Key Points
Question
Is the quality of reperfusion rated with the thrombolysis in cerebral infarction score associated with longer hospital arrival to groin puncture time?
Findings
In this meta-analysis, the rate of successful reperfusion, defined as a thrombolysis in cerebral infarction score of 2b-3 at the end of the procedure, decreased as time elapsed after arrival at the stroke endovascular center.
Meaning
Fast reperfusion is a major modifiable factor associated with better clinical outcome when successful reperfusion is achieved, and the intermediary outcome, the rate of successful reperfusion, is higher with faster in-hospital process times.
Abstract
Importance
Reperfusion is a key factor for clinical outcome in patients with acute ischemic stroke (AIS) treated with endovascular thrombectomy (EVT) for large-vessel intracranial occlusion. However, data are scarce on the association between the time from onset and reperfusion results.
Objective
To analyze the rate of reperfusion after EVT started at different intervals after symptom onset in patients with AIS.
Design, Setting, and Participants
We conducted a meta-analysis of individual patient data from 7 randomized trials of the Highly Effective Reperfusion Using Multiple Endovascular Devices (HERMES) group. This is a multicenter cohort study of the intervention arm of randomized clinical trials included in the HERMES group. Patients with anterior circulation AIS who underwent EVT for M1/M2 or intracranial carotid artery occlusion were included. Each trial enrolled patients according to its specific inclusion and exclusion criteria. Data on patients eligible but not enrolled (eg, refusals or exclusions) were not available. All analyses were performed by the HERMES biostatistical core laboratory using the pooled database. Data were analyzed between December 2010 and April 2015.
Main Outcomes and Measures
Successful reperfusion was defined as a modified thrombolysis in cerebral infarction score of 2b/3 at the end of the EVT procedure adjusted for age, occlusion location, pretreatment intravenous thrombolysis, and clot burden score and was analyzed in relation to different intervals (onset, emergency department arrival, imaging, and puncture) using mixed-methods logistic regression.
Results
Among the 728 included patients, with a mean (SD) age of 65.4 (13.5) years and of whom 345 were female (47.4%), decreases in rates of successful reperfusion defined as a thrombolysis in cerebral infarction score of 2b/3 were observed with increasing time from admission or first imaging to groin puncture. The magnitude of effect was a 22% relative reduction (odds ratio, 0.78; 95% CI, 0.64-0.95) per additional hour between admission and puncture and a 26% relative reduction (odds ratio, 0.74; 95% CI, 0.59-0.93) per additional hour between imaging and puncture.
Conclusions and Relevance
Because the probability of reperfusion declined significantly with time between hospital arrival and groin puncture, we provide additional arguments for minimizing the intervals after symptom onset in anterior circulation acute ischemic stroke.
This meta-analysis of individual patient data from 7 randomized clinical trials analyzes the rate of reperfusion after endovascular thrombectomy started at different intervals after symptom onset in patients with acute ischemic stroke.
Introduction
The challenges in the field of acute ischemic stroke (AIS) owing to large-vessel occlusion (LVO) focus on reducing time to reperfusion, optimizing imaging methods for patient selection, and evaluating the best technical approach.1,2 Reperfusion is significantly associated with clinical outcome in patients undergoing endovascular thrombectomy (EVT).3,4 Reperfusion is commonly scored with the modified thrombolysis in cerebral infarction (mTICI) grading scale, with 0 indicating persistent complete occlusion and 3 indicating complete reperfusion.5 Because grade 2b was shown to be the best cutoff for predicting favorable outcome at 90 days, grades 2b and 3 are termed successful reperfusion.6,7 A pooled analysis of the first 5 randomized clinical stroke trials, which predominantly used stent retrievers as the primary approach, demonstrated that successful reperfusion was obtained in 71% of patients, whereas the rate of mTICI 0 to 2a varied from 12% to 41%.1 Successful reperfusion is influenced by device choice and strategy, use of intravenous (IV) alteplase, collateral status, and thrombus size, location, or composition.8,9,10,11,12,13,14,15 Thrombus composition and characteristics may change rapidly over time after occlusion.16 Although time to successful reperfusion strongly affects clinical outcome,2 few data exist describing the effect of time on the rate of successful reperfusion. In this meta-analysis of the HERMES population, we aimed to analyze the rate of successful reperfusion as a function of interval times in patients with AIS-LVO treated with EVT.
