Abstract
Porphyromonas gingivalis is one of the most important Gram-negative anaerobe bacteria involved in the pathogenesis of periodontitis. P. gingivalis has an arsenal of specialized virulence factors that contribute to its pathogenicity. Among them, fimbriae play a role in the initial attachment and organization of biofilms. Different genotypes of fimA have been related to length of fimbriae and pathogenicity of the bacterium.
Objectives
The aim of this study was to identify 5 types of fimA genotype strains in smokers and nonsmokers with periodontitis, before and after periodontal therapy.
Material and Methods
Thirty-one patients with periodontitis harboring P. gingivalis were selected: 16 nonsmokers (NS) and 15 smokers (SM). Clinical and microbiological parameters were evaluated at baseline and 3 months after periodontal treatment, namely: plaque index, bleeding on probe, probing depth, gingival recession and clinical attachment level. The frequency of P. gingivalis and fimA genotype strains were determined by polymerase chain reaction.
Results
Type I fimA was detected in the majority of SM and NS at baseline, and the frequency did not diminish after 3 months of treatment. The frequency of type II genotype was higher in SM than NS at baseline. After 3 months, statistical reduction was observed only for types II and V fimA genotypes in SM. The highest association was found between types I and II at baseline for NS (37.5%) and SM (53.3%).
Conclusion
The most prevalent P. gingivalis fimA genotypes detected in periodontal and smoker patients were genotypes I and II. However, the presence of fimA genotype II was higher in SM. Periodontal treatment was effective in controlling periodontal disease and reducing type II and V P. gingivalis fimA.
Keywords: Periodontal disease, Smoking, Porphyromonas gingivalis
Introduction
Recent advances in DNA sequencing and bioinformatics technologies have provided an overview of microbiomes associated with health and disease, thereby expanding the knowledge on putative pathogenic species. Differences between periodontitis and health now are detected at the level of phylum and genus, in addition to confirming previous association of specific species with periodontal disease, including P. gingivalis and T. denticola, which are two of the main species associated with the disease, strongly related to severe forms of periodontitis 1 . P. gingivalis has an arsenal of specialized virulence factors that contribute to its pathogenicity. Among them, fimbriae play a role in the initial attachment and organization of biofilms, and act as adhesins that mediate invasion and colonization of host epithelial cells 2 . P. gingivalis generally expresses two distinct fimbriae, called FimA and Mfa1, which are composed of polymerized FimA and Mfa1 proteins encoded by the fimA and mfa1 genes, respectively 3 . The P. gingivalis gene fim cluster consists of seven genes, fimX, pgmA and fimA-E, encoding FimX, PgmA and FimA-E proteins, respectively 4 . Six genotypes of fimA (I-V) and Ib were identified in P. gingivalis strains, and the genotype has been especially related to fimbriae length and pathogenicity of the bacterium 4 .
Previous studies have evaluated the association between the frequency of fimA genotypes and periodontal health status in adults 5 , 6 . P. gingivalis was detected in 36.8% of the healthy subjects and in 87.1% of the patients with periodontitis. Among the P. gingivalis-positive healthy adults, the most prevalent fimA was genotype I (76.1%), followed by genotype V (29.7%). In contrast, most patients with periodontitis carried fimA genotype II (66.1%), followed by genotype IV (28.9%) 6 . These findings indicate that there are both disease-associated and non-disease-associated strains of P. gingivalis, and their infectious traits, which influence periodontal health status, could be differentiated based on the clonal variation of fimA genes 6 .
Tobacco consumption is a risk factor for periodontal disease. Smoking is associated with higher clinical attachment loss and gingival recession, reduced bone height and density, and, consequently, with increased tooth loss 5 . The mechanism by which tobacco affects the periodontal tissue is related to toxic substances such as nicotine and cotinine, which has been associated with various cellular changes that may contribute to the onset and subsequent progression of periodontal disease 7 . Tobacco use promotes several adverse effects, such as reduced gingival blood flow 8 , oxidative stress and alterations in immunoinflammatory responses, reducing the functional activity of neutrophils such as chemotaxis, glycolytic activity and phagocytosis 9 ; it also impairs wound healing 10 and interferes on bacterial acquisition and host response to colonization in biofilms 11 . The frequency of periodontopathogens was previously investigated in smokers and nonsmokers. P. gingivalis has been detected more frequently in the periodontal pockets of smokers (66.7% – pocket depth of 3-5 mm) in comparison with nonsmokers (52.2%), and it has been found in high levels in sites with periodontitis 12 .
