Glioblastoma multiforme (GBM) is the most lethal primary brain tumor. This article examines the effect of medical comorbidities and their stability on postoperative complications and overall survival in GBM patients at a single large cancer institute.
Keywords: Blood pressure, Blood glucose, Serum albumin, Postoperative complications, Survival, Glioblastoma
Abstract
Background.
The impact of noncancerous factors on the morbidity and mortality of glioblastoma multiforme (GBM) has not been well studied. Using a large surgical cohort, we examined the association between multiple clinical characteristics and postoperative morbidities and survival in patients with GBM.
Materials and Methods.
The study included 404 consecutive GBM patients who underwent initial tumor resection at MD Anderson Cancer Center between January 1, 2010, and December 31, 2014. Data about clinical characteristics, treatments, and postoperative complications were collected. The associations between clinical parameters and postoperative complications and survival were analyzed.
Results.
Charlson Comorbidity Index was positively related to a higher incidence of postoperative total (odds ratio [OR] = 1.20; p = .002) and neurological (OR = 1.18; p = .011) complications. Preoperative systolic blood pressure (SBp) over 140 mmHg was associated with a higher incidence of postoperative intracranial hemorrhage (OR = 4.42; p = .039) and longer hospital stay (OR = 2.48; p = .015). Greater postoperative fluctuation of SBp (OR = 1.14; p = .025) and blood glucose (mmol/L; OR = 1.48; p = .023) were related to a higher incidence of neurological complications, whereas higher postoperative blood glucose (OR = 0.64; p < .001) was related to a lower incidence. Long‐term lower SBp (<124 mmHg; hazard ratio [HR] = 1.47; p = .010) and higher blood glucose (HR = 1.12; p < .001) were associated with shorter survival. Long‐term serum albumin level (g/dL; HR = 0.32; p < .001) was positively associated with survival.
Conclusion.
Short‐term SBp and blood glucose levels and fluctuations are associated with postoperative complications in GBM patients. Their long‐term optimization may impact survival of these patients. Future clinical trials are needed to confirm the benefit of optimizing medical comorbidities on GBM patients' outcomes.
Implications for Practice.
Glioblastoma multiforme (GBM) is one of the most feared cancer diagnoses because of its limited survival and treatment. This study revealed significant associations of noncancerous factors on the morbidity and mortality of GBM. The complexity of medical comorbidities, as well as short‐term postoperative levels and fluctuations of blood pressure and blood glucose, was associated with postoperative complications, but not overall survival. However, long‐term levels of these common clinical parameters were significantly associated with survival. Optimization of medical conditions may be critical for reducing the morbidity and mortality of GBM patients. Future clinical trials are needed to validate the observed associations in an independent cohort.
Introduction
Glioblastoma multiforme (GBM) is the most lethal primary brain tumor [1]. Although survival in GBM has improved with the new standard treatment of maximum safe resection followed by radiotherapy with concomitant and adjuvant temozolomide, it is grimly measured in months after the diagnosis [2], [3]. Improvements in survival and quality of life in these patients are critically needed. The number of lesions, treatment strategy, and completeness of tumor resection are well‐known factors that impact GBM survival [4]. Age, performance status, and corticosteroid use have also been identified as prognostic variables [5]. A few retrospective studies reported an adverse association between hyperglycemia and survival in GBM patients [6], [7], [8], [9]. In addition, postoperative complications after GBM tumor resection were associated with a lower likelihood of adjuvant therapy administration [10], which could impair survival. However, risks for developing those complications have not been well defined. The complexity of medical comorbidities is a known risk factor for morbidity and mortality of many disease conditions including cancer [11], [12]. Its impact on clinical outcomes of GBM patients has not been reported. The condition of medical comorbidities is routinely monitored through common clinical parameters including blood pressure, blood glucose, and serum albumin. These clinical parameters are known to significantly predict morbidity and mortality among critically ill patients and those who undergo major surgeries [13], [14]. However, their effects on postoperative outcomes and overall survival of GBM patients are not thoroughly explored. We believe that identifying morbidity and survival associating factors will orient future research and clinical strategies to productive targets that impact quality of life and survival in patients with GBM. Our study aims to examine the impact of medical comorbidities and their stability on postoperative complications and overall survival in GBM patients at a single large cancer institute.
Materials and Methods
Study Design and Patients
This retrospective study was approved by The University of Texas MD Anderson Cancer Center's Institutional Review Board in accordance with an assurance filed with, and approved by, the Department of Health and Human Services. Using the Brain and Spine Center Database, we searched for GBM patients who are 18 years or older and underwent initial tumor resection and primary treatment at MD Anderson Cancer Center between January 1, 2010, and December 31, 2014. The final study cohort consisted of 404 consecutive patients.
