Abstract
Implants are in the pre-clinical stage for long-acting HIV pre-exposure prophylaxis (PrEP), with an opportunity to solicit end-users' feedback early in development. Health care providers (HCPs) have been key gatekeepers for contraceptive implant uptake, and uniquely understand both technical considerations and the social context of use. Given their influential role, we gathered South African HCP perspectives on contraceptive implant implementation and features of PrEP implant prototypes that may influence future provider and patient acceptability. We conducted in-depth interviews with 30 HCPs (20 nurses and 10 doctors) in Cape Town and Soshanguve, South Africa. Interviews were conducted by a bioengineer and later transcribed, coded, and analyzed for key themes. HCPs described health system barriers such as understaffed clinics and inadequate training on contraceptive implant removal as major influences to their PrEP implant design preferences. They preferred a PrEP implant that is long lasting (>6 months) to minimize patient–clinic interactions, biodegradable to avoid need for removal, and flexible (but still palpable in case of removal). Commenting on negative experiences with contraceptive implant rollout, they recommended prioritizing both HCP and community education on the PrEP implant, with emphasis on expected side effects, and planning ahead for adequate training of HCPs before rollout. Challenges experienced with past contraceptive implant rollout may taint perspectives on future PrEP implants and must be carefully considered during product development and planning for clinical studies. Particular consideration should be given to the health system context of future distribution, including staff who would be providing and monitoring implants.
Keywords: HIV pre-exposure prophylaxis, contraceptive, implant, health care providers, product development, South Africa
Introduction
Several research groups are currently developing pre-exposure prophylaxis (PrEP) implants for HIV prevention,1–6 a strategy that aims to increase the number of safe, effective, and long-acting options for prevention. Contraceptive implants have been in use across sub-Saharan Africa for nearly two decades, but were only introduced in the public sector in South Africa in 2014 in the form of Implanon NXT®.7 The few existing studies on acceptability of contraceptive implants in South Africa have reported decreasing acceptability after the initial high uptake due to implant side effects, negative community perception, and service delivery barriers.8–11 Attitudes toward and experiences with contraceptive implant introduction in South Africa may offer important insights for the design and implementation of PrEP implants.
Since PrEP implants are still in pre-clinical development, there are several “modifiable” attributes such as duration, number of rods, rod stiffness (i.e., palpability), and appearance that could still be changed before PrEP implants advance to human clinical trials. For this work, we focused on a biodegradable PrEP implant being developed by RTI International and collaborators that would dissolve in the body and not require removal.5,6
While acceptability studies are often performed alongside or after clinical trials, many have called for the need to seek end-users' perspectives on product design before reaching clinical trials such that preferences can be incorporated into design.12–14 Importantly, assessing potential “acceptability” of a new technology such as a PrEP implant goes beyond product design and technical specifications, but includes social and cultural considerations based on the context of use. Past clinical trials of PrEP strategies have pointed toward the need for deeper consideration of the product use context, including not just physical attribute preferences but also how the product may fit within existing practices and everyday life of the user.15–18
In this study, we sought the dual perspectives of health care providers (HCPs) as both service providers for their understanding of the technical requirements of an implant and members of the community who have interacted with hundreds of contraceptive implant end-users and potential future PrEP implant end-users. In this study, we present HCPs' perspectives on the design of a biodegradable PrEP implant, as well as their perspectives on contraceptive implant rollout and recommendations for future PrEP implant implementation.
Methods
Setting
This research was conducted between March 2017 and June 2017 at two locations in South Africa: Cape Town in Western Cape Province and Soshanguve in Gauteng Province. These sites were selected to represent two geographically and culturally distinct settings in South Africa with high HIV prevalence.19 Research was conducted in collaboration with the iPrevent study with the Desmond Tutu HIV Centre/Foundation in Cape Town20 and the TRIO study with the Setshaba Research Centre in Soshanguve.21
Study design
We conducted 30 in-depth interviews with HCPs (15 per site). Semistructured interviews were selected to allow for open-ended questions to explore reasons behind preferences in PrEP implant design. During the interview, the HCP handled an Implanon NXT contraceptive device system (trocar and implant rods) and several prototypes of a PrEP implant in development (Fig. 1). HCPs were also shown pictures of various trocars and a model of how Implanon NXT feels under mock skin to facilitate conversation around implant stiffness and palpability. Topics discussed included familiarity with HIV PrEP, community perceptions of contraceptive implants, HCP experience with insertion and removal technique, preferences for PrEP implant attributes (dimensions, number of rods inserted at one time, duration, biodegradability, stiffness, appearance), social adoption considerations (discreet use, target audience), and service delivery considerations.
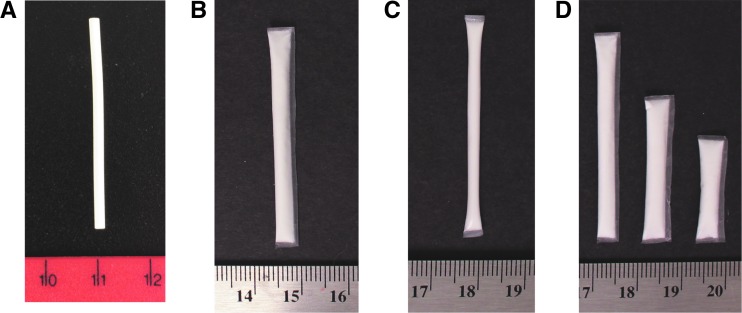
FIG. 1.