Methods
Patients in the intervention (EVT) arms of randomized clinical trials of the HERMES group (Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands [MR CLEAN]; Endovascular Treatment for Small Core and Anterior Circulation Proximal Occlusion with Emphasis on Minimizing CT to Recanalization Times [ESCAPE]; Thrombectomie des Artères Cerebrales [THRACE] trial; The Pragmatic Ischaemic Thrombectomy Evaluation [PISTE]; Extending the Time for Thrombolysis in Emergency Neurological Deficits–Intra-Arterial [EXTEND-IA]; Randomized Trial of Revascularization with Solitaire FR Device versus Best Medical Therapy in the Treatment of Acute Stroke Due to Anterior Circulation Large Vessel Occlusion Presenting Within Eight Hours of Symptom Onset [REVASCAT]; and Solitaire with the Intention for Thrombectomy as Primary Endovascular Treatment [SWIFT PRIME] trial) were included. Patients with AIS-LVO who had M1/M2 or intracranial carotid artery (ICA) occlusion for whom reperfusion results were assessed by a separate core laboratory for HERMES (not only the core laboratory of each individual study) were also included. A complete list of investigators in the HERMES group can be found in the eAppendix in the Supplement. Each trial enrolled patients according to its specific inclusion and exclusion criteria: SWIFT PRIME, with 195 patients from December 2012 through November 2014 in the United States and Europe; ESCAPE, with 315 patients from February 2013 through October 2014 in Canada, the United States, South Korea, Ireland, and the United Kingdom; EXTEND-IA: with 70 patients from August 2012 through October 2014 in Australia and New Zealand; REVASCAT, with 207 patients from November 2012 through December 2014 in Catalonia, Spain; MR CLEAN, with 500 patients between December 2010 and March 2014 in the Netherlands; PISTE, with 65 patients between April 2013 and April 2015 in the United Kingdom; and THRACE, with 412 patients between June 2010 and February 2015 in France. Data on patients eligible but not enrolled (eg, refusals or exclusions) were not available. The primary end point was the rate of successful reperfusion, defined as an mTICI 2b/3 at the end of the EVT procedure (eTable 1 in the Supplement). We also evaluated the potential association between different intervals from onset to groin puncture and the clot burden score (CBS). The CBS was scored on a scale of 0 to 10, according to Puetz et al,17 with a score of 2 subtracted if the thrombus was found in either of the supraclinoid ICAs or the proximal or the distal half of the MCA trunk, and a score of 1 subtracted if the thrombus was found in the infraclinoid ICA, anterior cerebral artery, and for each affected M2 branch. Thus, a score of 10 indicates absence of thrombus and a score of 0 indicates a complete multisegment occlusion of the anterior circulation. Interval times were defined according to individual trial definitions. All participants provided written informed consent according to each trial protocol, and each study was approved by the local ethics board.
Statistical Analysis
Probability of successful reperfusion as a function of time was analyzed using mixed-methods logistic regression, with trial as a random effect. Models were constructed for the dependence of the log odds of reperfusion on time intervals including time from stroke onset,1 arrival at the emergency department,2 and imaging to arterial puncture.3 Potential nonlinear effects of time were also investigated, including exploratory nonlinear models using locally weighted scatterplot smoother regression.
Logistic regression models were adjusted for age (a linear variable), centrally adjudicated target occlusion location (a 3-level categorical variable: ICA, M1 middle cerebral artery [MCA], and M2 MCA), and pretreatment IV tissue plasminogen activator (binary variable). In addition to the full cohort, subgroup analyses were conducted to examine the association between reperfusion and time in patients imaged either with computed tomography or magnetic resonance imaging.
