Abstract
Liver recovery after hepatic ischemia-reperfusion (I/R) injury is characterized by clearance of dead tissue and its replacement with functional liver parenchyma. Previous reports have observed fibrosis after liver I/R. To determine whether liver fibrosis after I/R was a pathologic consequence of the injury response, we assessed the development of liver fibrosis after I/R and its impact on subsequent insult. A murine model of partial I/R was used to induce liver injury and study the reparative response. During liver remodeling after I/R, expression of the profibrotic genes increased in the ischemic liver. Histologically, α-smooth muscle actin (α-SMA)-positive hepatic stellate cells (HSCs)/myofibroblasts increased, and collagen deposition was enhanced along the injured site. Selective staining experiments showed that HSCs, not portal fibroblasts, were the major source of myofibroblasts. During liver repair after I/R, liver fibrosis was readily observed at the interface between necrotic tissue and regenerating liver in association with HSCs/myofibroblasts. The number of HSCs/myofibroblasts decreasing shortly after the full resolution of necrotic injury and restoration are normal liver architecture. However, liver fibrosis persisted for several more weeks before gradually resolving. Resolution of liver fibrosis was accompanied by upregulated expression of matrix metalloproteinase-13. After resolution of fibrosis, the administration of CCl4 did not result in exacerbated liver injury, suggesting that I/R injury does not predispose the liver to future fibrotic insults. The data suggest that liver fibrosis is a component of tissue repair after I/R, is caused by myofibroblasts derived from HSC, and does not increase susceptibility of the liver to subsequent hepatic injury.
NEW & NOTEWORTHY This study is the first to assess pathology of liver fibrosis during the reparative process after ischemia-reperfusion (I/R) injury. Here we show that profibrotic gene expression increased in the liver after I/R, and collagen accumulation produced by hepatic stellate cells (HSCs)/myofibroblasts enhanced at the interface between necrotic tissue and regenerating liver. Liver fibrosis gradually resolved concomitant with decreasing activation of HSC and upregulating matrix metalloproteinase-13. After resolution of fibrosis, the liver was not more susceptible to subsequent hepatic injury.
Keywords: hepatic stellate cells, liver fibrosis, liver regeneration, liver repair
INTRODUCTION
Hepatic ischemia-reperfusion (I/R) injury occurs during liver resection surgery, liver transplantation, and trauma and contributes to significant morbidity and mortality (8, 16). During ischemia, interruption of the blood flow to the liver causes oxidative stress that upon reperfusion triggers a cascade of proinflammatory mediators that culminates in the recruitment of leukocytes. Reactive oxygen species and proteases from neutrophils are the main causes of hepatocyte damage, which results in acute liver injury (13). Although the mechanisms leading to I/R injury have been studied for decades and are largely known, far less is understood about the mechanism by which the liver repairs itself, through tissue remodeling and regeneration, to clear the damaged and/or dead tissue and replace it with functional liver mass. Understanding these processes may allow for development of therapeutics that can accelerate liver repair after acute liver injuries.
Hepatic stellate cells (HSCs) are located in the space of Disse and are quiescent in the normal liver. In response to injury, HSCs differentiate into myofibroblasts and serve a variety of functions depending on the insult. In models of hepatectomy, HSCs contribute to liver regeneration through the release of hepatic growth factor and transforming growth factor-β (TGF-β) (20), whereas, in models of chronic liver injury, HSCs are the primary contributor to liver fibrosis (19). In liver I/R injury, HSCs appear to promote the inflammatory response by increasing proinflammatory mediator production (23). The role of HSCs in liver repair and regeneration after I/R injury is not well understood. Our laboratory recently reported that, after the injury response to liver I/R, there is significant HSC proliferation, and they locate spatially in close association with Kupffer cells and infiltrating macrophages at the interface with necrotic tissue (14). Those observations suggest that activated HSCs/myofibroblasts contribute to liver regeneration by working in concert with macrophages to clear necrotic cells and tissue debris (macrophages) and promoting tissue remodeling by producing extracellular matrix (HSCs/myofibroblasts). However, if not properly regulated, this process may lead to fibrosis and scar formation. For example, in chronic liver injury, HSCs contribute to fibrosis, which exacerbates liver dysfunction and can progress to cirrhosis and hepatocellular carcinoma. Liver fibrosis has been investigated mainly in chronic liver injury models; however, little is known about fibrosis that may occur after acute liver injury, such as caused by I/R. In this study, we investigated whether the activation of HSCs that occurs after I/R injury results in liver fibrosis and whether any fibrotic scar tissue predisposes the liver to injury from a subsequent insult.