Contraceptive implant and PrEP implant prototypes shown during in-depth interviews. (A) Implanon NXT® contraceptive implant. (B) PrEP implant prototype #1: TFPD, a reservoir device with a biodegradable polycaprolactone membrane fabricated using a solvent casting technique. (C) PrEP implant prototype #2: EXPD, fabricated using an extruded tube fabrication technique. (D) Multiple sizes of PrEP implant prototype #1 (TFPD). All PrEP implant prototypes shown during interviews contained cellulose powder as placebo in place of active drug. Actual PrEP implants in development contain a liquid excipient, resulting in a drug slurry paste within the implant instead of a white powder. TFPD prototypes were fabricated with a thinner membrane compared with EXPD prototypes, affecting stiffness (from flexible to stiff: TFPD prototype < EXPD prototype < Implanon NXT). Scale is in cm. EXPD, extruded tube polymer device; PrEP, pre-exposure prophylaxis; TFPD, thin film polymer device. Color images available online at www.liebertpub.com/apc
This research design is rooted within a socioecological framework in that (1) HCPs play important roles in influencing individual access and decision-making around HIV prevention method use and (2) within the interview guide, questions were structured to explore factors at the individual, organizational, and community levels.
Participant selection
The HCPs were purposively selected to reflect a range of clinic settings (public, private, research) and job function (nurse, doctor) in Cape Town and Soshanguve. Inclusion criteria included having previous experience inserting a contraceptive implant.
Data collection and analysis
Participants provided informed consent and completed an interviewer-administered demographics questionnaire that assessed sociodemographic characteristics before beginning the interview. Interviews were facilitated in English by a bioengineer with experience in PrEP drug delivery system design (E.A.K.). In-depth interviews lasted approximately 1–1.5 h each and were audiorecorded and transcribed. Within 24 h of interview completion, the interviewer wrote a debriefing report that summarized participant responses to key research questions and themes.
Coding was performed using Dedoose software22 by a multidisciplinary team of three researchers. Code definitions were created a priori to analysis, and discussed and agreed on among the coding team. To ensure consistency in how codes were applied, four inter-rater agreement tests were completed throughout coding on key codes with an average Cohen's kappa statistic of 0.85, considered to be a high level of agreement.22,23 Analysis was primarily completed through content analysis using a framework matrix-based approach as described previously,24,25 allowing for large volumes of qualitative data to be distilled and compared across sites.
Briefly, key findings from debriefing reports were organized into matrices, with each participant as an individual row and each theme as an individual column, and results were compared and summarized across interviews by site. Code reports of extracted data from transcripts were also compiled for key codes and/or their combinations, including “PARTICIPANT RECOMMENDATIONS,” “CONTRACEPTIVE IMPLANT,” “DEVICE,” “CONCERNS,” and “UPTAKE,” and reviewed as part of the analysis. The coding team held regular telephonic and in-person discussions to reconcile differences in code applications and discuss key findings from interviews.
Ethics approval
This study was approved by the institutional review boards (IRB) at all participating institutions. All participants provided written consent and were reimbursed for their time and transportation in accordance with local IRB requirements.
Results
Participant characteristics are shown in Table 1. We interviewed 20 nurses and 10 medical doctors (MBChB degree or equivalent). HCPs interviewed were mostly female (n = 26, 87%). HCPs worked in a diverse range of health care settings, including public clinics (50%), research clinics (43%), and private clinics (10%). All 30 HCPs had experience inserting Implanon NXT (single-rod, 3-year implant available through the public sector in South Africa), and 13 HCPs (43%) also had experience inserting Jadelle (two-rod, 5-year implant available through research studies in South Africa). Twenty-six HCPs (87%) had removed at least one implant within the past year, and 8 HCPs (27%) had removed more than 100 implants within the past year.
Table 1.
Participant Characteristics and Experience with Contraceptive Implants, Stratified by Site
| Characteristics | Cape Town | Soshanguve | Total |
|---|---|---|---|
| Total participants, n | 15 | 15 | 30 |
| Age (years), median (range) | 41 (28–57) | 43 (30–71) | 42 (28–71) |
| Gender, n (%) | |||
| Female | 12 (80) | 14 (93) | 26 (87) |
| Male | 3 (20) | 1 (7) | 4 (13) |
| Educational degree, n (%) | |||
| Nursing diploma | 8 (53) | 12 (80) | 20 (67) |
| MBChB (medical doctor) | 7 (47) | 3 (20) | 10 (33) |
| Type of clinic currently working in,a,bn (%) | |||
| Public | 9 (60) | 6 (40) | 15 (50) |
| Private | 2 (13) | 1 (7) | 3 (10) |
| Research | 6 (40) | 7 (47) | 13 (43) |
| Nongovernmental organization (NGO) | 0 (0) | 1 (7) | 1 (3) |
| Type of clients served,an (%) | |||
| HIV/AIDS patients | 12 (80) | 11 (73) | 23 (77) |
| Family planning patients | 15 (100) | 15 (100) | 30 (100) |
| Adults (>24 years old) | 13 (87) | 15 (100) | 28 (93) |
| Young women (18–24 years old) | 15 (100) | 15 (100) | 30 (100) |
| Young men (18–24 years old) | 11 (73) | 14 (93) | 25 (83) |
| Adolescent girls (12–18 years old) | 14 (93) | 11 (73) | 25 (83) |
| Adolescent boys (12–18 years old) | 8 (53) | 7 (47) | 15 (50) |
| Children | 3 (20) | 14 (93) | 17 (57) |
| Experience as implant provider (years), median (range) | 3 (1–4) | 2 (1–17) | 3 (1–17) |
| Type of implants previously inserteda | |||
| Norplant | 1 (7) | 1 (7) | 2 (7) |
| Jadelle | 5 (33) | 8 (53) | 13 (43) |
| Implanon NXT® | 15 (100) | 15 (100) | 30 (100) |
| Number of insertions performed within past year | |||
| 0 | 1 (7) | 1 (7) | 2 (7) |
| 1–10 | 2 (13) | 4 (27) | 6 (20) |
| 10–100 | 10 (67) | 7 (47) | 17 (57) |
| >100 | 2 (13) | 3 (20) | 5 (17) |
| Number of removals performed within past year | |||
| 0 | 2 (13) | 2 (13) | 4 (13) |
| 1–10 | 4 (27) | 4 (27) | 8 (27) |
| 10–100 | 3 (20) | 7 (47) | 10 (33) |
| >100 | 6 (40) | 2 (13) | 8 (27) |
| Reasons for implant removal (ever removed for this)a | |||
| Indication period finished (end of device lifetime) | 12 (80) | 11 (73) | 23 (77) |
| No longer wants contraception | 8 (53) | 13 (87) | 21 (70) |
| Irregular menstrual bleeding | 11 (73) | 12 (80) | 23 (77) |
| Insertion complications (e.g., infection, expulsion) | 1 (7) | 0 (0) | 1 (3) |
| Pregnant while on implantc | 2 (13) | 1 (7) | 3 (10) |
| Headachesc | 4 (27) | 6 (40) | 10 (33) |
| Nauseac | 0 (0) | 2 (13) | 2 (7) |
| Weight gainc | 4 (27) | 3 (20) | 7 (23) |
| HIV positive while on implantc | 4 (27) | 1 (7) | 5 (17) |
| Partner or family concerns with implantc | 1 (7) | 2 (13) | 3 (10) |
| Hair lossc | 2 (13) | 1 (7) | 3 (10) |
| Fears of implant robberyc | 5 (33) | 0 (0) | 5 (17) |
More than one response allowed.
