Accurate identification of seizure-generating brain tissue is challenging, particularly when MRI shows no clear abnormality or extensive abnormality. Plummer et al. achieve this non-invasively by analysing the earliest detectable part of the electromagnetic seizure signal recordable across the head surface. Their findings challenge current practice with its reliance on invasive intracranial monitoring.
Keywords: high density electroencephalography, electroencephalographic source localization, combined electromagnetoencephalographic source localization, magnetoencephalography, magnetoencephalographic source localization
Abstract
Drug-resistant focal epilepsy is a major clinical problem and surgery is under-used. Better non-invasive techniques for epileptogenic zone localization are needed when MRI shows no lesion or an extensive lesion. The problem is interictal and ictal localization before propagation from the epileptogenic zone. High-density EEG (HDEEG) and magnetoencephalography (MEG) offer millisecond-order temporal resolution to address this but co-acquisition is challenging, ictal MEG studies are rare, long-term prospective studies are lacking, and fundamental questions remain. Should HDEEG-MEG discharges be assessed independently [electroencephalographic source localization (ESL), magnetoencephalographic source localization (MSL)] or combined (EMSL) for source localization? Which phase of the discharge best characterizes the epileptogenic zone (defined by intracranial EEG and surgical resection relative to outcome)? Does this differ for interictal and ictal discharges? Does MEG detect mesial temporal lobe discharges? Thirteen patients (10 non-lesional, three extensive-lesional) underwent synchronized HDEEG-MEG (72–94 channel EEG, 306-sensor MEG). Source localization (standardized low-resolution tomographic analysis with MRI patient-individualized boundary-element method) was applied to averaged interictal epileptiform discharges (IED) and ictal discharges at three phases: ‘early-phase’ (first latency 90% explained variance), ‘mid-phase’ (first of 50% rising-phase, 50% mean global field power), ‘late-phase’ (negative peak). ‘Earliest-solution’ was the first of the three early-phase solutions (ESL, MSL, EMSL). Prospective follow-up was 3–21 (median 12) months before surgery, 14–39 (median 21) months after surgery. IEDs (n = 1474) were recorded, seen in: HDEEG only, 626 (42%); MEG only, 232 (16%); and both 616 (42%). Thirty-three seizures were captured, seen in: HDEEG only, seven (21%); MEG only, one (3%); and both 25 (76%). Intracranial EEG was done in nine patients. Engel scores were I (9/13, 69%), II (2/13,15%), and III (2/13). MEG detected baso-mesial temporal lobe epileptogenic zone sources. Epileptogenic zone OR [odds ratio(s)] were significantly higher for earliest-solution versus early-phase IED-surgical resection and earliest-solution versus all mid-phase and late-phase solutions. ESL outperformed EMSL for ictal-surgical resection [OR 3.54, 95% confidence interval (CI) 1.09–11.55, P = 0.036]. MSL outperformed EMSL for IED-intracranial EEG (OR 4.67, 95% CI 1.19–18.34, P = 0.027). ESL outperformed MSL for ictal-surgical resection (OR 3.73, 95% CI 1.16–12.03, P = 0.028) but was outperformed by MSL for IED-intracranial EEG (OR 0.18, 95% CI 0.05–0.73, P = 0.017). Thus, (i) HDEEG and MEG source solutions more accurately localize the epileptogenic zone at the earliest resolvable phase of interictal and ictal discharges, not mid-phase (as is common practice) or late peak-phase (when signal-to-noise ratios are maximal); (ii) from empirical observation of the differential timing of HDEEG and MEG discharges and based on the superiority of ESL plus MSL over either modality alone and over EMSL, concurrent HDEEG-MEG signals should be assessed independently, not combined; (iii) baso-mesial temporal lobe sources are detectable by MEG; and (iv) MEG is not ‘more accurate’ than HDEEG—emphasis is best placed on the earliest signal (whether HDEEG or MEG) amenable to source localization. Our findings challenge current practice and our reliance on invasive monitoring in these patients.
Introduction
Drug refractory focal epilepsy is a major clinical problem. Despite the expanding array of medications available to treat patients with epilepsy, at least 30% of this group have seizures that are not controlled with two or more medications. An increasing proportion of such patients are being referred for epilepsy surgery when the MRI shows no clear abnormality (up to 40% now in some centres) (So and Ryvlin, 2015), a complex lesion, or multiple potential lesions. For these cases, functional imaging with PET, single photon emission computed tomography (SPECT), and simultaneous electroencephalography functional MRI (EEG-fMRI) are more relied upon for non-invasive surgical localization but they generally lack sufficient temporal resolution to distinguish spike and seizure discharge onset from propagation in the attempt to characterize the epileptogenic zone—the anatomical ‘zone’ of brain tissue needing resection to obtain long-term seizure freedom (Rosenow and Luders, 2001). Magnetoencephalography (MEG) and scalp EEG offer millisecond-order temporal resolution to track rapidly evolving interictal (spikes, sharp waves) and ictal (seizure) patterns but lack the spatial resolution to guide surgery without the use of source localization modelling techniques (such as dipole and distributed modelling) (Ebersole, 2003). MEG and EEG see focal sources differently. MEG has a higher spatial resolution, whereas scalp EEG needs a comparatively larger cortical surface area to see an intracranially recorded spike, mainly because EEG potentials measured at the scalp from extracellular volume currents are dampened by skull impedance while complementary MEG fields from intracellular currents are not (Ebersole and Ebersole, 2010). The MEG sensor array is more sensitive to sources that are tangential to the plane of the cortical surface (sulcal wall depolarization) while EEG sees radially oriented sources (gyral crest depolarization) as well as tangential sources (Ebersole and Ebersole, 2010). It is surprising that few studies combine MEG and EEG source localization (MSL, ESL) for epilepsy surgery; most that have are retrospective or use lower density EEG (<64 electrodes) (Ochi et al., 2001; Lin et al., 2003; Jin et al., 2007; Scheler et al., 2007; Kaiboriboon et al., 2010; Wennberg et al., 2011; Pellegrino et al., 2016). Retrospective epilepsy surgery studies that apply source localization carry a bias; the ESL or MSL solution is calculated in the context of known surgical margins and histopathology results, and post-surgical outcome is reliant on patient recall of pre- and postoperative seizure events. Low density EEG (19–32 electrodes) is standard practice in epilepsy surgical centres for video EEG telemetry, but this is inadequate for reliable ESL without an inferior electrode array to view the basal and inner surfaces of the frontal, temporal and occipital lobes. Further, commercial EEG caps, which come with MEG scanners that do have an inbuilt-EEG operating system, are routinely devoid of an inferior electrode array, with electrode coverage only from the ‘ears-up’. It has been shown that the greatest gain in the accuracy and spatial resolution of ESL is seen as the electrode count increases from 32 to 64 (provided an inferior array is included); the gain falls off between 64 and 128 electrodes (Lantz et al., 2003a; Song et al., 2015). This reveals a bias in studies that have argued for the superiority of MEG over EEG for discharge detection and source localization accuracy when a 150–300 MEG sensor array is pitted against low-density EEG. Given that the present, albeit imperfect, gold standard for presurgical delineation of the epileptogenic focus is intracranial EEG (and given that MEG sees sources differently) it seems rational that the use of a like-modality in the form of scalp EEG should more fully inform ESL—by the use of at least 64 electrodes with the inclusion of an inferior array—to guide, limit, or even avoid the placement of intracranial electrodes alongside MSL in centres where MEG is available. Higher spatial sampling improves the signal-to-noise ratio (SNR) (Song et al., 2015) of the scalp EEG recorded spike and seizure rhythm. Optimizing the SNR around discharge onset (‘take-off’ from baseline) is relevant because it has been shown that scalp EEG potentials at earlier spike components (take-off and mid-upstroke phases of the initial spike deflection, where the SNR is usually lower) better reflect the synchronously measured intracranial EEG potential at seizure onset (Ray et al., 2007). Solutions based on the spike peak (where the SNR is usually higher) are more likely to be removed from the original cortical generator due to the effects of cortico-cortical propagation of the discharge (Ray et al., 2007). It has also been noted that EEG spikes can peak just before, at the same time, or just after the MEG spike peak for a given cortical generator (Merlet et al., 1997; Ebersole and Ebersole, 2010) arguably from differences in signal detection owing to differences in the orientation, depth, and extent of the source configuration, but the clinical importance of this observation is unclear. With these factors in mind, we hypothesize that synchronously-acquired high-density EEG (HDEEG) with MEG for source localization will capture the epileptogenic zone with greater accuracy when two conditions are met: (i) temporal, the earliest component of the interictal or ictal discharge is modelled; and (ii) spatial, source localization is performed on spatially combined complementary HDEEG-MEG signals (‘EMSL’), not on spatially independent signals (ESL, MSL). That is, EMSL should outperform ESL and MSL.
Materials and methods
Patients and procedures
Subjects’ consent was obtained according to the Declaration of Helsinki. Ethics approval for the research protocol was given by The Human Research Ethics Committees of St Vincent’s Hospital Melbourne and Swinburne University of Technology. Selection criteria were: no visible lesion or an extensive lesion on MRI; insufficient localizing data from routine non-invasive tests including video EEG monitoring, PET, and SPECT; interictal epileptiform discharges (IEDs) during week-long video EEG monitoring; frequent medically refractory seizures (at least monthly). Patients were not required to have a seizure during the recording but at least two monomorphic IEDs had to be present in at least one modality. Thirteen consecutive, prospectively enrolled patients with drug-resistant focal epilepsy (seven males, six females, age range 10–54 years, median 33 years; disease duration 3–32 years) underwent 1-h synchronously-acquired HDEEG-MEG, seated in a comfortable reclined position. MRI brain showed no clear lesion in 10 patients and a complex lesion in three patients. Patients were sleep deprived and medications withheld 12 h from the recording. Scalp HDEEG (ANT Waveguard® 72–94 electrode cap with 12-electrode inferior temporal array, 10–10 positions) acquired on one of two EEG amplifier systems (ANT ASAlab®, Enschede; Compumedics SynampRT®, Melbourne) and MEG (Elekta Triux® 306 sensors, 102 magnetometers, 204 planar gradiometers) (Fig. 1) were sampled at 1000 Hz or 5000 Hz with anti-aliasing filter set at 330 Hz or 1650 Hz, respectively. Bad channels for all MEG data were checked prior to applying temporal extension to signal source separation (tSSS) using Maxfilter® v2.2.10–15 (Elekta Oy) for interference suppression (correlation limit 0.98 and sliding window of 10 s). Independent 1-s interval clock triggers acquired on each system were used to synchronize HDEEG and MEG offline, verified by comparing ECG channel signal phase from each independent modality. MEG head coils, HDEEG electrode positions, and PAN (pre-auricular, nasion) co-ordinates were digitized (Polhemus Fastrak®) in common space for MRI co-registration; digitized points were cross-validated with optical sensor tracking (NDI Polaris Vicra®). Applying a bandpass Hann-shaped FFT filter from 1 to 100 Hz (slope of 2 Hz, 50% transmission at 1 Hz; slope of −20 Hz, 50% transmission at 100 Hz), IEDs and seizures were manually identified by a neurologist (C.P.) on HDEEG and MEG independent files using Curry 7® (Compumedics Neuroscan, Hamburg). Independent-modality source localization (ESL, MSL) was performed before marking of the corresponding synchronized HDEEG-MEG file for EMSL of the common HDEEG-MEG signal space. Ictal and interictal discharge marking and source localization were therefore always done blind to the synchronized file data. Monomorphic discharges were marked at ‘take-off’ (first clear disruption of background rhythm that reached >50% amplitude of preceding baseline activity) (Ray et al., 2007) and, for averaging, at the negative-peak (IEDs and early ictal rhythm). For reliable discharge SNR calculation, a baseline noise interval of at least double the number of samples (relative to modelled discharge) was taken prior to earliest signal take-off. Due to frequent asynchrony between HDEEG and MEG negative-peaks for a given interictal or ictal discharge, one modality (HDEEG or MEG) was priority selected for averaging on the synchronized file (selection criteria total discharge count, SNR maxima); the alternate modality was then used for averaging to assess reproducibility of respective ESL, MSL, EMSL solutions. ESL and MSL solutions from both synchronized-file averaged datasets were likewise compared with their corresponding independent-file HDEEG- and MEG-based results. Seizures were classified as either localizing or non-localizing. Ictal source solutions were assessed for reproducibility if more than one seizure was captured during a patient’s recording. All source localization operations were performed with the boundary element method (Fuchs et al., 1998a) from patient-specific MRI (forward model) (Fig. 1) and standardized low resolution tomographic analysis (sLORETA) (Pascual-Marqui, 2002) constrained to cortex (rotating sources) but without source extension (inverse model) to avoid over-constraining of solutions. Distributed source orientations were represented by a current density reconstruction moving dipole at the sLORETA maximum of each solution. To allow valid comparison between EMSL and ESL/MSL solutions: (i) only source solutions from IEDs and ictal rhythms common to HDEEG and MEG were used in the statistical analyses; and (ii) conductivity matching was applied to the combined HDEEG-MEG signal to account for the differential influence of tissue compartments (particularly the skull) on lead-field conductivity for HDEEG relative to MEG for the boundary element method (Fuchs et al., 1998a, b); a multiple tangential-dipole fits procedure was applied to overlapping EEG-MEG signal maxima. SNR transformation of the datasets allows HDEEG and MEG unitless signals to be matched when the MEG dominant tangential-source lead-field, which is relatively unaffected by the boundary element method (brain/skull/skin) conductivity, weights the synchronous HDEEG lead-field, which is affected by these tissue interfaces. Conductivity weighting scales the nominal tissue conductivity (S/m) values (brain 0.33/skull 0.0042/skin 0.33) up or down (with brain/skull/skin S/m proportions fixed) to account for individual skull effects (for age, shape, thickness) on the combined lead-field. Simulation data and case studies have validated this approach based on somatosensory evoked responses with combined EEG and MEG (Fuchs et al., 1998b; Huang et al., 2007; Choi et al., 2013). Source solutions had to explain at least 90% of the signal variance (above noise) at the following time points; ‘early’ (first solution from take-off along the millisecond incremental time course of the discharge to reach 90% explained variance); ‘mid’ [50% dominant negative-peak rising phase or 50% mean global field power (MGFP), whichever occurred first]; and ‘late’ (discharge dominant negative-peak) phases. Due to lead-lag differences between respective HDEEG-MEG MGFP waveforms (shown in the bottom-left of Figs 3 and 4), EMSL latencies were as follows: take-off (first-latency take-off, HDEEG or MEG), early (first EMSL latency with 90% explained variance using combined HDEEG-MEG signal), mid (EMSL at 50% combined HDEEG-MEG MGFP), late (later dominant negative-peak, HDEEG or MEG). ‘Earliest’ solutions were defined as the first of the three early-phase ESL, MSL, or EMSL solutions, whether isolated (one leading early-phase solution), or simultaneous (two or all early-phase solutions leading at the same latency).
