Key Points
Question
How do 12-month visual outcomes and injection loads compare between ranibizumab and aflibercept for neovascular age-related macular degeneration in a treat-and-extend regimen?
Findings
In this randomized clinical trial, a 12-month interim analysis of data from 278 participants found a mean best-corrected visual acuity letter score change from baseline to month 12 of 7.2 for ranibizumab and 4.9 for aflibercept. The mean number of injections was 9.7 in both arms.
Meaning
Neither aflibercept nor ranibizumab were superior to the other regarding 12-month average visual acuity gains and injection numbers using a treat-and-extend regimen.
Abstract
Importance
To our knowledge, this is the first randomized clinical trial to compare visual outcomes and injection loads between ranibizumab and aflibercept using an identical treat-and-extend (TE) regimen for neovascular age-related macular degeneration (nAMD).
Objective
To report the results of the preplanned 12-month interim analysis of 2 predefined secondary efficacy end points of a randomized clinical trial.
Design, Setting, and Participants
The Comparison of Ranibizumab and Aflibercept for the Development of Geographic Atrophy in (Wet) AMD Patients (RIVAL) trial was conducted in 24 sites in Australia and included 281 treatment-naive eyes from 281 participants with active choroidal neovascularization secondary to nAMD and a visual acuity letter score of 23 or greater who were recruited between April 11, 2014, and October 31, 2015. A preplanned interim analysis was performed at month 12. Best-corrected visual acuity (BCVA) assessors and the central reading center, which determined treatment intervals, were masked to treatment assignments.
Interventions
Participants were randomized (1:1) to receive intravitreal injections of 0.5 mg of ranibizumab or 2.0 mg of aflibercept. After receiving 3 initial monthly injections, participants entered the TE phase.
Main Outcomes and Measures
Mean change in BCVA and the number of injections from baseline to month 12.
Results
Of 281 participants, 148 (52.7%) were women and the mean (SD) age was 77.7 (8.1) years. The baseline mean BCVA letter score (approximate Snellen equivalent) was 65.3 (20/50) in the ranibizumab arm and 65.1 (20/50) in the aflibercept arm. One hundred twenty-seven ranibizumab participants (90.1%) and 121 aflibercept participants (88.3%) completed month 12 with a mean (SD [Snellen equivalent]) BCVA letter score of 72.9 (15.5 [20/32]) and 70.5 (14.6 [20/40]), respectively. The mean change in BCVA letter scores from baseline to month 12 was 7.2 (95% CI, 5.5-8.9) for ranibizumab and 4.9 (95% CI, 3.1-6.6) for aflibercept (letter score difference, 2.3; 95% CI, −0.1 to 4.7; P = .06). The mean number of injections from baseline to month 12 was 9.7 in both the ranibizumab (SD, 2.8) and aflibercept (SD, 2.6) arms with a rate ratio of 1.00 (95% CI, 1.0-1.1; P = .86).
Conclusions and Relevance
Our findings suggest that neither aflibercept nor ranibizumab for nAMD are superior to the other regarding the average visual acuity gains and number of injections during 1 year in a TE regimen. Further follow-up to 2 years may determine if advantages of one over the other can be identified.
Trial Registration
Clinicaltrials.Gov identifier: NCT02130024
This randomized clinical trial explores the effect of ranibizumab vs aflibercept in treating neovascular age-related macular degeneration with a treat-and-extend regimen for Australian participants in the RIVAL trial.
Introduction
Ranibizumab is a small recombinant, humanized, monoclonal antibody that neutralizes all forms of vascular endothelial growth factor (VEGF) A,1 whereas aflibercept is a larger recombinant fusion protein that neutralizes all VEGF-A isoforms as well as VEGF-B and placental growth factor.2 These differences in receptor inhibition and biologic characteristics may confer different efficacy and safety profiles in treating neovascular age-related macular degeneration (nAMD).
Long-term VEGF inhibition might cause macular atrophy.3,4 A long-term study reported an overall mean reduction in letter scores from a baseline of 8.6 after 7 years of treatment, with more than 90% reported to have developed macular atrophy.5 The Fight Retinal Blindness (FRB) registry reported a mean reduction in letter scores from baseline of 2.6 after 7 years of treatment, with 39% of eyes that had a 10-letter loss reported to have had macular atrophy.6 Similar results were seen from the Comparison of Age-Related Macular Degeneration Treatment Trials follow-up study.7 There are limited published data on the risk of macular atrophy in eyes receiving aflibercept.
The Development of New Macular Atrophy In Patients With Neovascular Age-Related Macular Degeneration: a Comparison of Ranibizumab and Aflibercept (RIVAL) randomized clinical trial (RCT) studied the difference in this risk between ranibizumab and aflibercept when using a treat-and-extend (TE) injection regimen. The primary end point of the study, the mean change in area of macular atrophy from baseline to month 24, as well as safety results, will be reported in the full analysis at study completion.