Two HCPs in Cape Town reported currently working in both public and private clinics.
These reasons were specified by participant as “other”; not provided as given choices on demographic form.
HCP, health care provider.
In the following section, we present results exploring HCP perspectives on contraceptive implant implementation and uptake, recommendations for PrEP implant and applicator design, and suggestions for future service delivery of PrEP implants. Direct quotations from HCPs are shown in italics, with pseudonym initial, job function, age in years, type of clinic, and site in brackets.
Negative community perception of contraceptive implants
Although most HCPs agreed that contraceptive implants have benefits of high efficacy and high user compliance, HCPs described a predominantly negative community reaction to the contraceptive implant in both Cape Town and Soshanguve, which has eroded their own and the community's initial optimism about the product. They attributed this negative reaction to side effects (especially changes in menstrual bleeding patterns), myths and misconceptions circulating in the community, and service delivery challenges.
Several HCPs said that the concept of side effects affecting individuals differently was not well understood in the community, resulting in some women complaining of problems with bleeding changes because of other women's experiences, or misattributing unrelated health issues to the implant. HCPs also commented on clinic pressures, including long queues, overburdened staff, and inadequate training on the implant removal procedure. Given such time pressures, there is often not enough time to counsel patients on what family planning options are available or what side effects are possible, which several HCPs identified as another factor underlying the implant's poor public perception.
“We are so swamped with our work in the facilities that we end up missing that point of educating clients, we really do. We so under pressure sometimes that we don't even… not that we don't care, you do think about it, heyee, ‘I'm not supposed to just give something to the person without knowing, without them knowing what it is and what to expect and how it works', but you feel so swamped that, ‘I must just give if she came to ask, it that means if she wants it. Let me give it to them, and let that person goes'. [Nurse A, 41 years, public clinic, Cape Town]
HCPs also attributed community misconceptions, stories, and rumors about the contraceptive implant to poor uptake and requests for implant removal. Common stories included that the implant moves or migrates in the body to the heart, lungs, or abdomen, that one can get pregnant while on the implant, and that women are being assaulted by drug users who physically cut implants out of women's arms and smoke them (Cape Town only). Based on their challenges with providing contraceptive implants in the clinic context, HCPs described how these same health system-level challenges also influenced their preferences for PrEP implant attributes.
Recommendations for PrEP implant design
Health care system barriers influence PrEP implant attribute preferences
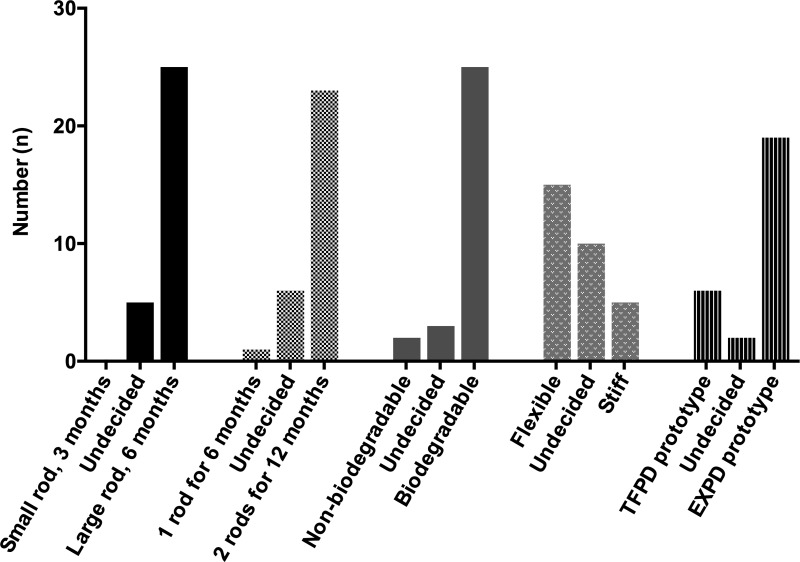
Minimizing interactions with the health care system for both HCPs and their patients came across as a strong preference for the PrEP implant attributes of duration and biodegradability. HCPs strongly agreed that the PrEP implant should be long lasting (minimally 3 months, but ideally 3 years or more) and biodegradable to avoid the removal process (Fig. 2).
FIG. 2.
HCP preferences for physical attributes of PrEP implants. Preferences from in-depth interviews with n = 30 HCPs when presented with choices for (1) small 3-month implant versus large 6-month implant; (2) one rod for 6 months versus two rods inserted at once for 12 months; (3) nonbiodegradable versus biodegradable implant; (4) flexible rod (less palpable) versus stiff rod (more palpable); and (5) TFPD prototype (more flexible, more visible “plastic”-like overhang) versus EXPD prototype (medium stiffness, less visible “plastic” overhang). See Fig. 1 for images of TFPD versus EXPD prototypes. “Undecided” represents when HCPs expressed uncertainty between the two options and did not state an explicit preference for one option versus the other option. EXPD, extruded tube polymer device; HCP, health care provider; PrEP, pre-exposure prophylaxis; TFPD, thin film polymer device.
