma increases the risk of post-traumatic stress disorder in response to first-episode psychosis. Aust N Z J Psychiatry 46, 35–39. [DOI] [PubMed] [Google Scholar]
- 6.Bendall Sarah S, 2013. Testing a model of the relationship between childhood sexual abuse and psychosis in a first-episode psychosis group: the role of hallucinations and delusions, posttraumatic intrusions, and selective attention. J. Nerv. Ment. Dis 201, 941–947. [DOI] [PubMed] [Google Scholar]
- 7.Benedek DM, Friedman MJ, Zatzick D, Ursano RJ, 2009. Guideline Watch (March 2009): Practice Guideline for the Treatment of Patients with Acute Stress Disorder and Posttraumatic Stress Disorder. Focus (Madison). 7, 204–213. [Google Scholar]
- 8.Bernard M, Jackson C, Jones C, 2006. Written emotional disclosure following first-episode psychosis: effects on symptoms of post-traumatic stress disorder. Br J Clin Psychol 45, 403–415. [DOI] [PubMed] [Google Scholar]
- 9.Bhui K, Warfa N, 2010. Trauma, khat and common psychotic symptoms among Somali immigrants: a quantitative study. J Ethnopharmacol 132, 549–553. [DOI] [PubMed] [Google Scholar]
- 10.Boden MT, Gaudiano BA, Walser RD, Timko C, Faustman W, Yasmin S, Cronkite RC, Bonn-Miller MO, McCarthy JF, 2016. Feasibility and challenges of inpatient psychotherapy for psychosis: lessons learned from a veterans health administration pilot randomized controlled trial. BMC Res Notes 9, 376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braakman MH, 2009. Validity of posttraumatic stress disorder with secondary psychotic features: a review of the evidence. Acta Psychiatr. Scand 119, 15. [DOI] [PubMed] [Google Scholar]
- 12.Braakman MH, Kortmann FA, van den Brink W, Verkes RJ, 2008. Posttraumatic stress disorder with secondary psychotic features: neurobiological findings. Prog Brain Res 167, 299–302. [DOI] [PubMed] [Google Scholar]
- 13.Braakman MH, Kortmann FAM, van den Brink W, 2009. Validity of ‘post-traumatic stress disorder with secondary psychotic features’: a review of the evidence. Acta Psychiatr. Scand 119, 15–24. [DOI] [PubMed] [Google Scholar]
- 14.Brewin CR, Patel T, 2010. Auditory pseudohallucinations in United Kingdom war veterans and civilians with posttraumatic stress disorder. J Clin Psychiatry 71, 419–425. [DOI] [PubMed] [Google Scholar]
- 15.Buhmann CB, 2014. Traumatized refugees: morbidity, treatment and predictors of outcome. Dan Med J 61, B4871. [PubMed] [Google Scholar]
- 16.Busner J, Targum SD, 2007. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont) 4, 28–37. [PMC free article] [PubMed] [Google Scholar]
- 17.Butterfield MI, 2001. Olanzapine in the treatment of post-traumatic stress disorder: a pilot study. Int. Clin. Psychopharmacol 16, 197. [DOI] [PubMed] [Google Scholar]
- 18.Calhoun PS, Stechuchak KM, Strauss J, Bosworth HB, Marx CE, Butterfield MI, 2007. Interpersonal trauma, war zone exposure, and posttraumatic stress disorder among veterans with schizophrenia. Schizophr Res 91, 210–216. [DOI] [PubMed] [Google Scholar]
- 19.Cerbone A, Sautter FJ, Manguno-Mire G, Evans WE, Tomlin H, Schwartz B, Myers L, 2003. Differences in smooth pursuit eye movement between posttraumatic stress disorder with secondary psychotic symptoms and schizophrenia. Schizophr Res 63, 59–62. [DOI] [PubMed] [Google Scholar]