Effect size estimates are provided with their corresponding 95% confidence intervals; P values are 2-sided with values less than .05 considered statistically significant. Statistical analyses were performed in SAS, version 9.4 (SAS Institute Inc). Graphic output was obtained from R, version 3.3 (R Foundation for Statistical Computing).
Results
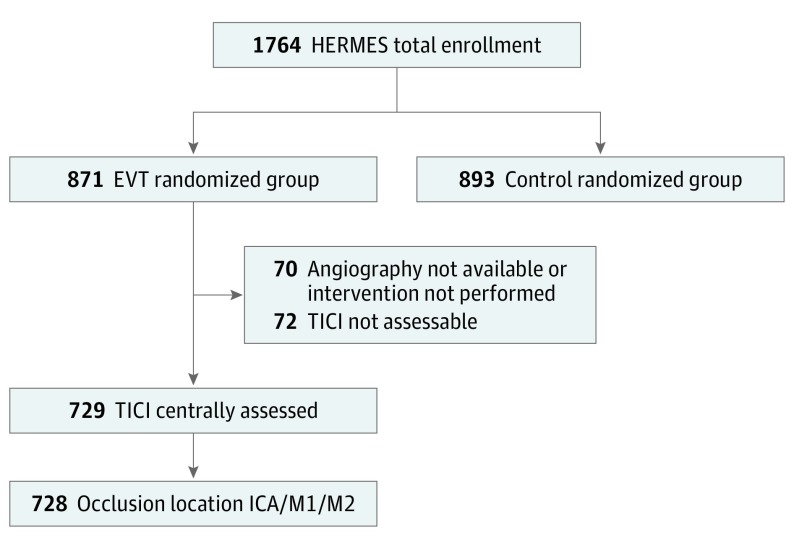
Among 871 patients assigned to endovascular therapy across the 7 HERMES trials, 729 had mTICI scores assessed by the central imaging core laboratory and 728 had ICA or MCA occlusion; these 728 patients constituted the primary analysis set for this report (Figure 1). Demographics and baseline characteristics are presented in Table 1. Median onset to arterial access time was 239 minutes (interquartile range [IQR], 184-299 minutes).
Figure 1. Study Flowchart.
EVT indicates endovascular treatment; ICA, intracranial carotid artery; M1/M2, first and second segment of the middle cerebral artery; TICI, thrombolysis in cerebral infarction.
Table 1. Baseline Characteristics of Included Patients.
| Characteristic | Mean (SD) | No. | Median (IQR) | No./Total No. (%) |
|---|---|---|---|---|
| Age, y | 65.4 (13.5) | 728 | 67.0 (56.8-76.0) | NA |
| Female | NA | 728 | NA | 345/728 (47.4) |
| Hypertension | NA | 728 | NA | 388/726 (53.4) |
| Hyperlipidemia | NA | 728 | NA | 246/712 (34.6) |
| Diabetes mellitus | NA | 728 | NA | 107/727 (14.7) |
| Atrial fibrillation | NA | 728 | NA | 205/591 (34.7) |
| NIHSS score at baseline | NA | 728 | 17.0 (14.0-20.0) | NA |
| ASPECTS at baseline | NA | 728 | 8.0 (7.0-9.0) | NA |
| Onset to arterial puncture, min | 250.4 (95.6) | 714 | 239.0 (184.3-299.0) | NA |
Abbreviations: ASPECTS, Alberta Stroke Program Early CT Score; IQR, interquartile range; NA, not applicable; NIHSS, National Institutes of Health Stroke Scale.
Successful reperfusion was associated with shorter times to arterial access (Figure 2; Table 2). Onset to arterial access time was not associated with mTICI 2b/3 reperfusion, but both computed tomography to arterial access and door to arterial access were. This effect persisted in subgroups of patients imaged with computed tomography and magnetic resonance imaging, where odds ratios within the subgroups were similar to those observed in the overall cohort.
Figure 2. Modeled Probability of Thrombolysis in Cerebral Infarction (TICI) Score of 2b/3 by Workflow Time.
