The therapeutic potential of the cannabis plant in cancer treatment has recently been considered. This article evaluates the clinical influence of cannabis use during immunotherapy treatment with nivolumab on response rate, progression‐free survival, and overall survival.
Keywords: Cancer, Cannabis, Immunotherapy, Nivolumab, Response rate
Abstract
Background.
There has been a significant increase in the use of immunotherapy and cannabis recently, two modalities that have immunomodulatory effects and may have possible interaction. We evaluated the influence of cannabis use during immunotherapy treatment on response rate (RR), progression‐free survival (PFS), and overall survival (OS).
Subjects, Materials, and Methods.
In this retrospective, observational study, data were collected from the files of patients treated with nivolumab in the years 2015–2016 at our hospital, and cannabis from six cannabis‐supplying companies. Included were 140 patients (89 nivolumab alone, 51 nivolumab plus cannabis) with advanced melanoma, non‐small cell lung cancer, and renal clear cell carcinoma. The groups were homogenous regarding demographic and disease characteristics. A comparison between the two arms was made.
Results.
In a multivariate model, cannabis was the only significant factor that reduced RR to immunotherapy (37.5% RR in nivolumab alone compared with 15.9% in the nivolumab‐cannabis group (p = .016, odds ratio = 3.13, 95% confidence interval 1.24–8.1). Cannabis use was not a significant factor for PFS or OS. Factors affecting PFS and OS were smoking (adjusted hazard ratio [HR] = 2.41 and 2.41, respectively (and brain metastases (adjusted HR = 2.04 and 2.83, respectively). Low performance status (adjusted HR = 2.83) affected OS alone. Tetrahydrocannabinol and cannabidiol percentages did not affect RR in any group (p = .393 and .116, respectively).
Conclusion.
In this retrospective analysis, the use of cannabis during immunotherapy treatment decreased RR, without affecting PFS or OS and without relation to cannabis composition. Considering the limitations of the study, further prospective clinical study is needed to investigate possible interaction.
Implications for Practice.
Although the data are retrospective and a relation to cannabis composition was not detected, this information can be critical for cannabis users and indicates that caution is required when starting immunotherapy.
Introduction
Immunomodulation is a major technique used by cancer cells to silence the immune system's natural response and to continue cancer cell proliferation. The field of immunotherapy in general, and checkpoint antibody inhibitors in particular, has progressed significantly during the past several years and is perceived as a new promise for cancer patients. It includes various therapeutic mechanisms that harness the immune system in order to control malignancy. One major class of immunotherapy that has been approved for clinical use are antibodies of the programmed death receptor 1 (PD‐1) or its ligand (PD‐L1), which directly inhibits the PD‐1/PD‐L1 interaction. This inhibition causes apoptosis of the tumor cell, promotes peripheral T‐effector cell exhaustion, and promotes conversion of T‐effector cells to T‐regulatory cells (T‐regs) [1], [2]. Anti PD‐1 agents have U.S. Food and Drug Administration approval for use in patients with advanced melanoma [3], [4], non‐small cell lung cancer (NSCLC) [5], and renal cell carcinoma (RCC) [6].
This growing field had led to increased knowledge regarding immunologic mechanisms and therapies that alter their function. One of the most commonly used therapies that modulates the immunologic balance is glucocorticosteroids. Glucocorticosteroids are extremely potent anti‐inflammatory and immunosuppressive hormones. Their actions are mediated by a variety of mechanisms that alter cell numbers and function [7], [8]. Because of their immune suppressive effect, these drugs are not recommended to be used together with immunotherapy and may alter the effectiveness of the treatment. Glucocorticosteroids could even work against the immunotherapy (anti PD‐1) by enhancing the expression of PD‐1 [9].