The patients’ demographic data, including age, sex, race, and vital status, were obtained from the MD Anderson Tumor Registry. The patients’ height, weight, blood pressure records, laboratory test results, pharmacy information, and billing data were obtained through the MD Anderson Data Warehouse. Status of isocitrate dehydrogenase 1 (IDH1) and/or 2 (IDH2) mutations and O6‐methylguanine‐DNA methyltransferase (MGMT) promoter methylation were obtained from electronic pathology reports. Postoperative complications were recorded in the Brain and Spine Center Database.
Systolic blood pressure (SBp, mmHg), blood glucose (mmol/L), serum albumin (g/dL), and the Charlson Comorbidity Index (CCI) were the major study factors. Serum albumin data were only available for the analysis for survival, but insufficient for postoperative complications. Baseline was defined as within 1 week before surgery; short‐term as within 7 days after tumor resection, counting the surgical day as day 1; and long‐term as occurred after postoperative day 31. Body mass index was calculated using the formula weight in kg/(height in m)2. Plasma and finger stick glucose data were both used to calculate blood glucose levels at baseline, postoperatively, and long term. Fluctuation was the absolute difference between two consecutive measures; for instance, postoperative SBp fluctuation was the mean of the differences between the first and second, second and third, etc. measurements. The units for SBp and glucose fluctuations were mmHg and mmol/L, respectively. Median values of all variables were used in the analysis unless otherwise noted. The International Classification of Diseases, Ninth Revision codes for each patient were used to calculate CCI [15]. Treatment information, including radiation therapy and temozolomide administration, was obtained from billing and pharmacy data.
The extent of tumor resection, expressed as a percentage, had been calculated on the basis of the differences between preoperative and postoperative tumor volumes by magnetic resonance imaging of brain and recorded in the Brain and Spine Center Database [16]. Gross total resection refers to ≥95% tumor resection, and subtotal resection <95%. Karnofsky Performance Scale (KPS) score at the time of surgery, tumor location, duration of postoperative hospital stay, and postoperative complications within 30 days of surgery were also obtained from the Brain and Spine Center Database.
Statistical Analysis
The two primary outcomes were postoperative complications and overall survival (OS). OS was defined as the number of days a patient survived between the date of radiographic diagnosis of GBM and the date of death. If a patient was not known to be dead, survival time was censored at their last confirmed contact with the health care system. We used descriptive statistics to analyze the frequencies, medians, means, and standard deviations of the study variables for the overall cohort. Student's t test and the Mann‐Whitney rank sum tests assessed statistical significance of continuous variables, and chi‐square test for categorical variables. Odds ratios with 95% confidence intervals (CIs) for the association between clinical factors and hospital stay or postoperative complications were estimated from logistic regression models. The models were further adjusted for age, sex, CCI, preoperative KPS, radiation therapy, and tumor resection extent.
Univariate analysis of the association between individual clinical characteristics and OS was performed using the Kaplan‐Meier method followed by the log‐rank test to compare the survival distributions between the groups. Multivariate regression analyses of survival data were based on Cox proportional hazards modeling, and the hazard ratio was calculated with 95% CIs. Results were considered significant when the p value was below .05. All statistical analyses were performed using R software (version 3.3.3, The R Foundation, http://www.r-project.org).
Results
Patient Characteristics
Patient demographics, clinical characteristics, and tumor and treatment information are illustrated in Table 1. At the time of our analysis, the mortality rate was 76%. The median age of the study patients was 59 (range: 20–91) years. The median CCI and KPS were 4 (range: 2–14) and 90 (range: 10–100), respectively. Most patients had gross total resection (60%), with 48% having underwent the treatment combination of surgery, radiation therapy, and temozolomide. IDH1 status was available in 218 (54%) patients, and mutations were detected in 25 patients. Eighteen patients underwent testing for MGMT promoter methylation, which was positive in 11 patients.
Table 1. Patient demographics and clinical and treatment characteristics.