Scaling and root planing is the most common and well-recognized nonsurgical periodontal therapy for promoting improvement in clinical and microbiological parameters 13 . Few studies have assessed the longitudinal clinical and microbiological evaluation of smokers undergoing periodontal maintenance therapy, and controversial results have been found when comparing smokers with nonsmokers 13 , 14 . A reduction of 93% in P. gingivalis frequency for nonsmokers, compared to 88% for smokers, was observed in subgingival sites after periodontal treatment 15 . Considering the presence of different genotypes of P. gingivalis fimA, Teixeira, et al. 16 (2009) suggested an association between the genotype fimA IV and disease severity in smoker-chronic periodontitis subjects. However, no study was found evaluating the influence of periodontal treatment on the frequency of different fimA genotypes in smokers and nonsmokers with periodontitis. The aim of this study was to compare the frequency of different genotypes of P. gingivalis fimA in smokers and nonsmokers with periodontitis, before and after 3 months of nonsurgical periodontal therapy. The null hypotheses are: 1) there is no difference in the periodontal status and frequency of P. gingivalis fimA genotypes between smokers and nonsmokers; and 2) smoking did not interfere in the response to periodontal treatment and levels of P. gingivalis fimA genotypes.
Material and methods
Study population
Thirty-one patients (15 smokers – SM and 16 nonsmokers – NS) with positive polymerase chain reaction (PCR) for P. gingivalis, from a total of 48 patients (24 in each group), aged 27 to 70 years, were selected to participate in this study. All participants were recruited from the Department of Periodontology, School of Dentistry, Fluminense Federal University, Nova Friburgo, RJ, Brazil, for a period of 3 months, between 2014 and 2015. The study protocol was approved by the Research Ethics Committee of the Fluminense Federal University – Nova Friburgo, RJ (CAAE: 55894816.2.0000.5626), and registered in the clinical trials (NCT02879903). Prior to participation, the purpose and procedures were fully explained to all participants, who gave their written informed consent in accordance with the Helsinki Declaration. An initial sample size of 16 participants per group was chosen, considering the standard deviations from a previous study 17 , the effect size [gingival index (GI), a minimum detectable change of 10%], a power of 80%, a significance level of 5%, and a loss to follow-up rate of up to 20% of the participants.
The following inclusion criteria were observed: presence of severe generalized chronic periodontitis in at least two teeth in different arches, including bleeding when probing these sites, probing depth (PPD) ≥5 mm, clinical attachment level (CAL) ≥5 mm and radiographic bone loss involving >30% of site 18 . The participants were considered heavy SM if they smoked ten or more cigarettes a day for at least 2 years. The smoking habit was confirmed at each visit, and the individuals who stopped smoking were excluded. Former SM were not included in the control group. The exclusion criteria were: patients with systemic diseases, diabetes or osteoporosis, pregnant and lactating females, use of immune suppressive medication, phenytoin, cyclosporine, calcium channel blockers or any antibiotics or nonsteroidal anti-inflammatory drugs in the past 3 months, and any medical conditions requiring immunotherapy or people diagnosed with HIV+ or AIDS, which could interfere with the periodontium status 19 .
Clinical examination and periodontal therapy
Prior to clinical examination, a questionnaire was applied to collect information on the smoking habits of the participants (years of cigarette consumption and number consumed daily), initially at baseline and then after a 3-month follow-up examination. An experienced periodontist (GACGC) determined the clinical periodontal parameters, including plaque index (PI), bleeding on probing (BOP), pocket probing depth (PPD), gingival recession (GR), and clinical attachment level (CAL), using a periodontal probe PCP15 (PCP-UNC15, Hu-Friedy, Chicago, IL, USA), at six sites per tooth on all the teeth, excluding third molars. The intraexaminer agreement of the categorical variables (PI, GI) using the kappa calculation, at the tooth level, was 0.75. Reproducibility of continuous variables (PPD, GR and CAL) was 0.70, as evaluated by the intraclass correlation coefficient (ICC).
Quadrant scaling and root planing (SRP) were performed weekly at baseline on each participant under local anesthesia, using periodontal curettes (American Eagle, Gracey Access Curettes, Missoula, MT, USA) and ultrasonic scalers (Cavitron, Dentsply, York, PA, USA). The maintenance therapy included professional plaque control and SRP in recurrent periodontal pockets monthly during the 6 months of the study. No teeth had to be extracted during therapy. A different clinician (MGLA) conducted the periodontal treatment. The examiner (GACGC) had no access to previous recordings. All participants received oral hygiene instructions for home care procedures (tooth-brushing technique, interdental cleaning and use of tongue scrapers).
Microbiological samples
After performing the clinical measurements, the supragingival biofilm was removed with sterile gauze. Subgingival samples of each participant were taken from the sites with the deepest PPD (≥5 mm), using a sterile periodontal curette. Pooled biofilms from each site were separated in microtubes containing Tris-EDTA buffer (10 mM Tris– HCl, 0.1 mM EDTA, pH 7.5), and stored at -20°C to be analyzed microbiologically using the PCR assay.