Recently, the therapeutic potential of the cannabis plant in the area of cancer treatment has been discovered and the use of cannabis by cancer patients has increased significantly [10], [11]. In Israel alone, ~31,000 people are treated with medical cannabis for several illnesses, including ~8,500 oncologic patients. The beneficial effects of cannabis are related to symptoms of the disease: pain, nausea and vomiting, loss of appetite, weight loss, mood swings, and sleep disorders [12], [13]. However, cannabis was found to have immunomodulatory properties as well. Studies examining the effects of cannabinoid‐based drugs on immunity have shown that various cellular and cytokine mechanisms are suppressed by these agents, mainly by four mechanisms: induction of apoptosis (of T cells, macrophages, splenocytes, and thymocytes) [14], inhibition of cell proliferation, inhibition of production of chemokines and cytokines, and induction of T‐regs [15], [16].
Despite knowing the immunomodulatory potential effects of cannabinoids, which may work in the same immunosuppressive mechanism as glucocorticosteroids, there is no contraindication for using them in combination with immunotherapy. To the best of our knowledge, the possible interaction between cannabis use and immunotherapy has not been studied yet. Hence, the purpose of this study was to evaluate retrospectively the clinical influence of cannabis use during immunotherapy treatment with nivolumab on response rate (RR), progression‐free survival (PFS), and overall survival (OS).
Subjects, Materials, and Methods
Patient Recruitment
The study took place in the Division of Oncology at Rambam Health Care Campus, Haifa, Israel. After approval of the study protocol by the institutional ethics committee (Certificate 0647‐16), a retrospective analysis was conducted of all the medical records of patients treated with nivolumab in the Division of Oncology from August 2015 to August 2016.
Inclusion criteria were patients over 18 years of age, stage IV cancer, histological diagnosis of NSCLC or RCC or advanced melanoma, initiation of treatment with nivolumab in the study period, and use of cannabis during the study period (in the arm using cannabis). All patients included an intention to treat analysis. However, analysis was also done with exclusion of patients with survival of less than 2 months on nivolumab treatment, to neutralize other factors that could affect the results.
Patients were deidentified after data were collected from digital and nondigital records. Data extracted from the medical files included demographics and medical history: age, type of cancer, stage (American Joint Committee on Cancer, seventh edition), diagnosis date, smoking status, metastases location, treatment line number, treatment details (start date, end date, adverse events), and cannabis start date, dosage, and way of administration. The response rate was evaluated using RECIST criteria based on imaging assessments carried out every 11–14 weeks. The results relied on the official Radiology reports.
Data related to the cannabinoid products used were collected from six of eight companies that supply these products to patients. Data collected included the product's tetrahydrocannabinol (THC) percentage, the product's cannabidiol (CBD) percentage, the way of administration, and the duration and dates of the product's supply to the patient.
HPLC‐DAD Identification of Phytocannabinoids (Table 1)
Table 1. Cannabis characteristics using high‐performance liquid chromatography‐diode array detector identification of phytocannabinoids for 37/59 patients.
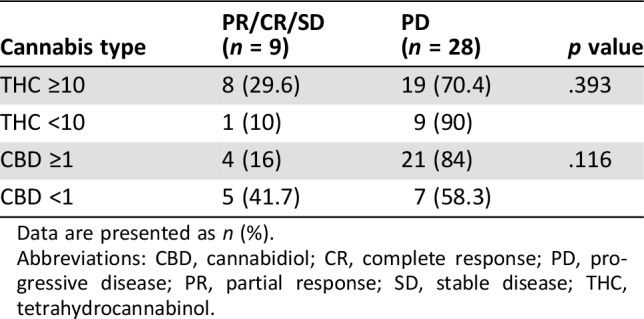
Data are presented as n (%).
Abbreviations: CBD, cannabidiol; CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease; THC, tetrahydrocannabinol.