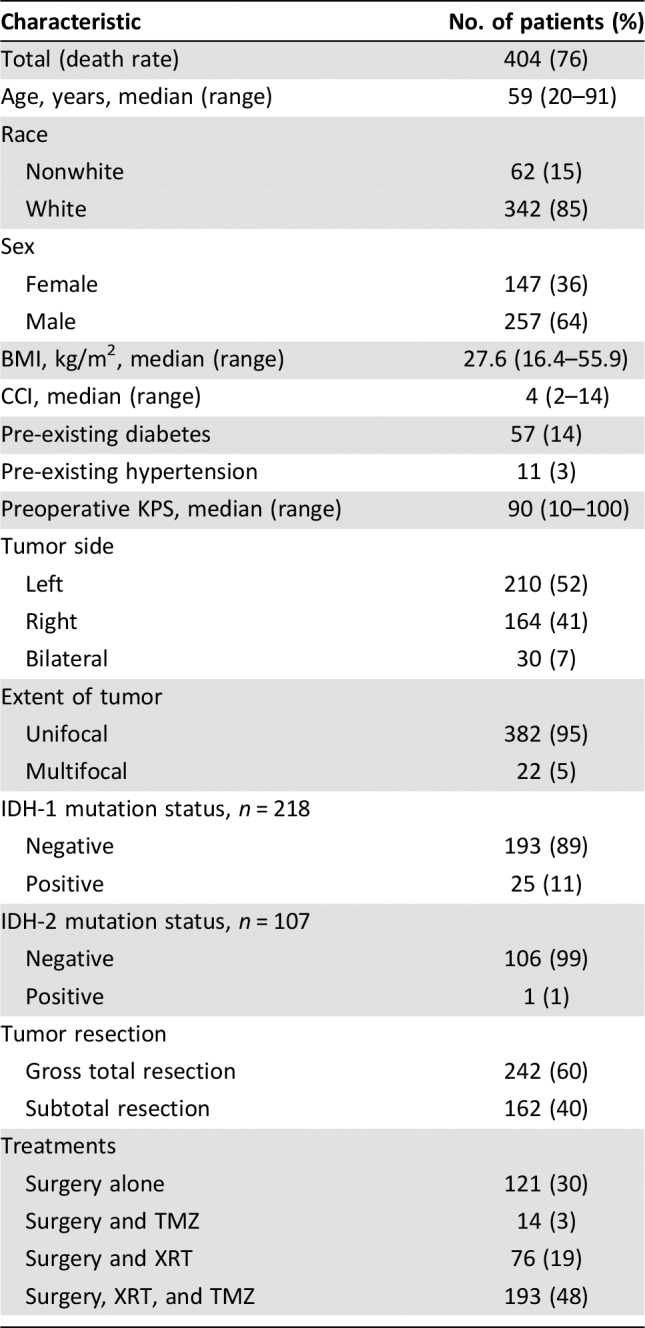
Abbreviations: BMI, body mass index; CCI, Charlson Comorbidity Index; IDH, isocitrate dehydrogenase; KPS, Karnofsky Performance Status, TMZ, temozolomide; XRT, radiotherapy.
Postoperative Complications
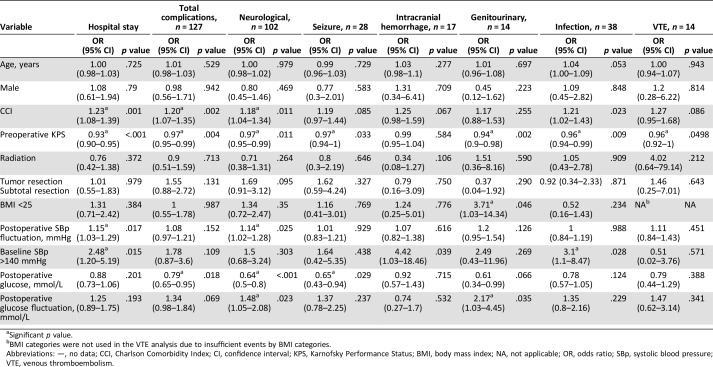
Table 2 reports the multivariate analysis of the association between perioperative clinical factors and postoperative hospital stay and complications. Postoperative hospital stay was categorized by the median (4 days). We excluded postoperative SBp in the multiple regression analysis to prevent multicollinearity of the model due to its close correlation with SBp fluctuation (coefficient = 0.20, p < .001). Higher CCI was significantly associated with a higher incidence of overall postoperative complications, neurological complications, and longer (>4 days) hospital stay, whereas higher preoperative KPS was associated with a lower incidence of overall complications, neurological complications, infection, venous thromboembolism, and shorter (≤4 days) hospital stay. Higher postoperative blood glucose level (mmol/L) was associated with a lower incidence of postoperative overall complications, neurological complications, and seizure events, and greater blood glucose fluctuation was related to a higher incidence of neurological complications. Baseline elevated SBp (>140 mmHg) was associated with a higher incidence of intracranial hemorrhage and longer hospital stay. Greater fluctuation of postoperative SBp level was positively related to higher incidences of neurological complications and longer hospital stay.
Table 2. Association between clinical factors and hospital stay or postoperative complications.
Significant p value.
BMI categories were not used in the VTE analysis due to insufficient events by BMI categories.
Abbreviations: —, no data; CCI, Charlson Comorbidity Index; CI, confidence interval; KPS, Karnofsky Performance Status; BMI, body mass index; NA, not applicable; OR, odds ratio; SBp, systolic blood pressure; VTE, venous thromboembolism.
The median blood glucose levels were as follows: patients with postoperative complications 7.74 mmol/L (interquartile range [IQR]: 6.97–9.09), without complications 7.83 mmol/L (IQR: 7.08–8.097); with postoperative infections 8.25 mmol/L (IQR: 7.11–9.83), without infections 7.79 mmol/L (IQR: 7.06–8.94); with postoperative seizure 7.65 mmol/L (IQR: 6.99–8.40), without seizure 7.83 mmol/L (IQR: 7.08–9.06); and with postoperative neurologic complications 7.56 mmol (IQR: 6.94–8.81), without neurologic complications 7.86 mmol/L (IQR: 7.11–9.08).
