This article focuses on the efficacy and safety of bevacizumab through multiple lines in patients with recurrent glioblastoma who progressed after first‐line treatment with radiotherapy, temozolomide, and bevacizumab.
Keywords: Clinical trial, Continuous bevacizumab, Overall survival, Recurrent glioblastoma
Abstract
Background.
We assessed the efficacy and safety of bevacizumab (BEV) through multiple lines in patients with recurrent glioblastoma who had progressed after first‐line treatment with radiotherapy, temozolomide, and BEV.
Patients and Methods.
TAMIGA (NCT01860638) was a phase II, randomized, double‐blind, placebo‐controlled, multicenter trial in adult patients with glioblastoma. Following surgery, patients with newly diagnosed glioblastoma received first‐line treatment consisting of radiotherapy plus temozolomide and BEV, followed by six cycles of temozolomide and BEV, then BEV monotherapy until disease progression (PD1). Randomization occurred at PD1 (second line), and patients received lomustine (CCNU) plus BEV (CCNU + BEV) or CCNU plus placebo (CCNU + placebo) until further disease progression (PD2). At PD2 (third line), patients continued BEV or placebo with chemotherapy (investigator's choice). The primary endpoint was survival from randomization. Secondary endpoints were progression‐free survival in the second and third lines (PFS2 and PFS3) and safety.
Results.
Of the 296 patients enrolled, 123 were randomized at PD1 (CCNU + BEV, n = 61; CCNU + placebo, n = 62). The study was terminated prematurely because of the high drop‐out rate during first‐line treatment, implying underpowered inferential testing. The proportion of patients receiving corticosteroids at randomization was similar (BEV 33%, placebo 31%). For the CCNU + BEV and CCNU + placebo groups, respectively, median survival from randomization was 6.4 versus 5.5 months (stratified hazard ratio [HR], 1.04; 95% confidence interval [CI], 0.69–1.59), median PFS2 was 2.3 versus 1.8 months (stratified HR, 0.70; 95% CI, 0.48–1.00), median PFS3 was 2.0 versus 2.2 months (stratified HR, 0.70; 95% CI, 0.37–1.33), and median time from randomization to a deterioration in health‐related quality of life was 1.4 versus 1.3 months (stratified HR, 0.76; 95% CI, 0.52–1.12). The incidence of treatment‐related grade 3 to 4 adverse events was 19% (CCNU + BEV) versus 15% (CCNU + placebo).
Conclusion.
There was no survival benefit and no detriment observed with continuing BEV through multiple lines in patients with recurrent glioblastoma.
Implications for Practice.
Previous research suggested that there may be value in continuing bevacizumab (BEV) beyond progression through multiple lines of therapy. No survival benefit was observed with the use of BEV through multiple lines in patients with glioblastoma who had progressed after first‐line treatment (radiotherapy + temozolomide + BEV). No new safety concerns arose from the use of BEV through multiple lines of therapy.
Introduction
Glioblastomas account for almost 50% of malignant central nervous system (CNS) tumors and have an annual incidence of 3.2 per 100,000 in the U.S. [1]. Glioblastomas are characterized by overexpression of vascular endothelial growth factor (VEGF)‐A (a key tumor‐associated regulator of angiogenesis) and a high degree of vascularization. Despite first‐line treatment with surgical resection followed by radiotherapy (RT) plus concomitant and adjuvant temozolomide (TMZ) [2], [3], the prognosis remains poor, with 1‐ and 5‐year glioblastoma survival rates of 39.3% and 5.5%, respectively [1]. In the recurrent setting, chemotherapy remains the main treatment option [4]; nitrosourea‐based regimens, such as lomustine (CCNU), are the preferred choice [5], [6], [7], [8]. The efficacy of chemotherapeutic agents is limited, and there remains a high unmet medical need to establish effective glioblastoma treatments; anti‐VEGF agents, such as bevacizumab (BEV), have been investigated.
BEV is approved in more than 60 countries worldwide, including the U.S., for use in adults with recurrent glioblastoma [9], [10], [11]. At the time of the protocol conception and initiation, two randomized, phase III trials showed that the addition of BEV to RT and TMZ extended progression‐free survival (PFS) in adult patients with newly diagnosed glioblastoma but did not improve overall survival (OS) [12], [13].
Tumors may be less likely to develop resistance to antiangiogenic agents than to chemotherapy [14]. VEGF has been implicated in the acquisition of drug resistance in tumor endothelial cells [15], and resistance to BEV would need to occur through development of alternative angiogenesis pathways. Thus, there may be value in continuing BEV through multiple lines [16], [17], [18], [19]. The strategy of sustained VEGF inhibition has been extensively investigated in metastatic colorectal cancer, in which continuing BEV through multiple lines improved OS [16], [17]. A retrospective pooled analysis of five consecutive, single‐arm, phase II clinical studies evaluating BEV‐based treatments for recurrent glioblastoma suggested that BEV continuation beyond initial progression was an independent predictor of improved OS [19].