MATERIALS AND METHODS
Animals.
Male Balb/c mice were purchased from Jackson Laboratory (Bar Harbor, ME). Mice used for these experiments were 8–12 wk of age. This project was approved by the University of Cincinnati Animal Care and Use Committee and was in compliance with the National Institutes of Health guidelines.
Hepatic I/R injury model.
Partial hepatic ischemia was performed as described previously (17). Briefly, mice were anesthetized with sodium pentobarbital (60 mg/kg ip). A midline laparotomy was performed, and an atraumatic clip was used to interrupt blood supply to the left lateral and median lobes of the liver. After 45 or 90 min of partial hepatic ischemia, the clip was removed to initiate hepatic reperfusion. Mice were euthanized after the indicated periods of reperfusion, and blood and samples of ischemic lobe of the liver were taken for analysis. In some experiments, mice were injected intraperitoneally with vehicle (10% DMSO in PBS) or 0.75 mg/kg gliotoxin 48, 72, and 96 h after reperfusion. In other experiments, mice were injected intraperitoneally with 1 ml/kg CCl4 (Sigma-Aldrich, St Louis, MO) diluted in three volumes of mineral oil twice weekly after 2 wk or 8 wk of I/R. Control (sham operated) mice underwent the same protocol without vascular occlusion.
Tissue analyses.
Liver tissues were fixed in 10% neutral-buffered formalin, processed, and then embedded in paraffin for light microscopy. Sections were stained with hematoxylin-eosin for histological examination and processed further for immunostaining. Sections were also stained with Sirius red for determination of collagen deposition. Immunohistochemical staining was performed as described previously (15). The antibodies were as follows: α-smooth muscle actin (α-SMA) (ab5694; Abcam, Cambridge, MA), desmin (ab15200; Abcam), Thy1 (ab92574; Abcam). Quantitative morphometric analysis of staining was performed with histological sections at low power (×50) using ImageJ (NIH, Bethesda, MD). Necrosis area, fibrosis area by Sirius red staining, α-SMA staining area, and desmin staining area were expressed as percentages of total area examined as described previously (2).
RT-PCR analysis.
Total RNA was extracted from liver tissue using TRIzol (Invitrogen, Carlsbad, CA). cDNA was generated through the reverse transcription of 2 μg of RNA using High-Capacity cDNA Reverse-Transcription kits (Applied Biosystems, Foster City, CA). Samples were incubated at 25°C for 10 min, 37°C for 120 min, and 85°C for 5 min to inactive the reverse transcriptase and then cooled at 5°C for 5 min. Four microliters of diluted cDNA samples were used for quantitative two-step PCR using SYBR Green PCR Master Mix (Applied Biosystems). Each sample was analyzed in duplicate. 18S was used as a housekeeping control gene. Threshold cycles were automatically calculated by the iCycler iQ Real-Time Detection System. Threshold cycle values were normalized to the housekeeping control (18S) to give relative genomic equivalence. Primers utilized were as follows: α-SMA: sense GTCCCAGACATCAGGGAGTAA, antisense TCGGATACTTCAGCGTCAGGA; collagen-1α1: sense GAGCGGAGAGTACTGGATCG, antisense GCTTCTTTTCCTTGGGGTTC; tissue inhibitor of metalloproteinase-1 (TIMP-1): sense CCTTGCAAACTGGAGAGTGACA, antisense AAGCAAAGTGACGGCTCTGGT; TNF-α: sense TCGTAGCAAACCACCAAGTG, antisense AGATAGCAAATCGGCTGACG; 18S: sense AGTCCCTGCCCTTTGTACACA, antisense GATCCGAGGGCCTCAAAC.
Western blot analyses.