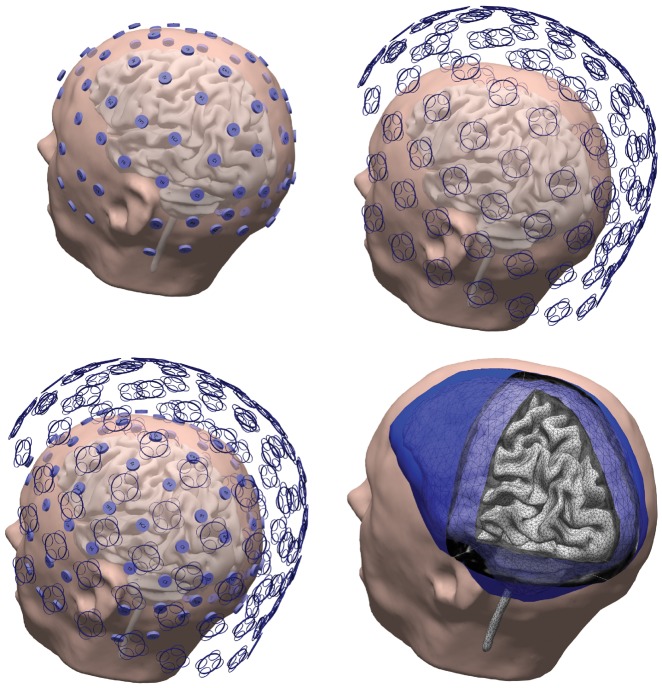
Figure 1.
Individualized HDEEG-MEG and boundary element method setup. Top left: Digitized locations of 94 electrode HDEEG configuration, including the 12-electrode inferior temporal array. Top right: 306 MEG sensors (102 magnetometers and 204 planar gradiometers). Bottom left: Spatial coverage of combined HDEEG-MEG from simultaneously acquired and synchronized data. Bottom right: Boundary element method (BEM) three-compartment tessellated head mode with skin (outer shell, smoothed), skull (dark-blue outer-skull shell, light-blue inner-skull shell showing intersecting vertices), boundary element method and cortically-constrained sources (vertices from tessellation superimposed on grey cortex surface) used for distributed current density reconstruction, generated from individual patient MRI (taken from Patient 1).
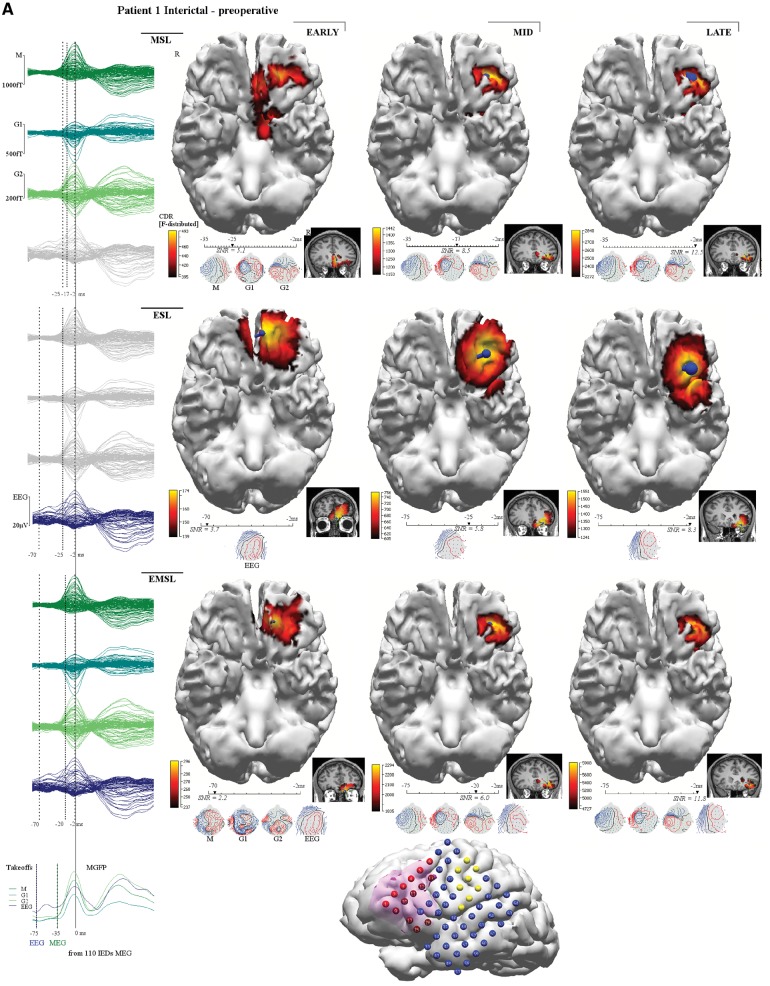
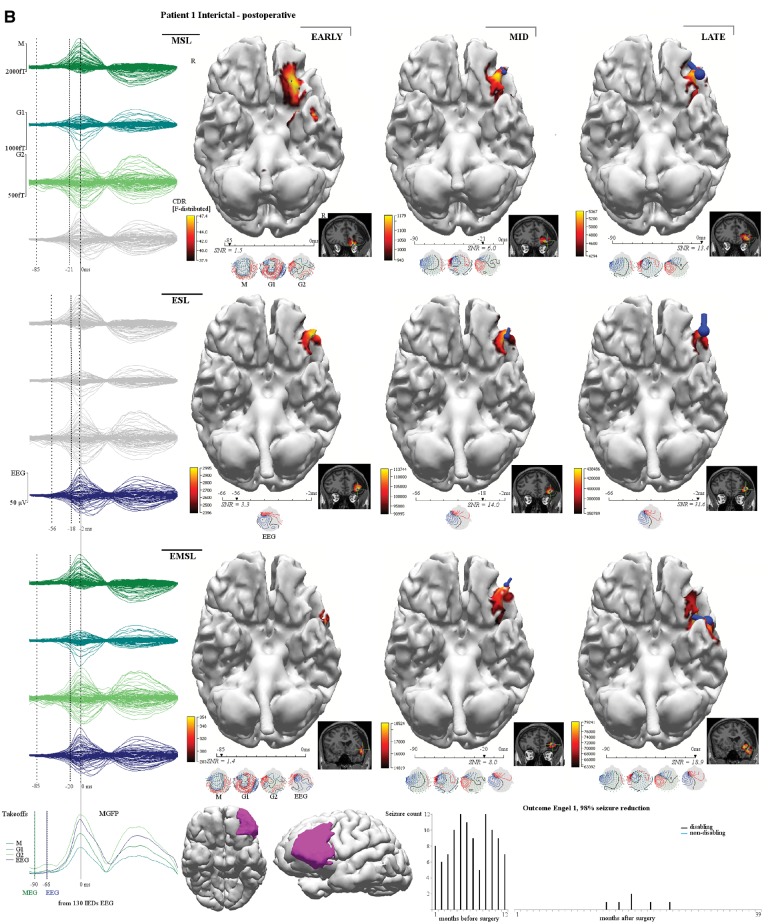
Figure 3.