Microbiological evaluation – PCR primers and amplification
DNA was extracted using a protocol originally described by Sardi, et al. 19 (2011) and quantified in a spectrophotometer at 260 nm (Genesys 10UV, Rochester, NY, USA), to obtain a standard concentration of 0.1 µg/mL, and then stored at −20°C to test subsequent PCR reactions. The detection of P. gingivalis and fimA genotypes was performed as previously described 6 , 19 . Primers for the 16S rRNA gene were used as a positive control and P. gingivalis species-specific primers were used for the fimA genotypes 6 . All primers were custom-made by IDT (Integrated DNA Technologies, Sintese Biotecnologia, Belo Horizonte, MG, Brazil). PCR amplification was performed with a thermocycler AmpliTherm TX96 Gradient (Axygen, Corning, NY, USA) under thermal conditions specific for each pair of primers. The PCR products were separated by electrophoresis in 2% agarose gels and Tris-borate-EDTA running buffer (8.0 pH). The molecular mass ladder (100 bp DNA ladder, Gibco, Grand Island, NY, USA) was included for running the agarose gel. The DNA was stained with 0.1 µl of SYBR Safe/mL (Invitrogen, CA, USA) and visualized under UV light (Pharmacia LKB-MacroVue, San Gabriel, CA, USA). The images were photographed (ImageMaster – LISCAP, VDS, Pharmacia Biotech, Piscataway, NJ, USA) and analyzed. The amplification reaction was performed with the following cycling parameters: initial denaturation at 95°C for 10 min, PCR cycling consisting of 40 cycles, 94°C for 30 s, 58°C for 30 s, and 72°C for 30 s, followed by an extension at 72°C for 2 min. Positive and negative controls were included in each PCR set, and in each sample process.
Statistical analysis
The statistical analysis was performed using SPSS Statistics 17.1 (IBM Inc., Chicago, IL, USA). All variables were tested for the normality of data (Shapiro-Wilk). Variables from participant’s characteristics and clinical analyses were compared between SM and NS using the Mann-Whitney test. Comparisons between the periods of time (baseline × 3 months) considering each group (SM and NS) separately were conducted using Wilcoxon tests. Differences were considered significant when p<0.05.
Results
Clinical results
There was no statistical difference between SM and NS regarding age, gender and race (Table 1). Median cigarette consumption was 20 cigarettes per day, and the duration (median) of the smoking habit was 23 years. Comparing SM and NS, at baseline, both groups had a similar periodontal status (Table 2). There was a statistically significant reduction in the clinical parameters for the NS group, with a decrease in PI (p=0.004) and BOP (p=0.011) indexes, and in PPD (p<0.001) and CAL (p=0.007) measurements, and also for the SM group, with a decrease in PPD (p<0.001) and CAL (p=0.007), comparing baseline with 3 months after periodontal treatment. SM exhibited a higher level of PI (50% -p<0.001) and CAL (5.68 mm – p=0.041) after 3 months than NS (PI: 9.69% and CAL: 3.51 mm).
Table 1. Characteristics of the participants.
| Variables | Groups | |
|---|---|---|
| Nonsmokers (NS) n=16 | Smokers (SM) n=15 | |
| Age (years)† | 49 (44.25-61.75) | 47 (33-52) |
| Gender n (%) | ||
| Female | 10 (62.5) | 6 (40) |
| Male | 6 (37.5) | 9 (60) |
| Ethnicity n (%) | ||
| White | 11 (73.3) | 8 (50) |
| Black | 4 (26.6) | 8 (50) |
| Cigarettes per day† | 0 | 20 (10-20) |
| Duration of smoking habit† | 0 | 23 (10-32) |
*Statistically significant differences between NS and SM groups for baseline (Mann Whitney test; p<0.05)
†Values expressed in medians (percentile 25-percentile 75)
Table 2. Clinical evaluation of smokers versus nonsmokers at baseline and after 3 months of periodontal therapy. Values are expressed in medians (percentile 25-percentile 75).