- 20.Coentre R, 2011. A diagnostic dilemma between psychosis and post-traumatic stress disorder: a case report and review of the literature. J. Med. Case Rep. 5, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Courtois Sonis, Jeffrey Brown, Laura Cook, Joan Fairbank, John Friedman, Matthew Gone, Joseph Jones, Russell La Greca, Annette C., 2017. Clinical Practice Guideline for the Treatment of Posttraumatic Stress Disorder (PTSD) in Adults 72. [Google Scholar]
- 22.David D, Kutcher GS, Jackson EI, Mellman TA, 1999. Psychotic symptoms in combat-related posttraumatic stress disorder. J Clin Psychiatry 60, 29–32. [DOI] [PubMed] [Google Scholar]
- 23.de Bont PA, van den Berg DP, van der Vleugel BM, de Roos C, Mulder CL, Becker ES, de Jongh A, van der Gaag M, van Minnen A, 2013. A multi-site single blind clinical study to compare the effects of prolonged exposure, eye movement desensitization and reprocessing and waiting list on patients with a current diagnosis of psychosis and co morbid post traumatic stress disorder: study pro. Trials 14, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sautter FJ, Cornwell J, Johnson JJ, Wiley J,FS., 2002. Family history study of posttraumatic stress disorder with secondary psychotic symptoms. Am. J. Psychiatry 159, 1775. [DOI] [PubMed] [Google Scholar]
- 25.Frueh BC, Cusack KJ, Grubaugh AL, Sauvageot JA, Wells C, 2006. Clinicians’ perspectives on cognitive-behavioral treatment for PTSD among persons with severe mental illness. Psychiatr Serv 57, 1027–1031. [DOI] [PubMed] [Google Scholar]
- 26.Frueh BC, Hamner MB, Bernat JA, Turner SM, Keane TM, Arana GW, 2002. Racial differences in psychotic symptoms among combat veterans with PTSD. Depress. Anxiety 16, 157. [DOI] [PubMed] [Google Scholar]
- 27.Frueh BC, Hamner MB, Cahill SP, Gold PB, Hamlin KL, 2000. Apparent symptom overreporting in combat veterans evaluated for PTSD. Clin. Psychol. Rev 20, 853. [DOI] [PubMed] [Google Scholar]
- 28.Gaudiano BA, Zimmerman M, 2010. Evaluation of evidence for the psychotic subtyping of post-traumatic stress disorder. Br J Psychiatry 197, 326–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gayle MC, Raskin JD, 2017. DSM-5. J. Humanist. Psychol. 57, 650–666. [Google Scholar]
- 30.Goodnight J, 2018. Psychotherapy for PTSD: An Evidence-Based Guide to a Theranostic Approach to Treatment. [DOI] [PubMed] [Google Scholar]
- 31.Grella CE, 2003. Effects of gender and diagnosis on addiction history, treatment utilization, and psychosocial functioning among a dually-diagnosed sample in drug treatment. J Psychoact. Drugs 35 Suppl 1, 169–179. [DOI] [PubMed] [Google Scholar]
- 32.Hamner MB, Frueh Christopher, Ulmer Helen, Huber Michael, Twomey Timothy, Tyson Clare, Arana G., 2000. Psychotic features in chronic posttraumatic stress disorder and schizophrenia: comparative severity. J. Nerv. Ment. Dis 188, 217. [DOI] [PubMed] [Google Scholar]
- 33.Hamner MB, 1997. Psychotic features and combat-associated PTSD. Depress Anxiety 5, 34–38. [DOI] [PubMed] [Google Scholar]
- 34.Hamner MB, 2011. Psychotic Symptoms in Posttraumatic Stress Disorder. Focus J. Lifelong Learn. Psychiatry IX, 278–285. [Google Scholar]
- 35.Hamner MB, Faldowski RA, Ulmer HG, Frueh BC, Huber MG, Arana GW, 2003. Adjunctive risperidone treatment in post-traumatic stress disorder: a preliminary controlled trial of effects on comorbid psychotic symptoms. Int. Clin. Psychopharmacol 18, 1–8. [DOI] [PubMed] [Google Scholar]