When presented with three trade-offs of (1) size versus duration (small rod for 3 months vs. large rod for 6 months), (2) number of rods versus duration (one rod for 6 months vs. two rods for 12 months), and (3) dosage form versus duration (injection for 3 months vs. implant for 6–12 months), long duration mattered most over other attributes. HCPs explained that a long duration device would result in less burden on the health care system, save on costs, and would be better for patients who dislike coming to the clinic. Several HCPs commented on challenges with women not being able to adhere to a 3-monthly dosing regimen for contraceptive injections, thus preferring a duration longer than 3 months for PrEP implants, as this HCP from Cape Town explained:
“Again, it goes back to compliance and coming back for repeat injections. […] I have lost count of the number of women that I've seen in the clinic who have fallen pregnant on the three month injectable. […] It means that after that three months when that thing is worn off and she's another part of the country where there are stock outs and she doesn't get that and is not protected, boom! Baby goes in. So, in case of HIV, boom! HIV goes in. […] That is why I am thinking that three months is way too short, from my experience with DMPA, no. You really want something that's long lasting, absolutely.” [Doctor D, 38 years, public clinic, Cape Town]
HCPs strongly preferred a biodegradable PrEP implant over a PrEP implant that requires removal to overcome barriers of overburdened clinics (less clinic time for both HCP and patients by avoiding removal) and inadequate HCP training on the removal process, as this doctor described:
“From like the patient perspective […], once it's in [biodegradable PrEP implant] they don't have to worry about coming back to remove it and then also from a provider perspective, you don't need to be a trained professional to be able to remove this thing especially if it's a single thing with a preloaded device like the Implanon. Literally anybody would be able–like any healthcare professional will be able to insert that [PrEP implant] completely.” [Doctor E, 28 years, research clinic, Cape Town]
Balancing patient acceptability with technical feasibility
When considering their preferences for PrEP implant design, HCPs also described balancing both the technical constraints from their perspective of administering the implants with social considerations such as perceived preferences of their patients (Table 2). This balancing act came through particularly strongly during discussion on preferred stiffness for a PrEP implant. While a stiffer, more palpable implant would likely be easier to locate for removal if necessary, HCPs commented that a less palpable, more flexible PrEP implant may be better for patients who want to use it discreetly.
Table 2.
Technical and Social Considerations Underlying Health Care Provider Preferences for Pre-Exposure Prophylaxis Implant Attributes
| Attributes | Technical considerations | Social considerations | Overall HCP preferences |
|---|---|---|---|
| Duration | Longer for less burden on health care system to provide. | Longer for less burden on patients to visit health facilities and adhere to a frequent dosing schedule. | Long duration matters more than size of implant or number of rods Minimum duration: 3 months Ideal duration: 3 years |
| Biodegradability | Biodegradable to reduce burden on health care system (less time required to remove implants). Biodegradable to avoid removal technique (challenging for some HCPs) and specialized training required. | Biodegradable to reduce burden on user to return to clinic for another visit. Biodegradable to counter negative community perception of removal procedure; may require more education for community to understand this concept. | Biodegradable to avoid technical challenges with removal, reduce burden on clinic to train and offer removal services, and reduce burden on patient to return to clinic for removal procedure. |
| Palpability | Must be palpable (or detectable in alternate way) such that it can be removed if necessary: safety concern from medical standpoint. | Nonpalpable to untrained people, such that it can be used discreetly (to partners, community, family) by end-user if desired. | Mixed: medium palpability Should be palpable to trained professionals for removal if necessary (or detectable in an alternate way), but also be nonpalpable so it can be used discreetly by end-user. |
| Location on body | Easily accessible by HCP for insertion and removal. Should not interfere with drug PK/PD in body. | Should be in a hidden, discreet location so can be used discreetly if desired. | Arm, thigh, or abdomen/side preferred to meet both technical and social requirements. |
| Applicator | Easy to use. Inserts at correct depth under skin. Safely inserts implant such that rod will not break during insertion. | Acceptable to end-users, as some users watch the insertion procedure and may see the applicator. | Easy to use; inserts implant safely at correct depth under skin. |
HCP, health care provider; PD, pharmacodynamics; PK, pharmacokinetics.
Besides allowing for more discreet use, some HCPs felt that patients would be reassured by a softer PrEP implant. One HCP described a perception in the community that “something that is hard is painful” and shared how patients have reacted positively when shown an example intrauterine device, remarking, “it's so soft, even the strings they're soft” [Nurse B, 67 years, nonprofit clinic, Soshanguve].
On the contrary, some HCPs noted that a stiffer implant such as Implanon might be easier to remove if necessary: “The cool thing about the Implanon being slightly firm is that when you're taking it out, the firmness helps in manipulating it, playing around with it. Uhm, as opposed to Jadelle, […] it twists a lot, you know? It's not as firm as the Implanon so taking it out can be a bit of a challenge because you are not able to push it from one end.” [Doctor D, 38 years, public clinic, Cape Town]. One doctor summed up this tension in balancing the contrasting perspectives of both HCP and patients in considering preferences for design, saying “The softness [of the PrEP implant prototype] is almost great for privacy and almost too soft for effective clinical use” [Doctor F, 54 years, public clinic, Cape Town].