Modeled probabilities of TICI score of 2b/3 outcome by workflow time, computed using logistic regression with covariates analogous to those in Table 2. Results are displayed as the point estimate for probability of TICI 2b/3 modeled by time along with the corresponding 95% confidence interval. ED indicates emergency department.
Table 2. Association of Delays in Workflow With TICI 2b/3 Outcomea.
| Outcome/Predictor | No. | OR (95% CI) | P Value |
|---|---|---|---|
| TICI 2b/3 by workflow predictors: all patients (adjustments: age, alteplase, and occlusion location) | |||
| Onset to arterial puncture, per 60 min | 714 | 0.92 (0.81-1.03) | .15 |
| Imaging to arterial puncture, per 60 min | 670 | 0.74 (0.59-0.93) | .01 |
| ED arrival to arterial puncture, per 60 min | 680 | 0.78 (0.64-0.95) | .01 |
| TICI 2b/3 by workflow predictors: CT (adjustments: age, alteplase, occlusion location, and collateral grade) | |||
| Onset to arterial puncture, per 60 min | 541 | 0.93 (0.82-1.06) | .26 |
| Imaging to arterial puncture, per 60 min | 499 | 0.83 (0.64-1.08) | .17 |
| ED arrival to arterial puncture, per 60 min | 508 | 0.81 (0.65-1.02) | .07 |
| TICI 2b/3 by workflow predictors: MR (adjustments: occlusion location only due to sample size) | |||
| Onset to arterial puncture, per 60 min | 117 | 0.97 (0.64-1.48) | .89 |
| Imaging to arterial puncture, per 60 min | 117 | 0.54 (0.30-0.99) | .05 |
| ED arrival to arterial puncture, per 60 min | 117 | 0.60 (0.34-1.07) | .09 |
Abbreviations: CT, computed tomography; ED, emergency department; MR, magnetic resonance; OR, odds ratio; TICI, thrombolysis in cerebral infarction.
The association of time with the binary outcome of TICI 2b/3 following the procedure, including odds ratios (where values <1 represent diminishing rates of TICI 2b/3 as time increases), their corresponding 95% confidence intervals, and P values. Odds ratios are scaled per 60 minutes of delay in each listed interval and are computed via logistic regression including the listed covariate adjustments.
Observed rates of reperfusion in various time subgroups are summarized in Table 2, while modeled probabilities of reperfusion with time as a continuous predictor are displayed in Figure 2. Similar to the analysis of odds ratios in previous paragraphs, rates of TICI 2b/3 decreased with longer time since onset, with odds ratio 0.78 (95% CI, 0.64-0.95) per additional hour in admission to puncture and odds ratio of 0.74 (95% CI, 0.59-0.93) per additional hour in imaging-to-puncture delays. In this study, every additional hour between arrival at the emergency department and groin puncture was associated with a 22% reduction in the odds of TICI 2b/3 reperfusion. Furthermore, every additional hour between imaging and groin puncture was associated with a 26% reduction in the odds of TICI 2b/3 reperfusion; this time interval had the strongest association with final reperfusion. One notable exception was the subgroup of onset to arterial puncture of more than 360 minutes (6 hours), for which reperfusion rates were substantially higher than for patients with onset to puncture between 300 and 360 minutes. An increased reperfusion rate in late time intervals was not observed with intervals beginning with imaging or emergency department arrival times. Last, the CBS did not vary by intervals from onset to groin puncture (eTable 2 in the Supplement).
Discussion
The main finding of this study is that the rate of successful reperfusion, defined as an mTICI 2b/3 at the end of the procedure, decreased as time elapsed after arrival at the stroke endovascular center. Hence, time is doubly important in the setting of AIS-LVO. First, the time to effective reperfusion is a major potentially modifiable factor associated with better clinical outcomes, and second, the intermediary outcome, the rate of successful reperfusion, is higher with faster in-hospital process times. A component of improved outcomes owing to overall faster onset to reperfusion times is faster in-hospital treatment times. Our results could be confusing compared with the results of the late time studies.18,19 Importantly, even if the reperfusion rate declines as time elapses, patients recanalized in later times continue to have better clinical outcome compared with those without reperfusion.