The placebo‐controlled TAMIGA study aimed to assess the efficacy and safety of BEV through multiple lines (plus CCNU in second line and chemotherapy of investigator's choice in third line and beyond) in patients with glioblastoma who had received first‐line treatment with RT, TMZ, and BEV prior to randomization.
Materials and Methods
Study Design
TAMIGA (study number MO28347; ClinicalTrials.gov NCT01860638) was a randomized, double‐blind, placebo‐controlled, multicenter, phase II trial in patients with recurrent glioblastoma. The study was originally designed as a phase III trial, following the phase III AVAglio study (BEV in newly diagnosed glioblastoma) [12], in which one of the coprimary endpoints (improved PFS with BEV + RT + TMZ vs. placebo + RT + TMZ) was met. However, the other coprimary endpoint (improved OS) was not met, and approval for the BEV indication to include adult patients with newly diagnosed glioblastoma was not granted by the Committee for Medicinal Products for Human Use. Thus, the sponsor amended the TAMIGA design to a phase II trial, stopping recruitment with front‐line RT plus TMZ and BEV but allowing already enrolled patients to continue in the study, maintaining the same endpoints as the planned phase III trial.
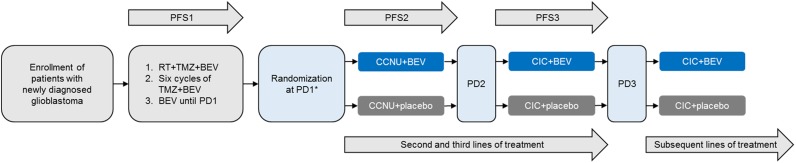
First‐line treatment included RT plus TMZ and BEV, followed by maintenance treatment with six cycles of TMZ plus BEV, then BEV monotherapy until first disease progression (PD1; Fig. 1). At PD1, patients eligible for second‐line therapy were randomized 1:1 to receive CCNU + BEV or CCNU + placebo through subsequent lines of treatment until second PD (PD2). The CCNU dose was 90 mg/m2 (maximum of 160 mg) every 6 weeks, increasing to 110 mg/m2 (maximum of 200 mg) in the absence of hematologic toxicity grade >1 during the first cycle. The BEV dose was 10 mg/kg every 2 weeks (intravenous). At PD2, patients continued to receive BEV or placebo (according to randomization at PD1) in addition to chemotherapy of investigator's choice. Stratification factors included PD1 timing (before vs. after completion of first‐line maintenance therapy) and Eastern Cooperative Oncology Group performance status (ECOG PS, 0 vs. 1 or 2). Investigators and patients were blinded to treatment assignment.
Figure 1.
TAMIGA study design. Stratification factors: occurrence of PD1 relative to completion of first‐line maintenance therapy (before vs. after completion) and Eastern Cooperative Oncology Group performance status at randomization (0 vs. 1 or 2). Abbreviations: *, start of TAMIGA study; BEV, bevacizumab; CCNU, lomustine; CIC, chemotherapy (investigator's choice); PD1, first disease progression; PD2, second disease progression; PD3, third disease progression; PFS1, first‐line progression‐free survival; PFS2, second‐line progression‐free survival; PFS3, third‐line progression‐free survival; RT, radiotherapy; TMZ, temozolomide.
Disease status was assessed by response assessment in neuro‐oncology (RANO) criteria [20]. All patients were followed until death, withdrawal of consent, loss to follow‐up, or study termination, whichever occurred first. The study end was defined as the date when the last visit of the last patient occurred (planned as 30 days after the 130th OS event was reported, estimated to be approximately 36 months after the first patient enrolled).
The protocol was approved by institutional review boards/ethics committees, and the study was conducted in accordance with individual countries’ regulations, International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Good Clinical Practice guidelines, and the principles of the Declaration of Helsinki. Written informed consent was obtained from all patients.
Patients
Patients aged ≥18 years with newly diagnosed glioblastoma histologically confirmed with surgical resection or biopsy, who had not been previously treated with chemotherapy or RT, were enrolled to receive front‐line treatment according to the regimen used in the AVAglio trial [12]. Key exclusion criteria included prior chemotherapy or immunotherapy for glioblastoma or low‐grade astrocytoma, or prior RT to the brain; prior or current antiangiogenic treatment; treatment with another investigational agent within 28 days or two investigational agent half‐lives (whichever was longer) prior to first study treatment; and history or evidence of CNS disease unrelated to cancer unless adequately treated with standard medical therapy.
At PD1 (by RANO criteria), patients were randomized if they had an ECOG PS of 0–2 and no BEV interruptions lasting >60 consecutive days during first‐line treatment. Randomization occurred within 28 days from PD1. Second surgery at PD1 was allowed (no BEV at least ±28 days from surgery). Tissue submission was mandatory before the start of second‐line treatment.
Study Assessments
The primary endpoint was OS, defined as the time from randomization at PD1 until death from any cause. Secondary endpoints included 6‐, 12‐, and 18‐month OS rates (as measured from randomization at PD1); PFS in the second (PFS2) and third (PFS3) lines of treatment; corticosteroid use; and health‐related quality of life (HRQoL). See supplemental online Appendix 1 for further definitions and details. Exploratory analyses of OS from enrollment and PFS from enrollment (PFS1) were also conducted.