Factoring in both provider and patient perspectives, most HCPs preferred a soft or medium stiffness for a PrEP implant, particularly if it was biodegradable and would not require removal for most patients. When comparing two PrEP implant prototypes of varying stiffness levels, the majority of HCPs preferred the medium stiffness extruded tube polymer device (EXPD) prototype compared with the softer thin film polymer device (TFPD) prototype (Fig. 1). The level of stiffness of the EXPD may be a “sweet spot” of stiffness: stiff enough to still be palpable for removal if necessary, but more flexible (and potentially more discreet) than Implanon NXT. HCPs also preferred the appearance of the EXPD prototype over the TFPD prototype, since the EXPD prototype has less of a visible “plastic” overhang (Fig. 1).
Besides having a medium level of stiffness to allow for palpability, several HCPs suggested making only a small portion of the device (e.g., only the ends) palpable to balance the needs for HCP palpability with a user's ability to use discreetly. Many HCPs agreed that for safety reasons, there should be some means of detecting the PrEP implant for removal if necessary, whether by imaging, palpation, or another method. As an alternate strategy to reduce requests for early removals, some HCPs suggested offering multiple options for implant duration to allow patients to test a shorter duration implant first for side effects:
“The other suggestion that I would make is that maybe they should introduce it [PrEP implant] in a way that a person must start a shorter duration method and go to a medium duration, then to a longer duration—just to test and see if the person could stand the side effects of the method.” [Nurse A, 41 years, public clinic, Cape Town]
Foreseeing potential implementation challenges during design
In considering what the optimal design should be for a PrEP implant, HCPs also thought ahead to predict what challenges might be encountered with the existing reproductive health technology landscape in South Africa. For example, perhaps the biggest unanswered questions raised by HCPs regarding the PrEP implant were how to monitor how long a PrEP implant had been in a patient to prevent double dosing (especially if it dissolves and is not palpable), and how to distinguish a PrEP implant from a contraceptive implant to avoid unintentional removal of the wrong rod.
“I wonder for a health professional, eish would it be safe for me not to feel it [PrEP implant]? […] Because if then I don't feel it as a health professional, it means I can double dose this–the very same drug, so there […] should be a system where these records can be recorded nationally, not in one clinic.” [Nurse C, 38 years, public clinic, Soshanguve]
Some HCPs suggested having the two different implants feel differently under the skin (i.e., make one implant more stiff than the other) to differentiate them. Another suggested creating some type of handheld scanner or imaging device that could be used to visualize where the PrEP implant was in the body—perhaps simpler and more accessible than ultrasound or X-ray. Finally, a few HCPs recommended having a unique, strict location on the body for insertion of a contraceptive implant versus a PrEP implant so as not to confuse the two implants in case removal is needed. Suggested locations for PrEP implants included the arm (recommended by n = 20 HCPs), thigh (n = 19 HCPs), and abdomen/side (n = 7 HCPs). One HCP, an expert in implant removals, thought the anterior abdominal wall would be best if the PrEP implant is intended to dissolve.
“That [PrEP implant] is probably almost predestined to go in the anterior abdominal wall. Seriously. […] And if you stick it in the anterior abdominal wall, it's going to disappear, it's going to be forgotten.” [Doctor F, 54 years, public clinic, Cape Town]
They recommended that the location be discreet and hidden, not modify the drug release or pharmacodynamics, and be easily accessible for the HCP for insertion and removal if necessary.
Guidance for future service delivery of PrEP implants
Engaging HCPs as key stakeholders and gatekeepers in PrEP implant provision
HCPs expressed tension in how much influence they actually hold in patients' decision-making on sexual reproductive health matters relative to information received by word-of-mouth in the community. For example, this HCP described counseling provided by HCPs on the contraceptive implant as largely ineffective in comparison with counseling “by the streets” (word-of-mouth from the community):
“As I said, most women tend to remove this [contraceptive implant] because they have been counselled by the streets and that ‘it will do this, it will do that' […] which are just myths, unfortunately. They tend to believe those more than […] what they are told in the hospital. So, in a well-counselled patient, it's a fantastic product, yes. However, if somebody comes in and they have set their mind that, “You know what? I want this thing out!” Whatever it is that they were told elsewhere and they believe that then, no matter how much you talk, you'll talk until you're blue in the face. They'll say, ‘Doctor, take this thing out.' What can you do?” [Doctor D, 38 years, public clinic, Cape Town]
In contrast, some HCPs described having a strong influencing role regarding decisions on family planning methods, if they have adequate time to provide counseling, such as this nurse in Cape Town:
“When you get time to sit them down and educate them […] they go out of the clinic with a smile, because sometimes they do want to keep it [contraceptive implant] but because they are not reassured, there is no reassurance, they do not know what to do, so they come and say they want to take it out.” [Nurse A, 41 years, public clinic, Cape Town]
Another HCP expanded on this sentiment of being influential gatekeepers in patient's health decisions, describing a lack of agency from patients regarding their own health. As such, he felt that the sub-Saharan African context was better suited for a provider-administered product such as injections or implants, not a user-controlled product.
“A culture almost that prevails in Sub-Saharan Africa which is unfortunately a legacy of colonialism that someone else is in charge of your health. Someone else tells you what to take, when to take it, and that's, that's it so you, you're not really a stakeholder in your own health. […]. It's you go to this place, the doctor tells you what to do, you do what the doctor says. It's not about what you understanding, you wanting to get better.” [Doctor G, 29 years, research clinic, Soshanguve]
Considering that HCPs described both themselves and the community as having a strong influence in health decisions, they highlighted the need to reach both audiences with clear educational messaging for the PrEP implant to be accepted (Table 3 for list of recommendations). They advised that special attention should be given to ensure that HCPs are adequately trained, educated, and onboard with PrEP implants in order for uptake to be high in the community. In particular, they recommended that training for HCPs on both insertion and removal of PrEP implants should be offered together to avoid challenges experienced with contraceptive implants on a lack of HCPs trained to remove implants. Several HCPs commented on how HCPs act as gatekeepers for what family planning methods are presented to women as options at the clinic, and that accordingly, it would critical to convince HCPs that offering PrEP implants would save them time in the long run:
Table 3.