Notably, the total onset-to-arterial access time was not statistically associated with reperfusion outcomes in this analysis, possibly because several of the trials used imaging selection criteria to choose patients (thereby selecting those more likely to be slow progressors), and 1 trial examined an extended 12-hour eligibility window from stroke onset. Long times from onset to arterial access in some cases could be dominated by onset to hospital arrival time even when in-hospital process times were short, and this patient group may have had additionally rigorous selection prior to transfer, diluting any possible demonstration of effect. Similarly, patients in later time epochs might have had blood flow stasis and new thrombus formation, increasing the total thrombus burden around the original thrombus.20 However, in our analysis, we did not find any association between CBS and time between onset and imaging.
Evolution of thrombus composition and properties could also explain an increase rate of EVT failure over time. Studies on human thrombus retrieved from patients with AIS-LVO have revealed varying compositions.21,22,23 As time elapses, the biochemical composition of thrombus changes,24 the hemoglobin passes through several forms prior to red blood cell lysis and break down into ferritin and hemosiderin.16 Afterwards, activation of coagulation pathways results in the formation of hemostatic fibrin plugs, and red blood cells become trapped within a fibrin mesh.25,26 This modification of thrombus properties might account for the difference in reperfusion rates with increasing time from onset. Indeed, a fibrin-rich thrombus can be difficult to engage in the stent retriever and is more adherent to the vessel wall.11,12
Neutrophil extracellular traps form through the release of decondensed chromatin that is lined with granular components. Apart from thrombus modification over time, neutrophil extracellular traps have been identified as key players involved in the formation of thrombi of various origins and in their adhesion to the vessel wall.27 Neutrophil extracellular traps are fibrous networks that form through the release of extracellular DNA from neutrophils, contributing to the scaffold of thrombus. As time elapses after occlusion, extracellular DNA and histones modify the structure of fibrin, rendering it more resistant to mechanical and enzymatic destruction. Neutrophil extracellular traps may participate in the interaction between the thrombus and the arterial wall or between the thrombus and the EVT device, thus increasing the difficulty of thrombus removal during EVT. The link between occlusion duration and neutrophil extracellular trap content was supported by a previous study,28 demonstrating the need for a higher number of device passes to achieve a successful reperfusion.
While the probability of successful reperfusion decreased in our study with all intervals, the association was much more pronounced when arrival at the emergency department or imaging to groin puncture were considered compared with onset to groin puncture. These results are similar to those described in a previous study2 that analyzed intervals and clinical outcomes. A potential explanation is the differential reliability of documented times for stroke onset vs emergency department arrival. Time of emergency department arrival is generally accurately documented in patient medical records. In contrast, the time of stroke onset (last known well) is often imprecisely determined or documented.29 This emphasizes the need for improvement of modifiable in-hospital delays and may include intervals for patient transfer, imaging, or procedural factors, although it is not possible to define from the data available which specific components might be most usefully modified.
One notable exception to the association between time and successful reperfusion was the subgroup in whom onset to arterial puncture was more than 360 minutes (6 hours), for which the reperfusion rate was substantially higher than for patients with onset-to-puncture times between 300 and 360 minutes. This pattern of substantially increased reperfusion in late time windows was not observed with the imaging or emergency department arrival-to-puncture intervals. This observation may be an artifact of the definition of stroke-onset time as the last time the patient was seen well. Two trials, ESCAPE and REVASCAT, enrolled patients later than 6 hours from stroke onset and in so doing included patients with unwitnessed stroke, whose true stroke onset to treatment time was likely shorter.