Liver samples were homogenized in lysis buffer consisting of 10 mM HEPES (pH 7.9), 150 mM NaCl, 1 mM EDTA, 0.6% NP-40, 0.5 mM PMSF, 1 μg/ml leupeptin, 1 μg/ml aprotinin, 10 μg/ml soybean trypsin inhibitor, 1 μg/ml pepstatin, and 1% phosphatase inhibitor cocktail 2 (from Sigma-Aldrich) on ice. Homogenates were centrifuged at 10,000 rpm to remove cellular debris. Protein concentrations of each sample were determined using BCA protein assay kit (Pierce/ThermoFisher, Rockford, IL). Samples containing equal amounts of protein were resuspended in 5× SDS sample buffer and separated in a denaturing 7.5–10% polyacrylamide gel and transferred to a 0.1-μm pore polyvinylidene difluoride membrane. Nonspecific binding sites were blocked with Tris-buffered saline (40 mM Tris, pH 7.6, 300 mM NaCl) with 0.1% Tween 20 containing 5% nonfat dry milk for 1 h at room temperature. Membranes were then incubated with antibodies to matrix metalloproteinase 2 (MMP-2) (ab37150; Abcam), MMP-9 (ab38898; Abcam), MMP-13 (ab39012; Abcam), β-actin (ab8227; Abcam) in Tris-buffered saline with 0.1% Tween 20. Membranes were washed and incubated with secondary antibodies conjugated to horseradish peroxidase. Immunoreactive proteins were detected by enhanced chemiluminescence.
Blood analysis.
Blood was obtained by cardiac puncture for analysis of serum alanine aminotransferase (ALT) as an index of hepatocellular injury. Measurements of serum ALT were made using a diagnosis kit by bioassay (Wiener Laboratories, Rosario, Argentina) according to the manufacturer’s instructions.
Statistical analysis.
All data are expressed as the means ± SE. Data were analyzed with a one-way ANOVA with subsequent Student’s t-test. Differences were considered significant when P < 0.05.
RESULTS
Liver fibrosis after I/R.
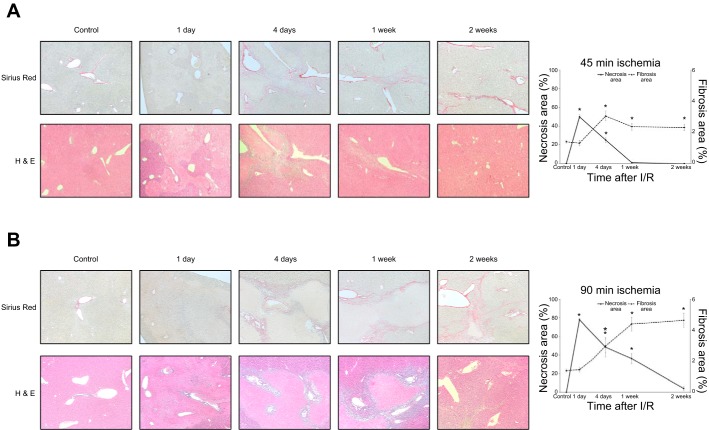
We previously reported that the expression of PDGF-BB, TGF-β, and connective tissue growth factor (CTGF) were increased in the liver after I/R injury (14). These cytokines are mitogens for HSCs and potent fibrogenic signals. To assess liver fibrosis after I/R injury, we measured collagen deposition by Sirius red staining in liver sections from mice undergoing 45 or 90 min of ischemia followed by reperfusion. In both ischemia models, we observed marked collagen deposition 4 days after I/R, which increased significantly over the subsequent 2 wk (Fig. 1, A and B). However, mice undergoing 90 min of ischemia displayed a higher amount of collagen deposition after 1 wk than mice undergoing 45 min of ischemia, suggesting that increased periods of ischemia result in greater liver fibrosis. We also assessed hepatocellular necrosis and, as expected, found massive necrosis 1 day after I/R, which gradually resolved over time (Fig. 1, A and B). We quantitated necrotic and fibrotic areas and noted an inverse relationship between necrotic area and extent of liver fibrosis, particularly in mice undergoing 90 min of ischemia (Fig. 1B). Interestingly, liver architecture was largely normal 2 wk after 90 min of I/R despite the presence of significant liver fibrosis, suggesting an uncoupling of HSC activation from tissue homeostasis. Because the I/R model using 90 min of ischemia resulted in more robust injury and fibrotic responses, we used this model exclusively for subsequent experiments.