Bivariate correlation analysis revealed that SBp fluctuation positively correlated with frequency of antihypertensive administration per day (Pearson's r = .30 [95% CI: 0.20–0.39], p < .001), as did blood glucose fluctuation with that of antidiabetic administration (Pearson's r = .24 [95% CI: 0.14–0.34], p < .001). Postoperative glucose level did not correlate with dexamethasone dose (Pearson's r = .018, p = .725).
Postoperative Complications and GBM Treatments
Patients with postoperative complications had a longer interval between surgery and radiation treatment (25 days, p = .018) than those without complications (24 days). The presence of postoperative complications was not significantly associated with delay of chemotherapy, 22 days (with complications) versus 21 days (without complications), p = .202. Patients with postoperative complications were less likely to receive standard treatment (combination of surgery, radiation, and temozolomide), p = .029.
Overall Survival
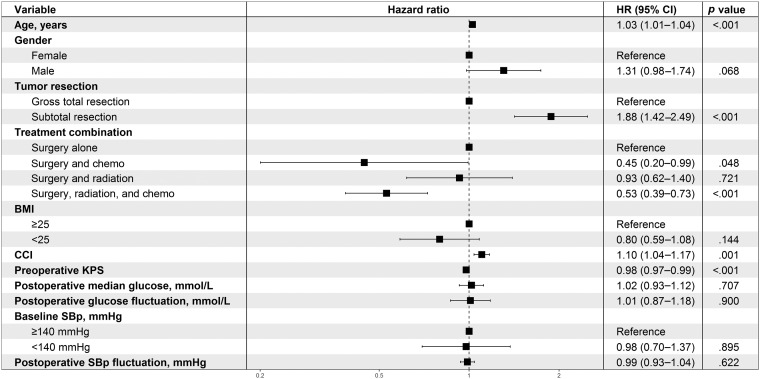
Using a multiple Cox regression model, we analyzed baseline and short‐term factors along with the known survival predictors of age, KPS, extent of tumor resection, and treatment (Fig. 1). CCI and the treatment combination of surgery, radiation therapy, and temozolomide were significantly associated with survival, whereas baseline SBp and postoperative levels and fluctuations of blood glucose and SBp were not.
Figure 1.
Multivariate analysis of associations between postoperative factors and survival.
Abbreviations: BMI, body mass index; CCI, Charlson Comorbidity Index; CI, confidence interval; KPS, Karnofsky Performance Status; SBp, systolic blood pressure.
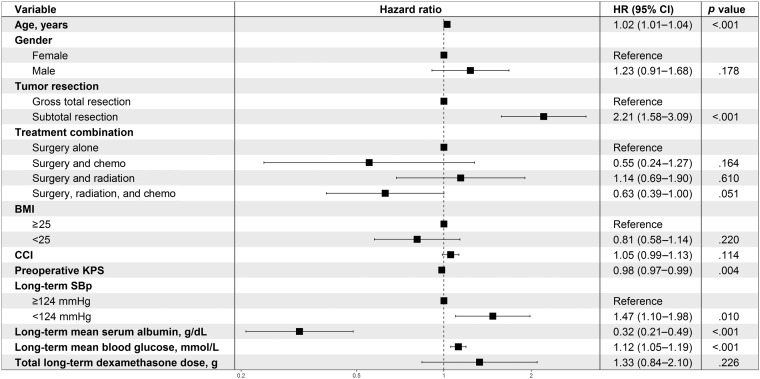
The multivariate analysis of the associations between long‐term factors and survival are demonstrated in Figure 2. Mean SBp was categorized by the median of 124 mmHg. SBp under 124 mmHg was a significant factor associated with shorter survival compared with otherwise. The effect of hypertension over long term was not assessed due to inadequate number of patients with SBp ≥140 mmHg. A higher mean long‐term blood glucose level was adversely associated with survival, independent of the total long‐term dexamethasone dose. A higher serum albumin level was associated with longer survival. The extent of tumor resection remained a significant survival factor, whereas the treatment combinations became statistically nonsignificant for survival after adjusting for the long‐term clinical factors.
Figure 2.
Multivariate analysis of associations between long‐term factors and survival.
Abbreviations: BMI, body mass index; CCI, Charlson Comorbidity Index; CI, confidence interval; KPS, Karnofsky Performance Status; SBp, systolic blood pressure.