Safety
Patients were monitored throughout the study for adverse events (AEs), serious AEs (SAEs), AEs of special interest (AESIs), and any AEs requiring drug interruption or discontinuation. Safety monitoring was performed every 3–6 months by an independent data monitoring committee.
Statistical Analysis
Full statistical methods are described in supplemental online Appendix 1.
Results
Patients
Overall, 296 patients were enrolled at 51 sites between August 2013 and December 2014. At the clinical cutoff date (January 13, 2017), 54% of patients discontinued first‐line treatment with RT + TMZ + BEV, and 123 patients were randomized at PD1 (CCNU + BEV, n = 61; CCNU + placebo, n = 62). Reasons for treatment discontinuation included AEs/SAEs (24.0%), investigator decision (8.1%), withdrawal of patient consent (5.1%), death (4.7%), administrative/other (4.7%), patient decision (4.1%), nonadherence (3.0%), and protocol violation (0.3%). During the second line, 56% of CCNU + BEV‐treated patients and 58% of CCNU + placebo‐treated patients discontinued study treatment. The most common reasons for treatment discontinuation in the CCNU + BEV and CCNU + placebo groups included investigator decision (16% and 18%), SAEs (10% and 13%), and withdrawal of patient consent (7% and 10%). At PD2, 25 patients in each group received third‐line treatment (supplemental online Fig. 1).
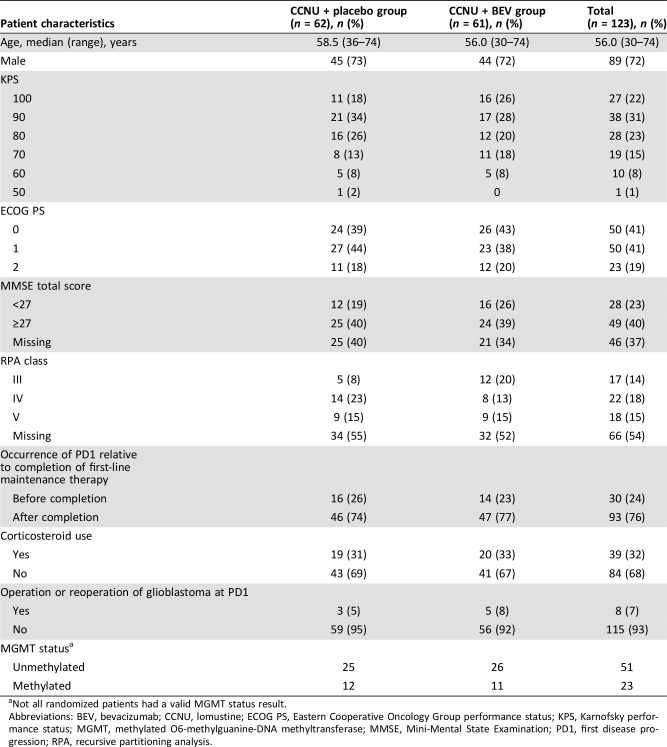
Patient characteristics at randomization were balanced between the treatment groups (Table 1), although more patients in the CCNU + BEV group were in recursive partitioning analysis class III than those in the CCNU + placebo group (20% and 8%, respectively). The majority of patients did not undergo reoperation at PD1 (CCNU + BEV, 92%; CCNU + placebo, 95%). Both treatment arms had a similar number of patients with (un‐)methylated O6‐methylguanine‐DNA methyltransferase. Gene expression profiling is currently ongoing (supplemental online Appendix 1).
Table 1. Patient demographics and clinical characteristics at randomization (intent‐to‐treat population).
Not all randomized patients had a valid MGMT status result.
Abbreviations: BEV, bevacizumab; CCNU, lomustine; ECOG PS, Eastern Cooperative Oncology Group performance status; KPS, Karnofsky performance status; MGMT, methylated O6‐methylguanine‐DNA methyltransferase; MMSE, Mini‐Mental State Examination; PD1, first disease progression; RPA, recursive partitioning analysis.
Exposure to BEV or Placebo
Among patients randomized to receive second‐line treatment, the median number of total administrations per patient was 4.0 (range, 1–17) and 3.0 (range, 1–35) in the CCNU + BEV and CCNU + placebo groups, respectively, and 81% and 80% of patients completed ≥90% of planned doses. Corresponding median values among patients who received third‐line treatment were 4.0 (range, 1–12) and 4.0 (range, 1–58) administrations, with 100% and 88% of patients completing ≥90% of planned doses.
A summary of the exposure to treatment in second and subsequent lines is shown in supplemental online Table 1.
OS (Primary Endpoint)
The study was terminated prematurely because of a high drop‐out rate during first‐line treatment, implying underpowered (i.e., 60%) inferential testing; only 98 of the targeted 130 OS events for the primary endpoint were reached in the 123 randomized patients; therefore, no p values are reported and only 95% confidence intervals (CIs) are shown.