The reversed phase high‐performance liquid chromatography (HPLC) method was used for detection of phytocannabinoids. The chromatographic separation was achieved using a UHPLC Rapid Separation system (Thermo Scientific, Bremen, Germany), coupled to a diode array detector (DAD). The system was equipped with a HALO C18 column (2.7 μm, 150 × 2.1 mm i.d.) with a guard column (0.5 μm depth filter × 0.1 mm; Phenomenex, Torrance, CA) and a ternary A/B/C multistep gradient (solvent A: 0.1% acetic acid in Milli Q water, solvent B: 0.1% acetic acid in acetonitrile, and solvent C: methanol; all solvents were of liquid chromatography/mass spectrometry grade). Solvent C was kept constant at 5% throughout the run. The multistep gradient program was established as follows: initial conditions were 50% B raised to 67% B until 3 minutes, held at 67% B for 5 minutes (until 8 minutes), and then raised to 90% B until 12 minutes, held at 90% B until 15 minutes, decreased to 50% B over the next minute, and held at 50% B until 18 minutes for re‐equilibration of the system prior to the next injection. A flow rate of 0.5 mL/minute was used, the column temperature was 30°C, and the injection volume was 1 μL. Data acquisition was performed in full ultraviolet‐visible scan mode.
Statistical Analysis
Chi‐square test was used to determine the difference between patients' characteristics in both groups. Logistic regression was used to calculate the odds ratios (OR) with 95% confidence intervals (95% CI) and p values in bivariate analysis to determine associations between patients' characteristics and response rate. Multivariable Forward Stepwise Logistic Regression analysis was performed. Bivariate Cox regression was then used to determine associations between patients' PFS and OS. A multivariate Cox Regression analysis was performed. Two‐tailed p values of .05 were considered statistically significant. Statistical analysis was performed using SPSS 21.0 software (IBM, Armonk, NY) for Windows.
Results
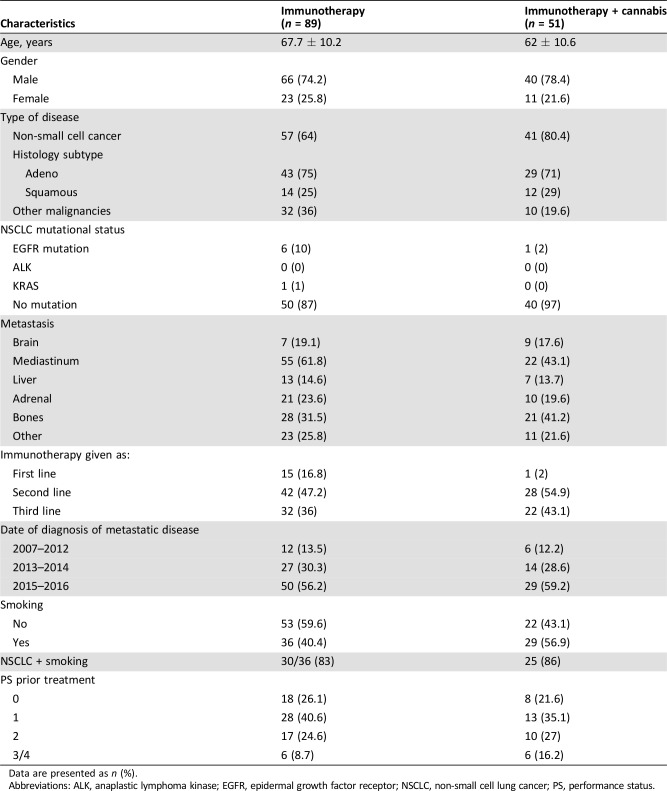
Over a period of 1 year (August 2015 to August 2016), 140 cancer patients were given nivolumab (89 nivolumab alone, 51 nivolumab plus cannabis). A comparison of patients treated only with nivolumab with those treated with nivolumab plus cannabis was made. As shown in Table 2, no significant difference was found between the two groups in aspects of demographic and medical characteristics.
Table 2. The demographic and medical characteristics of the study sample (n = 140).
Data are presented as n (%).
Abbreviations: ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; NSCLC, non‐small cell lung cancer; PS, performance status.