The results presented are from the prespecified 12-month interim analysis of the mean number of injections and the mean change in best-corrected visual acuity (BCVA) from baseline to month 12. To our knowledge, the comparative outcomes of a TE regimen between ranibizumab and aflibercept have not been previously investigated in an RCT.
Methods
Study Design
RIVAL was a 24-month, randomized, multicenter, phase IV study that was conducted at 24 sites across Australia. Eligible participants were randomly assigned 1:1 to receive either 0.5 mg of ranibizumab or 2.0 mg of aflibercept following a masked reading center-controlled TE protocol (Supplement 1). The prespecified secondary efficacy outcomes reported in this article include the mean number of injections and the mean change in BCVA from baseline to month 12.
Participant Selection and Outcomes
Participants who were 50 years or older with a baseline BCVA letter score (approximate Snellen equivalent) of 23 (20/400) or more in a treatment-naive eye diagnosed by investigators with active subfoveal choroidal neovascularization (CNV) secondary to nAMD (or evidence of fluid or hemorrhage involving the fovea for CNV that was not subfoveal) without a restriction of lesion size or type were considered for the study. Markers of lesion activity included retinal thickening with evidence of intraretinal, subretinal, or subpigment epithelial fluid accumulation confirmed by optical coherence tomography (OCT); the presence of intraretinal or subretinal hemorrhage; leakage shown on fluorescein angiography (FA); and deterioration in BCVA attributed to CNV activity.
Participants with 1 or more patches of macular atrophy that were greater than 250 µm in the greatest linear dimension in either eye (measured with multimodal imaging, which included OCT, FA, color fundus photography, and autofluorescence) were ineligible. Inclusion and exclusion criteria are detailed in the eTable 1 in Supplement 2.
Written informed consent was obtained from all participants and the study protocol was approved by the ethics committee for each study site. Recruitment started on April 11, 2014, and was completed on October 31, 2015. Data were collected using an electronic case report form. The study was conducted in accordance with International Conference on Harmonization Good Clinical Practice guidelines and the Declaration of Helsinki and was registered at ClinicalTrials.gov (NCT02130024). The prospectively specified secondary end points that were assessed in this interim analysis were the mean number of injections and the mean change in BCVA from baseline to month 12.
Measurement of BCVA
Best-corrected visual acuity was measured using a logMAR 4-m chart at all visits, including protocol refraction at screening/baseline, week 8, and month 12 by BCVA assessors who were accredited by a third-party vendor. The BCVA testing and refraction techniques were standardized for all of the study sites.
Imaging and Central Reading Centre
Investigators made the diagnosis of active CNV using FA and OCT imaging. Spectral-domain OCT images, taken at all injection visits to assess the status of disease activity, were graded by the Bern Photographic Reading Centre.
Treatments and Masking
Eligible participants were randomized using a dynamic allocation method in an interactive web-based response system (Medidata Rave; Medidata Solutions, Inc.) 1:1 to receive either ranibizumab, 0.5 mg, or AFL, 2.0 mg. Participants were stratified at randomization by current treatment or no current treatment of the nonstudy fellow eye to account for any potential contralateral effect on the study eye.
Participants received 3 monthly doses (at baseline, week 4, and week 8) followed by a TE regimen. Subsequent treatment intervals were determined according to the following disease activity criteria: a loss of visual acuity of 5 or more letters from the best visual acuity recorded since treatment started, which was attributed by the investigator to disease activity; the presence of a new retinal hemorrhage as determined by the investigator; the presence of any intraretinal fluid or subretinal fluid on spectral-domain OCT as determined by the Central Reading Centre (CRC). During the TE phase, OCT images were uploaded by study sites within 3 days of being taken for the CRC to grade within 7 days of receipt. The CRC had to approve or adjudicate the injection interval that was initially determined by investigators so that the appointment for the next injection could be made. After the third injection (week 8), the injection interval was kept at 4 weeks if there were any signs of disease activity. If none were present, the injection interval was extended by 2 weekly increments to a maximum of 12 weekly intervals. If there was 1 sign of disease activity, the interval was reduced by 2 weeks; if there were 2 or more signs of disease activity, the interval reverted to 4 weekly injections, which was the shortest injection interval permitted. Extension occurred at subsequent visits if there were no signs of disease activity. However, on the fourth extension cycle, the maximum interval allowed was 2 weeks less than the maximum interval at which disease activity had previously recurred (referred to as the break point). Participants continued at the break point treatment interval unless they showed signs of disease activity. The investigator was not masked to the treatment that was administered; however, the CRC, BCVA assessors, and participants were masked to the treatment allocation.
Statistical Methods
The sample size of 278 participants was calculated based on the primary end point at 2 years. Assuming a 13% dropout rate over 12 months, a sample size of 278 eyes per arm would provide a power of 80% or greater at the .05 significance level to test a difference in change in visual acuity between ranibizumab and aflibercept of 5 letters (common SD, 12) and to test a difference in injection frequency between ranibizumab and AFL of 0.7 injections (common SD, 2).