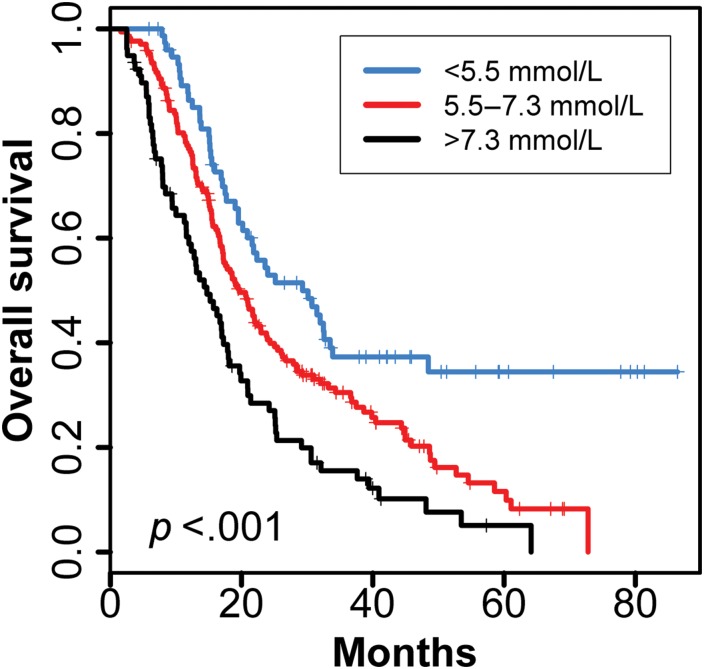
Figure 3 depicts the Kaplan‐Meier survival plot for long‐term blood glucose level in quartiles. Patients with a mean long‐term blood glucose level of <5.5 mmol/L (the lowest quartile) demonstrated the longest survival, compared with those with blood glucose levels between 5.5 and 7.3 mmol/L (the middle 50%) and >7.3 mmol/L (the highest quartile). The median survival of the 38 patients with IDH wild type and long‐term glucose level <5.5 mmol/L was 24 months (18–48).
Figure 3.
Kaplan‐Meier estimates of overall survival for glioblastoma patients by long‐term mean blood glucose levels. The mean blood glucose level was divided into three groups: <5.5 mmol/L (the lowest quartile), >7.3 mmol/L (the highest quartile), and 5.5–7.3 mmolL (middle 50%).
Discussion
Our study is the first to report the impact of medical comorbidities, blood pressure, and glycemic control on postoperative complications in patients with GBM. Our results show that the complexity of medical comorbidities (as measured by the CCI) and the levels and stability of systolic blood pressure and blood glucose significantly predict postoperative morbidities in GBM patients. Previous studies reported associations between the extent of tumor resection and resection methods and postoperative complications [10], [16], [17]. Our study results also concurred with the current literature that postoperative complications may negatively affect the delivering of adjuvant treatments for GBM patients [10]. The findings provide an important clinical implication that optimization of medical comorbidities may also be a useful interventional target that can improve glioblastoma patients’ postoperative outcomes. Future studies are necessary to define the optimal range for postoperative blood pressure and blood glucose to maximally reduce complications, which play an important role in determining the quality of life and functional outcomes of GBM patients.
Higher complexity of pre‐existing medical comorbidities and lower functional capacity are known predisposing factors for numerous postoperative complications and longer hospital stay after cardiac surgeries and craniotomies [18], [19], [20]. Our study echoes the same findings in GBM patients after tumor resection. Lower KPS was also prominently associated with increased incidence of postoperative seizure, genitourinary complications, and venous thromboembolism. These findings support the use of preoperative rehabilitation measures before craniotomy to maximize patients’ functional capacity.
Glycemic control can be challenging during postoperative in‐hospital care. Hyperglycemia occurs frequently in glioblastoma patients due to steroid use. It is a well‐known predictor of in‐hospital mortality and morbidity among critically ill patients and those who have undergone cardiac surgeries, regardless of pre‐existing diabetes [13], [14]. Glycemic control is therefore an essential strategy to reduce adverse outcomes. However, strict glucose control increases the risk for hypoglycemia, which is linked to both mortality and morbidity after cardiac operations [21]. There is no clear definition of postoperative hyperglycemia, and the optimal blood glucose range is not established for patients after major surgeries. Egi and colleagues reported both blood glucose level and fluctuation were significantly associated with intensive care unit mortality [22]. The median blood glucose level within 7 days after surgery was 8.44 mmol/L (95% CI: 8.26–8.63; data not shown) among our study patients. Lower postoperative blood glucose level and higher fluctuation were both associated with increased neurological complications. Higher glucose fluctuation had a positive correlation with more frequent administration of antidiabetic drugs. Lower postoperative blood glucose level was also associated with increased overall postoperative complications and seizure incidence. Our study supports stable glycemic control and less strict intervention thresholds for hyperglycemia in glioblastoma patients in the postoperative setting.