- 36.Hamner MB, Frueh BC, Ulmer HG, Arana GW, 1999. Psychotic features and illness severity in combat veterans with chronic posttraumatic stress disorder. Biol Psychiatry 45, 846–852. [DOI] [PubMed] [Google Scholar]
- 37.Hamner MB, Gold PB, 1998. Plasma dopamine beta-hydroxylase activity in psychotic and non-psychotic post-traumatic stress disorder. Psychiatry Res 77, 175–181. [DOI] [PubMed] [Google Scholar]
- 38.Hamner MB, Deitsch SE, Brodrick PS, Ulmer HG, L J., 2003. Quetiapine treatment in patients with posttraumatic stress disorder: an open trial of adjunctive therapy. J. Clin. Psychopharmacol 23, 15. [DOI] [PubMed] [Google Scholar]
- 39.Ivezic S, Bagaric A, Oruc L, Mimica N, Ljubin T, 2000. Psychotic symptoms and comorbid psychiatric disorders in Croatian combat-related posttraumatic stress disorder patients. Croat Med J 41, 179–183. [PubMed] [Google Scholar]
- 40.Jackson C, Knott C, Skeate A, Birchwood M, 2004. The trauma of first episode psychosis: the role of cognitive mediation. Aust. N. Z. J. Psychiatry 38, 327–333. [DOI] [PubMed] [Google Scholar]
- 41.Kaštelan A, Frančišković T, Moro L, Rončević-Gržeta I, Grković J, Jurcan V, Lesica T, Graovac M, Girotto I, 2007. Psychotic Symptoms in Combat-Related Post-Traumatic Stress Disorder. Mil. Med 172, 273–277. [DOI] [PubMed] [Google Scholar]
- 42.Kastelan A, Franciskovic T, Moro L, Roncevic GI, Grkovic J, Jurcan V, Lesica T, Graovac M, Girotto I, 2007. Psychotic symptoms in combat-related post-traumatic stress disorder. Mil Med 172, 273–277. [DOI] [PubMed] [Google Scholar]
- 43.Katzman MA, Bleau P, Blier P, Chokka P, Kjernisted K, Van Ameringen M, the Canadian Anxiety Guidelines Initiative Group on behalf of the Anxiety Disorders Association of Canada/Association Canadienne des troubles, anxieux, McGill U., 2014. Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC Psychiatry 14, S1–S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kessler RC, 1995. Posttraumatic Stress Disorder in the National Comorbidity Survey. Arch. Gen. Psychiatry 52, 1048. [DOI] [PubMed] [Google Scholar]
- 45.Kilcommons AM, Morrison AP, 2005. Relationships between trauma and psychosis: an exploration of cognitive and dissociative factors. Acta Psychiatr Scand 112, 351–359. [DOI] [PubMed] [Google Scholar]
- 46.Kirmayer LJ, 2003. Cultural consultation: A model of mental health service for multicultural societies. Can. J. Psychiatry, 48, 145–153. [DOI] [PubMed] [Google Scholar]
- 47.Kozaric-Kovacic D, Borovecki A, 2005. Prevalence of psychotic comorbidity in combat-related post-traumatic stress disorder. Mil Med 170, 223–226. [DOI] [PubMed] [Google Scholar]
- 48.Kozaric-Kovacic D, Pivac N, 2007. Quetiapine treatment in an open trial in combat-related post-traumatic stress disorder with psychotic features. Int J Neuropsychopharmacol 10, 253–261. [DOI] [PubMed] [Google Scholar]
- 49.Kroll JL, 2007. New directions in the conceptualization of psychotic disorders. Curr Opin Psychiatry 20, 573–577. [DOI] [PubMed] [Google Scholar]
- 50.Kroll J, Yusuf AI, Fujiwara K, 2011. Psychoses, PTSD, and depression in Somali refugees in Minnesota. Soc Psychiatry Psychiatr Epidemiol 46, 481–493. [DOI] [PubMed] [Google Scholar]