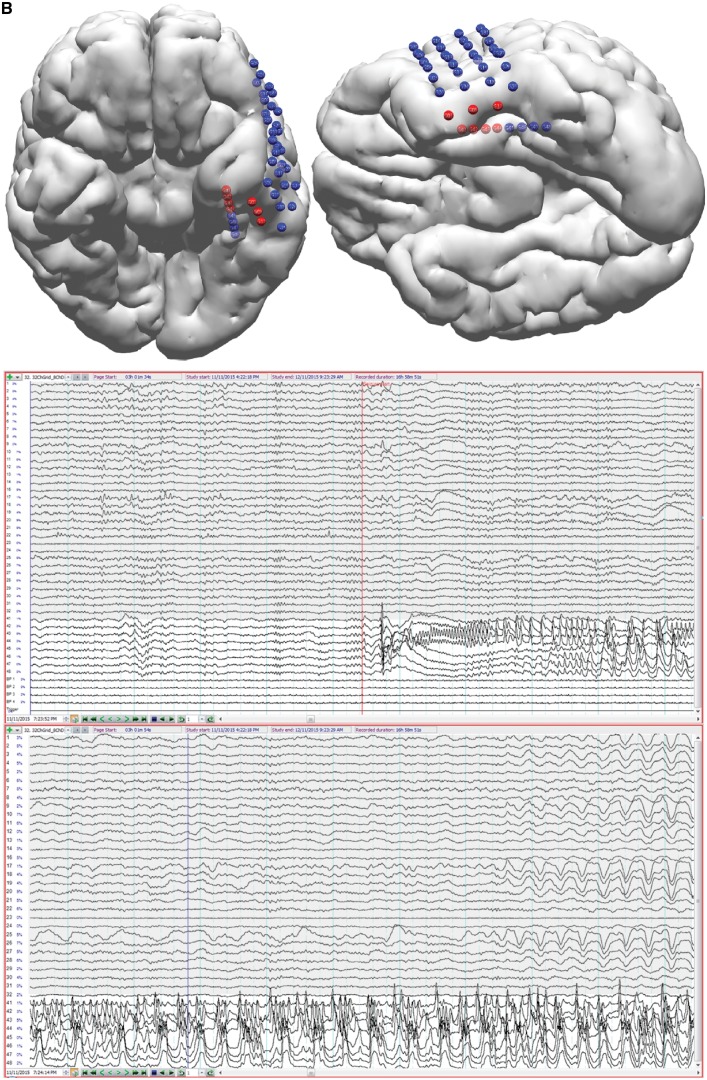
Patient 1. This patient (MRI negative), had incomplete resection of the epileptogenic zone with six postoperative seizures around medication weaning (bottom right seizure chart). Left inferior frontal gyrus (IFG) and orbitofrontal gyrus (OFG) corticectomy showed type 1 dysplasia involving resection margins. (A) Preoperative interictal MSL (top row), ESL (middle row), EMSL (bottom row) suggested medial orbitofrontal gyrus and rectal gyrus (RG) onset with propagation to lateral orbitofrontal gyrus. Early-solutions (left column) for ESL and EMSL preceded early-MSL by 45 ms. (B) Post-operative interictal localization showed a similar pattern of onset and propagation but early-MSL preceded early ESL by 29 ms (early-late-EMSL localized to the superior temporal gyrus). Preoperative, mid-phase solutions (middle column in A) for MSL, ESL, and EMSL fell within the resection bed (magenta shading reconstructed cortex at the bottom of B) and were concordant with ICEEG (A) (red electrodes seizure onset, blue electrodes inactive at seizure onset, yellow electrodes map eloquent cortex for speech, motor) but the medial orbitofrontal gyrus was not covered by the grid; ICEEG position directed by broad fronto-temporal localization given by PET, video EEG monitoring and by lateral inferior frontal gyrus focus given by SPECT (Table 1) and by EEG-fMRI (not shown). The preoperative ictal results (not shown) reflect a similar pattern of discharge onset and propagation. Preoperative ictal early-MSL preceded early-ESL by 14 ms. ESL localized to the anterior inferior frontal gyrus and medial orbitofrontal gyrus while the earlier latency MSL solutions localized to the medial orbitofrontal gyrus and rectal gyrus. Taken together, the results are consistent with a left frontal source starting at the medial orbitofrontal gyrus and rectal gyrus and propagating to the lateral orbitofrontal gyrus and anterior inferior frontal gyrus, the latter supported by ICEEG. The patient is seizure free on re-instituted medication (months 26–39) with consideration of extended resection (involving medial orbitofrontal gyrus) should seizures return. Corresponding averaged waveforms are seen at left margin for MEG (top left, M = magnetometer; G1 = first planar gradiometer; G2 = second planar gradiometer), for HDEEG (middle left), and for combined HDEEG-MEG (bottom left). Corresponding early-phase, mid-phase, late-phase source localization latencies are marked by dotted vertical lines. MGFP (mean global field power) take-offs for HDEEG and MEG are shown at the bottom left corner. Take-off was defined as the first clear disruption of the background that achieved >50% amplitude of preceding baseline activity. The early-phase solution was the first solution to reach 90% explained variance along the millisecond-incremental time-course of the discharge from take-off. sLORETA (standardized low resolution tomographic analysis) current density reconstruction (CDR) maps are represented as F-distribution heat maps, with dominant orientation of distributed sources represented by a dark blue current density reconstruction moving ‘dipole’ (surface negative at spherical end of dipole), at reconstructed cortical surfaces and at MRI scans. HDEEG potentials and MEG fields are shown below the latency bars along with the corresponding SNR value of the signal at the solution time point. EZ = epileptogenic zone; VEM = video EEG monitoring.
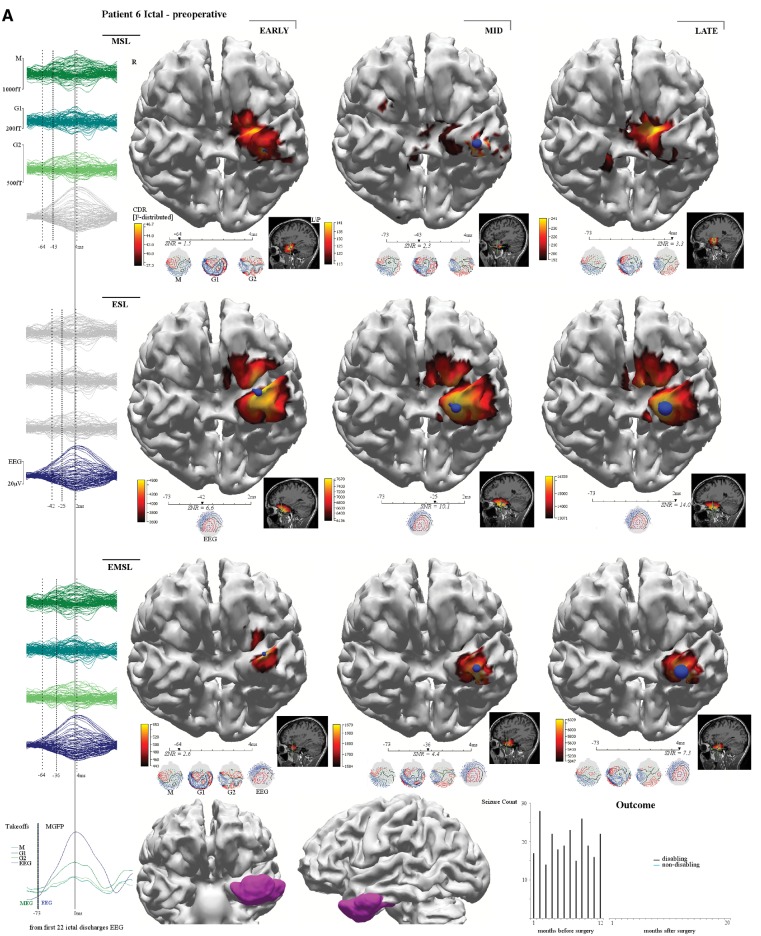
Figure 4.
Patient 6. This patient (MRI negative) had complete epileptogenic zone resection with 240 seizures over 12 months pre-surgery and no seizures to 20 months post-surgery. As a right-handed speech therapist, a left anterolateral temporal grid was planned based on PET (temporal pole) and SPECT (anterolateral temporal cortex) to avoid unwarranted resection of mesial temporal structures. Ictal early-MSL (mesial temporal) preceded early-ESL (anterior temporal pole) by 22 ms (A). Interictal (not shown) early-MSL and early-EMSL preceded early-ESL by 33 ms (all solutions baso-mesial temporal) with propagation to basolateral temporal cortex. These results led to the decision to place a left hippocampal depth electrode (B) (in addition to the grid). The seizure subsequently captured starts at the tip of the hippocampal depth electrode (four anterior red hippocampal electrodes, white background channels) and propagates to the inferior grid margin (three lower grid red electrodes, grey background channels). Standard (antero-mesial) temporal lobectomy (type 1C dysplasia entorhinal cortex) was not complicated by a language deficit. EZ = epileptogenic zone.
Outcomes
Clinical validation of each source localization modality (ESL, MSL, EMSL) was based on agreement between the source solution (location, orientation of current density maxima for a given time point) with the epileptogenic zone. The epileptogenic zone was defined by intracranial electrode depth or grid (ICEEG)-localized seizure onset and by surgical resection margins (SU) associated with improved postoperative seizure control (minimum 12-month post-operative versus preoperative monthly seizure counts) for interictal and ictal discharge datasets (herein abbreviated as IED-ICEEG, IED-SU, ictal-ICEEG, ictal-SU). Patients with seizure recurrence underwent post-operative HDEEG-MEG to compare with preoperative source solutions. Each patient had one outcome for each combination of phases (earliest, early, mid, late) and methods (ESL, MSL, EMSL). While all patients subsequently underwent surgery and all patients recorded IEDs, not all patients had seizures during the recording and not all patients underwent ICEEG—four separate analyses for outcome were therefore required (namely, IED-ICEEG, IED-SU, ictal-ICEEG, ictal-SU).
Statistical analysis
Epileptogenic zone agreement (outcome) was examined using binomial regression on interictal (IED-ICEEG/SU) and ictal (ictal-ICEEG/SU) data separately. Discharge phase was entered in the model as a statistical predictor, namely, ‘earliest’, ‘early’, ‘mid’ or ‘late’, and method as ‘EMSL’, ‘ESL’ or ‘MSL’. Metrics of association were presented as odds ratios (OR) and 95% confidence intervals (CI). Fisher’s exact test was used to explore a potential confounding relationship between modality used for priority averaging on the synchronized file (HDEEG or MEG) and the method (ESL, MSL, or EMSL) returning the earliest solution. For single IEDs seen by both HDEEG and MEG, respective take-off and negative peak latencies were compared and recorded as ‘simultaneous’ if lead or lag was <10 ms (to allow for any marking errors related to digital resolution). Note that this 10-ms cut-off rule was not applied to averaged discharges due to the superior (millisecond-level) signal-resolution of the averaged waveform MGFP at HDEEG and MEG take-off and negative-peak latencies. Methods were also compared across the following source solution variables: latency (signal take-off relative to early, mid, late phase solutions) to characterize time-lag differences between corresponding ESL, MSL, EMSL solutions; SNR (signal-to-noise ratio); F-distribution maximum (scaled probability map of current source distribution), using Wilcoxon matched-pairs signed-ranks test. To isolate significant differences in strength of association of predictor influences on outcomes, alpha risk for all statistical tests was set to 5%. Stata/IC® 15.1 (Statacorp, College Station Texas) was used for all statistical analyses. Because of the small number of subjects ultimately studied, datasets were independently analysed by two statisticians, S.V. and R.B., (see ‘Acknowledgements’ section), blinded to each other’s results, using binomial regression (with outcomes based, as above, on average agreement within the combination of phase and method) and binary regression (based on each participant’s epileptogenic zone agreement for each combination of phase and method).
Data availability
All data are available on request including raw HDEEG and MEG independent file and synchronized file recordings, results for Fischer’s exact test on the potential confounding relationship between modality used for averaging and the method returning the earliest source solution (no significant effect seen), and the following images omitted due to submission restrictions for Supplementary material (Patient 1 ictal, Patient 3 ictal, Patient 4 ictal, Patient 6 interictal, Patient 7 ictal, Patient 9 interictal, Patient 10 interictal, Patient 11 ictal, Patient 12 ictal, Patient 13 interictal); however, these images are described in the figure legend for each patient’s corresponding interictal/ictal displayed result with the related data given in Tables 2–4 and Supplementary Table 1.
Table 2.