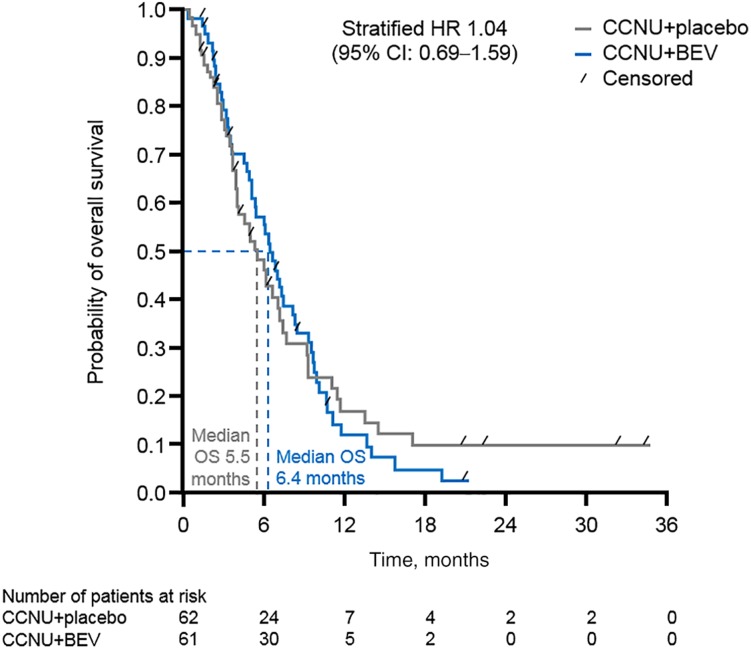
In the intent‐to‐treat population, median OS from randomization was 6.4 (95% CI, 5.1–8.1) and 5.5 months (95% CI, 3.9–7.2) in the CCNU + BEV and CCNU + placebo groups, respectively (stratified hazard ratio [HR], 1.04; 95% CI, 0.69–1.59; Fig. 2). The OS for the per protocol population (sensitivity analysis) showed comparable results (median 6.0 and 4.9 months, respectively; HR, 1.01; 95% CI, 0.62–1.64).
Figure 2.
OS from randomization with multiple‐line BEV treatment versus placebo (primary efficacy endpoint). Abbreviations: BEV, bevacizumab; CCNU, lomustine; CI, confidence interval; HR, hazard ratio; OS, overall survival.
OS Rates and PFS (Secondary Endpoints)
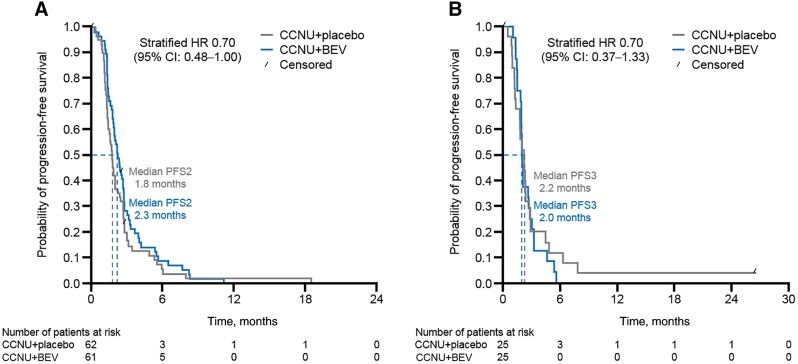
The Kaplan‐Meier‐estimated event‐free rates (CCNU + BEV vs. CCNU + placebo, respectively) for OS at 6, 12, and 18 months were 55.4% (95% CI, 41.4–67.3) versus 46.2% (95% CI, 32.8–58.5), 11.7% (95% CI, 4.6–22.5) versus 16.5% (95% CI, 7.5–28.5), and 4.7% (95% CI, 0.9–13.7) versus 9.4% (95% CI, 3.1–20.1). For CCNU + BEV versus CCNU + placebo, respectively, values for median PFS2 were 2.3 months (95% CI, 1.9–2.7) and 1.8 months (95% CI, 1.4–2.1; stratified HR, 0.70; 95% CI, 0.48–1.04; Fig. 3A), and median PFS3 was 2.0 months (95% CI, 1.8–2.7) and 2.2 months (95% CI, 1.3–2.7, stratified HR, 0.70; 95% CI, 0.37–1.33; Fig. 3B). Definitions of PFS2 and PFS3 are described in supplemental online Appendix 1.
Figure 3.
PFS2 and PFS3 with multiple‐line BEV treatment versus placebo. (A): PFS2. (B): PFS3. Abbreviations: BEV, bevacizumab; CCNU, lomustine; CI, confidence interval; HR, hazard ratio; PFS2, second‐line progression‐free survival; PFS3, third‐line progression‐free survival.