| Variables | Groups | |
|---|---|---|
| Nonsmokers (NS) n=16 | Smokers (SM) n=15 | |
| PI (%) | ||
| Baseline | 46.04 (14.12-71.98) | 49.24 (38-86) |
| 3 months | 9.69(5.04-25.37)* | 50 (19.84-72.22)‡ |
| BOP (%) | ||
| Baseline | 29.91 (17.71-63.15) | 23.07 (12.34-64.74) |
| 3 months | 13.15 (3.54-30)* | 24.6 (13.49-30.2) |
| PD (mm) | ||
| Baseline | 5.18 (5-5.29) | 5.1 (5-5.31) |
| 3 months | 2.93 (2.65-3.81)* | 3.8 (3-4.12)*‡ |
| GR (mm) | ||
| Baseline | 0.87 (0-2.62) | 2 (1-2.66) |
| 3 months | 0.5 (0-2.66) | 2 (1-2.57) |
| CAL (mm) | ||
| Baseline | 5.9 (5-8.16) | 7 (6.11-7.91) |
| 3 months | 3.51 (2.75-6.29)* | 5.68 (4-6.74)*‡ |
*Statistically significant difference between baseline and 3 months (Wilcoxon test; p<0.05)
†Statistically significant differences between NS and SM groups at baseline (Mann Whitney test; p<0.05)
‡Statistically significant differences between NS and SM groups at 3 months (Mann Whitney test; p<0.05
fimA genotype results
P. gingivalis was detected in 16 NS and 15 SM, and distributed according to 5 genotypes of fimA P. gingivalis at baseline and 3 months after periodontal therapy, as described in Table 3. Genotype I was detected in the majority of SM (60%) and NS (87.5%) at baseline, and the frequency did not diminish after 3 months of treatment (SM – 60% and NS – 93.8%). The frequency of genotypes II, III and V fimA was higher in SM than NS at baseline; however, a statistical difference was found only in the frequency of fimA genotype II for SM (93.3%) compared with NS – (47.8%) (p=0.017). After 3 months, statistical reduction was observed only for fimA genotypes II and V in SM (p=0.001).
Table 3. Distribution of 5 fimA types of P. gingivalis at baseline and 3 months after periodontal therapy.
| fimA types | Frequency in % (number of patients) | |
|---|---|---|
| Nonsmokers (NS) | Smokers (SM) | |
| n=16 | n=15 | |
| I | ||
| Baseline | 87.5(14) | 60(9) |
| 3 months | 93.8(15) | 60(9) |
| II | ||
| Baseline | 47.8(7)† | 93.3(14) * |
| 3 months | 12.5(2) | 13.3(2) |
| III | ||
| Baseline | 18.8(3) | 40(6) |
| 3 months | 18.8(3) | 33.3(5) |
| IV | ||
| Baseline | 0 | 0 |
| 3 months | 0 | 0 |
| V | ||
| Baseline | 25(4) | 40(6) * |
| 3 months | 18.8(3) | 0 |
*Statistically significant difference between baseline and 3 months (Wilcoxon test; p<0.05)
†Statistically significant differences between NS and SM groups at baseline (Mann Whitney test; p<0.05)
‡Statistically significant differences between NS and SM groups at 3 months (Mann Whitney test; p<0.05)
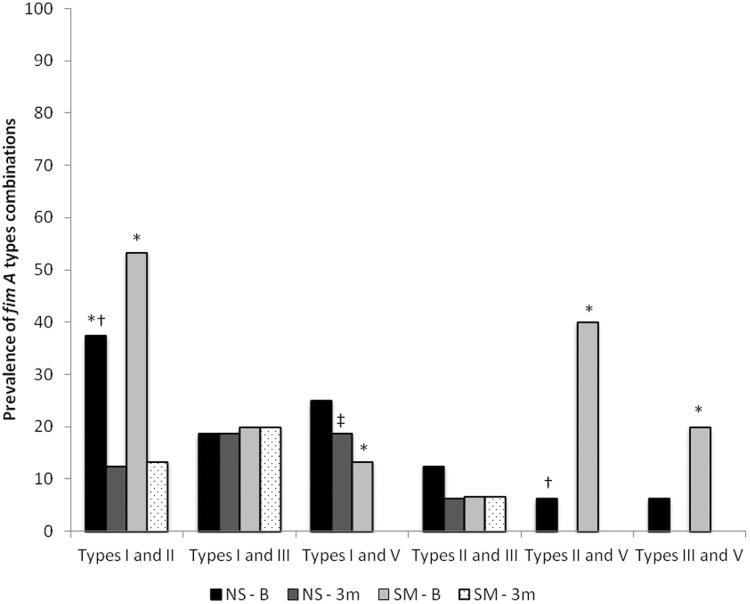
The frequency of two different genotypes of fimA in NS and SM participants, at baseline and after 3 months of treatment, are shown in Figure 1. The highest association was found between genotypes I and II at baseline for NS (37.5%) and SM (53.3%). Statistical differences were found between NS and SM, considering genotypes I and II and genotypes II and V, at baseline. The combination of genotypes I and V in SM was not detected after treatment, unlike NS. Comparing baseline with 3 months of treatment, there was a statistical reduction in the combination of fimA genotypes I and III for both groups. The combinations of genotypes I and V, genotypes II and V and genotypes III and V were not detected after treatment of SM participants.