Strengths and Limitations
Our analysis has several strengths because we were able to adjust our results for known factors of reperfusion, including intravenous alteplase treatment prior to EVT30,31,32 and (in the computed tomography–selected subgroup) collateral status.33,34 Potential limitations include differences in study entry criteria and patient characteristics among the trials that are a source of potential bias. Second, owing to multiple imaging modalities performed in the different trials, thrombus imaging characteristics were not analyzed but may have influenced reperfusion rates.35,36,37 Third, a 2018 study9 demonstrates that the number of EVT passes, an unreported variable in these studies, is itself associated with the probability of successful reperfusion.9 Last, we were not able to analyze EVT procedural detail such as device selection, use of contact aspiration alone or combined with stent-retriever, or the use of balloon guide catheter.
Conclusions
In this post hoc analysis of the HERMES population, the probability of reperfusion declined significantly with time between hospital arrival and groin puncture. We provide additional arguments for minimizing the time intervals after symptom onset in anterior circulation AIS-LVO. Hence, the importance of reducing intrahospital delays after onset of symptom is highlighted because the rate of successful reperfusion itself is directly affected by shorter time intervals before groin puncture.
eAppendix. The HERMES Investigator List
eTable 1. Thrombolysis In Cerebral Infarction (TICI) Scale
eTable 2. Clot Burden Score by Time Interval of Onset to Arterial Puncture
References
- 1.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES collaborators . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 2.Saver JL, Goyal M, van der Lugt A, et al. ; HERMES Collaborators . Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA. 2016;316(12):1279-1288. doi: 10.1001/jama.2016.13647 [DOI] [PubMed] [Google Scholar]
- 3.Manning NW, Chapot R, Meyers PM. Endovascular stroke management: key elements of success. Cerebrovasc Dis. 2016;42(3-4):170-177. doi: 10.1159/000445449 [DOI] [PubMed] [Google Scholar]
- 4.Rha J-H, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007;38(3):967-973. doi: 10.1161/01.STR.0000258112.14918.24 [DOI] [PubMed] [Google Scholar]
- 5.Higashida R, Furlan A, Roberts H, et al. ; Technology Assessment Committees of the American Society of Interventional and Therapeutic Neuroradiology and the Society of Interventional Radiology . Trial design and reporting standards for intraarterial cerebral thrombolysis for acute ischemic stroke. J Vasc Interv Radiol. 2003;14(9 Pt 2):S493-S494. [DOI] [PubMed] [Google Scholar]
- 6.Yoo AJ, Simonsen CZ, Prabhakaran S, et al. ; Cerebral Angiographic Revascularization Grading Collaborators . Refining angiographic biomarkers of revascularization: improving outcome prediction after intra-arterial therapy. Stroke. 2013;44(9):2509-2512. doi: 10.1161/STROKEAHA.113.001990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jayaraman MV, Grossberg JA, Meisel KM, Shaikhouni A, Silver B. The clinical and radiographic importance of distinguishing partial from near-complete reperfusion following intra-arterial stroke therapy. AJNR Am J Neuroradiol. 2013;34(1):135-139. doi: 10.3174/ajnr.A3278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoo AJ, Andersson T. Thrombectomy in acute ischemic stroke: challenges to procedural success. J Stroke. 2017;19(2):121-130. doi: 10.5853/jos.2017.00752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seker F, Pfaff J, Wolf M, et al. . Correlation of thrombectomy maneuver count with recanalization success and clinical outcome in patients with ischemic stroke. AJNR Am J Neuroradiol. 2017;38(7):1368-1371. doi: 10.3174/ajnr.A5212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turk AS, Frei D, Fiorella D, et al. . ADAPT FAST study: a direct aspiration first pass technique for acute stroke thrombectomy. J Neurointerv Surg. 2014;6(4):260-264. doi: 10.1136/neurintsurg-2014-011125 [DOI] [PubMed] [Google Scholar]