Health Care Providers Recommendations for Future Service Delivery of Pre-Exposure Prophylaxis Implants
| • Convince health care providers (HCPs) of the value of pre-exposure prophylaxis (PrEP) implant by emphasizing long duration and reduced time burden for both HCPs and patients in clinic. |
| • Tailor educational messaging toward young people in places they go: taverns/pubs, live entertainment shows, in schools. |
| • Advertise through multiple media venues, particularly TV (e.g., soap operas). |
| • Shift mass media campaign messaging from messaging around HIV treatment to HIV prevention. |
| • Address misinformation and misconceptions around contraceptive implants. |
| • Preemptively address potential confusion between contraceptive implants and PrEP implants, particularly around menstrual bleeding changes (would not occur with PrEP implants). |
| • Use analogies to clearly explain the concept of a biodegradable implant, such as dissolving stitches, oral capsules, or contraceptive “depot” injections. |
“If you minimize the resistance from the health care providers you have won half the battle. Because once they are convinced, they could convince others. They could convince the community leaders […] That's the key, […] making them think about it differently and not thinking about it as a waste of my time, but rather the positive impact it will have. And this—This is actually how much time you'll save by giving this person prevention instead of them coming here every three months for the next 20 years. You know, just making someone aware that, that five minutes is worth 50 hours in the next 20 years.” [Doctor G, 29 years, research clinic, Soshanguve]
Messaging on PrEP implants targeting the community as key stakeholders
HCPs also emphasized the importance of providing accurate, targeted education to the community. For PrEP implants, they recommended preemptively addressing potential confusion between the contraceptive implant and PrEP implant, especially that a PrEP implant will not cause menstrual changes for women. HCPs urged that community rumors, misinformation, and fears about contraceptive implants need to be addressed, particularly fears of contraceptive implant robbery in Cape Town. Otherwise, these same fears and misconceptions may become the baseline foundation for introduction of the PrEP implant.
Finally, some HCPs were concerned that the community may have a hard time accepting the concept of a biodegradable implant or “dissolving plastic,” and suggested this concept will require clear explanation. HCPs in both Cape Town and Soshanguve suggested comparing a biodegradable implant to existing products such as dissolving stitches (e.g., chromic sutures used after childbirth), injections such as Depo-Provera in which a depot of drug slowly dissolves, and oral capsules with a covering that dissolves as it is digested.
Discussion
Experiences from the introduction of contraceptive implant in South Africa offer important lessons salient to the development of future PrEP implants for HIV prevention. HCPs described an overall negative community reaction to contraceptive implants in both Cape Town and Soshanguve, underpinned by side effects of menstrual bleeding, community misconceptions and myths, service delivery barriers, and technical challenges with implant removal. Considering these challenges with the contraceptive implant, HCPs recommended that future PrEP implants be long lasting (>6 months preferred), biodegradable, and of medium stiffness, to be discreet but still be palpable in case removal is required. Planning for future PrEP implant implementation, they commented on the need to engage HCPs as key stakeholders (including adequate training) and preemptively address potential concerns and misconceptions about the PrEP implant (e.g., expected side effects; clearly explain the biodegradability process).
Strikingly, the description of the overall negative perception of contraceptive implants in this study resonates with other recent studies in South Africa,8,9,11 but diverges from reports of growing implant uptake in other countries in sub-Saharan Africa.26 While contraceptive implant insertions in the public sector have decreased year-on-year since introduction in South Africa,27 in contrast, contraceptive implant use has increased in the past decade in other sub-Saharan African countries, from a prevalence of 1.1% in 2011 to higher than 6% in 2017 in 10 of 12 countries in one report, and as high as 18.1% in Kenya.26
Several studies of both women end-users and nurses in public clinics in Gauteng, North West, and Eastern Cape Provinces in South Africa reported findings consistent with results from this study, including implant discontinuation fueled by side effects (especially changes in menstrual bleeding) and misconceptions, inadequate counseling and insufficient HCP training (particularly on removals), and overall poor HCP perceptions of the implant.8,9,11 Challenges described in this study with inadequate training and overburdened clinics have been similarly described in other reports on health care system-level barriers in South Africa.27–29
Given the pronounced differences in contraceptive implant uptake in South Africa relative to other countries in sub-Saharan Africa, it will be essential to solicit perspectives from HCPs in other sub-Saharan African countries on PrEP implant design and implementation who likely have varied patient experiences. In planning for a PrEP implant targeted for use across much of sub-Saharan Africa, a broad array of opinions from other HCPs, not just South Africa specifically, will be crucial in guiding development.
Despite the initial challenges with contraceptive implant implementation in South Africa, HCPs were optimistic toward the continued development of a PrEP implant, with many saying they would prefer an implant for their patients over other possible PrEP dosage forms, such as pills, vaginal products, or injections, if implants are able to provide longer lasting protection. Reducing time spent at the clinic for patients, and, in parallel, reducing the burden on HCPs to provide care came across as a major theme throughout interviews.
This desire for a long-lasting, biodegradable product to minimize interactions with health clinics mirrored results from focus groups conducted with 105 potential PrEP implant end-users (HIV-negative young men and women, ages 18–24 years) on similar topics in Cape Town and Soshanguve.24 Many design preferences of potential end-users were the same as those of HCPs: long duration mattered more than size of implant or number of rods, choice in duration was preferred, a biodegradable implant was strongly preferred, and both suggested to reduce the “plastic”-like appearance of the implant. The main difference between HCPs and potential end-users was that HCPs felt more strongly that there should be a way to remove the PrEP implant if needed (e.g., medium stiffness implant that would be more palpable) compared with end-users, who prioritized flexibility and discreetness over removability. While this comparison study was conducted among South African young people, women from different contexts may have different needs and preferences for PrEP products, such as partners of miners in Mozambique who desired short-term PrEP methods given their variable seasons of risk.30
Overall, South African HCPs' recommendations for the design of future PrEP implants centered on service delivery. This included modifying product attributes of PrEP implants to be suitable for the health system context in which they will be delivered such as a biodegradable implant that reduces burden on the clinic to provide removal services. Further, recommendations also included nonattribute-related considerations, such as adequate training for HCPs on how to counsel patients on what side effects to expect, managing side effects that do occur, and training on how to remove implants if necessary.