- 51.Monnier J, Elhai JD, Frueh BC, Sauvageot JA, Magruder KM, 2002. Replication and expansion of findings related to racial differences in veterans with combat-related PTSD. Depress Anxiety 16, 64–70. [DOI] [PubMed] [Google Scholar]
- 52.Morgan C, Fisher H, 2007. Environment and schizophrenia: environmental factors in schizophrenia: childhood trauma--a critical review. Schizophr Bull 33, 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morrison AP, Frame L, Larkin W, 2003. Relationships between trauma and psychosis: a review and integration. Br J Clin Psychol 42, 331–353. [DOI] [PubMed] [Google Scholar]
- 54.Mortimer AM, 2007. Symptom rating scales and outcome in schizophrenia. [DOI] [PubMed] [Google Scholar]
- 55.Mueser KT, Gottlieb JD, Xie H, Lu W, Yanos PT, Rosenberg SD, Silverstein SM, Duva SM, Minsky S, Wolfe RS, McHugo GJ, 2015. Evaluation of cognitive restructuring for post-traumatic stress disorder in people with severe mental illness. Br J Psychiatry 206, 501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mueser KT, Rosenberg SD, Goodman LA, Trumbetta SL, 2002. Trauma, PTSD, and the course of severe mental illness: an interactive model. Schizophr Res 53, 123–143. [DOI] [PubMed] [Google Scholar]
- 57.Murphy J, Shevlin M, Houston JE, Adamson G, 2014. Modelling the cooccurrence of psychosis-like experiences and childhood sexual abuse. Soc Psychiatry Psychiatr Epidemiol 49, 1037–1044. [DOI] [PubMed] [Google Scholar]
- 58.Mustapic M, 2007. Dopamine betahydroxylase (DBH) activity and1021C/T polymorphism of DBH gene in combatrelated posttraumatic stress disorder. Am. J. Med. Genet. Part B Neuropsychiatr. Genet 144, 1087. [DOI] [PubMed] [Google Scholar]
- 59.Mustapić M, Pivac N, Kozarić-Kovačić D, Deželjin M, Cubells JF, Mück-Šeler D, 2007. Dopamine beta-hydroxylase (DBH) activity and −1021C/T polymorphism ofDBH gene in combat-related post-traumatic stress disorder. Am. J. Med. Genet. Part B Neuropsychiatr. Genet 144B, 1087–1089. [DOI] [PubMed] [Google Scholar]
- 60.Nygaard M, Sonne C, Carlsson J, 2017. Secondary psychotic features in refugees diagnosed with post-traumatic stress disorder: a retrospective cohort study. BMC Psychiatry 17, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Odenwald M, Hinkel H, Schauer E, Schauer M, Elbert T, Neuner F, Rockstroh B, 2009. Use of khat and posttraumatic stress disorder as risk factors for psychotic symptoms: a study of Somali combatants. Soc Sci Med 69, 1040–1048. [DOI] [PubMed] [Google Scholar]
- 62.Pae CU, Lim HK, Peindl K, Ajwani N, Serretti A, Patkar AA, Lee C, 2008. The atypical antipsychotics olanzapine and risperidone in the treatment of posttraumatic stress disorder: a meta-analysis of randomized, double-blind, placebo-controlled clinical trials. Int Clin Psychopharmacol 23, 1–8. [DOI] [PubMed] [Google Scholar]
- 63.Petty F, Brannan S, Casada J, Davis LL, Gajewski V, Kramer GL, Stone RC, Teten AL, Worchel J, Young KA, 2001. Olanzapine treatment for post-traumatic stress disorder: an open-label study. Int Clin Psychopharmacol 16, 331–337. [DOI] [PubMed] [Google Scholar]
- 64.Pivac N, Kozaric-Kovacic D, Muck-Seler D, 2004. Olanzapine versus fluphenazine in an open trial in patients with psychotic combat-related post-traumatic stress disorder. Psychopharmacol. 175, 451–456. [DOI] [PubMed] [Google Scholar]
- 65.Pivac N, Kozaric-Kovacic D, 2006a. Pharmacotherapy of treatment-resistant combat-related posttraumatic stress disorder with psychotic features. Croat Med J 47, 440–451. [PMC free article] [PubMed] [Google Scholar]