Interictal data and earliest solution source localization results
| Patient | IEDs | IED lead time | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total, n | EEG only, n | MEG only, n | Both, n | Peaks averaged, n (modalitya) | Earliest source solution | Earliest EZ agreement | EEG take-off to early ESL, ms | Earliest source to ESL, ms | MEG take-off to early MSL, ms | Earliest source to MSL, ms | EMEG take-off to early EMSL, ms | Earliest source to EMSL, ms | |
| Preoperative | |||||||||||||
| 1 | 176 | 66 | 40 | 70 | 110 (MEG) | ESL, EMSL | ESL | 5 | 0 | 10 | 45 | 5 | 0 |
| 2 | 11 | 0 | 0 | 11 | 8 (EEG) | MSL | MSL | 8 | 4 | 14 | 0 | 15 | 1 |
| 3 | 63 | 17 | 37 | 9 | 46 (MEG) | MSL, EMSL | MSL, EMSL | 7 | 22 | 6 | 0 | 6 | 0 |
| 4 | 98 | 35 | 23 | 40 | 63 (MEG) | MSL, EMSL | MSL | 4 | 15 | 6 | 0 | 6 | 0 |
| 5 | 34 | 11 | 6 | 17 | 23 (MEG) | MSL, EMSL | MSL | 0 | 43 | 6 | 0 | 6 | 0 |
| 6 | 36 | 16 | 10 | 10 | 26 (EEG) | MSL, EMSL | MSL, EMSL | 9 | 33 | 0 | 0 | 0 | 0 |
| 7 | 279 | 234 | 7 | 38 | 272 (EEG) | MSL | ESL | 12 | 12 | 5 | 0 | 8 | 3 |
| 8 | 87 | 30 | 42 | 15 | 57 (MEG) | MSL, ESL, EMSL | MSL, EMSL | 0 | 0 | 0 | 0 | 0 | 0 |
| 9 | 84 | 50 | 8 | 26 | 76 (EEG) | ESL | ESL | 4 | 0 | 13 | 1 | 14 | 2 |
| 10 | 122 | 70 | 11 | 41 | 111 (EEG) | ESL, EMSL | ESL, EMSL | 0 | 0 | 5 | 5 | 0 | 0 |
| 11 | 133 | 0 | 2 | 131 | 131 (EEG) | MSL, EMSL | MSL, EMSL | 1 | 23 | 3 | 0 | 3 | 0 |
| 12 | 181 | 94 | 46 | 41 | 135 (EEG) | MSL, EMSL | MSL | 14 | 14 | 2 | 0 | 2 | 0 |
| 13 | 170 | 3 | 0 | 167 | 170 (EEG) | MSL,ESL, EMSL | MSL, ESL, EMSL | 24 | 0 | 24 | 0 | 24 | 0 |
| All | 1474 | 626 (42%) | 232 (16%) | 616 (42%) | Median | 5 | 12 | 6 | 0 | 6 | 0 | ||
| Range | 0–24 | 0–43 | 0–24 | 0–45 | 0–24 | 0–3 | |||||||
| Postoperative | |||||||||||||
| 1 | 151 | 10 | 21 | 120 | 130 (EEG) | MSL | 10 | 29 | 5 | 0 | 5 | 0 | |
| 5 | 92 | 64 | 6 | 22 | 28 (MEG) | ESL, EMSL | 0 | 0 | 0 | 5 | 0 | 0 | |
| 10 | 155 | 41 | 40 | 74 | 114 (MEG) | ESL | 0 | 0 | 3 | 8 | 7 | 7 | |
A higher proportion of discharges were seen in HDEEG alone against MEG alone. EMSL solutions that agreed with the epileptogenic zone (based on ICEEG or SU concordance) never occurred in isolation as opposed to ESL (three patients) and MSL (four patients).
aModality (HDEEG or MEG) chosen for averaging was based on relative discharge count and SNR maxima. Note that the modality used for averaging had no bearing on the modality that gave rise to the earliest source solution (Fisher’s exact test, not shown).
Results
Table 1 shows patient characteristics and surgical outcomes for 13 patients (seven males, six females, age at surgery 10–54 years, median 33 years). Median duration of epilepsy to surgery was 20 years (range 4–33 years). All patients had frequent disabling seizures before surgery. Prospective seizure counts before surgery (over median 12 months, range 3–21 months) and after surgery (over median 21 months, range 14–39 months), gave Engel scores and percentage seizure reductions as follows: I 98–100% (nine patients), II 80%, 99% (two patients) and III 59%, 60% (two patients).
Table 1.
Patient characteristics and outcome and standard clinical work-up
| Patient | Sex | Duration epilepsy to surgery, years | Age at surgery, years | Seizure pattern | Follow-up, months | Post-surgery seizure reduction, % | Engel outcome | Semiology suspected focus | MRI | VEM | PET | SPECT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 12 | 25 | Weekly to monthly | 39 | 98 | I | L Fronto-temporal | Normal | L Fronto-temporal (spikes/seizures) | L Frontal (orbital) L Temporal (mesial) | L Frontal (posterior) |
| 2 | F | 33 | 54 | Weekly to monthly | 30 | 80 | II | L Temporal | Normal | L Anterior quadrant (spikes/seizures) | R Temporal (anteromesial) L Temporal (mesial) | L Temporal (anterior) |
| 3 | M | 31 | 51 | Weekly to monthly | 26 | 100 | I | R Temporal | Normal | R Anterior quadrant (spikes/seizures) | R Temporal (mesial, anterolateral) | R Temporal (lateral) |
| 4 | F | 25 | 27 | Multiple daily | 23 | 99 | I | L Frontal (hand/leg) | Normal | L Fronto-central (spikes/seizures) | L Frontal (lateral)L Parietal (lateral) | L Frontal (lateral) L Parietal (lateral) |
| 5 | F | 8 | 29 | Daily to weekly | 21 | 59 | III | R Temporal | Normal | R Lateral temporal (spikes/seizures) | Normal | Not captured |
| 6 | F | 4 | 36 | Daily to weekly | 20 | 100 | I | L Temporal | Normal | L Anterior temporal (spikes/seizures) | L Temporal (anterior pole) | L Temporal (lateral) |
| 7 | M | 32 | 39 | Multiple daily | 21 | 99 | I | R Frontal (face/hand) | Normal | R Frontal (spikes/seizures) | L Temporal (mesial) | R Frontal (lateral) R Temporal (lateral) |
| 8 | M | 5 | 20 | Daily to weekly | 20 | 100 | I | L Parietal | Normal | L Parieto-occipital (spikes/seizures) | Vertex (bilateral, sensorimotor strip) | L Temporal (lateral) |
| 9 | M | 6 | 25 | Daily to weekly | 22 | 98 | I | L Temporal | Normal | L Anterior quadrant (spikes) | L Temporal (anterior pole) | Not captured |
| 10 | F | 4 | 10 | Multiple daily | 26 | 99 | II | R Parietal (paracentral lobule) | Normal | L Fronto-central (spikes/seizures) | R Frontal (superior frontal gyrus) R Parietal (paracentral lobule) | Not captured |
| 11 | F | 33 | 36 | Multiple daily | 17 | 99 | I | R Frontal (face/hand/leg) | Multilobar dysplasia | R Fronto-central (spikes/seizures) | Not done | Not captured |
| 12 | M | 32 | 49 | Daily to weekly | 15 | 60 | III | L Temporo-parietal | Multilobar dysplasia | L Posterior quadrant (spikes/seizures) | L Occipito-parietal junction | Not captured |
| 13 | M | 20 | 33 | Daily to weekly | 14 | 100 | I | R Frontal | R Frontal gliosis | R Frontal (spikes/seizures) | Not done | Not captured |
All patients had severe refractory disabling seizures that improved after surgery, most with an Engel I outcome. Although Patient 10, a young female, had a 99% seizure reduction after surgery, her postoperative focal seizures, which carried a different semiology, were still disabling (Engel II outcome). No clear MRI abnormality was seen in 10 patients while three patients had complex lesions. ICEEG was performed in nine patients: 64-electrode array (Patients 1, 10, 11, 12, and 13); 32-electrode array plus ipsilateral 8-electrode hippocampal depth (Patients 5 and 6); an intra-operative depth electrode (Patient 8); and bilateral hippocampal-depth electrodes (Patient 2).
ICEEG = intracranial EEG.
Table 1 also shows the standard pre-surgical work-up. Ten patients did not have a clear abnormality on MRI. Three patients had extensive lesions. Nine patients required ICEEG because of non-localizing or discordant results from video EEG monitoring, PET or SPECT. ICEEG placements were as follows: 64-electrode array (5/9 patients), 32-electrode array plus ipsilateral 8-electrode hippocampal depth (2/9), an intraoperative depth electrode (1/9), bilateral hippocampal-depth electrodes (1/9). Four patients had surgery without ICEEG based on concordance between source localization and video EEG monitoring (Patients 4 and 7) or PET (Patients 3 and 9).
A total of 1474 IEDs were preoperatively recorded across all patients (Table 2), seen in: HDEEG only, 626 (42%); MEG only, 232 (16%); both, 616 (42%). A total of 33 seizures were recorded from 11 patients (Table 3), seen in: HDEEG only, seven (21%); MEG only, one (3%); both, 25 (76%); and were localizable in 10 patients (non-localizable single seizure in Patient 8) with 75% (24/32) localizable by ESL, 54% (14/26) by MSL, and 76% (25/33) by EMSL.
Table 3.
Ictal data and earliest solution source localization results
| Patient | Ictal events | Ictal lead time | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total, n | EEG only, n | MEG only, n | Both, n | Peaks averaged, n (modalitya) | Earliest source solution | Earliest EZ agreement | EEG take-off to early ESL, ms | Earliest source to ESL, ms | MEG take-off to early MSL, ms | Earliest source to MSL, ms | EMEG take-off to early EMSL, ms | Earliest source to EMSL, ms | |
| 1 | 6 | 0 | 0 | 6 | 7 (EEG) | MSL, EMSL | MSL | 2 | 14 | 0 | 0 | 0 | 0 |
| 2 | 0 | ||||||||||||
| 3 | 3 | 0 | 1 | 2 | 7 (EEG) | ESL | ESL | 0 | 0 | 15 | 10 | 13 | 8 |
| 4 | 3 | 0 | 0 | 3 | 28 (MEG) | MSL, EMSL | MSL, EMSL | 9 | 24 | 0 | 0 | 0 | 0 |
| 5 | 0 | ||||||||||||
| 6 | 3 | 0 | 0 | 3 | 22 (EEG) | MSL, EMSL | MSL | 31 | 22 | 9 | 0 | 9 | 0 |
| 7 | 5 | 4 | 0 | 1 | 38 (EEG) | EMSL | ESL | 1 | 3 | 8 | 3 | 5 | 0 |
| 8 | 1 | 0 | 0 | 1 | Non-localizing | ||||||||
| 9 | 2 | 1 | 0 | 1 | 25 (EEG) | ESL, EMSL | ESL | 20 | 0 | 45 | 25 | 20 | 0 |
| 10 | 2 | 0 | 0 | 2 | 16 (EEG) | ESL | ESL | 0 | 0 | 26 | 26 | 10 | 10 |
| 11 | 1 | 0 | 0 | 1 | 9 (MEG) | MSL, EMSL | MSL, EMSL | 0 | 4 | 0 | 0 | 0 | 0 |
| 12 | 4 | 2 | 0 | 2 | 6 (EEG) | MSL | None | 8 | 3 | 3 | 0 | 7 | 2 |
| 13 | 3 | 0 | 0 | 3 | 6 (EEG) | ESL, EMSL | ESL, EMSL | 5 | 0 | 8 | 3 | 5 | 0 |
| All | 33 | 7 (21%) | 1 (3%) | 25 (76%) | Median | 4 | 3 | 8 | 2 | 6 | 0 | ||
| Range | 0–31 | 0–24 | 0–45 | 0–26 | 0–20 | 0–10 | |||||||
A higher proportion of discharges were seen in HDEEG alone against MEG alone. EMSL solutions that agreed with the epileptogenic zone (based on ICEEG or SU concordance) never occurred in isolation as opposed to ESL (four patients) and MSL (two patients).
aModality (HDEEG or MEG) chosen for averaging was based on relative discharge count and SNR maxima. Note that the modality used for averaging had no bearing on the modality that gave rise to the earliest source solution (Fisher’s exact test).
Of the 616 single IEDs seen in both HDEEG and MEG, take-off was earlier in HDEEG for 116 (19%) discharges (medians 11–28 ms), earlier in MEG for 339 (55%) discharges (medians 15–44 ms), and simultaneous (within 10 ms) for 161 (26%) discharges (Supplementary Table 1). Negative-peak was earlier in HDEEG for 50 (8%) discharges (medians 11–36 ms), earlier in MEG for 397 (64%) discharges (medians 16–60 ms), and simultaneous for 169 (28%) discharges. When not simultaneous, HDEEG-MEG leads and lags for respective take-offs and peaks were bidirectional for 10 patients but unidirectional for Patients 3 and 11 (MEG always earlier for take-offs and peaks) and Patient 9 (HDEEG always earlier for take-offs). Lead-lag patterns differed between pre- and postoperative datasets (Patients 1, 5, and 10).