Corticosteroid Intake
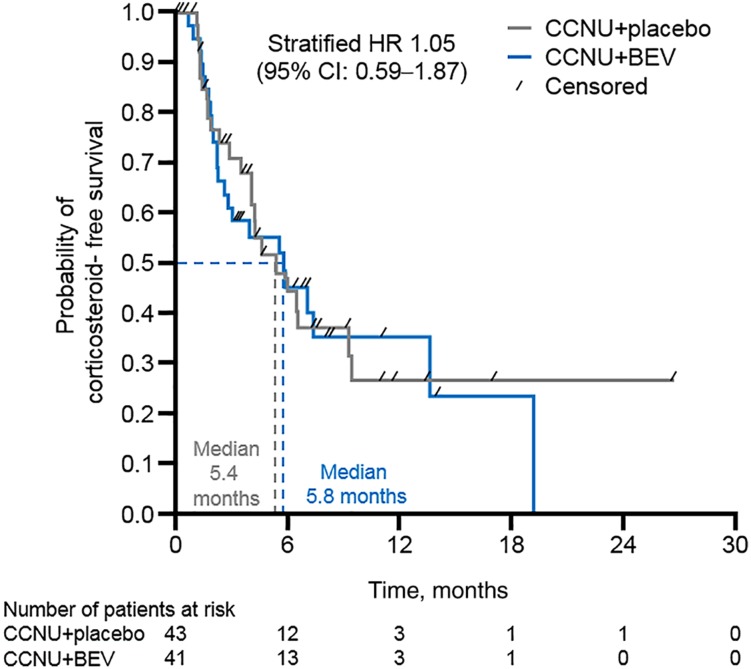
The proportion of patients receiving corticosteroids at the time of randomization was balanced between treatment arms (33% vs. 31%, CCNU + BEV vs. CCNU + placebo, respectively). For those patients not receiving corticosteroids at the time of randomization (BEV, n = 41; placebo, n = 43), the median time from randomization to corticosteroid initiation during second‐line treatment was 5.8 months (95% CI, 2.3–13.7) in the CCNU + BEV group, and 5.4 months (95% CI, 3.5–9.3) in the CCNU + placebo group (unstratified HR, 1.05; 95% CI, 0.59–1.87; Fig. 4).
Figure 4.
Time‐to‐event analysis of corticosteroid initiation in second‐line treatment with BEV versus placebo. A deterioration in health‐related quality of life (HRQoL) was defined as a decrease of ≥10 points from randomization on the respective functional scale/global health status or an increase of ≥10 points from randomization on the respective symptom/neurological deficit subscales; time to HRQoL deterioration was defined as the time from randomization to HRQoL deterioration or second‐line progression or death due to any cause. Abbreviations: BEV, bevacizumab; CCNU, lomustine; CI, confidence interval; HR, hazard ratio.
Analyses of OS from Enrollment and PFS from Enrollment (PFS1; Exploratory Analyses)
Median OS from enrollment, at time of diagnosis, was 18.1 (95% CI, 13.9–20.7) and 16.4 months (95% CI, 14.3–20.3) in the CCNU + BEV and CCNU + placebo groups, respectively (supplemental online Fig. 2). Median PFS1 was 10.8 months (95% CI, 10.3–11.8).
Safety and HRQoL
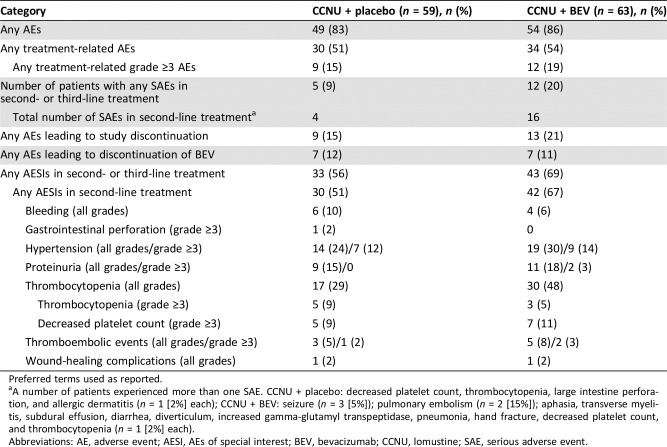
Across the second and third lines of treatment combined, in the CCNU + BEV and CCNU + placebo groups, respectively, 86% and 83% of patients experienced at least one AE, 19% and 15% experienced at least one treatment‐related grade ≥3 AE, 20% and 9% experienced at least one SAE, 21% and 15% experienced an AE leading to study discontinuation, and 69% and 56% had at least one AESI (Table 2). More patients in the CCNU + BEV group experienced thrombocytopenia (mostly grade 1–2) than those receiving CCNU + placebo (48% vs. 29%).
Table 2. Overview of safety in second‐ and third‐line treatments (safety population).
Preferred terms used as reported.
A number of patients experienced more than one SAE. CCNU + placebo: decreased platelet count, thrombocytopenia, large intestine perforation, and allergic dermatitis (n = 1 [2%] each); CCNU + BEV: seizure (n = 3 [5%]); pulmonary embolism (n = 2 [15%]); aphasia, transverse myelitis, subdural effusion, diarrhea, diverticulum, increased gamma‐glutamyl transpeptidase, pneumonia, hand fracture, decreased platelet count, and thrombocytopenia (n = 1 [2%] each).