These recommendations are consistent with what other contraceptive experts in South Africa have suggested for improving contraceptive implant uptake: improving training of HCPs for implant-related services,7 increasing pharmacovigilance on reasons for removal,7,10 and considering other service delivery venues besides public clinics to increase accessibility.27 Stigma-associated barriers for using PrEP product are context specific and should also be considered when planning for future PrEP implant rollout. For example, women from PrEP studies in sub-Saharan Africa reported stigma around being perceived as HIV positive by partners and communities because of their daily use of antiretroviral tablets,31,32 whereas in the United States, sexual orientation-related stigma experienced at health facilities has been reported.33
Taken together, these recommendations point toward the need to better consider the context where technologies are introduced during the product development phase, encompassing both the life context of the end-user themselves and the service delivery system that the product will be implemented within.
Limitations of this study include that the sampling strategy used for recruitment was not random, so views may not be representative of all providers in the community. Social desirability bias may have resulted in HCPs reacting more positively toward PrEP implants, as they were the focus of the interviews. In addition, inherent bias of HCPs may have resulted in the reporting of more negative than positive reactions to the contraceptive implant as (1) most women that HCPs see are those who are dissatisfied with Implanon due to side effects or requests for early removals (those who are satisfied do not return to the clinic for 3 years) and (2) HCPs are directly involved with implant discontinuation (provider required for removal), unlike pills and injections (patient can stop themselves).7 Therefore, HCPs likely interact with disproportionately more women who have had negative experiences with the contraceptive implant compared with those who have had positive experiences, resulting in possible overreporting of negative experiences.
In conclusion, HCPs' experiences with and community perceptions of the contraceptive implant in South Africa may inform the future success (or lack thereof) of PrEP implants. Service delivery challenges such as overburdened clinics and lack of adequate training on removals should be overcome as much as possible in PrEP implant design, such as by prioritizing long implant duration and implants that do not require removal. Perspectives from HCPs in South Africa and other sub-Saharan African countries should be thoughtfully considered both in addressing existing challenges in the rollout of contraceptive implants in South Africa, and in the design of PrEP implants currently in development.
Acknowledgments
The authors especially thank all research participants who took part in this study. The authors thank Kgahlisho Manenzhe, Tsholofelo Malapane, Sheily Ndwayana, and site staff at the Setshaba Research Centre and Desmond Tutu HIV Centre/Foundation for assistance with recruitment. The authors acknowledge Imogen Hawley, Lebogang Mpete, Nqaba Nkomana, Enyonam Odoom, Ketshepileng Seemise, Jaclyn Shea, Siyaxolisa Sindelo, Flora Thobela, and Ziyanda Yola for assistance with transcription. The authors also appreciate assistance from Erica Browne with demographics preparation and Mary Kate Shapley-Quinn with coding and data management.
Funding for this research was provided by the US Agency for International Development (USAID), through the US President's Emergency Plan for AIDS Relief (PEPFAR) under terms of Cooperative Agreement AID-OAA-A-14-00012. Research funding was also provided by the National Institutes of Mental Health under award number R01MH105262. The content is solely the responsibility of the authors and does not necessarily represent the official views of USAID or of the National Institutes of Health. E.A.K. was partially supported by a postdoctoral Whitaker Fellowship from the Institute for International Education.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Gunawardana M, Remedios-Chan M, Miller CS, et al. . Pharmacokinetics of long-acting tenofovir alafenamide (GS-7340) subdermal implant for HIV prophylaxis. Antimicrob Agents Chemother 2015;59:3913–3919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kiser P. Kiser Laboratory Website, Northwestern University. Long-Acting Antiretroviral Implants for Prevention and Treatment of HIV Infection. Available at: http://kiserlab.org/research.html (Last accessed December28, 2017)
- 3. Intarcia Therapeutics, Inc. Company Website. Revolutionizing HIV Prevention: Converging Medicine and Technology. Available at: http://intarcia.com/pipeline-technology/hiv-prevention.html (Last accessed January6, 2018)
- 4. Lykins WR, Luecke E, Johengen D, van der Straten A, Desai TA. Long acting systemic HIV pre-exposure prophylaxis: An examination of the field. Drug Deliv Transl Res 2017;7:805–816 [DOI] [PubMed] [Google Scholar]
- 5. Schlesinger E, Johengen D, Luecke E, et al. . A tunable, biodegradable, thin-film polymer device as a long-acting implant delivering tenofovir alafenamide fumarate for HIV pre-exposure prophylaxis. Pharmaceut Res 2016;33:1649–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gatto G, Girouard N, Brand R, et al. . Pharmacokinetics of tenofovir alafenamide by subcutaneous implant for HIV PrEP. Poster 486, P-F5 presented at the: Conference of Retroviruses and Opportunistic Infections; March 2018 Boston, MA [Google Scholar]
- 7. Pleaner M, Morroni C, Smit J, et al. . Lessons learnt from the introduction of the contraceptive implant in South Africa. S Afr Med J 2017;107:933–938 [DOI] [PubMed] [Google Scholar]
- 8. Adeagbo OA, Mullick S, Pillay D, et al. . Uptake and early removals of Implanon NXT in South Africa: Perceptions and attitudes of healthcare workers. S Afr Med J 2017;107:822. [DOI] [PubMed] [Google Scholar]