Table 4 shows the resection performed and the corresponding histopathology results for all patients. The most common pathology was cortical dysplasia (eight patients). Results for source localization-epileptogenic zone agreement and non-agreement are given by phase (early, mid, late) and method (ESL, MSL, EMSL). Early-phase solutions co-localized with ICEEG and SU more often than mid-phase and late-phase solutions.
Table 4.
ICEEG and surgical resection concordance
| Patient | Resection | Histology | PET | SPECT | Interictal | Ictal | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EARLY | MID | LATE | EARLY | MID | LATE | |||||||||||||||||
| MSL | ESL | EMSL | MSL | ESL | EMSL | MSL | ESL | EMSL | MSL | ESL | EMSL | MSL | ESL | EMSL | MSL | ESL | EMSL | |||||
| 1 | L Inferior frontal gyrus | CD 1A | * | – | – | – | – | + | + | + | + | – | – | + | + | – | – | + | – | – | + | – |
| > | – | – | – | – | × | × | × | × | – | – | × | × | – | – | × | – | – | × | – | |||
| 2 | L ATLE | Non-specific | * | – | + | + | – | + | – | – | + | – | – | · | · | · | · | · | · | · | · | · |
| > | × | × | × | – | × | × | – | × | × | – | · | · | · | · | · | · | · | · | · | |||
| 3 | R ATLE | CD 1 | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · |
| × | – | × | × | × | – | × | × | – | – | – | × | × | – | – | – | × | – | – | × | |||
| 4 | L Paracentral lobule | CD 2A | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · |
| – | – | × | – | – | – | – | – | – | – | – | × | – | × | – | – | – | – | – | – | |||
| 5 | R ATLE | Non-specific | – | · | + | – | – | + | – | – | – | – | – | · | · | · | · | · | · | · | · | · |
| – | · | – | – | – | × | – | – | – | – | – | · | · | · | · | · | · | · | · | · | |||
| 6 | L ATLE | CD 1C | – | – | + | + | + | – | – | – | – | – | – | + | – | – | – | – | – | – | – | – |
| × | × | × | × | × | – | – | – | – | – | – | × | × | × | × | × | × | – | × | × | |||
| 7 | R Premotor cortex | CD 2A | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · |
| – | – | – | × | – | – | – | – | – | – | – | – | × | – | – | – | – | – | – | – | |||
| 8 | L Superior parietal lobule | CD 2A | – | – | + | – | + | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| – | – | × | – | × | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | |||
| 9 | L ATLE (HC spared) | CD 1 | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · | · |
| × | · | – | × | – | – | × | – | – | × | – | – | × | – | – | × | – | – | × | – | |||
| 10 | R PCL, precuneus | CD 2B | * | · | + | + | + | + | + | + | + | – | + | + | + | + | – | + | – | – | + | – |
| > | · | × | × | × | – | – | – | – | – | – | × | × | × | – | × | – | – | × | – | |||
| 11 | R Premotor cortex | Normal | · | · | + | – | + | – | – | – | – | – | – | + | – | + | + | – | + | – | – | – |
| · | · | × | – | × | – | – | – | – | – | – | × | – | × | × | – | × | – | – | – | |||
| 12 | L TPO junction | Ischaemia | + | · | + | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| × | · | × | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | |||
| 13 | R Inferior frontal gyrus | Gliosis | · | · | + | + | + | – | – | – | – | – | – | + | + | + | – | – | – | – | – | – |
| · | · | × | × | × | – | × | × | × | – | × | × | × | × | – | × | – | – | × | – | |||
For each patient, there are two rows: upper row ICEEG concordance (bold plus symbol), lower row SU resection concordance (×, in bold), non-concordance (–). Asterisk = one of two PET abnormalities ICEEG-concordant; dot point (·) = ICEEG, PET, SPECT not done or no seizure. One of two PET abnormalities Resection-concordant is denoted by a greater than symbol (>). Note the early phase predominance of ICEEG- and Resection-concordant solutions (first third of interictal and ictal panels). Note that, unlike ESL and MSL solutions, EMSL solutions never occur as early concordant solutions in isolation; they only ever occur with ESL or MSL solutions. Refer to Table 2 (interictal), Table 3 (ictal) for ‘earliest’ solutions and ‘earliest concordant’ solutions.
ATLE = anterior temporal lobectomy; CD = cortical dysplasia; HC = hippocampus; PCL = paracentral lobule; TPO = temporo-parieto-occipital junction.
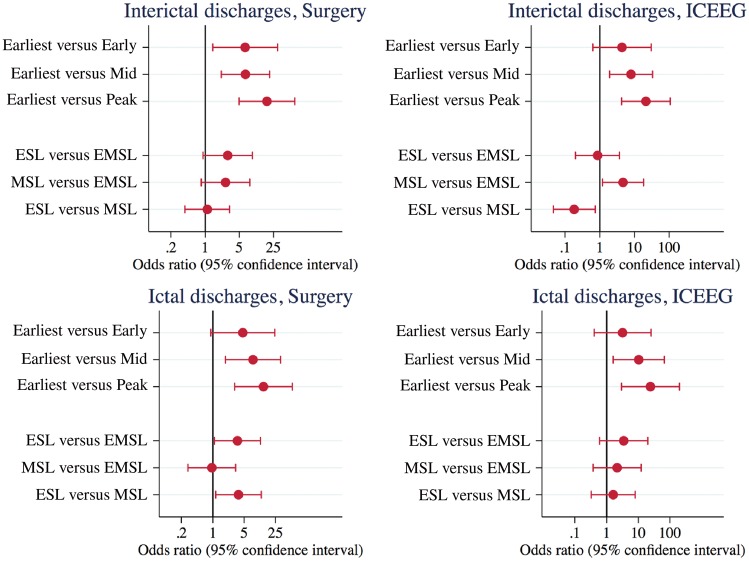
For the IED data, the earliest solution that agreed with the epileptogenic zone was ESL (three patients), MSL (four patients), and EMSL simultaneous with ESL or MSL (six patients) (Table 2). Three patients with seizure recurrence had post-operative studies with the earliest solution landing outside the resection bed but in the same location as the preoperative earliest solution (Patient 1, Fig. 3; Patient 5, Supplementary Fig. 3; and Patient 10, Supplementary Fig. 6). For the ictal data, the earliest solution that agreed with the epileptogenic zone was ESL (four patients), MSL (two patients), and EMSL simultaneous with ESL or MSL (three patients). By phase, epileptogenic zone agreement was significantly better for earliest versus all mid-phase and all late-phase solutions for IEDs and ictal discharges (ICEEG and SU), and for earliest versus early-phase IED-SU. By method, epileptogenic zone agreement was better for ESL versus EMSL for ictal-SU (OR 3.54, 95% CI 1.09–11.55, P = 0.036) and for MSL versus EMSL for IED-ICEEG (OR 4.67, 95% CI 1.19–18.34, P = 0.027). Epileptogenic zone agreement was better for ESL versus MSL for ictal-SU (OR 3.73, 95% CI 1.16–12.03, P = 0.028), but worse for ESL versus MSL for IED-ICEEG (OR 0.18, 95% CI 0.05–0.73, P = 0.017) (Fig. 2). The same OR values and CI ranges were arrived at (within 0.05 numerical increment) by the two statisticians (S.V., R.B.) using alternate methods for statistical analyses (binomial regression and binary regression). Choice of modality—HDEEG or MEG—for averaging discharges (Tables 2 and 3) on the synchronized file had no bearing on the likelihood that ESL, MSL, or EMSL returned the earliest epileptogenic zone-concordant solution (Fisher’s exact test, not shown). When the alternate modality (HDEEG or MEG) was used for averaging on the synchronized file, lead-lag relationships and current density reconstruction maps (for location, orientation) did not change, but SNR and F-statistic values were lower and MGFP take-off points were more difficult to define. Current density reconstruction maps for independent-file ESL and MSL (when all HDEEG and MEG interictal and ictal events were analysed) were the same as the respective synchronized-file ESL and MSL maps (when only events common to both modalities were analysed) except independent-file SNR and F-statistic values were marginally higher. Conductivity matching HDEEG/MEG fit factors across all patients ranged between 0.52 and 2.07 (median 1.22) for EMSL.
Figure 2.
Odds ratios and 95% confidence intervals for source solution agreement with epileptogenic zone as defined by intracranial EEG and surgical resection margins. By phase, the results indicate better epileptogenic zone agreement for earliest (as first modality early-phase solution/s) against early-phase (ictal-SU) solutions, and against all corresponding mid-phase (as 50% mean global field power or 50% upstroke phase discharge, whichever first) and late-phase (negative peak) solutions. By method, epileptogenic zone agreement was better for ESL versus EMSL and for ESL versus MSL for ictal-SU, while epileptogenic zone agreement was better for MSL versus EMSL and for MSL versus ESL for IED-ICEEG. This indicates a superiority of independent ESL plus MSL over either method alone and over combined EMSL for non-invasive epileptogenic zone characterization. EZ = epileptogenic zone; SU = surgical resection margins.
Supplementary Table 2 shows ESL, MSL, EMSL solution variables: SNR, F-distribution, and latencies for take-off, early-phase. mid-phase, and late-phase solutions (irrespective of epileptogenic zone agreement). Take-off latencies for EEG were later than EMEG (IED, ictal) and MEG (IED). Early-phase solution latencies were earliest for EMSL, but SNR values were similar across methods. Ictal F-distribution maxima were higher for ESL and EMSL. There were no differences between methods for take-off to early solution time-lags and for mid-phase and late-phase solution latencies.
Refer to Figs 3 (Patient 1) and 4 (Patient 6) for example source solution maps by method and by phase relative to ICEEG localization and surgical margins. Refer to Supplementary material for source solution images for remaining patients: Supplementary Fig. 1 (Patient 2), Supplementary Fig. 2 (Patients 3 and 4), Supplementary Fig. 3 (Patient 5), Supplementary Fig. 4 (Patients 7 and 8), Supplementary Fig. 5 (Patient 9 and 11), Supplementary Fig. 6 (Patient 10), Supplementary Fig. 7 (Patient 12 and 13).
Discussion
This long-term prospective epilepsy surgery study tests the clinical validity of synchronously-acquired high density EEG with MEG for interictal and ictal source localization of the epileptogenic zone. It is only with evidence of sustained post-operative seizure improvement that the surgical resection zone can be taken as a valid primary reference for the epileptogenic zone: here, prospectively recorded seizure-count follow-up before surgery was 3–21 months (median 12 months) and prospective follow-up after surgery was 14–39 months (median 21 months). All patients benefited from surgery, the majority (9/13) with an Engel I outcome (Table 1). Based on ordinal outcome (Engel class) and quantitative outcome (pre- versus post-operative disabling and non-disabling monthly seizure counts), we deduced that the epileptogenic zone was involved in the resection bed in all patients with major overlap in 11 patients (Engel I–II) and minor overlap in two patients (Engel III). From the intracranial EEG (ICEEG) patient data, all subjects (9/9) had at least one interictal or ictal source solution that overlapped with the intracranial electrodes defining seizure onset. ICEEG, however, should be regarded as a secondary reference for the epileptogenic zone because of its sampling bias; as only part of the cortical surface can be sampled by intracranial electrodes, seizure propagation can be misconstrued as seizure onset. This problem is highlighted by pre- and post-operative HDEEG-MEG studies on Patients 1 (Fig. 3) and 5 (Supplementary Fig. 3). For both patients, preoperative mid-phase source solutions agreed with the ICEEG and fell within the subsequent surgical resection margins but early-phase solutions were outside the grid margins (Patient 1 ESL, EMSL, Fig. 3) or at its posterior edge (Patient 5 MSL, EMSL, Supplementary Fig. 3) and outside the surgical margins. In each case, post-operative HDEEG-MEG for seizure recurrence recorded the same spike complex morphology and gave the same early-phase localization as the preoperative result (Patient 1 left inferior frontal gyrus, Patient 5 right superior temporal gyrus). This supports the hypothesis that it is early-phase—not mid-phase—source localization that more accurately captures the epileptogenic zone, which we argue was not fully covered by the ICEEG grid in both cases. The long-term surgical benefit to both patients does not invalidate this argument—incomplete seizure freedom has followed incomplete epileptogenic zone resection (indeed Patient 1 had cortical dysplasia involving resection margins, Table 4).