Baseline characteristics were assessed using the randomized set (consisting of all randomized participants). The 12-month interim analysis was performed on the full analysis set that included all participants who were randomized who had at least 1 postbaseline efficacy value for the primary end point.
The change in BCVA from baseline to month 12 was analyzed using a random-effects mixed model to account for correlations between repeated measures for the 2 treatment arms with no imputation for missing data. The model included changes in BCVA from baseline to week 4 week 8, and month 12 as a response variable and included baseline BCVA, treatment, visit, and treatment by visit interaction as covariates. The participant was modeled as a random effect. The least squares means and corresponding 95% confidence intervals were estimated for each treatment group. The difference between the ranibizumab and AFL treatment arms with corresponding 95% confidence intervals and P values were also calculated. Additional sensitivity analyses were conducted by imputing missing data using the last observation carried forward method.
The number of injections that were administered over the first 12 months was analyzed using a negative binomial regression model with the follow-up period used as an offset variable. The least squares means and corresponding 95% confidence intervals of the yearly injection rates were estimated for each treatment group. The treatment effect, defined as the rate ratio of ranibizumab vs AFL, and the corresponding 95% confidence intervals and P values were also calculated.
All analyses were performed using SAS, version 9.2 (SAS Institute). Multiplicity adjustment was not applied for multiple end points nor for the interim analysis, which was conducted for information purposes only with no stopping rules.
Results
Participant Dispositions
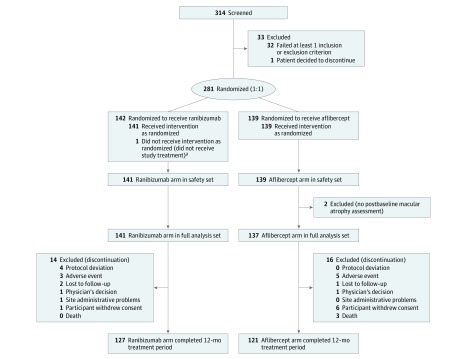
Three hundred fourteen participants were screened and 281 were randomized (ranibizumab, 142 [50.5%]; AFL, 139 [49.5%]; randomized set). Of these 281 participants, 278 (98.9%) were included in the full analysis set (ranibizumab, 141 [50.7%]; aflibercept, 137 [49.2%]). Thirty participants (10.8%) did not complete the first 12 months of the study (ranibizumab, 14 [9.9%]; aflibercept, 16 [11.7%]). Participant dispositions are summarized in the Figure.
Figure. Flow Diagram of Patient Disposition.
The randomized population consisted of all randomized participants. The safety set consists of all participants who received at least 1 application of the study treatment and had at least 1 postbaseline safety assessment. The statement that a participant had no adverse events also constitutes a safety assessment. The full analysis set is composed of all participants who were randomized who had at least 1 postbaseline efficacy value for the primary end point.
aOne participant was excluded from the safety set as the participant did not receive the study treatment.
Demographic and Baseline Characteristics
Baseline characteristics (randomized set) of the 2 treatment groups were similar (Table 1). Participants in the ranibizumab arm had a mean (SD) age of 76.6 (8.5) years and 72 (51%) were women and those in the aflibercept arm had a mean (SD) age of 78.7 (7.5) years and 76 (55%) were women.
Table 1. Demographics and Baseline Characteristics From the Randomized Populationa.
| Parameters | Ranibizumab (n=142) | Aflibercept (n=139) |
|---|---|---|
| Age, y | ||
| Mean (SD) | 76.6 (8.50) | 78.7 (7.45) |
| Median (range) | 78.0 (56.0-94.0) | 79.0 (59.0-94.0) |
| Sex, No. (%) | ||
| Male | 70 (49.3) | 63 (45.3) |
| Female | 72 (50.7) | 76 (54.7) |
| Family history of AMD, No. (%) | ||
| Yes | 30 (21.1) | 26 (18.7) |
| No | 112 (78.9) | 113 (81.3) |
| History of arterial thromboembolic events, No. (%) | ||
| Yes | 13 (9.2) | 23 (16.5) |
| No | 129 (90.8) | 116 (83.5) |
| History of smoking, No. (%) | ||
| Yes | 75 (52.8) | 72 (51.8) |
| No | 67 (47.2) | 67 (48.2) |
| Total BCVA letter score (logMAR) [approximate Snellen equivalent] | ||
| Mean (SD) | 65.0 (15.44) [20/50] | 65.2 (12.56) [20/50] |
| Visual acuity, categorical (20/40), No. (%) | ||
| ≥70 | 68 (47.9) | 60 (43.2) |
| <70 | 74 (52.1) | 79 (56.8) |
| Fellow eye, No. (%) | ||
| Right eye | 77 (54.2) | 73 (52.5) |
| Left eye | 65 (45.8) | 66 (47.5) |
| Treatment ever given to the fellow eye before screening, No. (%) | ||
| No | 118 (83.1) | 121 (87.1) |
| Yes | 24 (16.9) | 18 (12.9) |
Abbreviations: AMD, age-related macular degeneration; BCVA, best-corrected visual acuity; Max, maximum; Min, minimum.