- 9. Pillay D, Chersich MF, Morroni C, et al. . User perspectives on Implanon NXT in South Africa: A survey of 12 public-sector facilities. S Afr Med J 2017;107:815. [DOI] [PubMed] [Google Scholar]
- 10. Pillay D, Morroni C, Pleaner M, et al. . Gaps in monitoring systems for Implanon NXT services in South Africa: An assessment of 12 facilities in two districts. S Afr Med J 2017;107:827. [DOI] [PubMed] [Google Scholar]
- 11. Mrwebi KP, Ter Goon D, Owolabi EO, Adeniyi OV, Seekoe E, Ajayi AI. Reasons for discontinuation of Implanon among users in Buffalo City Metropolitan Municipality, South Africa: A cross-sectional study. Afr J Reprod Health 2018;22:113–119 [DOI] [PubMed] [Google Scholar]
- 12. Brady M, Tolley E. Aligning product development and user perspectives: Social-behavioural dimensions of multipurpose prevention technologies. BJOG 2014;121:70–78 [DOI] [PubMed] [Google Scholar]
- 13. Romano J, Van Damme L, Hillier S. The future of multipurpose prevention technology product strategies: Understanding the market in parallel with product development. BJOG 2014;121:15–18 [DOI] [PubMed] [Google Scholar]
- 14. Rausch DM, Grossman CI, Erbelding EJ. Integrating behavioral and biomedical research in HIV interventions: Challenges and opportunities. J Acquir Immune Defic Syndr 2013;63:S6–S11 [DOI] [PubMed] [Google Scholar]
- 15. Montgomery CM, Gafos M, Lees S, et al. . Re-framing microbicide acceptability: Findings from the MDP301 trial. Cult Health Sex 2010;12:649–662 [DOI] [PubMed] [Google Scholar]
- 16. Lin AH, Breger TL, Barnhart M, Kim A, Vangsgaard C, Harris E. Learning from the private sector: Towards a keener understanding of the end-user for microbicide introduction planning. J Int AIDS Soc 2014;17(3 Suppl 2):19162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Luecke EH, Cheng H, Woeber K, et al. . Stated product formulation preferences for HIV pre-exposure prophylaxis among women in the VOICE-D (MTN-003D) study. J Int AIDS Soc 2016;19:20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van der Straten A, Shapley-Quinn MK, Reddy K, et al. . Favoring “Peace of Mind”: A qualitative study of African women's HIV prevention product formulation preferences from the MTN-020/ASPIRE trial. AIDS Patient Care STDs 2017;31:305–314 [Google Scholar]
- 19. South African National Department of Health. The 2015 National Antenatal Sentinel HIV and Syphilis Survey Report. Pretoria, South Africa: 2015 [Google Scholar]
- 20. Montgomery E, Atujuna M, Ndwayana S, et al. . The invisible product: Preferences for long-acting injectable and implantable PrEP among South African youth. Poster presented at the: International Aids Society (IAS) Conference; July 2017 Paris, France [Google Scholar]
- 21. van der Straten A, Agot K, Ahmed K, et al. . The Tablets, Ring, Injections as Options (TRIO) study: What young African women chose and used for future HIV and pregnancy prevention. J Int AIDS Soc 2018;21:e25094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dedoose Version 8.0.41, Web Application for Managing, Analyzing, and Presenting Qualitative and Mixed Method Research Data. Los Angeles, CA: SocioCultural Research Consultants, LLC; 2018. Available at: www.dedoose.com (Last accessed June10, 2018). [Google Scholar]
- 23. Hruschka DJ, Schwartz D, St. John DC, Picone-Decaro E, Jenkins RA, Carey JW. Reliability in coding open-ended data: Lessons learned from HIV behavioral research. Field Methods 2004;16:307–331 [Google Scholar]
- 24. Krogstad EA, Atujuna M, Montgomery ET, et al. . Perspectives of South African youth in the development of an implant for HIV prevention. J Int AIDS Soc 2018;21:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ware NC, Wyatt MA, Geng EH, et al. . Toward an understanding of disengagement from HIV treatment and care in Sub-Saharan Africa: A qualitative study. PLoS Med 2013;10:e1001369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jacobstein R. Liftoff: The blossoming of contraceptive implant use in Africa. Global Health Sci Pract 2018;6:17–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rees H, Pillay Y, Mullick S, Chersich MF. Strengthening implant provision and acceptance in South Africa with the ‘Any woman, any place, any time' approach: An essential step towards reducing unintended pregnancies. S Afr Med J 2017;107:939. [DOI] [PubMed] [Google Scholar]
- 28. Mullick S, Chersich MF, Pillay Y, Rees H. Introduction of the contraceptive implant in South Africa: Successes, challenges and the way forward. S Afr Med J 2017;107:812. [DOI] [PubMed] [Google Scholar]
- 29. Lince-Deroche N, Pleaner M, Morroni C, et al. . Achieving universal access to sexual and reproductive health services: The potential and pitfalls for contraceptive services in South Africa. S Afr Health Rev 2016;2016:95–108 [Google Scholar]
- 30. Falcao J, Zerbe A, Lahuerta M, et al. . Factors associated with use of short-term pre-exposure prophylaxis for HIV among female partners of migrant miners in mozambique. AIDS Patient Care STDs 2017;31:528–534 [DOI] [PubMed] [Google Scholar]
- 31. van der Straten A, Stadler J, Luecke E, Laborde N, Hartmann M, Montgomery ET. Perspectives on use of oral and vaginal antiretrovirals for HIV prevention: The VOICE-C qualitative study in Johannesburg, South Africa. J Int AIDS Soc 2014;17(3 Suppl 2):19146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Van der Elst EM, Mbogua J, Operario D, et al. . High acceptability of HIV pre-exposure prophylaxis but challenges in adherence and use: Qualitative insights from a Phase I trial of intermittent and daily PrEP in at-risk populations in Kenya. AIDS Behav 2013;17:2162–2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aaron E, Blum C, Seidman D, et al. . Optimizing delivery of HIV preexposure prophylaxis for women in the United States. AIDS Patient Care STDs 2018;32:16–23 [DOI] [PMC free article] [PubMed] [Google Scholar]