Fig. 1.
Development of liver fibrosis after ischemia-reperfusion (I/R) injury. A: liver sections from mice undergoing 45-min ischemia followed by reperfusion were analyzed. Sirius red staining of liver sections shows increased collagen deposition 4 days after I/R injury and maintaining a similar level of fibrosis up to 2 wk after I/R. Hematoxylin-eosin staining shows significant liver injury 1 day after reperfusion with repair and resolution of injury to nearly normal liver architecture by 1 wk after reperfusion. Original magnification is ×100. B: liver sections from mice undergoing 90-min ischemia followed by reperfusion were analyzed. Sirius red staining of liver sections shows collagen deposition within 4 days after I/R injury and increasing fibrosis during the time of liver repair (up to 2 wk after I/R). Hematoxylin-eosin staining shows massive liver injury 1 day after I/R with repair and resolution of the injury to nearly normal liver architecture by 2 wk after reperfusion. There was much more liver injury and liver fibrosis in mice undergoing 90 min of ischemia compared with those undergoing 45 min of ischemia. Original magnification is ×100. Quantification of necrosis area and fibrosis area shows an inverse relationship between necrotic area and extent of liver fibrosis. Data are means ± SE with n = 3 per group. *P < 0.05 compared with control.
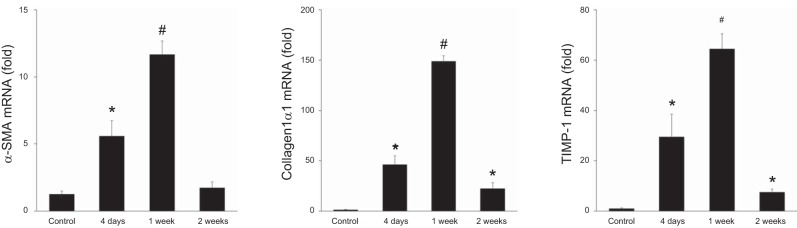
We next measured profibrotic gene expression in the ischemic liver. The expression of α-SMA, a marker of activated HSCs/myofibroblasts, was upregulated and peaked 1 wk after I/R (Fig. 2). Collagen-1α1 and TIMP-1 are released from myofibroblasts and accelerate scar production (11). Both of these mediators were expressed in a similar manner as α-SMA, elevated expression that peaked at 1 wk after I/R (Fig. 2). Collectively, these results suggest that myofibroblasts cause fibrotic change, whereas injured liver reconstructed after I/R injury. We also investigated the pattern of liver fibrosis after I/R.
Fig. 2.
Profibrotic gene expression after ischemia-reperfusion (I/R) injury. Liver mRNA expression of α-smooth muscle actin (α-SMA), collagen-1α1, and tissue inhibitor of metalloproteinase-1 (TIMP-1) after I/R injury were measured by qPCR. Expression of these genes increased during liver repair and returned to near baseline expression by 2 wk after reperfusion. Data are means ± SE with n = 4 per group. *P < 0.05 compared with control. #P < 0.05 compared with control and 4 days.
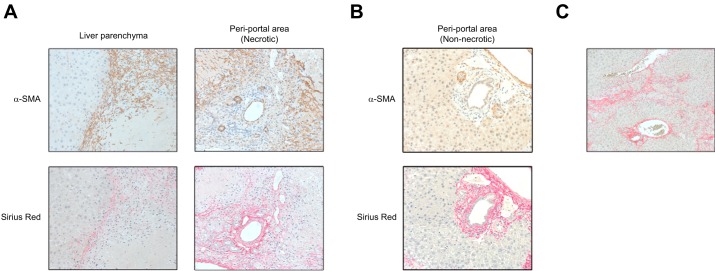
To assess the distribution of myofibroblasts, the regional expression of α-SMA in the postischemic liver was determined by immunohistochemistry. Myofibroblasts expanded at the border between viable liver parenchyma and necrotic areas at 1 wk after I/R (Fig. 3A). We also detected myofibroblast expansion in the periportal regions within necrotic areas (Fig. 3A). Sirius red staining showed that collagen deposition was enhanced in the same area of myofibroblast expansion (Fig. 3A). However, virtually no α-SMA-positive cells were observed around bile ducts in the nonnecrotic areas of liver although collagen deposition was detected in these areas (Fig. 3B). This suggests that the fibrosis observed in these areas may not be attributable to myofibroblasts. After the clearance of necrotic tissue and restoration of otherwise normal liver architecture, fibrosis was still readily observed 2 wk after I/R (Fig. 3C).