Our study also supports a similar recommendation for blood pressure control. Hypertension is the most common risk factor for intracranial hemorrhage [23], [24]. Current American Heart Association/American Stroke Association guidelines for the management of spontaneous intracranial hemorrhage recommend an SBp goal of <140 mmHg [25]. Among our study patients, an elevated SBp of 140 mmHg and above before tumor resection correlated with an increased incidence of postoperative intracranial hemorrhage. Greater postoperative SBp fluctuation was associated with increased neurological as well as overall complications among our patients. SBp fluctuation could be a result of aggressive antihypertensive treatments, as higher postoperative mean SBp and higher fluctuation, as well as higher fluctuation and use of antihypertensive agents, were correlated among our patients. Optimizing blood pressure control before the tumor resection and careful intervention of SBp elevation postoperatively are recommended for glioblastoma patients.
In the analysis for overall survival among our patient population, none of the postoperative blood pressure and blood glucose levels and fluctuations had a significant association with overall survival. This further supports the theory that in the short‐term postoperative period, tight intervention thresholds for both blood pressure and blood glucose may not be necessary and may in fact be harmful.
Our study is the first to report the significant adverse association of lower long‐term SBp levels (<124 mmHg) with shorter survival in GBM patients. Although we did not have data on the use of antihypertensive treatments in our patients, overzealous blood pressure control should be avoided in this population. In patients with prior lacunar stroke and relatively well‐preserved kidney function, intensive blood pressure lowering was associated with greater kidney function decline during the first year [26]. Significantly higher rates of serious adverse events including hypotension and syncope were observed in the intensive‐treatment group with SBp lower than 120 mmHg, as compared with the group with SBp lower than 140 mmHg [27]. The central nervous system, including the brain stem and higher brain levels, regulates blood pressure through modulating baroreceptor, chemoreceptor, and other cardiovascular reflexes [28]. Disruptions in these central mechanisms may result in cardiovascular dysfunctions. Abnormal echocardiography and hypotension were observed in 28% and 32%, respectively, of 50 patients who suffered from severe brain injuries [29]. Future studies are needed to decide the value of blood pressure as a marker of brain disease changes, such as disease recurrence or progression, in glioblastoma patients.
Previous studies have identified high blood glucose level as a negative survival predictor for glioblastoma patients [7]. In our study, the survival impact of blood glucose level remained significant even after adjustment for known survival predictors including age, extent of tumor resection, KPS, and treatment. The median long‐term random blood glucose level among our patients was 6.14 mmol/L (data not shown). The Kaplan‐Meier plot (Fig. 1) demonstrates that the long‐term blood glucose level associated with the lowest mortality risk was <5.5 mmol/L, which was consistent with the Derr and colleagues’ finding in GBM patients prior to the emergence of temozolomide [7]. The evidence from the two studies consistently suggests a much narrower optimum range than normally defined for blood glucose levels in patients with GBM; however, the optimum range of blood glucose level and risks of strict glycemic control need to be defined by future prospective studies.
Serum albumin is an established prognostic indicator in many acute and chronic diseases and has been incorporated into inflammation and nutrition scoring tools for predicting outcomes [30], [31], [32]. Schwartzbaum et al. found that preoperative hypoalbuminemia (<3.4 g/dL) was associated with a marked reduction in median survival from 494 to 62 days in 24 GBM patients [33]. Several subsequent studies also identified preoperative serum albumin as a survival predictor for GBM [34], [35]. In our retrospective review, lower long‐term serum albumin level was independently associated with shorter survival after adjustment for the significant confounders. Considering the current research evidence, serum albumin level should be incorporated into the prognostic evaluation at the initial diagnosis of GBM and during follow‐up. Improving patients’ nutritional status and reducing chronic inflammation are sensible interventional targets to improve GBM survival.
It is worth noting that treatment combination of surgery, radiation therapy, and temozolomide was significantly associated with survival in the analysis adjusted for short‐term factors. However, such significance disappeared in the survival analysis adjusted for long term factors. This may suggest that chronically uncontrolled medical comorbidities may compromise the efficacy of treatments for GBM. In summary, blood pressure, blood glucose levels, and serum albumin are commonly monitored clinical indices for assessing the stability of patients’ medical conditions and risks. Prospective studies are required to confirm the benefit of judicious recording and optimizing such clinical parameters and glioblastoma outcomes.