The data presented are reported from the Clinical Research Form.
Mean Changes in BCVA Letter Score From Baseline to Month 12
The mean BCVA letter score (SD [minimum-maximum]; approximate Snellen equivalent) at baseline from the full analysis set was 65.3 (15.1 [23-90]; 20/50; 141 [50.7%]) and 65.1 (12.5 [23-89]; 20/50; 137 [49.2%]) in the ranibizumab and aflibercept arms, respectively, which increased to 72.9 (15.5 [5-95]; 20/32; 127 [45.7%]) and 70.5 (14.6 [36-91]; 20/40; 121 [43.5%]) at month 12 (Table 2), with a mean (SD [minimum-maximum]) change in BCVA letter score of 6.9 (12.3 [−41 to 52]; 127 [45.7%]) in the ranibizumab arm and 5.2 (12.8 [−38 to 37]; 121 [43.5%]) in the aflibercept arm (Table 2; eFigure in Supplement 2). In participants who did not complete the first 12 months of the study, the mean BCVA letter score (approximate Snellen equivalent; mean change from baseline) at their last visit was 66.1 (20/50; 7.1) for the ranibizumab arm and 64.8 (20/50; 2.0) for the aflibercept arm (eTable 2 in Supplement 2).
Table 2. Best-Corrected Visual Acuity Letter Score and Change in BCVA From Baseline to Month 12 for the Full Analysis Set.
| Parameter/Visita | Ranibizumab (n=141) | Aflibercept (n=137) |
|---|---|---|
| Baseline | ||
| BCVA letter score (logMAR) [approximate Snellen equivalent] | ||
| No. | 141 | 137 |
| Mean (SD) | 65.3 (15.10) [20/50] | 65.1 (12.53) [20/50] |
| Median (range) | 68.0 (23 to 90) [20/40] | 67.0 (23 to 89) [20/50] |
| Stratification by BCVA letter score [approximate Snellen equivalent], No. (%) | ||
| <39 [<20/160] | 8 ( 5.7) | 6 ( 4.4) |
| 39-54 [20/160 to <20/80] | 27 ( 19.1) | 19 ( 13.9) |
| 55-69 [20/80 to <20/40] | 38 ( 27.0) | 53 ( 38.7) |
| 70-84 [20/40 to <20/20] | 58 ( 41.1) | 56 ( 40.9) |
| 85-100 [≥20/20] | 10 ( 7.1) | 3 ( 2.2) |
| Week 4 | ||
| BCVA letter score (logMAR) [approximate Snellen equivalent] | ||
| No. | 138 | 137 |
| Mean (SD) | 67.8 (13.53) [20/40] | 65.3 (13.19) [20/50] |
| Median (range) | 71.0 (34 to 94) [20/40] | 68.0 (22 to 88) [20/40] |
| Stratification by BCVA letter score [approximate Snellen equivalent], No. (%) | ||
| <39 [<20/160] | 5 ( 3.6) | 6 ( 4.4) |
| 39-54 [20/160 to <20/80] | 20 ( 14.5) | 25 ( 18.2) |
| 55-69 [20/80 to <20/40] | 40 ( 29.0) | 47 ( 34.3) |
| 70-84 [20/40 to <20/20] | 66 ( 47.8) | 54 ( 39.4) |
| 85-100 [≥20/20] | 7 ( 5.1) | 5 ( 3.6) |
| Change in BCVA letter score from baselineb | ||
| No. | 138 | 137 |
| Mean (SD) | 2.5 (8.47) | 0.2 (8.55) |
| Median (range) | 1.0 (−19 to 39) | 0.0 (−31 to 21) |
| Stratification by change in BCVA letter score, No. (%) | ||
| Gained ≥15 letters | 11 ( 8.0) | 5 ( 3.6) |
| Gained 10-14 letters | 11 ( 8.0) | 10 ( 7.3) |
| Gained 1-9 letters | 56 ( 40.6) | 52 ( 38.0) |
| Lost 0-9 letters | 53 ( 38.4) | 60 ( 43.8) |
| Lost 10-14 letters | 5 ( 3.6) | 3 ( 2.2) |
| Lost ≥15 letters | 2 ( 1.4) | 7 ( 5.1) |
| Week 8 | ||
| BCVA letter score (logMAR) [approximate Snellen equivalent] | ||
| No. | 137 | 137 |
| Mean (SD) | 70.4 (15.31) [20/40] | 68.7 (13.46) [20/40] |
| Median (range) | 76.0 (27 to 94) [20/32] | 72.0 (11 to 90) [20/40] |
| Stratification by BCVA letter score [approximate Snellen equivalent], No. (%) | ||
| <39 [<20/160] | 5 ( 3.6) | 4 ( 2.9) |
| 39-54 [20/160 to <20/80] | 19 ( 13.9) | 15 ( 10.9) |
| 55-69 [20/80 to <20/40] | 29 ( 21.2) | 41 ( 29.9) |
| 70-84 [20/40 to <20/20] | 64 ( 46.7) | 68 ( 49.6) |