- 66.Pivac N, Kozaric-Kovacic D, Mustapic M, Dezeljin M, Borovecki A, Grubisic-Ilic M., Muck-Seler D., 2006b. Platelet serotonin in combat related posttraumatic stress disorder with psychotic symptoms. J Affect Disord 93, 223–227., [DOI] [PubMed] [Google Scholar]
- 67.Pivac N, Knezevic J, Kozaric-Kovacic D, Dezeljin M, Mustapic M, Rak D, Matijevic T, Pavelic J, Muck-Seler D, 2007. Monoamine oxidase (MAO) intron 13 polymorphism and platelet MAO-B activity in combat-related posttraumatic stress disorder. J Affect Disord 103, 131–138. [DOI] [PubMed] [Google Scholar]
- 68.Pivac N, Kozaric-Kovacic D, Grubisic-Ilic M, Nedic G, Rakos I, Nikolac M, Blazev M, Muck-Seler D, 2012. The association between brain-derived neurotrophic factor Val66Met variants and psychotic symptoms in posttraumatic stress disorder. World J. Biol. Psychiatry, 13, 306. [DOI] [PubMed] [Google Scholar]
- 69.Read J, 2005. Childhood trauma, psychosis and schizophrenia: a literature review with theoretical and clinical implications. Acta Psychiatr. Scand 112, 330. [DOI] [PubMed] [Google Scholar]
- 70.Roffman JL, Silverman BC, Stern TA, 2010. Diagnostic Rating Scales and Laboratory Tests. Massachusetts Gen. Hosp. Handb. Gen. Hosp. Psychiatry [Google Scholar]
- 71.Ronconi JM, Shiner B, Watts BV, 2014. Inclusion and exclusion criteria in randomized controlled trials of psychotherapy for PTSD. J Psychiatr Pr. 20, 25–37. [DOI] [PubMed] [Google Scholar]
- 72.Rothbaum BO, Killeen TK, Davidson JR, Brady KT, Connor KM, Heekin MH, 2008. Placebo-controlled trial of risperidone augmentation for selective serotonin reuptake inhibitor-resistant civilian posttraumatic stress disorder. J Clin Psychiatry 69, 520–525. [DOI] [PubMed] [Google Scholar]
- 73.Sautter FJ, Brailey K, Uddo MM, Hamilton MF, Beard MG, B A., 1999. PTSD and comorbid psychotic disorder: comparison with veterans diagnosed with PTSD or psychotic disorder. J. Trauma. Stress 12, 73. [DOI] [PubMed] [Google Scholar]
- 74.Sautter FJ, Bissette G, Wiley J, Manguno-Mire G, Schoenbachler B, Myers L, Johnson JE, Cerbone A, M D., 2003. Corticotropin-releasing factor in posttraumatic stress disorder (PTSD) with secondary psychotic symptoms, nonpsychotic PTSD, and healthy control subjects. Biol. Psychiatry 54, 1382. [DOI] [PubMed] [Google Scholar]
- 75.Schäfer I, Fisher HL, 2011a. Childhood trauma and psychosis - what is the evidence? Dialogues Clin Neurosci 13, 360–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schäfer I, Fisher HL, 2011b. Childhood trauma and posttraumatic stress disorder in patients with psychosis: clinical challenges and emerging treatments. Curr Opin Psychiatry 24, 514–518. [DOI] [PubMed] [Google Scholar]
- 77.Seedat S, Stein MB, Oosthuizen PP, Emsley RA, S D, 2003. Linking posttraumatic stress disorder and psychosis: a look at epidemiology, phenomenology, and treatment. J. Nerv. Ment. Dis 191, 675–681. [DOI] [PubMed] [Google Scholar]
- 78.Shaw K, McFarlane AC, Bookless C, Air T, 2002. The aetiology of postpsychotic posttraumatic stress disorder following a psychotic episode. J Trauma Stress 15, 39–47. [DOI] [PubMed] [Google Scholar]
- 79.Shevlin M, Armour C, Murphy J, Houston JE, Adamson G, 2011. Evidence for a psychotic posttraumatic stress disorder subtype based on the National Comorbidity Survey. Soc Psychiatry Psychiatr Epidemiol 46, 1069–1078. [DOI] [PubMed] [Google Scholar]