Abbreviations: AE, adverse event; AESI, AEs of special interest; BEV, bevacizumab; CCNU, lomustine; SAE, serious adverse event.
A similar number of patients died during the study in the CCNU + BEV (n = 42 [67%]) and CCNU + placebo (n = 41 [70%]) groups, with the majority of deaths in both treatment groups due to disease progression (41 of 42 [98%] and 39 of 41 [95%], respectively). None of the deaths was considered treatment related.
Analysis of median time to HRQoL deterioration in second‐line treatment did not show a difference between the treatment groups: 1.3 months (95% CI, 1.0–1.6) in the CCNU + placebo group and 1.4 months (95% CI, 1.2–2.0) in the CCNU + BEV group (stratified HR, 0.76, 95% CI, 0.52–1.12; supplemental online Fig. 3).
Discussion
Although this study was initially designed as a phase III study, the protocol was amended, and the design was changed to a phase II study with the same primary endpoint (OS). There was no observed benefit or detrimental effect on OS with the addition of BEV to second‐line CCNU and third‐line chemotherapy of investigator's choice in patients with recurrent glioblastoma; however, PFS2 was numerically longer with CCNU + BEV than with CCNU + placebo (although PFS3 was not).
Overall, these findings are in line with previous studies of BEV for newly diagnosed glioblastoma, in which improvements in PFS with the addition of BEV to RT and TMZ did not translate to an OS benefit [16], [17]. A recent meta‐analysis showed that BEV improved PFS, both alone and in combination with chemotherapy, and both as first‐line treatment or in recurrent glioblastoma, but no improvement in OS was observed [21]. The median OS of 6.4 months for BEV‐treated patients in the current study was shorter than that reported in a previous phase II trial of BEV at first recurrence (9 months) [14]; however, in that study (the BRAIN study), patients had no prior exposure to BEV. Median PFS1 and OS from enrollment in the current study (10.8 months and 18.1 months, respectively) are consistent with the PFS and OS observed in the AVAglio study (10.6 months and 16.8 months, respectively) [12]. The lack of OS benefit observed in the current study is also consistent with the European Organisation for Research and Treatment of Cancer 26101 trial of CCNU plus BEV versus CCNU alone for recurrent glioblastoma (median OS 9.1 vs. 8.6 months) [22].
A previous study of BEV through multiple lines (TML) for the treatment of patients with metastatic colorectal cancer who had progressed following first‐line BEV‐based therapy showed an OS benefit compared with second‐line chemotherapy alone (median OS 11.2 vs. 9.8 months; HR, 0.81; 95% CI, 0.69–0.94; p = .006) [12]. However, there is limited existing literature, mainly retrospective with relatively small study samples, on the efficacy of BEV through multiple lines for patients with glioblastoma. One retrospective study of patients with recurrent malignant glioma showed that continuing BEV and modifying the chemotherapy agent after disease progression only provided long‐term control in a small subset of patients [23]. Other studies have suggested that BEV continuation beyond initial progression was an independent predictor of improved OS in patients with recurrent glioblastoma [19]. The prospective phase II CABARET study of patients with recurrent glioblastoma who progressed on BEV did not demonstrate improvements in median PFS or OS with continuing BEV beyond disease progression compared with stopping BEV treatment [24].
In the current study, the proportion of patients who were receiving corticosteroids at randomization was similar between treatment groups (approximately one third) and lower than the proportion of patients using corticosteroids reported at study entry in previous trials of recurrent glioblastoma (49%–54%) [14], [15], [22]. This may suggest that patients who received first‐line BEV in addition to RT and TMZ benefitted in terms of reduced corticosteroid use. This is in line with the AVaglio study, in which more patients with newly diagnosed glioblastoma were able to discontinue corticosteroid use for ≥5 consecutive days in the BEV + RT + TMZ arm, versus placebo plus RT + TMZ (66.3% vs. 47.1%) [16]. In TAMIGA, there were no differences between treatment groups in the time of initiation and use of corticosteroids after randomization. Time‐to‐event analysis of HRQoL deterioration in second‐line treatment did not show a difference between the placebo and BEV groups (supplemental online Appendix 1), which is consistent with previous trials [22], [24].
Administration of BEV through multiple lines may raise concerns about increased toxicity; this was addressed by comparing BEV through multiple lines to a placebo‐treated control arm. No new safety signals were observed. The incidence of AEs in second and third lines of treatment was generally similar between the treatment groups, although the incidence of SAEs in second‐line treatment was higher with CCNU + BEV than with CCNU + placebo (18% vs. 7%). The higher rate of thrombocytopenia observed in the CCNU + BEV group than in the CCNU + placebo group is in line with data from the BELOB and AVAREG studies [6], [7]. The high rates of study treatment discontinuation in second and third lines of treatment observed in this study reflect the hastened clinical deterioration upon progression and the difficulty in managing these patients in clinical practice. Similarly, in the CABARET trial, only 40% of patients who were initially randomized to receive BEV were able to continue into the second part of the study after further disease progression (80% of the planned sample size) [24].