| 85-100 [≥20/20] | 20 ( 14.6) | 9 ( 6.6) |
| Change in BCVA letter score from baselineb | ||
| No. | 137 | 137 |
| Mean (SD) | 5.3 (9.07) | 3.6 (10.86) |
| Median (Range) | 4.0 (−15 to 49) | 3.0 (−33 to 42) |
| Stratification by change in BCVA letter score, No. (%) | ||
| Gained ≥15 letters | 18 ( 13.1) | 18 ( 13.1) |
| Gained 10-14 letters | 18 ( 13.1) | 21 ( 15.3) |
| Gained 1-9 letters | 69 ( 50.4) | 48 ( 35.0) |
| Lost 0-9 letters | 27 ( 19.7) | 40 ( 29.2) |
| Lost 10-14 letters | 4 ( 2.9) | 2 ( 1.5) |
| Lost ≥15 letters | 1 ( 0.7) | 8 ( 5.8) |
| Month 12 | ||
| BCVA letter score (logMAR) [approximate Snellen equivalent], No. (%) | ||
| No. | 127 | 121 |
| Mean (SD) | 72.9 (15.54) [20/32] | 70.5 (14.63) [20/40] |
| Median (range) | 78.0 (5 to 95) [20/25] | 74.0 (36 to 91) [20/32] |
| Stratification by BCVA letter score [approximate Snellen equivalent], No. (%) | ||
| <39 [<20/160] | 4 ( 3.1) | 5 ( 4.1) |
| 39-54 [20/160 to <20/80] | 13 ( 10.2) | 13 ( 10.7) |
| 55-69 [20/80 to <20/40] | 22 ( 17.3) | 25 ( 20.7) |
| 70-84 [20/40 to <20/20] | 63 ( 49.6) | 58 ( 47.9) |
| 85-100 [≥20/20] | 25 ( 19.7) | 20 ( 16.5) |
| Change in BCVA letter score from baselineb | ||
| No. | 127 | 121 |
| Mean (SD) | 6.9 (12.25) | 5.2 (12.83) |
| Median (range) | 6.0 (−41 to 52) | 5.0 (−38 to 37) |
| Stratification by change in BCVA letter score, No. (%) | ||
| Gained ≥15 letters | 28 ( 22.0) | 25 ( 20.7) |
| Gained 10-14 letters | 17 ( 13.4) | 20 ( 16.5) |
| Gained 1-9 letters | 52 ( 40.9) | 34 ( 28.1) |
| Lost 0-9 letters | 24 ( 18.9) | 28 ( 23.1) |
| Lost 10-14 letters | 2 ( 1.6) | 8 ( 6.6) |
| Lost ≥15 letters | 4 ( 3.1) | 6 ( 5.0) |
Abbreviation: BCVA, best-corrected visual acuity.
Mandatory visit times are included for the analysis: baseline, week 4, week 8, and month 12.
The last available nonmissing value collected before the start of treatment in the study eye. One participant in the aflibercept arm had baseline BCVA assessed using a Snellen chart instead of a logMAR chart. The BCVA total score for that participant was converted from Snellen to logMAR.
The random-effects mixed model estimated the mean changes in BCVA letter scores from baseline to month 12 to be 7.2 (95% CI, 5.5-8.9) for the ranibizumab arm and 4.9 (95% CI, 3.1-6.6) for the aflibercept arm, with an estimated difference in letter score between the 2 treatment arms of 2.3 (95% CI, −0.1 to 4.7; P=.06) (Table 3). Consistent results were found from a sensitivity analysis by imputing missing data using the last observation carried forward method: the difference in mean change in BCVA letter scores from baseline to month 12 between the 2 treatment arms was estimated to be 2.2 (95% CI, −0.1 to 4.6; P=.06).
Table 3. Mixed-Model Analysis of Changes in Visual Acuity From Baseline to Month 12 for the Full Analysis Seta.
| Characteristic | Ranibizumab (n=141) | Aflibercept (n=137) | Ranibizumab vs Aflibercept | P Value |
|---|---|---|---|---|
| LSM (95% CI) | ||||
| Week 4 | 2.53 (0.88 to 4.17) | 0.14 (-1.51 to 1.79) | NA | NA |
| Week 8 | 5.22 (3.57 to 6.87) | 3.55 (1.89 to 5.20) | NA | |
| Month 12 | 7.16 (5.47 to 8.85) | 4.85 (3.14 to 6.56) | NA | |
| Difference in letter scoreb (95% CI) | ||||
| Week 4 | NA | NA | 2.39 (0.05 to 4.72) | .05 |
| Week 8 | NA | NA | 1.67 (−0.67 to 4.01) | .16 |
| Month 12 | NA | NA | 2.31 (−0.09 to 4.72) | .06 |
Abbreviations: LSM, least squares means; NA, not applicable.