Specific attention was paid to the use of concomitant antibiotics or glucocorticosteroids. In both groups, fewer than 5% of patients were treated concomitantly with antibiotics. Regarding glucocorticosteroids, all patients in the first and second lines of treatment did not receive any concomitant glucocorticosteroids. In the third line of treatment, four patients in the immunotherapy only group and three patients who used cannabis also had glucocorticosteroids concomitantly. The maximal dose was 20 mg of prednisone.
In terms of side effects, a comparison between the two groups was made for the most common side effects of immunotherapy. With the limitation of a retrospective report, no significant difference between the two groups was found (Table 3). More than half the patients did not suffer from immunotherapy‐related side effects. Fewer than 10% of the patients required high‐dose glucocorticosteroids, without differences between the groups.
Table 3. Reported side effects during the immunotherapy treatment period.
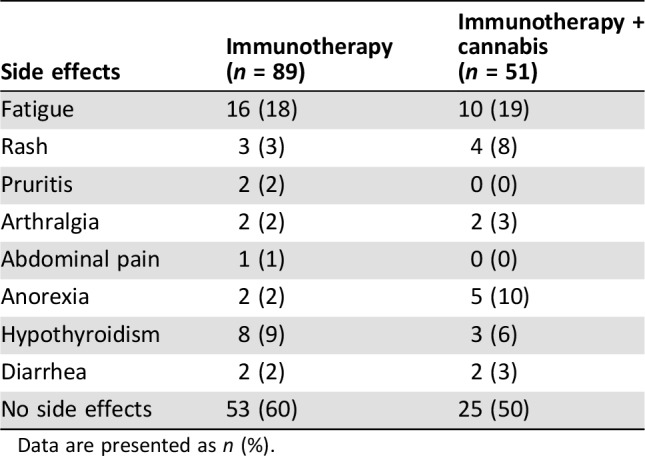
Data are presented as n (%).
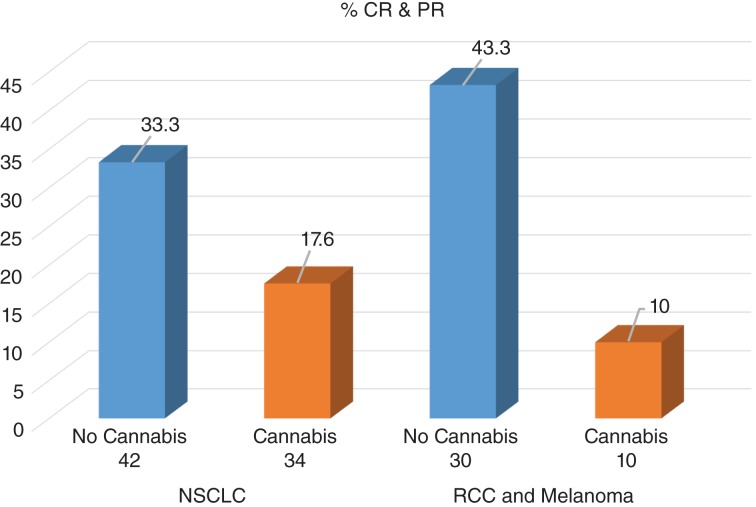
Given the high number of patients diagnosed with NSCLC, a comparison was made between them and the other malignancies (melanoma and clear cell RCC). In addition, to exclude patients with advanced and complicated clinical status, the statistical model was made on patients with overall survival of more than 2 months. Figure 1 demonstrates the RR of the NSCLC group and the other malignancies group when using nivolumab alone or nivolumab with cannabis. A decreased RR is shown in both groups among patients using cannabis with nivolumab. In the NSCLC group, the RR among patients using nivolumab alone was 33.3%, and 17.6% among patients using nivolumab with cannabis (p = .128, OR = 0.43, 95% CI 0.14–1.28). In the melanoma and clear cell RCC group, the RR among patients using nivolumab alone was 43.3%, and 10% among patients using nivolumab with cannabis (p = .084, OR = 0.15, 95% CI 0.02–1.3).