Fig. 3.
Expansion of myofibroblasts is restricted to areas of liver necrosis. The location of myofibroblasts and their proximity to areas of liver fibrosis were determined by immunohistochemistry for α-smooth muscle actin (α-SMA) and Sirius red staining in serial sections at 1 wk after ischemia-reperfusion (I/R). A: myofibroblasts expanded at the border between viable liver parenchyma and necrotic areas and also in the periportal regions within necrotic areas of the liver. Collagen deposition was observed in the same areas. Original magnification is ×400. B: virtually no α-SMA-positive cells were observed around bile ducts in nonnecrotic areas of liver, whereas collagen deposition was still detected in these areas. Original magnification is ×400. C: fibrosis was observed where the necrotic tissue was replaced with reconstructed liver parenchyma at 2 wk after I/R. Original magnification is ×200.
Origin of myofibroblasts after I/R.
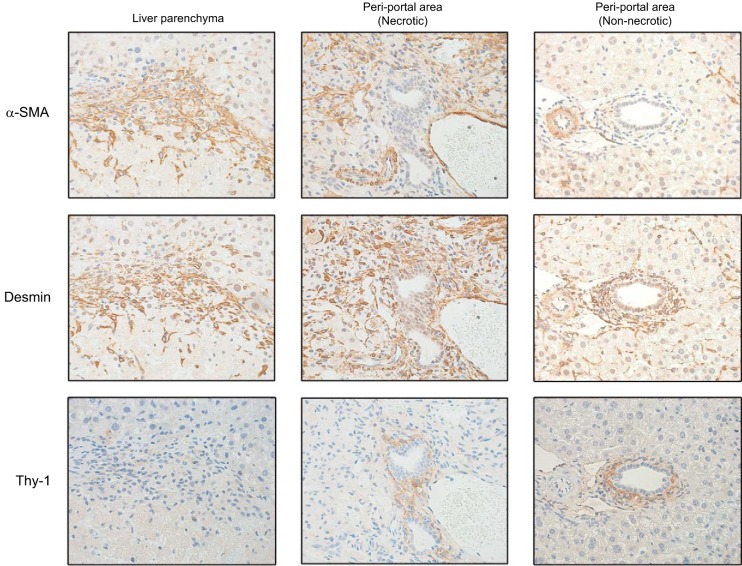
HSCs and portal fibroblasts are thought to be the primary cells that differentiate into myofibloblasts (26). To determine the origin for myofibloblasts after I/R, we stained for α-SMA (myofibroblast marker), desmin (HSC marker), and Thy-1 (portal fibroblast marker) in serial sections. In normal liver, HSCs exist in the liver parenchyma and around bile ducts, whereas portal fibroblasts exist in the portal tract stroma around bile duct. One week after I/R, α-SMA-positive myofibroblasts also stained positive for desmin at the border between viable liver and necrotic areas in the liver parenchyma, as well as in periportal regions within necrotic areas (Fig. 4). However, there was little, if any, α-SMA staining around bile ducts (Fig. 4). Thy-1-positive cells were restricted to areas around the bile ducts, and there was no notable colocalization of Thy-1 and α-SMA (Fig. 4). These data suggest that HSCs are the major source of myofibloblasts after I/R.
Fig. 4.
Origin of hepatic myofibroblasts after ischemia-reperfusion (I/R) injury. To determine the nature of myofibroblasts after I/R injury, serial sections of liver obtained 1 wk after I/R were stained by immunohistochemistry for α-smooth muscle actin (α-SMA) (myofibroblasts), desmin (hepatic stellate cells), and Thy-1 (portal fibroblasts). There is clear colocalization of α-SMA and desmin but no colocalization of α-SMA and Thy-1. Original magnification is ×630.