Mixed model: the change from the baseline best-corrected visual acuity = baseline best-corrected visual acuity + treatment + visit + treatment visit + participant (random effect). The baseline best-corrected visual acuity was treated as a continuous variable.
Difference in letter score: ranibizumab-aflibercept.
The estimated mean change in visual acuity letter score at week 4 was 2.5 for the ranibizumab arm (95% CI, 0.9-4.2) and 0.1 for the aflibercept arm (95% CI, 1.5-1.8), with an estimated difference in letter score between the 2 agents of 2.4 (P = .05) (Table 3). There was a slight disproportion in the number of participants who had lost 10 or more letters at week 4 (17 [6.1%]): 7 participants (2.5%) in the ranibizumab arm, and 10 participants (3.6%) in the AFL arm (Table 2), 5 of whom had the greatest visual loss at week 4 of the whole participant population. A review of the baseline and week-4 data of these participants did not identify any anomalies, such as clustering at study sites, baseline characteristics (the presence of macular atrophy or hemorrhage or lesion types), distribution of baseline BCVA, medical history, time to access study treatment, protocol deviations, or adverse events. The estimated mean change in BCVA letter scores at week 8 was 5.2 for the ranibizumab arm (95% CI, 3.6-6.9) and 3.6 for the AFL arm (95% CI, 1.9-5.3), with a difference in letter score estimated to be 1.7 (P = .16) (Table 3).
Number of Injections From Baseline to Month 12
The mean number (number of participants [%]; SD [minimum-maximum]) of intravitreal injections from baseline to month 12 was 9.7 in both the ranibizumab (141 [50.7%]; 2.8 [1-13]) and aflibercept arms (137 [49.2%]; 2.6 [3-13]). The numbers of injections in participants who withdrew before month 12 are described in eTable 3 Supplement 2. A negative binomial regression model was used to estimate the number of injections received per year for each treatment group, including the projected numbers for each participant in the full analysis set who did not complete the study. When applying the model, the estimated mean number of injections received per year remained comparable between the 2 arms: 11.2 (95% CI, 10.9-11.5) for ranibizumab and 11.2 (95% CI, 10.8-11.5) for aflibercept. The rate ratio between the 2 treatment arms was approximately 1.00 (95% CI, 1.0-1.1; P = .86) (Table 4). The distribution of injection intervals at month 12 found that almost half of participants were still on 4 weekly intervals at month 12 in both arms, while 27 eyes (21%) treated with ranibizumab and 16% of eyes treated with aflibercept were on a 12 weekly interval at month 12 (eTable 4 in Supplement 2). The numbers of visits and their distribution did not substantially differ (eTable 5 and eTable 6 in Supplement 2).
Table 4. Number of Injections and Negative Binomial Regression Analysis From Baseline to Month 12 for the Full Analysis Seta,b.
| Characteristic | Treatment Effectc | |
|---|---|---|
| Ranibizumab (n=141) | Aflibercept (n=137) | |
| No. of injections | ||
| Mean (SD) | 9.7 (2.77) | 9.7 (2.55) |
| Median (range) | 10.0 (1-13) | 10.0 (3-13) |
| LSM,d (95% CI) | 11.20 (10.88-11.53) | 11.16 (10.83-11.50) |
Abbreviation: LSM, least squares means.
Model: Number of Injections = Treatment + log (Follow-up Period in Years) (offset).
Follow-up period: from baseline (first dosing) to visit month 12 (exclusive).
Treatment effect: rate ratio of ranibizumab vs aflibercept; treatment effect, 1.00 (95% CI, 0.96-1.05; P = .86).
Unit for least squares means: number of injections per year.
Discussion
A TE regimen can achieve good outcomes with fewer visits in patients with nAMD who have a highly variable need for retreatment.8,9,10 Although TE has become one of the most commonly used regimens for treating nAMD with VEGF inhibitors, few large RCTs have evaluated its outcomes for different agents. The Lucentis Compared to Avastin Study compared bevacizumab and ranibizumab using a TE protocol over 12 months.9 Participants in the ranibizumab arm received a mean of 8.0 injections over 12 months. Participants who were randomized to the TE arm of the Treat and Extend study, which compared monthly with TE ranibizumab for nAMD over 12 months, received a mean of 8.7 injections.10 The overall mean number of injections in the FRB observational study of real-world outcomes was 7.5 injections of ranibizumab over the first 12 months for practitioners who used a TE regimen exclusively11 and 7.8 for eyes receiving aflibercept.12
The mean number of intravitreal injections from baseline to month 12 in this study is higher (9.7 injections, including the first 3 mandatory monthly injections) in both the ranibizumab and aflibercept arms than in other studies of the TE regimen.8,9,10,11,12 This difference is likely because the CRC may have applied retreatment criteria more strictly than in other studies and in routine clinical practice.