By quantifying the temporal relationship between HDEEG and MEG for single IEDs (when seen by both modalities), it is clear that—for both take-off and negative-peak latencies—corresponding HDEEG and MEG time points are not routinely simultaneous and there is a lead-lag relationship in the order of tens of milliseconds (Supplementary Table 1). The corresponding spatial relationship between HDEEG and MEG source solutions indicates that source propagation has typically occurred within this time-frame. For example, the early-phase ESL ictal solution at the left anterior temporal pole for Patient 9 preceded the early-phase MSL solution at the left superior temporal gyrus by 25 ms and the patient is seizure free OFF medication 22 months after a resection that did not include the MSL solution (histology confirmed type 1 cortical dysplasia with clear margins) (Table 4 and Supplementary Fig. 5A). In most patients, this lead-lag relationship is bidirectional (Supplementary Table 1); for a series of single IEDs in a given patient, sometimes HDEEG take-off is first, sometimes MEG is first. The exceptions for take-off are Patients 3 (Supplementary Fig. 2A) and 11 (Supplementary Fig. 5B) when HDEEG never precedes MEG, and Patient 9 when MEG never precedes HDEEG (Supplementary Table 1, figure not shown). The exceptions for negative-peak are Patients 3 (Supplementary Fig. 2A) and 11 (Supplementary Fig. 5B) when HDEEG never precedes MEG (Supplementary Table 1). Despite this within-patient lead-lag variability, it is noteworthy that when single IEDs are averaged for source localization (whether by HDEEG or MEG peak-alignment), the earliest solution that agrees with the epileptogenic zone (ICEEG or SU) typically belongs to the modality that leads for a majority of single IED take-offs (Table 2 and Supplementary Table 1). For example, Patient 6 has 8/10 MEG-led single IED take-offs and the earliest epileptogenic zone-concordant source solution (based on the averaged IED generated by HDEEG-peak alignment) is given by MSL and EMSL (33 ms earlier than ESL) (Table 2 and Supplementary Table 1); and Patient 10 has 19/41 HDEEG-led and 12/41 MEG-led single IED take-offs and the earliest epileptogenic zone-concordant source solution (based on the averaged IED generated by MEG-peak alignment) is given by ESL and EMSL (5 ms earlier than MSL) (Table 2 and Supplementary Table 1).
The unexpectedly large number of seizures captured across the 1-h recordings (33 from 11/13 patients) reflects the severity of the epilepsy suffered by the patients; 9/11 patients had more than one focal seizure during the recording (Patients 8 and 11 had single seizures). A majority (9/11 patients) had a localizable early ictal rhythm that agreed with the epileptogenic zone (Patient 8 non-localizing, Patient 12 ICEEG/SU discordant). From the patients who had more than one seizure that was localizable (by ESL 75%, MSL 54%, EMSL 76%), within-patient ictal current density reconstruction maps overlapped at F-maxima locations. With two exceptions (Patients 1 and 3), the modality that source localized the earliest IED activity also source localized the earliest ictal activity to the epileptogenic zone. While spike and seizure ‘zones’ are traditionally mapped as different spatial extensions of the epileptogenic zone (Rosenow and Luders, 2001), our results indicate that non-invasive HDEEG-MEG source localization of the earliest components of interictal and ictal discharges can give rise to interictal and ictal source maps that are more alike than different and that are more proximal to the epileptogenic zone than extensions of it. Our results are consistent with an earlier observation that the seizure onset zone defined by ICEEG is more accurately identified by synchronously-acquired scalp EEG potentials around spike onset and not around spike peak (Ray et al., 2007) and with more recent work suggesting that scalp EEG potentials around ictal onset can source localize the ICEEG-defined seizure onset zone (providing HDEEG is used along-with an inferior temporal array) (Nemtsas et al., 2017). This is reiterated by our consistent finding for both ictal and interictal discharges that the earliest source solution (to explain a minimum 90% signal variance)—whether given by ESL, MSL, or EMSL—was more likely than corresponding mid-phase and late-phase solutions to agree with the epileptogenic zone (as referenced by surgical margins and ICEEG seizure onset) (Fig. 2 and Table 4). Further, the earliest solution (as the first-occurring early-phase solution) was also more likely than corresponding alternate modality early-phase solutions to agree with the epileptogenic zone for interictal discharges (as referenced by surgical margins) (Fig. 2). The superiority of the ‘earliest localizable’ over ‘mid-phase localized’ solutions challenges a common default practice of source modelling at the 50% up-swing phase (Megevand and Seeck, 2018) of interictal discharges. The mid-upstroke has been regarded as a modelling ‘sweet spot’ because, it is argued, source analysis is less prone to both noise contamination around discharge take-off and to cortical propagation effects around the negative-peak. This finding was based on EEG-only data sampled at a lower frequency with fewer discharges used for signal averaging (Lantz et al., 2003b) compared to our study. Because we recorded HDEEG and MEG IEDs that were relatively complex with polyphasic components preceding a dominant negative-peak (examples are Patients 3, 7 and 8 in Supplementary Figs 2A, 4A, and B, respectively), a feature of IEDs described in cortical dysplasia (Noachtar et al., 2008), we broadened the definition of ‘mid-phase’ to include the 50%-MGFP (mean global field power) latency if it preceded the dominant spike 50% upswing. We argue this lends weight to the significance of our findings because earliest localizable source solutions were measured against ‘earliest mid-phase’ localized solutions. Similarly, our results cast doubt on the reliability of studies that lack long-term post-operative follow-up when source modelling epochs include the discharge peak (ostensibly to optimize the SNR) (Jin et al., 2007; Ossenblok et al., 2007; Tanaka et al., 2010; Wennberg et al., 2011; Pellegrino et al., 2016) when there is even greater risk that source solutions are further removed from the epileptogenic zone due to signal propagation. This is particularly relevant to the rapid propagation seen in extra-temporal neocortical epilepsy and applies to eight patients in our study, three of whom had large lesions (Patients 11–13 in Supplementary Fig. 5B, 7A and B, respectively). Previously denied surgery, these complex-lesional patients underwent ICEEG based on the localization given by early MSL (Patient 12), early MSL, EMSL (Patient 11), and early ESL, MSL, EMSL (Patient 13) before mid-phase and late-phase discharge propagation was evidenced across larger parts of the lesion; extensive ICEEG grids validated the early source solution in each case.
Our findings also challenge two common views: (i) spike yields are higher in MEG; and (ii) MEG is blind to deep cortical sources. First, several synchronized EEG-MEG studies have found that MEG sees a higher proportion of interictal discharges than EEG (Zijlmans et al., 2002; Lin et al., 2003; Iwasaki et al., 2005; Knake et al., 2006; Jin et al., 2007; Ossenblok et al., 2007; Heers et al., 2010). We found the opposite applied to both interictal and ictal discharges; of 1474 recorded IEDs, 42% were seen only by HDEEG, 16% seen only by MEG, 42% seen by both; of 33 seizures, 21% were seen only by HDEEG, 3% seen only by MEG, 76% seen by both (Tables 2 and 3). One explanation for the discrepancy is that some studies used lower density EEG (Lin et al., 2003; Iwasaki et al., 2005; Jin et al., 2007; Heers et al., 2010; Pellegrino et al., 2016) or a standard EEG cap with electrodes from the ‘ears-up’ (Knake et al., 2006; Tanaka et al., 2010). While our findings for relative HDEEG and MEG discharge detection await replication in a larger prospective study encompassing a broader spectrum of epilepsy-severity (all patients in our study had severe refractory disease), our observations held across two different HDEEG acquisition systems (ANT ASAlab®, Enschede; Compumedics SynampRT®, Melbourne). Moreover, our findings are consistent with a recently published large retrospective study (Ebersole and Wagner, 2018) that analysed 656 spike types from 270 patients; comparable spike yield ratios were found (36% seen only by EEG, 8% seen only by MEG, 56% seen by both). Despite the use of a 25-electrode set-up (against 306 MEG sensors), the authors argued that the inclusion of a six-electrode inferior temporal array was a key factor in improving the EEG spike yield. (They also point to the possible over-inflation of MEG spike tallies in previous studies by the inclusion of benign epileptiform transients, which are less well characterized in the MEG literature.) Second, several reports argue that MEG is blind to deep basal cortical sources, particularly mesial temporal sources (Baumgartner et al., 2000; Shigeto et al., 2002; Leijten et al., 2003; Agirre-Arrizubieta et al., 2009; Wennberg et al., 2011). Our results, validated by ICEEG and prospective long-term pre- and post-operative follow-up, discredit this position. Early-phase MSL consistently identified mesial temporal source onset in two patients: Patient 2 with bilateral mesial temporal discharges (Supplementary Fig. 1); and Patient 6 with left mesial temporal discharges (Fig. 4); and basal temporal source onset in a third patient (Patient 3 Supplementary Fig. 2A). The clearest evidence is given by Patient 6. Originally planned for a left antero-lateral temporal grid based on PET, video EEG monitoring, SPECT (Table 1), early-phase ictal (Fig. 4) and interictal (not shown) MSL identified a baso-mesial source that changed the pre-surgical plan with the decision to include an ipsilateral hippocampal depth electrode (Fig. 4B). This decision proved to be correct because ICEEG captured a seizure that began at the anterior tip of the hippocampal electrode and propagated 30 s later to the inferior margin of the lateral temporal grid (Fig. 4B). Left anterior temporal lobectomy with inclusion of mesial structures (entorhinal cortex type 1C dysplasia, Table 4) has given seizure freedom to 20 months follow-up (Table 1). Previous studies that argue against MEG detection of mesial temporal source activity have used fewer sensors and source localization epochs included the spike peak (Baumgartner et al., 2000; Shigeto et al., 2002; Leijten et al., 2003; Agirre-Arrizubieta et al., 2009; Wennberg et al., 2011). Interrogation of the earliest components of the interictal or ictal signal was also assisted in our study by the averaging of multiple monomorphic discharges (for shape and field topography) to heighten the SNR. One previous study on a group of patients with hippocampal sclerosis (Kaiboriboon et al., 2010) also found that MEG could see mesial temporal source activity but the results were inconsistent and the localization did not distinguish spike onset from spike peak. Our results do not infer that MSL directly detects hippocampal discharges but rather, as has been shown with ESL (Ebersole, 2003), MSL models early propagation from the hippocampus to neighbouring mesial temporal cortex such as uncus and entorhinal cortex.