Figure 1. Frequency (%) of combinations of P. gingivalis fimA genes in smokers (SM) and nonsmokers (NS) at baseline and after 3 months of periodontal treatment. Values express percentage of P. gingivalis harboring fimA types.
*Statistically significant difference between baseline and 3 months (Wilcoxon test; p<0.05)
†Statistically significant differences between NS and SM groups for baseline (Mann Whitney test; p<0.05)
‡Statistically significant differences between NS and SM groups for 3 months (Mann Whitney test; p<0.05)
Discussion
In this study, we confirmed that P. gingivalis is a common microorganism present in periodontal disease in SM and NS, corroborating other studies 16 , 20 . The virulence of P. gingivalis has been heavily associated with the presence of different types of fimbriae, which have been characterized as key factors in adhesion, invasion and colonization of this pathogen in the periodontal tissues 2 . However, the influence of periodontal treatment in the prevalence of fimA genotypes in SM compared with NS was not clear.
No difference was found among the groups of participants diagnosed with periodontal disease in relation to gender. Nevertheless, differences were found in literature with predominance of periodontal disease in males 21 or females 22 . Considering ethnicity, no significant differences were found between the groups, contradicting a study that found a prevalence of periodontal disease for the black ethnic group 23 . In this study, the participants smoked an average of 20 cigarettes per day, characterizing them as heavy SM. A study pointed that there was greater severity of periodontal disease in patients who consumed more than 20 cigarettes per day, with a 10% prevalence of insertion loss in heavy SM 24 . Light SM, who consumed fewer than 4 cigarettes/day had an alveolar bone loss of 3.3 mm, whereas heavy SM had a loss of 7.3 mm, showing a direct relation between the number of cigarettes consumed and the rate of progression of periodontal disease 6 .
Some studies have shown that SM have higher prevalence and severity of periodontal disease, and worse results after periodontal therapy than NS 7 , 25 . In this study, SM and NS were matched by periodontal parameters (PI, BOP, PPD, GR and CAL) at the baseline, since no statistical difference was observed between the groups. Besides, instruction on oral hygiene was given to all patients, regardless of the group, in order not to bias data collection. However, higher levels of PI were observed after 3 months of periodontal treatment only for the SM group. One hypothesis is that smokers may be less motivated to keep a high-quality oral hygiene throughout the period of study 26 . Due to plaque accumulation, smokers showed a higher GI mean over 3 months. This finding is opposite to what is found in literature data 15 , 27 , in which inflammatory levels generally are reduced in smokers by the influence of the vasoconstrictor effect of nicotine, regardless of the plaque index 28 . In addition, a significant reduction in the periodontal parameters PPD and CAL was detected for both groups, SM and NS, after periodontal treatment. However, SM showed lower reduction in probing depth and gain in clinical attachment in comparison with NS. The same was observed in other studies 26 , 29 , 30 . Trombelli and Scabbia 29 (1997) found that SM with furcation problems exhibited a less favorable healing pattern after surgery, caused by the interference of nicotine on collagen synthesis and bone formation. Jansson and Hagstrom 26 (2002) reported that SM have high risk of recurrence of periodontitis in the periodontal maintenance phase, and need more surgical intervention. In contrast, Zuabi, et al. 30 (1999) observed a higher reduction in probing depth in SM (0.81+0.11 mm) compared to NS (0.5+0.08 mm), after conventional therapy, showing that the effect of supragingival plaque control and clinical signs of periodontitis is yet controversial when smoking habits are considered.
Many variations in the distribution of P. gingivalis fimA genotypes can be found in literature, depending on the population examined. However, one finding is recurrent in periodontal studies, namely, greater frequency of the fimA genotype II in patients with chronic periodontitis 16 , 20 , 31 . This determined that fimA genotypes I and II were the most prevalent at baseline for both NS and SM groups, thus confirming the frequency of these types of fimbriae in patients with chronic periodontitis 6 . In agreement with our results, Beikler, et al. 7 (2003) found that predominant fimA genotypes in 26 P. gingivalis isolates from Caucasian patients with periodontitis were types I (25.5%), II (38.2%) and IV (18.6%), by using PCR and restriction analysis. However, there was no difference in the association of the various fimA genotypes and disease severity. On the contrary, Teixeira, et al. 29 (2000) found higher levels of genotype IV (69.6%) than II (28%), and the proportion of genotypes was associated with increasing probing depth only for genotype II.
Our study also found that genotype II was higher in SM compared to NS. The greater virulence of genotypes I and II can be attributed to their adhesiveness and invasiveness, which are key determinants for P. gingivalis virulence. FimA genotype I and II microorganisms are more adhesive to salivary proteins than other types, and their binding abilities are related to the sequence similarity of fimbrillin proteins 20 . This explains why genotypes I and II were more prevalent in this study, as well as in other studies 31 , 32 . High relative risk (RR) for the presence of genotype II, followed by genotypes I and III, was observed for SM compared with NS, at baseline, confirming the importance of these two genotypes for the development of periodontal disease.