- 80.Sokolski KN, 2003. Quetiapine for treatment of refractory symptoms of combat-related post-traumatic stress disorder. Mil. Med 168, 486. [PubMed] [Google Scholar]
- 81.Soosay I, Silove D, Bateman-Steel C, Steel Z, Bebbington P, Jones PB, Chey T, Ivancic L, Marnane C, 2012. Trauma exposure, PTSD and psychotic-like symptoms in post-conflict Timor Leste: an epidemiological survey. BMC Psychiatry 12, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stein MB, Kline NA, Matloff JL, 2002. Adjunctive olanzapine for SSRI-resistant combat-related PTSD: a double-blind, placebo-controlled study. Am J Psychiatry 159, 1777–1779. [DOI] [PubMed] [Google Scholar]
- 83.van den Berg DP, de Bont PA, van der Vleugel BM, de Roos C, de Jongh A, van Minnen A, van der Gaag M, 2016a. Trauma-Focused Treatment in PTSD Patients With Psychosis: Symptom Exacerbation, Adverse Events, and Revictimization. Schizophr Bull 42, 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van den Berg DPG, van der Vleugel BM, de Bont P, Staring ABP, Kraan T, Ising H, de Roos C, de Jongh A, van Minnen A, van der Gaag M, 2016b. Predicting trauma-focused treatment outcome in psychosis. Schizophr Res 176, 239–244. [DOI] [PubMed] [Google Scholar]
- 85.van Nierop M, Janssens M, Bruggeman R, Cahn W, de Haan L, Kahn RS, Meijer CJ, Myin-Germeys I, van Os J, Wiersma D, Investigators GR Ou. of P., 2013. Evidence that transition from health to psychotic disorder can be traced to semi-ubiquitous environmental effects operating against background genetic risk. PLoS One 8, e76690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van Praag HM, 2004. The cognitive paradox in posttraumatic stress disorder: a hypothesis. Prog Neuropsychopharmacol Biol Psychiatry 28, 923–935. [DOI] [PubMed] [Google Scholar]
- 87.Villarreal G, Hamner MB, Cañive JM, Robert S, Calais LA, Durklaski V, Zhai Y, Qualls C, 2016. Efficacy of Quetiapine Monotherapy in Posttraumatic Stress Disorder: A Randomized, Placebo-Controlled Trial. Am J Psychiatry 173, 1205–1212. [DOI] [PubMed] [Google Scholar]
- 88.Walsh Z, Gonzalez R, Crosby K, S Thiessen M, Carroll C, Bonn-Miller MO, 2017. Medical cannabis and mental health: A guided systematic review. Clin Psychol Rev 51, 15–29. [DOI] [PubMed] [Google Scholar]
- 89.Weathers Litz BT, Keane TM, Palmieri PA, Marx BP, & Schnurr PP, F. W, 2013. PTSD Checklist for DSM-5 (PCL-5) - PTSD: National Center for PTSD. [Google Scholar]
- 90.Widmann Marina M, 2014. Khat Use, PTSD and Psychotic Symptoms among Somali Refugees in Nairobi - A Pilot Study. Front. Public Heal. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wilkinson ST, Radhakrishnan R, D’Souza DC, 2016. A Systematic Review of the Evidence for Medical Marijuana in Psychiatric Indications. J Clin Psychiatry 77, 1050–1064. [DOI] [PubMed] [Google Scholar]
- 92.Zhang H, Ozbay F, Lappalainen J, Kranzler HR, van Dyck CH, Charney DS, Price LH, Southwick S, Yang BZ, Rasmussen A, Gelernter J, 2006. Brain derived neurotrophic factor (BDNF) gene variants and Alzheimer’s disease, affective disorders, posttraumatic stress disorder, schizophrenia, and substance dependence. Am J Med Genet B Neuropsychiatr Genet 141b, 387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]