While these results support our first hypothesis—that early-phase source localization of HDEEG-MEG recorded interictal and ictal discharges is more likely to agree with the epileptogenic zone than mid-phase and late-phase solutions—they do not support our second hypothesis that spatially combined HDEEG-MEG based source analysis (EMSL) is superior to spatially independent source analysis (ESL, MSL). In fact, the opposite was evidenced. Independent HDEEG and MEG signal modelling is superior to combined signal modelling of ictal and interictal discharges. EMSL never gave the only earliest epileptogenic zone concordant solution (it always featured with ESL or MSL) while ESL and MSL stood alone as earliest epileptogenic zone concordant solutions for several patients (IEDs seven patients, ictal six patients). EMSL was outperformed by ESL for ictal source localization-surgical resection margin (ictal-SU) agreement (Fig. 2). EMSL was also outperformed by MSL for interictal source localization-intracranial EEG localization (IED-ICEEG) agreement (Fig. 2). Perhaps not surprisingly, the use of both modalities for epileptogenic zone characterization was superior to the use of either modality alone. That is, ESL outperformed MSL for ictal-SU but was outperformed by MSL for interictal-ICEEG (Fig. 2). The use of spatially combined HDEEG-MEG signals for EMSL did not elevate early-phase SNR values relative to spatially independent signal modelling (ESL, MSL) as might have been anticipated (Supplementary Table 2). Inferior EMSL localization accuracy was not explained by delayed solution latencies (take-offs and early-phase latencies were earlier against ESL) or by current density decay (F-distributed maxima were higher against MSL) (Supplementary Table 2). One explanation for the superiority of independent ESL plus MSL over combined EMSL signal modelling might lie with our first hypothesis (the significance of the earliest localisable, and not simply the early-phase solution, in flagging the epileptogenic zone) coupled with the finding of a bidirectional lead-lag relationship between HDEEG and MEG take-offs and between their corresponding early ESL and MSL solutions. That is, when HDEEG-MEG signals are spatially mixed for EMSL around take-off, and if one modality is seeing source onset more clearly than the other due to a lead-lag effect, accuracy might suffer. This hypothesis is supported by the observation that in the few instances (Patients 8 and 13 interictal) when there are three earliest solutions (when early-phase EMSL, ESL, and MSL are simultaneous), EMSL survives as the earliest epileptogenic zone-concordant solution; however, when early-phase EMSL is the earliest solution with either ESL or MSL (when the corresponding early-phase ESL or MSL solution lags), EMSL survives as the earliest epileptogenic zone-concordant solution in only 50% of instances for both ictal and interictal datasets. We suspect this is because EMSL accuracy can be hindered by the lagging modality early-phase signal (whether from HDEEG or MEG). In such instances, corresponding early-phase ESL lags by up to 43 ms (interictal), 24 ms (ictal) and early-phase MSL lags by up to 45 ms (interictal), 26 ms (ictal). Conductivity matching might also be a source of error for EMSL. While we followed the approach described by Fuchs et al. (1998b), later adapted by others (Huang et al., 2007; Choi et al., 2013), who showed the benefit of combined EEG-MEG signal modelling for boundary element method-based source localization, we did not replicate their findings. These studies, which included whole-head test-dipole simulations and somatosensory-evoked recordings, generated EEG-MEG waveforms that appear to have greater spatiotemporal overlap compared to the temporal dispersion common to our HDEEG-MEG waveforms (when combined modelling is perhaps more likely to fail). Recent work with more advanced finite element method (FEM) head-modelling, however, did support the case for combined EEG-MEG signal modelling in two epilepsy case reports (Aydin et al., 2014, 2015) when anisotropic tissue conductivity was calibrated from somatosensory-evoked EEG-MEG recordings. While conductivity matching might be more accurate with this approach for neighbourhood sources subjacent to skull vertex, these values may not translate to other areas of the head (skull base for deep temporal lobe sources) and, as the authors point out, their use of a limited inferior temporal array might have hampered ESL accuracy. Future long-term prospective studies in combined modality modelling, or EMSL, will need to accommodate HDEEG-MEG differences across spatial (whole-head conductivity) and temporal (lead-lag variability) domains for clinical validation.
A possible explanation for our finding that MSL is superior to ESL for IED source localization is that take-offs were usually earlier for MEG than HDEEG as were corresponding early-phase solutions (MSL preceded ESL in 10/13 patients). Current density reconstruction maps were also more spatially discrete for MSL compared to ESL and, apart from Patient 11, the ICEEG-defined seizure onset zone was smaller than the surgical resection zone. This ability of MEG to capture small focal cortical sources, particularly those with a dominant early tangential field as seen in sulcal dysplasia (e.g. Patients 4 and 8 in Supplementary Figs 2B and 4B, respectively), is a well-recognized advantage of the modality over EEG along-side the relative indifference of the MEG lead-field to skull impedance. Our converse finding for ictal source localization, that ESL was superior to MSL for surgical resection agreement, appears to stem from more sustained ESL solution overlap with the resection across all phases of the averaged ictal discharge (early, mid, late), noting though that early-phase resection overlap was seen in 7/11 patients for both ESL and MSL, and in 5/11 patients for EMSL. While it is likely that the extent of the resection zone ‘accommodated’ potentially propagated mid-phase and late-phase ictal ESL sources in some cases (Patients 1, 10 and 13 in Fig. 3, Supplementary Figs 6 and 7B, respectively), it is equally plausible that the result stems from the ability of HDEEG to capture broader source configurations with a rapidly varying admixture of radial and tangential source orientations that typify an ictal discharge. Excessive head movement with seizures in the MEG dewer may have disadvantaged ictal MSL accuracy, but we would add that, in all cases, ictal discharge onset was seen in the MEG recording several seconds before any sudden head movement occurred on real-time video, all seizures were limited to focal events (without secondary generalization), and when ictal MSL analysis was rerun with an independent head-movement correction algorithm, source results were unchanged. While there were exceptions to the above findings for ictal-ESL and interictal-MSL (Patient 11 ictal-SU overlapped with MSL, not ESL for instance, Supplementary Fig. 5B) these cases only serve, in our view, to underscore the case-by-case value of synchronously acquired HDEEG-MEG for epileptogenic zone characterization.
A limitation of this study was the number of patients followed. We point out though that this was a prospective study with long-term, methodically recorded monthly pre- and postoperative follow up; this avoids the inherent bias of retrospective studies, a bias that cannot be overcome by increasing patient numbers. While the study was limited to a 13-patient cohort, the spectrum of epilepsy was heterogeneous with emphasis on two of the most challenging patient groups (non-lesional, complex-lesional). Moreover, as the first MEG epilepsy study to be performed in Australia, clinical teams regarded the technology as untested. Our results were not routinely considered in the pre-surgical work-up, for example, Patient 1 (Fig. 3), Patient 5 (Supplementary Fig. 3), and Patient 10 (Supplementary Fig. 6) MSL results were not fully factored into ICEEG placement. This is less likely to be the case in centres where MEG has a more established role in epilepsy surgery work-up and surgical decisions (such as ICEEG placement) are more likely to be biased by knowledge of the MEG result. Because the MSL result for our last three patients (Patients 11–13) might have spared the patients such extensive grids, surgeons at our centre have since routinely considered our HDEEG-MEG results in the surgical work-up. In our view, this potentially changes the experiment; hence, statistical analyses were done up to this point. While the patient count was ultimately sufficient to detect differences between early-phase and mid/late-phase source solutions, we suspect that the study was underpowered to replicate the findings of earlier studies that demonstrate the benefit of mid-phase over late-phase source analysis. We also intended to perform postoperative studies on Patients 2 (Engel II) and 12 (Engel III), but both deferred unless seizures worsened. Another criticism is lack of power to compare source results with PET and SPECT findings; only some of the patients had these routine tests. However, the recruitment process would have biased the results against PET and SPECT because a key indication for referral was non-concordance of findings from routine clinical work-up such as SPECT and PET (Table 1). Our finding that EMSL was outperformed by ESL and MSL applies to sLORETA-boundary element method, a commonly used inverse-forward modelling source localization approach. Similar long-term prospective studies are needed to assess whether this extends to other forward modelling methods, such as finite element method, and to other inverse modelling approaches.
In conclusion, we find that non-invasive source localization for epileptogenic zone characterization is more accurate when performed independently, not combined, on HDEEG and MEG synchronously-acquired interictal and ictal discharges. ESL plus MSL is superior to either modality alone and both outperform EMSL. The earliest localizable source solution (to explain 90% signal variance from the point of averaged discharge take-off), whether it be from HDEEG or MEG, is superior to mid-phase and late-phase discharge analysis. We believe this approach significantly improves the non-invasive surgical work-up of MRI lesion-negative and complex-lesional drug-resistant focal epilepsy. Our findings also challenge current practice of source localization with its emphasis on mid-phase discharge analysis and its common inclusion of the late-peak phase of the discharge in the source solution. And, contrary to several previous reports, we have shown that early-phase MSL does reveal the capacity of MEG to see deep basal and mesial temporal discharges. We propose that prioritization of early-phase source analysis of interictal and ictal discharges will produce HDEEG-MEG source solutions that better guide and limit reliance on invasive intracranial monitoring (with its high cost and allied morbidity) in the pre-surgical work-up of these challenging patients.
Supplementary Material
Acknowledgements
The authors acknowledge the facilities, and the scientific and technical assistance of the National Imaging Facility at the Swinburne Node, Swinburne University of Technology with particular thanks to Dr Rachel Batty, Ms Mahla Cameron-Bradley, and Ms Johanna Stephens for their technical support. We acknowledge the Australian National Imaging Facility for the support of W. Woods and the MEG system at Swinburne University of Technology. We thank Ms Sara Vogrin and Prof. Ray Boston for their invaluable statistical advice and assistance. We acknowledge the doctors who referred some of the patients – Dr Simon Harvey (Royal Children’s Hospital Melbourne), Dr John Archer (Austin Hospital Melbourne), A/Prof Wendyl D’Souza (St Vincent’s Hospital Melbourne), and A/Prof Ross Carne (St Vincent’s Hospital Melbourne).
Glossary
Abbreviations
- EMSL
combined electromagnetoencephalographic source localization
- ESL
electroencephalographic source localization
- HDEEG
high density electroencephalography
- IED
interictal epileptiform discharge
- MGFP
mean global field power
- MEG
magnetoencephalography
- MSL
magnetoencephalographic source localization
- SNR
signal-to-noise ratio
- SU
surgical resection margin
Funding
C.P. was supported by an Australian National Health Medical Research Council Post-Doctoral Fellowship APP1054520.
Competing interests
The authors report no competing interest.