Some studies have established that fimA genotype IV is considered an important virulence factor for the pathogenesis of periodontal disease 6 , 32 . However, in our study, there was no detection of type IV in the groups, disagreeing with some authors, who found not only genotypes I and II, but also genotype IV among the most frequently detected 30 , 31 . It is important to emphasize that genotyping was performed exclusively among participants with chronic periodontitis, and there was a variation in P. gingivalis genotypes regarding other types of periodontitis, a distinction that could change the course and evolution of the pathology.
In this study, we selected samples of P. gingivalis from our previous study 33 , in which the authors detected P. gingivalis in 50% of SM and 70.8% of NS, both groups with periodontal disease. The high frequency of P. gingivalis in patients with periodontitis is according with the results of Amano, et al. 6 (2000), which detected P. gingivalis in 87.1% of patients with periodontitis. Type II fimA is associated with deeper pockets, whereas genotypes III and V seem to be involved in periodontitis to a lesser extent 12 . The results of this study corroborated with these findings, because a strong correlation was found between probing depth (>5 mm) and presence of type II fimA. Likewise, when associations between the fimbriae were tested in SM and NS at baseline and after 3 months, statistical analysis revealed significant differences between SM and NS, when II and V fimA genotypes were associated. This may be attributed to deeper pockets in SM compared with NS. Results obtained by Darby, et al. 34 (2005) and Lee, et al. 35 (2012) corroborate these results.
One limitation of this study is the absence of biochemical validation of the smoking status. Self-reported smoking status can underestimate true smoking prevalence and quantity due to a variety of factors such as misunderstanding, intentional deception, embarrassment, denial, shame, etc., inducing socially desirable responses. Measurement of cotinine, a primary metabolite of nicotine, can be detected in urine, saliva or serum and could provide a reliable biochemical marker of smoking status and other tobacco use or exposure over a period of 2 to 3 days 36 .
Conclusion
Within the limitation of the relatively small sample size, this study concluded that the most prevalent P. gingivalis fimA genotypes detected in periodontal participants were genotypes I and II. However, the presence of fimA genotype II was higher in SM. Periodontal treatment was effective in controlling periodontal disease and in reducing P. gingivalis fimA type II and V. The authors suggest that more longitudinal studies are necessary to establish whether genotypes of P. gingivalis fimA can be maintained for long periods of time, and whether they influence the evolution of periodontal disease over time.
Acknowledgements
We wish to thank CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) for their financial support. The authors deny any conflict of interest related to this study.
References
- 1.Griffen AL, Beall CJ, Campbell JH, Firestone ND, Kumar PS, Yang ZK, et al. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME J. 2012;6(6):1176–1185. doi: 10.1038/ismej.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin X, Wu J, Xie H. Porphyromonas gingivalis minor fimbriae are required for cell-cell interactions. Infect Immun. 2006;74(10):6011–6015. doi: 10.1128/IAI.00797-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshimura F, Murakami Y, Nishikawa K, Hasegawa Y, Kawaminami S. Surface components of Porphyromonas gingivalis. J Periodontal Res. 2009;44(1):1–12. doi: 10.1111/j.1600-0765.2008.01135.x. [DOI] [PubMed] [Google Scholar]
- 4.Nagano K, Hasegawa Y, Abiko Y, Yoshida Y, Murakami Y, Yoshimura F. Porphyromonas gingivalis FimA fimbriae: fimbrial assembly by fimA alone in the fim gene cluster and differential antigenicity among fimA genotypes. PLoS One. 2012;7(9):e43722. doi: 10.1371/journal.pone.0043722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albandar JM. Global risk factors and risk indicators for periodontal diseases. J Periodontol. 2002;29(1):177–206. doi: 10.1034/j.1600-0757.2002.290109.x. [DOI] [PubMed] [Google Scholar]