The mean number of injections administered in this study was the same for the ranibizumab and aflibercept arms during the initial 12 months of treatment. The mean number of injections was also similar between the 2 agents in the second year of the VEGF Trap-Eye: Investigation of Efficacy and Safety in Wet AMD studies when a pro re nata regimen was applied, with 4.2 injections in the aflibercept group and 4.7 injections in the ranibizumab group.13 Similar results were also observed in the FRB 12-month head-to-head analysis (mean number of injections, 8.1 in the ranibizumab group vs 8.0 in the aflibercept group),14 suggesting that both agents are used similarly when an individualized treatment regimen is applied. Our study did not find any substantial difference between the 2 agents in the change in BCVA from baseline at 12 months, suggesting that neither ranibizumab nor aflibercept are superior to the other regarding their efficacy and duration when used in a TE regimen for nAMD.
The mean baseline BCVA in our study was higher than in other RCTs of patients with nAMD, with about 128 participants (46%) in our study having a BCVA letter score of 70 (20/40) or better at baseline.15,16,17,18,19 This is likely due to the absence of a restriction for how good the visual acuity could be in our study, in combination with the greater public and referrer awareness of nAMD in Australia, ensuring that patients are seen earlier in the course of the disease.20 It has been reported that baseline BCVA is associated with BCVA changes, with eyes having a lower initial BCVA that shows greater mean visual improvements and vice versa.21,22 Despite a high mean BCVA at the baseline, the participants in our study still achieved relatively good gains in visual acuity at month 12. This may be related to the higher frequency of treatment or the restrictions on the presence and size of macular atrophy at baseline in either eye that may have not been imposed in other studies.
The mean change in visual acuity letter score for the aflibercept group at week 4 in this study was low (0.1) compared with that of the ranibizumab group (2.5). We were unable to explain this result at week 4 based on available data. Considering the very good starting visual acuity for both groups, we do not believe that a 2-letter difference between the 2 groups after the first injection is clinically significant.
Limitations
Our study has some limitations. This is an interim analysis of prospectively specified secondary end points. The investigators were not masked; however, the participants and the BCVA assessors were, as was the CRC, which assessed the signs of disease activity on OCT. This interim analysis was prespecified by the study steering committee to look only at injections and visual acuity without reference to images from the CRC, limiting the interpretation of the results that are presented in this article. The full analysis of primary and secondary outcomes, including the structural data as reported by the CRC and safety data, will be reported in the full study analysis.
Conclusions
The main finding from this preplanned interim analysis of the RIVAL study is that visual acuity gains at 12 months were not substantially different between ranibizumab and aflibercept for nAMD when using an identical TE regimen, with both agents requiring the same mean number of injections during the first 12 months of the study. Further follow-up to 2 years may determine if advantages of one over the other can be identified.
Trial protocol.
eTable 1. Inclusion and exclusion criteria from the RIVAL study
eTable 2. Best-corrected visual acuity letter score (BCVA) and Change in BCVA from Baseline to the last visit of participants who withdrew from the study prior to Month 12
eTable 3. Injections in participants who withdrew from the study prior to Month 12.
eTable 4. Distribution injection intervals at Month 12
eTable 5. Number of visits in the first 12 months
eTable 6. Distribution of numbers of visits in the first 12 months.
eFigure. Mean Change in Best-Corrected Visual Acuity from Baseline to Month 12, by Treatment Arm.
eAppendix. List of RIVAL study investigators
Data sharing statement.
References
- 1.US Food and Drug Administration Highlights of prescribing information LUCENTIS® (ranibizumab injection). https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/125156s105lbl.pdf. Accessed August 2, 2017
- 2.U.S. Food and Drug Administration (2011). Highlights of prescribing information EYLEA™ (aflibercept) injection. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/125387lbl.pdf. Accessed August 2, 2017.