Figure 1.
Response rate among patients with overall survival ≥2 months (n = 116).
Abbreviations: CR, complete response; NSCLC, non‐small cell lung cancer; PR, partial response; RCC, renal cell carcinoma.
A multivariate stepwise analysis was made to all variables close to statistical significance and showed that the only factor affecting the response to treatment was cannabis (p = .016, OR = 3.17, 95% CI 1.24–8.1). Patients not using cannabis were 3.17 times more likely to respond to treatment.
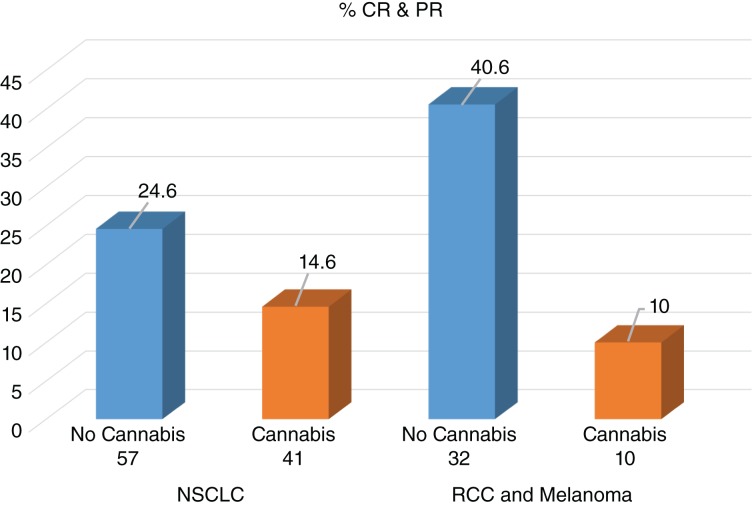
The same statistical model was used on all patients, including patients with OS of less than 2 months. The model again showed a decreased RR in both groups among patients using cannabis with nivolumab. In the NSCLC group, the RR among patients using nivolumab alone was 24.6%, and 14.6% among patients using nivolumab with cannabis (p = .234, OR = 0.53, 95% CI 0.18–1.51). In the melanoma and clear cell RCC group, the RR among patients using nivolumab alone was 40.6%, and 10% among patients using nivolumab with cannabis (p = .103, OR = 0.16, 95% CI 0.02–1.44). These findings are shown in Figure 2.
Figure 2.
Response rate among all patients (n = 140).
Abbreviations: CR, complete response; NSCLC, non‐small cell lung cancer; PR, partial response; RCC, renal cell carcinoma.
A multivariate stepwise analysis was made to all variables close to statistical significance and showed that the only factor affecting response to treatment was cannabis (p = .031, adjusted OR = 2.74, 95% CI 1.09–6.85).
Data related to cannabis were given by six of eight cannabis‐supplying companies in Israel for 37 of 51 patients in the nivolumab plus cannabis group. We could not obtain sufficient details for the remaining 14 patients and they were excluded from the statistics. All cannabis products in Israel are analyzed by the Laboratory for Cannabinoids Research at Technion‐Israel Institute of Technology in Haifa, which provided the data on the products that were used. Because the THC and CBD percentages range was wide, we divided the products to four subgroups depending on the substances' percentage cutoff. We chose 10% as the THC cutoff and 1% as the CBD cutoff.
As shown in Table 3, THC and CBD percentages did not affect the RR in any group (p = .393 and .116, respectively). The results might indicate a possible paradoxical interaction related to THC level, as patients who had high‐THC‐percentage products had a better RR to immunotherapy compared with those with low‐THC‐percentage products.
Most of the patients (75%) used the lowest prescribed monthly dose of cannabis (20 grams), and only 13 patients used a dose of 30 grams or higher during the immunotherapy treatment period. The patients who used cannabis were divided into three groups of users, each including one third of the patients: smoked or inhaled (cannabis flowers only), prepared cannabis oil, or combined use (Table 4). The cannabis dose or way of use did not have any significance on RR.