- 11.Machi P, Jourdan F, Ambard D, et al. . Experimental evaluation of stent retrievers’ mechanical properties and effectiveness. J Neurointerv Surg. 2017;9(3):257-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fennell VS, Setlur Nagesh SV, Meess KM, et al. . What to do about fibrin rich ‘tough clots’? comparing the Solitaire stent retriever with a novel geometric clot extractor in an in vitro stroke model. J Neurointerv Surg. 2018;10(9):907-910. [DOI] [PubMed] [Google Scholar]
- 13.Kim SK, Yoon W, Heo TW, Park MS, Kang HK. Negative susceptibility vessel sign and underlying intracranial atherosclerotic stenosis in acute middle cerebral artery occlusion. AJNR Am J Neuroradiol. 2015;36(7):1266-1271. doi: 10.3174/ajnr.A4280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broeg-Morvay A, Mordasini P, Bernasconi C, et al. . Direct mechanical intervention versus combined intravenous and mechanical intervention in large artery anterior circulation stroke: a matched-pairs analysis. Stroke. 2016;47(4):1037-1044. doi: 10.1161/STROKEAHA.115.011134 [DOI] [PubMed] [Google Scholar]
- 15.Bourcier R, Alexandre PL, Eugène F, et al. . Is bridging therapy still required in stroke due to carotid artery terminus occlusions? J Neurointerv Surg. 2018;10(7):625-628. [DOI] [PubMed] [Google Scholar]
- 16.Nosaka M, Ishida Y, Kimura A, Kondo T. Time-dependent organic changes of intravenous thrombi in stasis-induced deep vein thrombosis model and its application to thrombus age determination. Forensic Sci Int. 2010;195(1-3):143-147. doi: 10.1016/j.forsciint.2009.12.008 [DOI] [PubMed] [Google Scholar]
- 17.Puetz V, Dzialowski I, Hill MD, et al. ; Calgary CTA Study Group . Intracranial thrombus extent predicts clinical outcome, final infarct size and hemorrhagic transformation in ischemic stroke: the clot burden score. Int J Stroke. 2008;3(4):230-236. doi: 10.1111/j.1747-4949.2008.00221.x [DOI] [PubMed] [Google Scholar]
- 18.Nogueira RG, Jadhav AP, Haussen DC, et al. ; DAWN Trial Investigators . Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11-21. [DOI] [PubMed] [Google Scholar]
- 19.Albers GW, Marks MP, Kemp S, et al. ; DEFUSE 3 Investigators . Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qazi EM, Sohn SI, Mishra S, et al. . Thrombus characteristics are related to collaterals and angioarchitecture in acute stroke. Can J Neurol Sci. 2015;42(6):381-388. [DOI] [PubMed] [Google Scholar]
- 21.Brinjikji W, Duffy S, Burrows A, et al. . Correlation of imaging and histopathology of thrombi in acute ischemic stroke with etiology and outcome: a systematic review. J Neurointerv Surg. 2017;9(6):529-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marder VJ, Chute DJ, Starkman S, et al. . Analysis of thrombi retrieved from cerebral arteries of patients with acute ischemic stroke. Stroke. 2006;37(8):2086-2093. doi: 10.1161/01.STR.0000230307.03438.94 [DOI] [PubMed] [Google Scholar]
- 23.Simons N, Mitchell P, Dowling R, Gonzales M, Yan B. Thrombus composition in acute ischemic stroke: a histopathological study of thrombus extracted by endovascular retrieval. J Neuroradiol. 2015;42(2):86-92. doi: 10.1016/j.neurad.2014.01.124 [DOI] [PubMed] [Google Scholar]
- 24.Kirchhof K, Welzel T, Mecke C, Zoubaa S, Sartor K. Differentiation of white, mixed, and red thrombi: value of CT in estimation of the prognosis of thrombolysis phantom study. Radiology. 2003;228(1):126-130. doi: 10.1148/radiol.2273020530 [DOI] [PubMed] [Google Scholar]