- 3.Grunwald JE, Daniel E, Huang J, et al. ; CATT Research Group . Risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2014;121(1):150-161. doi: 10.1016/j.ophtha.2013.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grunwald JE, Pistilli M, Daniel E, et al. ; Comparison of Age-Related Macular Degeneration Treatments Trials Research Group . Incidence and growth of geographic atrophy during 5 years of comparison of age-related macular degeneration treatments trials. Ophthalmology. 2017;124(1):97-104. doi: 10.1016/j.ophtha.2016.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K; SEVEN-UP Study Group . Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology. 2013;120(11):2292-2299. doi: 10.1016/j.ophtha.2013.03.046 [DOI] [PubMed] [Google Scholar]
- 6.Gillies MC, Campain A, Barthelmes D, et al. ; Fight Retinal Blindness Study Group . Long-term outcomes of treatment of neovascular age-related macular degeneration: data from an observational study. Ophthalmology. 2015;122(9):1837-1845. doi: 10.1016/j.ophtha.2015.05.010 [DOI] [PubMed] [Google Scholar]
- 7.Maguire MG, Martin DF, Ying GS, et al. ; Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group . Five-year outcomes with anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration: the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2016;123(8):1751-1761. doi: 10.1016/j.ophtha.2016.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gemenetzi M, Patel PJ. A systematic review of the treat and extend treatment regimen with anti-VEGF agents for neovascular age-related macular degeneration. Ophthalmol Ther. 2017;6(1):79-92. doi: 10.1007/s40123-017-0087-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berg K, Pedersen TR, Sandvik L, Bragadóttir R. Comparison of ranibizumab and bevacizumab for neovascular age-related macular degeneration according to LUCAS treat-and-extend protocol. Ophthalmology. 2015;122(1):146-152. doi: 10.1016/j.ophtha.2014.07.041 [DOI] [PubMed] [Google Scholar]
- 10.Silva R, Berta A, Larsen M, Macfadden W, Feller C, Monés J; TREND Study Group . Treat-and-extend versus monthly regimen in neovascular age-related macular degeneration: results with ranibizumab from the TREND study. Ophthalmology. 2018;125(1):57-65. doi: 10.1016/j.ophtha.2017.07.014 [DOI] [PubMed] [Google Scholar]
- 11.Arnold JJ, Campain A, Barthelmes D, et al. ; Fight Retinal Blindness Study Group . Two-year outcomes of “treat and extend” intravitreal therapy for neovascular age-related macular degeneration. Ophthalmology. 2015;122(6):1212-1219. doi: 10.1016/j.ophtha.2015.02.009 [DOI] [PubMed] [Google Scholar]
- 12.Barthelmes D, Nguyen V, Daien V, et al. Two year outcomes of “treat and extend” intravitreal therapy using aflibercept preferentially for neovascular age-related macular degeneration. Retina. 2018;38(1):20-28. doi: 10.1097/IAE.0000000000001496 [DOI] [PubMed] [Google Scholar]
- 13.Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology. 2014;121(1):193-201. doi: 10.1016/j.ophtha.2013.08.011 [DOI] [PubMed] [Google Scholar]
- 14.Gillies MC, Nguyen V, Daien V, Arnold JJ, Morlet N, Barthelmes D. Twelve-month outcomes of ranibizumab vs. aflibercept for neovascular age-related macular degeneration: data from an observational study. Ophthalmology. 2016;123(12):2545-2553. doi: 10.1016/j.ophtha.2016.08.016 [DOI] [PubMed] [Google Scholar]
- 15.Martin DF, Maguire MG, Fine SL, et al. ; Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group . Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119(7):1388-1398. doi: 10.1016/j.ophtha.2012.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heier JS, Brown DM, Chong V, et al. ; VIEW 1 and VIEW 2 Study Groups . Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537-2548. doi: 10.1016/j.ophtha.2012.09.006 [DOI] [PubMed] [Google Scholar]
- 17.Busbee BG, Ho AC, Brown DM, et al. ; HARBOR Study Group . Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2013;120(5):1046-1056. doi: 10.1016/j.ophtha.2012.10.014 [DOI] [PubMed] [Google Scholar]
- 18.Rosenfeld PJ, Brown DM, Heier JS, et al. ; MARINA Study Group . Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419-1431. doi: 10.1056/NEJMoa054481 [DOI] [PubMed] [Google Scholar]
- 19.Brown DM, Kaiser PK, Michels M, et al. ; ANCHOR Study Group . Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432-1444. doi: 10.1056/NEJMoa062655 [DOI] [PubMed] [Google Scholar]
- 20.Heraghty J, Cummins R. A layered approach to raising public awareness of macular degeneration in Australia. Am J Public Health. 2012;102(9):1655-1659. doi: 10.2105/AJPH.2012.300657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Regillo CD, Busbee BG, Ho AC, Ding B, Haskova Z. Baseline predictors of 12-month treatment response to ranibizumab in patients with wet age-related macular degeneration. Am J Ophthalmol. 2015;160(5):1014-1023.e2. doi: 10.1016/j.ajo.2015.07.034 [DOI] [PubMed] [Google Scholar]
- 22.Ying GS, Huang J, Maguire MG, et al. ; Comparison of Age-related Macular Degeneration Treatments Trials Research Group . Baseline predictors for one-year visual outcomes with ranibizumab or bevacizumab for neovascular age-related macular degeneration. Ophthalmology. 2013;120(1):122-129. doi: 10.1016/j.ophtha.2012.07.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol.
eTable 1. Inclusion and exclusion criteria from the RIVAL study
eTable 2. Best-corrected visual acuity letter score (BCVA) and Change in BCVA from Baseline to the last visit of participants who withdrew from the study prior to Month 12
eTable 3. Injections in participants who withdrew from the study prior to Month 12.
eTable 4. Distribution injection intervals at Month 12
eTable 5. Number of visits in the first 12 months
eTable 6. Distribution of numbers of visits in the first 12 months.
eFigure. Mean Change in Best-Corrected Visual Acuity from Baseline to Month 12, by Treatment Arm.
eAppendix. List of RIVAL study investigators
Data sharing statement.