A strength of this study is that we evaluated the efficacy and safety of BEV through multiple lines of therapy in patients with recurrent glioblastoma with a placebo‐controlled design in all subsequent lines of chemotherapy. The design was intended to provide a representative comparison to the treatment path of patients with glioblastoma throughout the course of the disease and to minimize the confounding factor of crossover from the placebo arm in later lines. Limitations include the high rate of study treatment discontinuation during first and second lines of treatment, which led to the premature termination of the study, limiting our ability to draw strong conclusions regarding treatment effects.
Conclusion
This study was terminated prematurely and was underpowered to address the primary objective. Descriptive analyses showed that there was no benefit or detriment in OS with continuing multiple‐line BEV treatment in patients with glioblastoma who had progressed following first‐line treatment with RT, TMZ, and BEV. No new safety signals were observed with BEV through multiple lines.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
The authors thank the patients and their families for participating in this study, the study investigators and their staff, Jeremy Scarato (International Clinical Trial Manager of the study), Vesna Sneller (former Clinical Scientist of the study), and Dr. Jeffrey Wefel (MD Anderson Cancer Center) for his help on the steering committee for the study. Third‐party writing assistance, under the direction of the authors, was provided by Thomas Burton, BMBS, and Abigail Robertson, Ph.D., of Gardiner‐Caldwell Communications, Macclesfield, U.K., and was funded by F. Hoffmann‐La Roche Ltd. The results from this study were previously presented at the 22nd Annual Scientific Meeting of the Society for Neuro‐Oncology 2017.
Retired
Author Contributions
Conception/design: Alba A. Brandes, Miguel Gil Gil, Frank Saran, Antoine F. Carpentier, Anna K. Nowak, Warren Mason, Josef Pichler
Provision of study material or patients: Alba A. Brandes, Miguel Gil‐Gil, Frank Saran, Antoine F. Carpentier, Anna K. Nowak, Warren Mason, Vittorina Zagonel, François Dubois, Gaetano Finocchiaro, George Fountzilas, Dana Michaela Cernea, Oliver Chinot, Rodica Anghel, Francois Ghiringhelli, Patrick Beauchesne, Giuseppe Lombardi, Enrico Franceschi, Martina Makrutzki, Chiedzo Mpofu, Hans‐Joerg Urban, Josef Pichler
Collection and/or assembly of data: Alba A. Brandes, Miguel Gil‐Gil, Frank Saran, Antoine F. Carpentier, Anna K. Nowak, Warren Mason, Vittorina Zagonel, François Dubois, Gaetano Finocchiaro, George Fountzilas, Dana Michaela Cernea, Oliver Chinot, Rodica Anghel, Francois Ghiringhelli, Patrick Beauchesne, Giuseppe Lombardi, Enrico Franceschi, Martina Makrutzki, Chiedzo Mpofu, Hans‐Joerg Urban, Josef Pichler
Data analysis and interpretation: Hans‐Joerg Urban
Manuscript writing: Alba A. Brandes, Miguel Gil‐Gil, Frank Saran, Antoine F. Carpentier, Anna K. Nowak, Warren Mason, Vittorina Zagonel, François Dubois, Gaetano Finocchiaro, George Fountzilas, Dana Michaela Cernea, Oliver Chinot, Rodica Anghel, Francois Ghiringhelli, Patrick Beauchesne, Giuseppe Lombardi, Enrico Franceschi, Martina Makrutzki, Chiedzo Mpofu, Hans‐Joerg Urban, Josef Pichler
Final approval of manuscript: Alba A. Brandes, Miguel Gil‐Gil, Frank Saran, Antoine F. Carpentier, Anna K. Nowak, Warren Mason, Vittorina Zagonel, François Dubois, Gaetano Finocchiaro, George Fountzilas, Dana Michaela Cernea, Oliver Chinot, Rodica Anghel, Francois Ghiringhelli, Patrick Beauchesne, Giuseppe Lombardi, Enrico Franceschi, Martina Makrutzki, Chiedzo Mpofu, Hans‐Joerg Urban, Josef Pichler
Disclosures
Miguel Gil‐Gil: F. Hoffmann‐La Roche Ltd. (H, SAB); Frank Saran: F. Hoffmann‐La Roche Ltd., Bristol‐Myers Squibb (C/A); Antoine F. Carpentier: F. Hoffmann‐La Roche Ltd. (C/A, H); Anna Nowak: F. Hoffmann‐La Roche Ltd. (SAB); Warren Mason: F. Hoffmann‐La Roche Ltd. (C/A); Gaetano Finocchiaro: Bristol‐Meyers Squibb (C/A); George Fountzilas: Pfizer, Sanofi, Roche (SAB), AstraZeneca (H); Dana Michaela Cernea: F. Hoffmann‐La Roche Ltd. (H); Oliver Chinot: F. Hoffmann‐La Roche Ltd., Ipsen, AbbVie (C/A), Bristol‐Myers Squibb, Celldex Therapeutics, Immatics, Servier Laboratories (H); Martina Makrutzki: F. Hoffmann‐La Roche Ltd. (E); Chiedzo Mpofu: F. Hoffmann‐La Roche Ltd. (E, OI); Hans‐Joerg Urban: F. Hoffmann‐La Roche Ltd. (E); Josef Pichler: F. Hoffmann‐La Roche Ltd. (H). The other authors declared no conflicts of interest.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Ostrom QT, Gittleman H, Xu J et al. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2009‐2013. Neuro Oncol 2016; 18 (suppl 5):v1–v75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–996. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Hegi ME, Mason WP et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5‐year analysis of the EORTC‐NCIC trial. Lancet Oncol 2009;10:459–466. [DOI] [PubMed] [Google Scholar]