Table 4. Patients' ways of use and doses of cannabis.
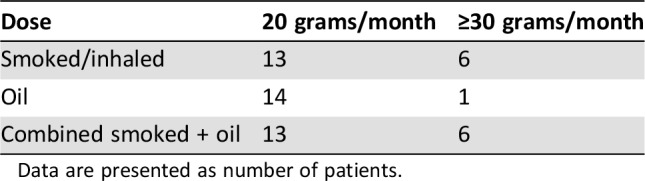
Data are presented as number of patients.
In univariate analysis, cannabis use was not a significant factor for PFS (p = .27, hazard ratio [HR] 1.25) but was a weak significant factor for OS (p = .045, HR = 1.58, 95% CI 1.01–2.46). In the multivariate analysis, cannabis was not a significant factor for PFS or OS. The significant factors affecting PFS were smoking status (p = .004, adjusted HR = 2.01, 95% CI for HR 1.25–3.24) and existing brain metastases (p = .013, adjusted HR = 2.04, 95% CI for HR 1.16–3.59). The factors that affected OS were smoking status (p < .0001, adjusted HR = 2.41, 95% CI for HR 1.33–4.37), existing brain metastases (p < .0001, adjusted HR = 2.83, 95% CI for HR 1.43–5.57), and low performance status—PS = 2 (p < .0001, adjusted HR = 4.25, 95% CI for HR 1.75–10.34).
To see if cannabis has any weak effect on PFS and OS, a Cox‐regression EnTER analysis technique was performed to evaluate the effect of cannabis on improving the quality of the statistical model. The analysis did not demonstrate any improvement on the quality of the statistical model and did not change the HR.
Discussion
The current study demonstrated a possible interaction between cannabis use and immunotherapy among cancer patients, as manifested by a decrease in RR to immunotherapy when using cannabis.
Whereas cannabinoids binding to CB1 receptors mainly result in psychoactive effects, such as analgesia and antiemesis, their binding to CB2 receptors is mainly associated with anti‐inflammatory and immunomodulatory effects. Modulation of the functional activities of immune cells results in suppression of cellular functions and cytokine production [17]. These suppressive actions have been studied in many disease models, mainly in animals, in both in vivo and in vitro systems. Suppression mechanisms have been also demonstrated in the treatment course of both preclinical and clinical models in humans. Modulatory immune responses were shown in various acute and chronic neuroinflammatory diseases, arthritis, and septic shock [18], [19], [20]. Moreover, preliminary clinical data support a beneficial role of cannabinoids in inflammatory bowel diseases [21].
PD‐1 receptor and its ligand PD‐L1 are highly expressed on T‐regs and exert a wide range of immunoregulatory roles in T‐cell activation and tolerance. The interaction between PD‐1 and PD‐L1 is involved in attenuating infectious immunity and tumor immunity, and in facilitating chronic infection and tumor progression. It also regulates the induction and maintenance of peripheral tolerance and protects tissues from autoimmune attack [21], [22], [23]. Inhibition of the interaction between PD‐1 and PD‐L1 harnesses the immune system through different mechanisms and enhances antitumor responses, delays tumor growth, and facilitates tumor rejection.
Patients using immunosuppressant drugs, such as glucocorticosteroids, are less recommended to use PD‐1 or PD‐L1 inhibitor drugs. Besides their profound effects on the cellular functions of leukocytes and endothelial cells, causing impairment entering to sites of infection and tissue injury and, therefore, resulting in suppression of the inflammatory response, glucocorticosteroids impair a variety of T‐cell functions and induce T‐cell apoptosis [24], [25]. Their effects in low‐moderate doses is more complicated, as they decrease counts of immature naïve CD4+ cells and slightly increase counts of CD8+, memory subsets, Th17+, and mature CD4+ cells [26].