Resolution of liver fibrosis after I/R.
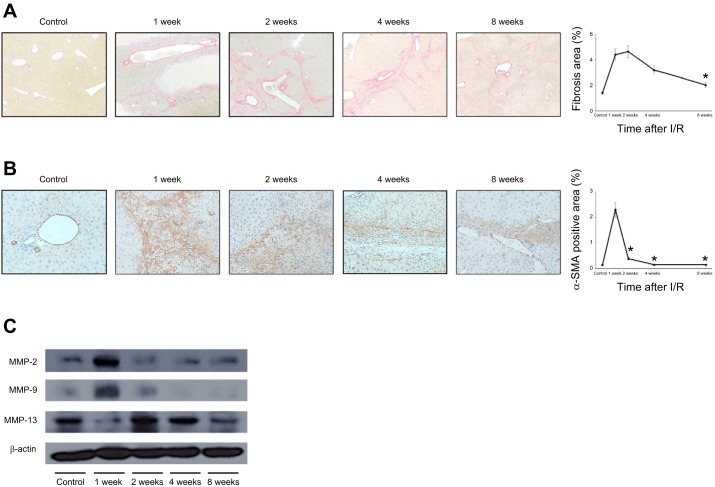
Despite all necrotic areas being cleared and otherwise normal tissue histology observed by 2 wk after I/R, there remained significant liver fibrosis (Fig. 1A). To determine whether this fibrosis persisted and constituted a more chronic phenotype, we assessed fibrosis at extended time points after I/R injury. Sirius red staining of liver sections showed that liver fibrosis was maximal at 2 wk after I/R and gradually resolved by 8 wk after I/R (Fig. 5A). Associated with this decrease in collagen deposition, we found the number of α-SMA-positive myofibroblasts decreased before regression of fibrosis (Fig. 5B). These findings are consistent with the reduced expression of profibrotic genes that we observed beginning 2 wk after I/R (Fig. 2). We next sought to investigate whether the reduction in fibrosis was simply related to a decrease in matrix-producing cells or might include other mechanisms. Because MMPs are critical for degradation of extracellular matrix components, we measured the expression of relevant MMPs in the postischemic liver by Western blot. MMP-2 and MMP-9 expression was upregulated at 1 wk after I/R and decreased thereafter (Fig. 5C). In contrast, the expression of MMP-13 was low 1 wk after I/R but markedly increased after 2 wk and remained increased up to 8 wk after I/R (Fig. 5C). Collectively, these data suggest that liver fibrosis gradually resolves many weeks after I/R injury and that this process involves a reduction in the number of HSCs/myofibroblasts as well as increased expression of MMP-13.
Fig. 5.
Resolution of liver fibrosis after ischemia-reperfusion (I/R). A: collagen deposition was determined by Sirius red staining. Liver fibrosis peaked 2 wk after reperfusion and was nearly resolved 8 wk after reperfusion. Fibrosis area was measured as a percentage of total area. Data are means ± SE with n = 3 per group. Original magnification is ×100. *P < 0.05 compared with 2 wk. B: myofibroblasts were identified by immunohistochemical staining for α-smooth muscle actin (α-SMA). The number of myofibroblasts decreased before the regression of fibrosis. α-SMA-positive area was measured as a percentage of total area. Data are means ± SE with n = 3 per group. Original magnification is ×400. *P < 0.05 compared with 1 wk. C: expression of matrix metalloproteinases (MMPs) was assessed in lysates of postischemic liver tissue by Western blot. The expression of MMP-2 and MMP-9 peaked 1 wk after I/R and then returned to baseline levels. MMP-13 expression decreased 1 wk after I/R and then was increased at 2, 4, and 8 wk after I/R. Figure is representative of 3 independent experiments.
Impact of liver fibrosis on liver repair after I/R injury.