- 4.Tosoni A, Franceschi E, Poggi R et al. Relapsed glioblastoma: Treatment strategies for initial and subsequent recurrences. Curr Treat Options Oncol 2016;17:49. [DOI] [PubMed] [Google Scholar]
- 5.Brandes AA, Bartolotti M, Tosoni A et al. Nitrosoureas in the management of malignant gliomas. Curr Neurol Neurosci Rep 2016;16:13. [DOI] [PubMed] [Google Scholar]
- 6.Taal W, Oosterkamp HM, Walenkamp AM et al. Single‐agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): A randomised controlled phase 2 trial. Lancet Oncol 2014;15:943–953. [DOI] [PubMed] [Google Scholar]
- 7.Brandes AA, Finocchiaro G, Zagonel V et al. AVAREG: A phase II, randomized, noncomparative study of fotemustine or bevacizumab for patients with recurrent glioblastoma. Neuro Oncol 2016;18:1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandes AA, Carpentier AF, Kesari S et al. A phase II randomized study of galunisertib monotherapy or galunisertib plus lomustine compared with lomustine monotherapy in patients with recurrent glioblastoma. Neuro Oncol 2016;18:1146–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen MH, Shen YL, Keegan P et al. FDA drug approval summary: Bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. The Oncologist 2009;14:1131–1138. [DOI] [PubMed] [Google Scholar]
- 10.Friedman HS, Prados MD, Wen PY et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 2009;27:4733–4740. [DOI] [PubMed] [Google Scholar]
- 11.Kreisl TN, Kim L, Moore K et al. Phase II trial of single‐agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 2009;27:740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chinot OL, Wick W, Mason W et al. Bevacizumab plus radiotherapy‐temozolomide for newly diagnosed glioblastoma. N Engl J Med 2014;370:709–722. [DOI] [PubMed] [Google Scholar]
- 13.Gilbert MR, Dignam JJ, Armstrong TS et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 2014;370:699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergers G, Hanahan D. Modes of resistance to anti‐angiogenic therapy. Nat Rev Cancer 2008;8:592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hida K, Akiyama K, Ohga N et al. Tumour endothelial cells acquire drug resistance in a tumour microenvironment. J Biochem 2013;153:243–249. [DOI] [PubMed] [Google Scholar]
- 16.Grothey A, Sugrue MM, Purdie DM et al. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: Results from a large observational cohort study (BRiTE). J Clin Oncol 2008;26:5326–5334. [DOI] [PubMed] [Google Scholar]
- 17.Bennouna J, Sastre J, Arnold D et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): A randomised phase 3 trial. Lancet Oncol 2013;14:29–37. [DOI] [PubMed] [Google Scholar]
- 18.Brandes AA, Mason W, Pichler J et al. Can bevacizumab prolong survival for glioblastoma patients through multiple lines of therapy? Future Oncol 2014;10:1137–1145. [DOI] [PubMed] [Google Scholar]
- 19.Reardon DA, Herndon JE 2nd, Peters KB et al. Bevacizumab continuation beyond initial bevacizumab progression among recurrent glioblastoma patients. Br J Cancer 2012;107:1481–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wen PY, Macdonald DR, Reardon DA et al. Updated response assessment criteria for high‐grade gliomas: Response assessment in neuro‐oncology working group. J Clin Oncol 2010;28:1963–1972. [DOI] [PubMed] [Google Scholar]
- 21.Lombardi G, Pambuku A, Bellu L et al. Effectiveness of antiangiogenic drugs in glioblastoma patients: A systematic review and meta‐analysis of randomized clinical trials. Crit Rev Oncol Hematol 2017;111:94–102. [DOI] [PubMed] [Google Scholar]
- 22.Wick W, Gorlia T, Bendszus M et al. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med 2017;377:1954–1963. [DOI] [PubMed] [Google Scholar]
- 23.Norden AD, Young GS, Setayesh K et al. Bevacizumab for recurrent malignant gliomas: Efficacy, toxicity, and patterns of recurrence. Neurology 2008;70:779–787. [DOI] [PubMed] [Google Scholar]
- 24.Hovey EJ, Field KM, Rosenthal MA et al. Continuing or ceasing bevacizumab beyond progression in recurrent glioblastoma: An exploratory randomized phase II trial. Neurooncol Pract 2017;4:171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]