References
- Agirre-Arrizubieta Z, Huiskamp GJM, Ferrier CH, van Huffelen AC, Leijten FSS. Interictal magnetoencephalography and the irritative zone in the electrocorticogram. Brain 2009; 132: 3060–71. [DOI] [PubMed] [Google Scholar]
- Aydin U, Vorwerk J, Dumpelmann M, Kupper P, Kugel H, Heers M, et al. Combined EEG/MEG can outperform single modality EEG or MEG source reconstruction in presurgical epilepsy diagnosis. PLoS One 2015; 10: e0118753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydin U, Vorwerk J, Kupper P, Heers M, Kugel H, Galka A, et al. Combining EEG and MEG for the reconstruction of epileptic activity using a calibrated realistic volume conductor model. PLoS One 2014; 9: e93154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner C, Pataraia E, Lindinger G, Deecke L. Neuromagnetic recordings in temporal lobe epilepsy. J Clin Neurophysiol 2000; 17: 177–89. [DOI] [PubMed] [Google Scholar]
- Choi JH, Jung YJ, Jung HK, Im CH. A new multimodal cortical source imaging algorithm for integrating simultaneously recorded EEG and MEG. Inverse Probl Sci Eng 2013; 21: 1074–89. [Google Scholar]
- Ebersole JS. EEG voltage topography and dipole source modeling of epileptiform potentials. In: Ebersole JS, Pedley TA, editors. Current practice of clinical electroencephalography. Philadelphia: Lippincott Williams and Wilkins; 2003. p. 732–52. [Google Scholar]
- Ebersole JS, Ebersole SM. Combining MEG and EEG Source modeling in epilepsy evaluations. J Clin Neurophysiol 2010; 27: 360–71. [DOI] [PubMed] [Google Scholar]
- Ebersole JS, Wagner M. Relative yield of MEG and EEG spikes in simultaneous recordings. J Clin Neurophysiol 2018; 35: 443–53. [DOI] [PubMed] [Google Scholar]
- Fuchs M, Drenckhahn R, Wischmann HA, Wagner M. An improved boundary element method for realistic volume-conductor modeling. IEEE Trans Biomed Eng 1998a; 45: 980–97. [DOI] [PubMed] [Google Scholar]
- Fuchs M, Wagner M, Wischmann HA, Kohler T, Theissen A, Drenckhahn R, et al. Improving source reconstructions by combining bioelectric and biomagnetic data. Electroencephalogr Clin Neurophysiol 1998b; 107: 93–111. [DOI] [PubMed] [Google Scholar]
- Heers M, Rampp S, Kaltenhaeuser M, Pauli E, Rauch C, Doelken MT, et al. Detection of epileptic spikes by magnetoencephalography and electroencephalography after sleep deprivation. Seizure 2010; 19: 397–403. [DOI] [PubMed] [Google Scholar]
- Huang MX, Song T, Hagler DJ Jr, Podgorny I, Jousmaki V, Cui L, et al. A novel integrated MEG and EEG analysis method for dipolar sources. Neuroimage 2007; 37: 731–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki M, Pestana E, Burgess RC, Luders HO, Shamoto T, Nakasato T. Detection of epileptiform activity by human interpreters: blinded comparison between electroencephalography and magnetoencephalography. Epilepsia 2005; 46: 59–68. [DOI] [PubMed] [Google Scholar]
- Jin K, Nakasato N, Shamoto H, Kanno A, Itoyama Y, Tominaga T. Neuromagnetic localization of spike sources in perilesional, contralateral mirror, and ipsilateral remote areas in patients with cavernoma. Epilepsia 2007; 48: 2160–6. [DOI] [PubMed] [Google Scholar]
- Kaiboriboon K, Nagarajan S, Mantle M, Kirsch HE. Interictal MEG/MSI in intractable mesial temporal lobe epilepsy: spike yield and characterization. Clin Neurophysiol 2010; 121: 325–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knake S, Halgren E, Shiraishi H, Hara K, Hamer HM, Grant PE, et al. The value of multichannel MEG and EEG in the presurgical evaluation of 70 epilepsy patients. Epilepsy Res 2006; 69: 80–6. [DOI] [PubMed] [Google Scholar]
- Lantz G, de Peralta RG, Spinelli L, Seeck M, Michel CM. Epileptic source localization with high density EEG: how many electrodes are needed? Clin Neurophysiol 2003a; 114: 63–9. [DOI] [PubMed] [Google Scholar]
- Lantz G, Spinelli L, Seeck M, Menendez RGD, Sottas CC, Michel CM. Propagation of interictal epileptiform activity can lead to erroneous source localizations: a 128-channel EEG mapping study. J Clin Neurophysiol 2003b; 20: 311–9. [DOI] [PubMed] [Google Scholar]
- Leijten FSS, Huiskamp GJM, Hilgersom I, van Huffelen AC. High-resolution source imaging in mesiotemporal lobe epilepsy: a comparison between MEG and simultaneous EEG. J Clin Neurophysiol 2003; 20: 227–38. [DOI] [PubMed] [Google Scholar]
- Lin YY, Shih YH, Hsieh JC, Yu HY, Yiu CH, Wong TT, et al. Magnetoencephalographic yield of interictal spikes in temporal lobe epilepsy—comparison with scalp EEG recordings. Neuroimage 2003; 19: 1115–26. [DOI] [PubMed] [Google Scholar]
- Megevand P, Seeck M. Electroencephalography, magnetoencephalography and source localization: their value in epilepsy. Curr Opin Neurol 2018; 31: 176–83. [DOI] [PubMed] [Google Scholar]
- Merlet I, Paetau R, GarciaLarrea L, Uutela K, Granstrom ML, Mauguiere F. Apparent asynchrony between interictal electric and magnetic spikes. Neuroreport 1997; 8: 1071–6. [DOI] [PubMed] [Google Scholar]
- Nemtsas P, Birot G, Pittau F, Michel CM, Schaller K, Vulliemoz S, et al. Source localization of ictal epileptic activity based on high-density scalp EEG data. Epilepsia 2017; 58: 1027–36. [DOI] [PubMed] [Google Scholar]
- Noachtar S, Bilgin O, Remi J, Chang N, Midi I, Vollmar C, et al. Interictal regional polyspikes in noninvasive EEG suggest cortical dysplasia as etiology of focal epilepsies. Epilepsia 2008; 49: 1011–7. [DOI] [PubMed] [Google Scholar]
- Ochi A, Otsubo H, Sharma R, Hunjan A, Rutka JT, Chuang SH, et al. Comparison of electroencephalographic dipoles of interictal spikes from prolonged scalp video-electroencephalography and magnetoencephalographic dipoles from short-term recording in children with extratemporal lobe epilepsy. J Child Neurol 2001; 16: 661–7. [DOI] [PubMed] [Google Scholar]
- Ossenblok P, de Munck JC, Colon A, Drolsbach W, Boon P. Magnetoencephalography is more successful for screening and localizing frontal lobe epilepsy than electroencephalography. Epilepsia 2007; 48: 2139–49. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui RD. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find Exp Clin Pharmacol 2002; 24 (Suppl D): 5–12. [PubMed] [Google Scholar]
- Pellegrino G, Hedrich T, Chowdhury R, Hall JA, Lina JM, Dubeau F, et al. Source localization of the seizure onset zone from ictal EEG/MEG data. Hum Brain Mapp 2016; 37: 2528–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A, Tao JX, Hawes-Ebersole SM, Ebersole JS. Localizing value of scalp EEG spikes: a simultaneous scalp and intracranial study. Clin Neurophysiol 2007; 118: 69–79. [DOI] [PubMed] [Google Scholar]
- Rosenow F, Luders H. Presurgical evaluation of epilepsy. Brain 2001; 124 (Pt 9): 1683–700. [DOI] [PubMed] [Google Scholar]
- Scheler G, Fischer MJM, Genow A, Hummel C, Rampp S, Paulini A, et al. Spatial relationship of source localizations in patients with focal epilepsy: comparison of MEG and EEG with a three spherical shells and a boundary element volume conductor model. Hum Brain Mapp 2007; 28: 315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeto H, Morioka T, Hisada K, Nishio S, Ishibashi H, Kira D, et al. Feasibility and limitations of magnetoencephalographic detection of epileptic discharges: simultaneous recording of magnetic fields and electrocorticography. Neurol Res 2002; 24: 531–6. [DOI] [PubMed] [Google Scholar]
- So EL, Ryvlin P. Scope and implications of MRI-negative refractory focal epilepsy. In: So EL, Ryvlin P, editors. MRI-negative epilepsy: evaluation and surgical management. Cambridge: Cambridge University Press; 2015. p. 1–5. [Google Scholar]
- Song J, Davey C, Poulsen C, Luu P, Turovets S, Anderson E, et al. EEG source localization: sensor density and head surface coverage. J Neurosci Methods 2015; 256: 9–21. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Hamalainen M, Ahlfors SP, Liu H, Madsen JR, Bourgeois BF, et al. Propagation of epileptic spikes reconstructed from spatiotemporal magnetoencephalographic and electroencephalographic source analysis. Neuroimage 2010; 50: 217–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennberg R, Valiante T, Cheyne D. EEG and MEG in mesial temporal lobe epilepsy: where do the spikes really come from? Clin Neurophysiol 2011; 122: 1295–313. [DOI] [PubMed] [Google Scholar]
- Zijlmans M, Huiskamp GM, Leijten FSS, van der Meij WM, Wieneke G, van Huffelen AC. Modality-specific spike identification in simultaneous magnetoencephalography/electroencephalography—a methodological approach. J Clin Neurophysiol 2002; 19: 183–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available on request including raw HDEEG and MEG independent file and synchronized file recordings, results for Fischer’s exact test on the potential confounding relationship between modality used for averaging and the method returning the earliest source solution (no significant effect seen), and the following images omitted due to submission restrictions for Supplementary material (Patient 1 ictal, Patient 3 ictal, Patient 4 ictal, Patient 6 interictal, Patient 7 ictal, Patient 9 interictal, Patient 10 interictal, Patient 11 ictal, Patient 12 ictal, Patient 13 interictal); however, these images are described in the figure legend for each patient’s corresponding interictal/ictal displayed result with the related data given in Tables 2–4 and Supplementary Table 1.
Table 2.
Interictal data and earliest solution source localization results
| Patient | IEDs | IED lead time | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total, n | EEG only, n | MEG only, n | Both, n | Peaks averaged, n (modalitya) | Earliest source solution | Earliest EZ agreement | EEG take-off to early ESL, ms | Earliest source to ESL, ms | MEG take-off to early MSL, ms | Earliest source to MSL, ms | EMEG take-off to early EMSL, ms | Earliest source to EMSL, ms | |
| Preoperative | |||||||||||||
| 1 | 176 | 66 | 40 | 70 | 110 (MEG) | ESL, EMSL | ESL | 5 | 0 | 10 | 45 | 5 | 0 |
| 2 | 11 | 0 | 0 | 11 | 8 (EEG) | MSL | MSL | 8 | 4 | 14 | 0 | 15 | 1 |
| 3 | 63 | 17 | 37 | 9 | 46 (MEG) | MSL, EMSL | MSL, EMSL | 7 | 22 | 6 | 0 | 6 | 0 |
| 4 | 98 | 35 | 23 | 40 | 63 (MEG) | MSL, EMSL | MSL | 4 | 15 | 6 | 0 | 6 | 0 |
| 5 | 34 | 11 | 6 | 17 | 23 (MEG) | MSL, EMSL | MSL | 0 | 43 | 6 | 0 | 6 | 0 |
| 6 | 36 | 16 | 10 | 10 | 26 (EEG) | MSL, EMSL | MSL, EMSL | 9 | 33 | 0 | 0 | 0 | 0 |
| 7 | 279 | 234 | 7 | 38 | 272 (EEG) | MSL | ESL | 12 | 12 | 5 | 0 | 8 | 3 |
| 8 | 87 | 30 | 42 | 15 | 57 (MEG) | MSL, ESL, EMSL | MSL, EMSL | 0 | 0 | 0 | 0 | 0 | 0 |
| 9 | 84 | 50 | 8 | 26 | 76 (EEG) | ESL | ESL | 4 | 0 | 13 | 1 | 14 | 2 |
| 10 | 122 | 70 | 11 | 41 | 111 (EEG) | ESL, EMSL | ESL, EMSL | 0 | 0 | 5 | 5 | 0 | 0 |
| 11 | 133 | 0 | 2 | 131 | 131 (EEG) | MSL, EMSL | MSL, EMSL | 1 | 23 | 3 | 0 | 3 | 0 |
| 12 | 181 | 94 | 46 | 41 | 135 (EEG) | MSL, EMSL | MSL | 14 | 14 | 2 | 0 | 2 | 0 |
| 13 | 170 | 3 | 0 | 167 | 170 (EEG) | MSL,ESL, EMSL | MSL, ESL, EMSL | 24 | 0 | 24 | 0 | 24 | 0 |
| All | 1474 | 626 (42%) | 232 (16%) | 616 (42%) | Median | 5 | 12 | 6 | 0 | 6 | 0 | ||
| Range | 0–24 | 0–43 | 0–24 | 0–45 | 0–24 | 0–3 | |||||||
| Postoperative | |||||||||||||
| 1 | 151 | 10 | 21 | 120 | 130 (EEG) | MSL | 10 | 29 | 5 | 0 | 5 | 0 | |
| 5 | 92 | 64 | 6 | 22 | 28 (MEG) | ESL, EMSL | 0 | 0 | 0 | 5 | 0 | 0 | |
| 10 | 155 | 41 | 40 | 74 | 114 (MEG) | ESL | 0 | 0 | 3 | 8 | 7 | 7 | |
A higher proportion of discharges were seen in HDEEG alone against MEG alone. EMSL solutions that agreed with the epileptogenic zone (based on ICEEG or SU concordance) never occurred in isolation as opposed to ESL (three patients) and MSL (four patients).
aModality (HDEEG or MEG) chosen for averaging was based on relative discharge count and SNR maxima. Note that the modality used for averaging had no bearing on the modality that gave rise to the earliest source solution (Fisher’s exact test, not shown).