The analysis did not show a significant difference in terms of OS or PFS due to the cannabis use. The factors affecting OS or PFS (smoking, brain metastases, and low performance status) are known to significantly affect these endpoints regardless of the use of cannabis.
In contrast to chemotherapy, RR is an eminent index for evaluation when using immunotherapy, because it usually shows an interpretation in terms of OS. This demonstrates the importance of being aware of this potential interaction, the need to bring it to the patient's attention, and the need to further investigate this interaction.
The findings of the current study should be evaluated in light of the study's limitations. It is a retrospective study that included a relatively small group of patients and a nonrepresentative sample, given the high number of lung cancer patients. Furthermore, the follow‐up period was relatively short.
Conclusion
The current preliminary findings demonstrated the effect of cannabis use on cancer patients' response to immunotherapy. Given the increased use of these two therapies in oncology, it is highly important to further investigate the interactions between them.
Author Contributions
Conception/design: Gil Bar‐Sela
Provision of study material or patients: Gil Bar‐Sela
Collection and/or assembly of data: Tarek Taha, David Meiri, Samira Talhamy, Mira Wollner, Avivit Peer
Data analysis and interpretation: Tarek Taha, David Meiri, Gil Bar‐Sela
Manuscript writing: Tarek Taha, David Meiri, Samira Talhamy, Mira Wollner, Avivit Peer, Gil Bar‐Sela
Final approval of manuscript: David Meiri, Gil Bar‐Sela
Disclosures
The authors indicated no financial relationships.
References
- 1.Francisco LM, Salinas VH, Brown KE et al. PD‐L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med 2009;206:3015–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amarnath S, Mangus CW, Wang JC et al. The PDL1‐PD1 axis converts human Th1 cells into regulatory T cells. Sci Transl Med 2011;3:111ra120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topalian SL, Sznol M, McDermott DF et al. Survival, durable tumor remission, and long‐term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014;32:1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hodi FS, Kluger H, Sznol M et al. Durable, long‐term survival in previously treated patients with advanced melanoma (MEL) who received nivolumab (NIVO) monotherapy in a phase I trial. Proceedings: AACR 107th Annual Meeting 2016; April 16–20, 2016; New Orleans, LA (abstract CT001).
- 5.Socinski M, Creelan B, Horn L et al. CheckMate 026: A phase 3 trial of nivolumab vs investigator's choice (IC) of platinum‐based doublet chemotherapy (PT‐DC) as first‐line therapy for stage iv/recurrent programmed death ligand 1 (PD‐L1)−positive NSCLC. Presented at: ESMO 2016; LBA7 PR.
- 6.Topalian SL, Hodi FS, Brahmer JR et al. Safety, activity, and immune correlates of anti‐PD‐1 antibody in cancer. N Engl J Med 2012;366:2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lichtenstein GR, Abreu MT, Cohen R et al. American Gastroenterological Association Institute technical review on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology 2006;130:940–987. [DOI] [PubMed] [Google Scholar]
- 8.Nelson RP Jr, Ballow M. Immunomodulation and immunotherapy: Drugs, cytokines, cytokine receptors, and antibodies. J Allergy Clin Immunol 2003;111(suppl 2):S720–S743. [DOI] [PubMed] [Google Scholar]