The primary strengths of our study include the large cohort and completeness of data records, which signify the power of the results. However, our study is limited due to its retrospective observational design and inherent biases. Vital sign and laboratory data collection were limited by availability and consistency of time frame; therefore, postoperative fluctuation of the observed factors may not have been timely captured. As such, the values of long‐term blood glucose were also random numbers, so fasting status or time elapsed between meals and testing time cannot be defined. Random blood glucose levels may underestimate the severity of glucose intolerance; however, its association with adverse survival was independent of outstanding confounders of age, performance status, and extent of tumor resection. Hemoglobin A1c reflects the mean glucose concentration over the previous 8–12 weeks and can be an excellent factor for evaluation of long‐term glycemic control, which, unfortunately, was largely unavailable to our study. Future studies are needed to delineate optimal glycemic control in this patient population. We were not able to assess the survival impact of the fluctuations of the observed factors over the long term due to lack of data. Data about treatments that patients might have received outside of our institution are not available to us. This may underestimate the number of patients who received standard treatments, which likely explains only 48% of patients in the standard treatment group. Data were also insufficient for tumor mutation status, recurrent tumor resections, and treatment agents other than temozolomide. Hence, our analysis was not able to include all potential survival factors.
Conclusion
The levels and stability of blood pressure, blood glucose, and serum albumin can be useful markers for predicting postoperative morbidities and survival in GBM patients. Optimization of medical comorbidities and nutritional status may improve postoperative outcomes and overall survival of glioblastoma patients. The optimal ranges of systolic blood pressure, blood glucose, and serum albumin will need to be determined in future research.
Author Contributions
Conception/design: Wenli Liu, Aiham Qdaisat, Eduardo Bruera, Sai‐Ching J. Yeung
Provision of study material or patients: Wenli Liu, Aiham Qdaisat, Jason Yeung, Jeffrey Weinberg, Sai‐Ching J. Yeung
Collection and/or assembly of data: Wenli Liu, Aiham Qdaisat, Jason Yeung
Data analysis and interpretation: Wenli Liu, Aiham Qdaisat, Eduardo Bruera, Shouhao Zhou, Sai‐Ching J. Yeung
Manuscript writing: Wenli Liu, Aiham Qdaisat, Gabriel Lopez, Jeffrey Weinberg, Lorenzo Cohen, Eduardo Bruera, Sai‐Ching J. Yeung
Final approval of manuscript: Wenli Liu, Aiham Qdaisat, Jason Yeung, Gabriel Lopez, Jeffrey Weinberg, Shouhao Zhou, Lorenzo Cohen, Eduardo Bruera, Sai‐Ching J. Yeung
Disclosures
The authors indicated no financial relationships.
References
- 1.Porter KR, McCarthy BJ, Berbaum ML et al. Conditional survival of all primary brain tumor patients by age, behavior, and histology. Neuroepidemiology 2011;36:230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Darefsky AS, King JT Jr, Dubrow R. Adult glioblastoma multiforme survival in the temozolomide era: A population‐based analysis of Surveillance, Epidemiology, and End Results registries. Cancer 2012;118:2163–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubrow R, Darefsky AS, Jacobs DI et al. Time trends in glioblastoma multiforme survival: The role of temozolomide. Neuro Oncol 2013;15:1750–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gorlia T, van den Bent MJ, Hegi ME et al.Nomograms for predicting survival of patients with newly diagnosed glioblastoma: Prognostic factor analysis of EORTC and NCIC trial 26981‐22981/CE.3. Lancet Oncol 2008;9:29–38. [DOI] [PubMed] [Google Scholar]
- 5.Michaelsen SR, Christensen IJ, Grunnet K et al. Clinical variables serve as prognostic factors in a model for survival from glioblastoma multiforme: An observational study of a cohort of consecutive non‐selected patients from a single institution. BMC Cancer 2013;13:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayer A, Vaupel P, Struss HG et al. Strong adverse prognostic impact of hyperglycemic episodes during adjuvant chemoradiotherapy of glioblastoma multiforme. Strahlenther Onkol 2014;190:933–938. [DOI] [PubMed] [Google Scholar]
- 7.Derr RL, Ye X, Islas MU et al. Association between hyperglycemia and survival in patients with newly diagnosed glioblastoma. J Clin Oncol 2009;27:1082–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagan K, Bhavsar S, Arunkumar R et al. Association between perioperative hyperglycemia and survival in patients with glioblastoma. J Neurosurg Anesthesiol 2017;29:21–29. [DOI] [PubMed] [Google Scholar]
- 9.Tieu MT, Lovblom LE, McNamara MG et al. Impact of glycemia on survival of glioblastoma patients treated with radiation and temozolomide. J Neurooncol 2015;124:119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gulati S, Jakola AS, Nerland US et al. The risk of getting worse: Surgically acquired deficits, perioperative complications, and functional outcomes after primary resection of glioblastoma. World Neurosurg 2011;76:572–579. [DOI] [PubMed] [Google Scholar]