- 6.Amano A, Kuboniwa M, Nakagawa I, Akiyama S, Morisaki I, Hamada S. Prevalence of specific genotypes of Porphyromonas gingivalis fimA and periodontal healthy status. J Dent Res. 2000;79(9):1664–1668. doi: 10.1177/00220345000790090501. [DOI] [PubMed] [Google Scholar]
- 7.Beikler T, Peters U, Prajaneh S, Prior K, Ehmke B, Flemmig TF. Prevalence of Porphyromonas gingivalis fimA genotypes in Caucasians. Eur J Oral Sci. 2003;111(5):390–394. doi: 10.1034/j.1600-0722.2003.00065.x. [DOI] [PubMed] [Google Scholar]
- 8.Morozumi T, Kubota T, Sato T, Okuda K, Yoshie H. Smoking cessation increases gingival blood flow and gingival crevicular fluid. J Clin Periodontol. 2004;31(4):267–272. doi: 10.1111/j.1600-051X.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 9.Zappacosta B, Persichilli S, Minucci A, Fasanella S, Scribano D, Giardina B, et al. Effects of aqueous cigarette smoke extract on the chemiluminescence kinetics of polymorphonuclear leukocytes and on their glycolytic and phagocytic activity. Luminescence. 2001;16(5):315–319. doi: 10.1002/bio.661. [DOI] [PubMed] [Google Scholar]
- 10.Sorensen LT. Wound healing and infection in surgery. The clinical impact of smoking and smoking cessation: a systematic review and meta-analysis. Arch Surg. 2012;147(4):373–383. doi: 10.1001/archsurg.2012.5. [DOI] [PubMed] [Google Scholar]
- 11.Kumar PS, Matthews CR, Joshi V, Jager M, Aspiras M. Tobacco smoking affects bacterial acquisition and colonization in oral biofilms. Infect Immun. 2011;79(11):4730–4738. doi: 10.1128/IAI.05371-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carvalho LH, D'Avila GB, Leão A, Gonçalves C, Haffajee AD, Socransky SS, et al. Scaling and root planing, systemic metronidazole and professional plaque removal in the treatment of chronic periodontitis in a Brazilian population II - Microbiological results. J Clin Periodontol. 2005;32(4):406–411. doi: 10.1111/j.1600-051X.2005.00720.x. [DOI] [PubMed] [Google Scholar]
- 13.Patel RA, Wilson RF, Palmer RM. The effect of smoking on periodontal bone regeneration: a systematic review and meta-analysis. J Periodontol. 2012;83(2):143–155. doi: 10.1902/jop.2011.110130. [DOI] [PubMed] [Google Scholar]
- 14.Fisher S, Kells L, Picard JP, Gelskey SC, Singer DL, Lix L, et al. Progression of periodontal disease in a maintenance population of smokers and non-smokers: a 3-year longitudinal study. J Periodontol. 2008;79(3):461–468. doi: 10.1902/jop.2008.070296. [DOI] [PubMed] [Google Scholar]
- 15.Gomes S, Puccinin FB, Susin C, Oppermann RV, Marcantonio RA. Effect of supragingival plaque control in smokers and never-smokers: 6-month evaluation of patients with periodontitis. J Periodontol. 2007;78:1515–1521. doi: 10.1902/jop.2007.060462. [DOI] [PubMed] [Google Scholar]
- 16.Teixeira SR, Mattarazo F, Feres M, Figueiredo LC, Faveri M, Simionato MR, et al. Quantification of Porphyromonas gingivalis and fimA genotypes in smoker chronic periodontitis. J Clin Periodontol. 2009;36(6):482–487. doi: 10.1111/j.1600-051X.2009.01411.x. [DOI] [PubMed] [Google Scholar]
- 17.Pereira AL, Cortelli SC, Aquino DR, Franco GC, Cogo K, Rodrigues E, et al. Reduction of salivary arginine catabolic activity through periodontal therapy. Quintessence Int. 2012;43(9):777–787. [PubMed] [Google Scholar]
- 18.International Workshop for a Classification of Periodontal Diseases and Conditions. Papers. Oak Brook, Illinois, October 30- November 2, 1999. Ann Periodontol. 1999;4:1–112. doi: 10.1902/annals.1999.4.1.i. [DOI] [PubMed] [Google Scholar]
- 19.Sardi JC, Duque C, Camargo GA, Hofling JF, Gonçalves RB. Periodontal conditions and prevalence of putative periodontopathogens and Candida spp. in insulin-dependent type 2 diabetic and non-diabetic patients with chronic periodontitis - a pilot study. Arch Oral Biol. 2011;56(10):1098–1105. doi: 10.1016/j.archoralbio.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 20.Puig-Silla M, Dasí-Fernández F, Montiel-Company JM, Almerich-Silla JA. Prevalence of fimA genotypes of Porphyromonas gingivalis and other periodontal bacteria in a Spanish population with chronic periodontitis. Med Oral Patol Oral Cir Bucal. 2012;17(6):1047–1053. doi: 10.4317/medoral.17009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brennan DM, Spencer AJ, Slade GD. Prevalence of periodontal conditions among public-funded dental patients in Australia. Aust Dent J. 2002;46(2):114–121. doi: 10.1111/j.1834-7819.2001.tb00566.x. [DOI] [PubMed] [Google Scholar]