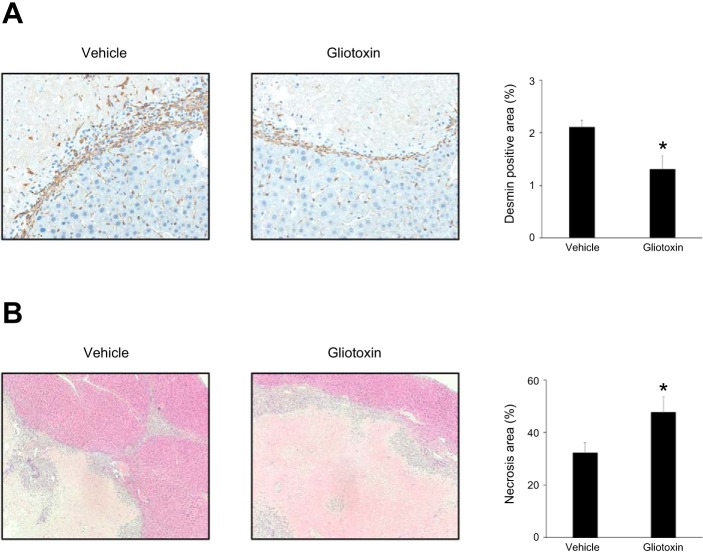
We next sought to determine whether the fibrosis that occurs after liver I/R is beneficial or detrimental to liver repair and recovery. For these experiments, mice were injected with gliotoxin, an antifibrotic agent known to cause stellate cell depletion (27), 48, 72, and 96 h after reperfusion, and liver sections were obtained 4 days and 7 days after reperfusion. Administration of gliotoxin resulted in a significant reduction in stellate cell numbers, as determined by desmin staining at 4 days (Fig. 6A). In addition, gliotoxin significantly increased liver necrosis area at 7 days (Fig. 6B). These data suggest that stellate cell-mediated fibrosis occurring after I/R injury is a reparative response that promotes liver recovery.
Fig. 6.
Impact of fibrosis on liver repair after ischemia-reperfusion (I/R). Mice were injected intraperitoneally with vehicle or 0.75 mg/kg gliotoxin 48, 72, and 96 h after reperfusion. A: stellate cells in the ischemic liver were assessed 4 days after I/R by desmin staining. Desmin-positive area was measured as a percentage of total area. Gliotoxin significantly reduced desmin-positive area. Data are means ± SE with n = 4 per group. *P < 0.05 compared with vehicle group. B: liver recovery, assessed by area of liver necrosis, was determined 7 days after I/R by hematoxylin and eosin staining. Gliotoxin significantly increased necrosis area. Data are means ± SE with n = 6–7 per group. *P < 0.05 compared with vehicle group.
Liver function in the reconstructed liver after I/R injury.
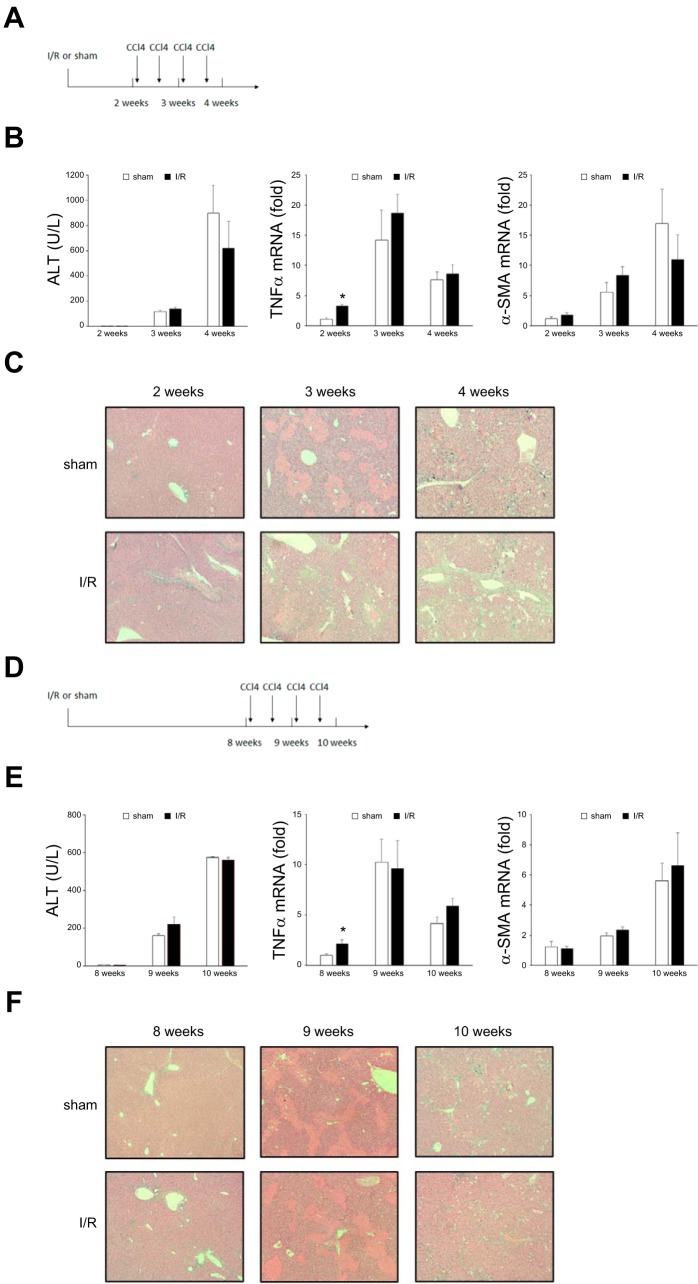
Because we observed liver fibrosis that lingered long after liver repair and the return of appropriate liver architecture, we sought to determine whether prolonged, yet transient, fibrosis predisposed the liver to injury by subsequent insults. To address this issue, mice underwent I/R or sham operation, were allowed to recover for 2 wk (peak of fibrosis) or 8 wk (resolution of fibrosis), and then were subjected to liver injury induced by carbon tetrachloride (CCl4) injection twice weekly for 1 or 2 wk (Fig. 7, A and D). Serum and liver samples were obtained 24 h after the last injection. Administration of CCl4 resulted in significant