- 11.Orcutt ST, Massarweh NN, Li LT et al. Patterns of care for colorectal liver metastasis within an integrated health system: Secular trends and outcomes. Ann Surg Oncol 2017;24:23–30. [DOI] [PubMed] [Google Scholar]
- 12.Paleri V, Wight RG, Silver CE et al. Comorbidity in head and neck cancer: A critical appraisal and recommendations for practice. Oral Oncol 2010;46:712–719. [DOI] [PubMed] [Google Scholar]
- 13.Ascione R, Rogers CA, Rajakaruna C et al. Inadequate blood glucose control is associated with in‐hospital mortality and morbidity in diabetic and nondiabetic patients undergoing cardiac surgery. Circulation 2008;118:113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van den Berghe G, Wouters P, Weekers F et al. Intensive insulin therapy in critically ill patients. N Engl J Med 2001;345:1359–1367. [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 16.Li YM, Suki D, Hess K et al. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: Can we do better than gross‐total resection? J Neurosurg 2016;124:977–988. [DOI] [PubMed] [Google Scholar]
- 17.Wright J, Chugh J, Wright CH et al. Laser interstitial thermal therapy followed by minimal‐access transsulcal resection for the treatment of large and difficult to access brain tumors. Neurosurg Focus 2016;41:E14. [DOI] [PubMed] [Google Scholar]
- 18.Guenther U, Theuerkauf N, Frommann I et al. Predisposing and precipitating factors of delirium after cardiac surgery: A prospective observational cohort study. Ann Surg 2013;257:1160–1167. [DOI] [PubMed] [Google Scholar]
- 19.Reponen E, Tuominen H, Korja M. Evidence for the use of preoperative risk assessment scores in elective cranial neurosurgery: A systematic review of the literature. Anesth Analg 2014;119:420–432. [DOI] [PubMed] [Google Scholar]
- 20.Lakomkin N, Goz V, Lajam CM et al. Higher modified Charlson index scores are associated with increased incidence of complications, transfusion events, and length of stay following revision hip arthroplasty. J Arthroplasty 2017;32:1121–1124. [DOI] [PubMed] [Google Scholar]
- 21.Johnston LE, Kirby JL, Downs EA et al. Postoperative hypoglycemia is associated with worse outcomes after cardiac operations. Ann Thorac Surg 2017;103:526–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egi M, Bellomo R, Stachowski E et al. Variability of blood glucose concentration and short‐term mortality in critically ill patients. Anesthesiology 2006;105:244–252. [DOI] [PubMed] [Google Scholar]
- 23.McCormick WF, Rosenfield DB. Massive brain hemorrhage: A review of 144 cases and an examination of their causes. Stroke 1973;4:946–954. [DOI] [PubMed] [Google Scholar]
- 24.Walsh KB, Woo D, Sekar P et al. Untreated hypertension: A powerful risk factor for lobar and nonlobar intracerebral hemorrhage in whites, blacks, and Hispanics. Circulation 2016;134:1444–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hemphill JC 3rd, Greenberg SM, Anderson CS et al. Guidelines for the management of spontaneous intracerebral hemorrhage: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015;46:2032–2060. [DOI] [PubMed] [Google Scholar]
- 26.Peralta CA, McClure LA, Scherzer R et al. Effect of intensive versus usual blood pressure control on kidney function among individuals with prior lacunar stroke: A post hoc analysis of the secondary prevention of small subcortical strokes (SPS3) randomized trial. Circulation 2016;133:584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wright JT Jr, Williamson JD, Whelton PK et al. A randomized trial of intensive versus standard blood‐pressure control. N Engl J Med 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dampney RA. Central neural control of the cardiovascular system: Current perspectives. Adv Physiol Educ 2016;40:283–296. [DOI] [PubMed] [Google Scholar]
- 29.Hasanin A, Kamal A, Amin S et al. Incidence and outcome of cardiac injury in patients with severe head trauma. Scand J Trauma Resusc Emerg Med 2016;24:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Honda Y, Nagai T, Iwakami N et al. Usefulness of geriatric nutritional risk index for assessing nutritional status and its prognostic impact in patients aged >/=65 years with acute heart failure. Am J Cardiol 2016;118:550–555. [DOI] [PubMed] [Google Scholar]
- 31.Mohri T, Mohri Y, Shigemori T et al. Impact of prognostic nutritional index on long‐term outcomes in patients with breast cancer. World J Surg Oncol 2016;14:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stenvinkel P, Gillespie IA, Tunks J et al. Inflammation modifies the paradoxical association between body mass index and mortality in hemodialysis patients. J Am Soc Nephrol 2016;27:1479–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwartzbaum JA, Lal P, Evanoff W et al. Presurgical serum albumin levels predict survival time from glioblastoma multiforme. J Neurooncol 1999;43:35–41. [DOI] [PubMed] [Google Scholar]
- 34.Borg N, Guilfoyle MR, Greenberg DC et al. Serum albumin and survival in glioblastoma multiforme. J Neurooncol 2011;105:77–81. [DOI] [PubMed] [Google Scholar]
- 35.Han S, Huang Y, Li Z et al. The prognostic role of preoperative serum albumin levels in glioblastoma patients. BMC Cancer 2015;15:108. [DOI] [PMC free article] [PubMed] [Google Scholar]