- 22.Hugoson A, Laulell L. A prospective longitudinal study on periodontal bone height changes in a Swedish population. J Clin Periodontol. 2000;27:665–674. doi: 10.1034/j.1600-051x.2000.027009665.x. [DOI] [PubMed] [Google Scholar]
- 23.Moore S, Ide M, Wilson RF, Coward PY, Borkowska E, Baylis R, et al. Periodontal health of London women during early pregnancy. Br Dent J. 2001;191(10):570–573. doi: 10.1038/sj.bdj.4801236. [DOI] [PubMed] [Google Scholar]
- 24.Martinez-Canut P, Lorca A, Magán R. Smoking and periodontal disease severity. J Clin Periodontol. 1995;22(10):743–749. doi: 10.1111/j.1600-051x.1995.tb00256.x. [DOI] [PubMed] [Google Scholar]
- 25.Miura M, Hamachi T, Fujise O, Maeda K. The prevalence and pathogenic differences of Porphyromonas gingivalis fimA genotypes in patients with aggressive periodontitis. J Period Res. 2005;40(2):147–152. doi: 10.1111/j.1600-0765.2005.00779.x. [DOI] [PubMed] [Google Scholar]
- 26.Jansson LE, Hagström KE. Relationship between compliance and periodontal treatment outcome in smokers. J Periodontol. 2002;73(6):602–607. doi: 10.1902/jop.2002.73.6.602. [DOI] [PubMed] [Google Scholar]
- 27.Labriola A, Needleman I, Moles DR. Systematic review of the effect of smoking on nonsurgical periodontal therapy. Periodontal 2000. 2005;37(1):124–137. doi: 10.1111/j.1600-0757.2004.03793.x. [DOI] [PubMed] [Google Scholar]
- 28.Gonçalves RB, Coletta RD, Silvério KG, Benevides L, Casati MZ, Silva JS, et al. Impact of smoking on inflammation: overview of molecular mechanisms. Inflamm Res. 2011;60(5):409–424. doi: 10.1007/s00011-011-0308-7. [DOI] [PubMed] [Google Scholar]
- 29.Trombelli L, Scabbia A. Healing response of gingival recession defects following guided tissue regeneration procedures in smokers and non-smokers. J Clin Periodontol. 1997;24(8):529–533. doi: 10.1111/j.1600-051x.1997.tb00224.x. [DOI] [PubMed] [Google Scholar]
- 30.Zuabi O, Machtei EE, Ben-Aryeh H, Ardekian L, Peled M, Laufer D. The effect of smoking and periodontal treatment on salivary composition in patients with established periodontitis. J Periodontol. 1999;70(10):1240–1246. doi: 10.1902/jop.1999.70.10.1240. [DOI] [PubMed] [Google Scholar]
- 31.Missailidis CG, Umeda JE, Ota-Tsuzuki C, Anzai D, Mayer MP. Distribution of FimA genotypes of Porphyromonas gingivalis in subjects with various periodontal conditions. Oral Microbiol Immunol. 2004;19(4):224–229. doi: 10.1111/j.1399-302X.2004.00140.x. [DOI] [PubMed] [Google Scholar]
- 32.Zhao L, Wu YF, Meng S, Yang H, OuYang YL, Zhou XD. Prevalence of FimA genotypes of Porphyromonas gingivalis and periodontal health status in Chinese adults. J Periodontal Res. 2007;42:511–517. doi: 10.1111/j.1600-0765.2007.00975.x. [DOI] [PubMed] [Google Scholar]
- 33.Camargo GA, Abreu MG, Cordeiro RS, Wenderoscky LF, Duque C. Prevalence of periodontopathogens and Candida spp. in smokers after nonsurgical periodontal therapy - a pilot study. e92Braz Oral Res. 2016;30(1) doi: 10.1590/1807-3107BOR-2016.vol30.0092. [DOI] [PubMed] [Google Scholar]
- 34.Darby IB, Angkasa F, Duong C, Ho D, Legudi S, Pham K, et al. Factors influencing the diagnosis and treatment of periodontal disease by dental practitioners in Victoria. Aust Dent J. 2005;50(1):37–41. doi: 10.1111/j.1834-7819.2005.tb00083.x. [DOI] [PubMed] [Google Scholar]
- 35.Lee HJ, Kim JK, Cho JY, Lee JM, Hong SH. Quantification of subgingival bacterial pathogens at different stages of periodontal diseases. Curr Microbiol. 2012;65(1):22–27. doi: 10.1007/s00284-012-0121-8. [DOI] [PubMed] [Google Scholar]
- 36.Caraballo RS, Giovino GA, Pechacek TF, Mowery PD. Factors associated with discrepancies between self-reports on cigarette smoking and measured serum cotinine levels among persons aged 17 years or older: Third National Health and Nutrition Examination Survey, 1988 –1994. Am J Epidemiol. 2001;153:807–814. doi: 10.1093/aje/153.8.807. [DOI] [PubMed] [Google Scholar]