- 9.Xing K, Gu B, Zhang P et al. Dexamethasone enhances programmed cell death 1 (PD‐1) expression during T cell activation: An insight into the optimum application of glucocorticoids in anti‐cancer therapy. BMC Immunol 2015;16:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bar‐Sela G, Vorobeichik M, Drawsheh S et al. The medical necessity for medicinal cannabis: Prospective, observational study evaluating treatment in cancer patients on supportive or palliative care. Evid Based Complement Alternat Med 2013;2013:510392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bar‐Sela G, Avisar A, Batash R et al. Is the clinical use of cannabis by oncology patients advisable? Curr Med Chem 2014;21:1923–1930. [DOI] [PubMed] [Google Scholar]
- 12.Cannabis‐In‐Cachexia‐Study‐Group , Strasser F, Luftner D et al. Comparison of orally administered cannabis extract and delta‐9‐tetrahydrocannabinol in treating patients with cancer‐related anorexia‐cachexia syndrome: A multicenter, phase III, randomized, double‐blind, placebo‐controlled clinical trial from the Cannabis‐In‐Cachexia‐Study‐Group. J Clin Oncol 2006;24:3394–3400. [DOI] [PubMed] [Google Scholar]
- 13.Brisbois TD, de Kock IH, Watanabe SM. Delta‐9‐tetrahydrocannabinol may palliate altered chemosensory perception in cancer patients: Results of a randomized, double‐blind, placebo‐controlled pilot trial. Ann Oncol 2011;22:2086–2093. [DOI] [PubMed] [Google Scholar]
- 14.McKallip RJ, Lombard C, Martin BR et al. Delta(9)‐tetrahydrocannabinol induced apoptosis in the thymus and spleen as a mechanism of immunosuppression in vitro and in vivo. J Pharmacol Exp Ther 2002;302:451–465. [DOI] [PubMed] [Google Scholar]
- 15.Klein TW, Cabral GA. Cannabinoid‐induced immune suppression and modulation of antigen‐presenting cells. J Neuroimmune Pharmacol 2006;1:50–64. [DOI] [PubMed] [Google Scholar]
- 16.Friedman H, Klein TW, Newton C et al. Marijuana, receptors and immunomodulation. Adv Exp Med Biol 1995;373:103–113. [DOI] [PubMed] [Google Scholar]
- 17.Rieder SA, Chauhan A, Singh U et al. Cannabinoid‐induced apoptosis in immune cells as a pathway to immunosuppression. Immunobiology 2010;215:598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Croxford JL, Yamamura T. Cannabinoids and the immune system: Potential for the treatment of inflammatory diseases? J Neuroimmunol 2005;166:3–18. [DOI] [PubMed] [Google Scholar]
- 19.Katz‐Talmor D, Katz I, Porat‐Katz BS, Shoenfeld Y. Cannabinoids for the treatment of rheumatic disease—Where do we stand? Nat Rev Rheumatol 2018;14:488–498. [DOI] [PubMed] [Google Scholar]
- 20.Rom S, Persidsky Y. Cannabinoid receptor 2: Potential role in immunomodulation and neuroinflammation. J Neuroimmune Pharmacol 2013;8:608–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naftali T, Mechulam R, Lev LB et al. Cannabis for inflammatory bowel disease. Dig Dis 2014;32:468–474. [DOI] [PubMed] [Google Scholar]
- 22.Sui X, Ma J, Han W et al. The anticancer immune response of anti‐PD‐1/PD‐L1 and the genetic determinants of response to anti‐PD‐1/PD‐L1 antibodies in cancer patients. Oncotarget 2015;6:19393–19404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Topalian SL, Drake CG, Pardoll DM. Targeting the PD‐1/B7‐H1(PD‐L1) pathway to activate anti‐tumor immunity. Curr Opin Immunol 2012;24:207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin HT, Ahmed R, Okazaki T. Role of PD‐1 in regulating T‐cell immunity. Curr Top Microbiol Immunol 2011;350:17–37. [DOI] [PubMed] [Google Scholar]
- 25.Fauci AS, Murakami T, Brandon DD et al. Mechanisms of corticosteroid action on lymphocyte subpopulations. VI. Lack of correlation between glucocorticosteroid receptors and the differential effects of glucocorticosteroids on T‐cell subpopulations. Cell Immunol 1980;49:43–50. [DOI] [PubMed] [Google Scholar]
- 26.Olnes MJ, Kotliarov Y, Biancotto A et al. Effects of systemically administered hydrocortisone on the human immunome. Sci Rep 2016;6:23002. [DOI] [PMC free article] [PubMed] [Google Scholar]