- 25.Blinc A, Keber D, Lahajnar G, Zupancic I, Zorec-Karlovsek M, Demsar F. Magnetic resonance imaging of retracted and nonretracted blood clots during fibrinolysis in vitro. Haemostasis. 1992;22(4):195-201. [DOI] [PubMed] [Google Scholar]
- 26.Lipinski B, Pretorius E, Oberholzer HM, van der Spuy WJ. Interaction of fibrin with red blood cells: the role of iron. Ultrastruct Pathol. 2012;36(2):79-84. doi: 10.3109/01913123.2011.627491 [DOI] [PubMed] [Google Scholar]
- 27.Laridan E, Denorme F, Desender L, et al. . Neutrophil extracellular traps in ischemic stroke thrombi. Ann Neurol. 2017;82(2):223-232. doi: 10.1002/ana.24993 [DOI] [PubMed] [Google Scholar]
- 28.Ducroux C, Di Meglio L, Loyau S, et al. . Thrombus neutrophil extracellular traps content impair tpa-induced thrombolysis in acute ischemic stroke. Stroke. 2018;49(3):754-757. doi: 10.1161/STROKEAHA.117.019896 [DOI] [PubMed] [Google Scholar]
- 29.Spokoyny I, Raman R, Ernstrom K, Kim AJ, Meyer BC, Karanjia NP. Accuracy of first recorded “last known normal” times of stroke code patients. J Stroke Cerebrovasc Dis. 2015;24(11):2467-2473. doi: 10.1016/j.jstrokecerebrovasdis.2015.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Behme D, Kabbasch C, Kowoll A, et al. . Intravenous thrombolysis facilitates successful recanalization with stent-retriever mechanical thrombectomy in middle cerebral artery occlusions. J Stroke Cerebrovasc Dis. 2016;25(4):954-959. doi: 10.1016/j.jstrokecerebrovasdis.2016.01.007 [DOI] [PubMed] [Google Scholar]
- 31.Leker RR, Pikis S, Gomori JM, Cohen JE. Is bridging necessary? a pilot study of bridging versus primary stentriever-based endovascular reperfusion in large anterior circulation strokes. J Stroke Cerebrovasc Dis. 2015;24(6):1163-1167. doi: 10.1016/j.jstrokecerebrovasdis.2015.01.008 [DOI] [PubMed] [Google Scholar]
- 32.Pfefferkorn T, Holtmannspötter M, Patzig M, et al. . Preceding intravenous thrombolysis facilitates endovascular mechanical recanalization in large intracranial artery occlusion. Int J Stroke. 2012;7(1):14-18. doi: 10.1111/j.1747-4949.2011.00639.x [DOI] [PubMed] [Google Scholar]
- 33.Leng X, Fang H, Leung TWH, et al. . Impact of collateral status on successful revascularization in endovascular treatment: a systematic review and meta-analysis. Cerebrovasc Dis. 2016;41(1-2):27-34. doi: 10.1159/000441803 [DOI] [PubMed] [Google Scholar]
- 34.Liebeskind DS, Jahan R, Nogueira RG, Zaidat OO, Saver JL; SWIFT Investigators . Impact of collaterals on successful revascularization in Solitaire FR with the intention for thrombectomy. Stroke. 2014;45(7):2036-2040. doi: 10.1161/STROKEAHA.114.004781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bourcier R, Brecheteau N, Costalat V, et al. . MRI quantitative T2* mapping on thrombus to predict recanalization after endovascular treatment for acute anterior ischemic stroke. J Neuroradiol. 2017;44(4):241-246. doi: 10.1016/j.neurad.2017.03.006 [DOI] [PubMed] [Google Scholar]
- 36.Bourcier R, Volpi S, Guyomarch B, et al. . Susceptibility vessel sign on MRI predicts favorable clinical outcome in patients with anterior circulation acute stroke treated with mechanical thrombectomy. AJNR Am J Neuroradiol. 2015;36(12):2346-2353. doi: 10.3174/ajnr.A4483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bourcier R, Mazighi M, Labreuche J, et al. ; ASTER Trial Investigators . Susceptibility vessel sign in the ASTER trial: higher recanalization rate and more favourable clinical outcome after first line stent retriever compared to contact aspiration. J Stroke. 2018;20(2):268-276. doi: 10.5853/jos.2018.00192 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. The HERMES Investigator List
eTable 1. Thrombolysis In Cerebral Infarction (TICI) Scale
eTable 2. Clot Burden Score by Time Interval of Onset to Arterial Puncture