liver injury, as measured by serum ALT levels, but there was no difference between I/R and sham-operated groups regardless of when the CCl4 was administered (Fig. 7, B and E). Likewise, CCl4 induced significant increases in the expression of the inflammatory cytokine, TNF-α (Fig. 7B). Although there was a significantly higher baseline of TNF-α expression in the I/R groups, there were no differences in TNF-α expression between I/R and sham-operated groups after CCl4 administration (Fig. 7, B and E). CCl4 injection also caused increased expression of the fibrotic marker, α-SMA, but there was no difference between I/R and sham-operated groups (Fig. 7, B and E). Finally, CCl4 administration did result in liver injury assessed histologically (Fig. 7, C and F). Mice in which CCl4 was administered 2 wk after I/R showed less necrosis by histology than sham mice at the 3-wk time point but no difference in liver architecture between sham and I/R groups after 4 wk (Fig. 7C). In mice in which CCl4 was administered 8 wk after I/R, no differences in histology were observed between the I/R and sham-operated groups (Fig. 7, C and F). These data suggest that previous I/R injury and the associated transient fibrosis do not render the liver more susceptible to subsequent injury.
Fig. 7.
Prior ischemia-reperfusion (I/R) injury and fibrosis do not predispose the liver to injury from subsequent insults. A–C: mice subjected to I/R injury were allowed to partially recover (2 wk) before being injected intraperitoneally with CCl4 twice weekly for 2 wk. Tissues and samples were analyzed at 3 wk and 4 wk after I/R. B: liver injury was measured by serum alanine aminotransferase (ALT), and expression of inflammatory and profibrotic genes was assessed by measuring liver TNF-α and α-smooth muscle actin (α-SMA) mRNA expression by qPCR. Data are means ± SE with n = 4 per group. *P < 0.05 compared with sham group. C: liver histology was assessed by hematoxylin-eosin staining. Original magnification is ×100. D–F: mice subjected to I/R injury were allowed to recover fully (8 wk) before being injected intraperitoneally with CCl4 twice weekly for 2 wk. Tissues and samples were analyzed at 9 wk and 10 wk after I/R. E: serum ALT and liver TNF-α and α-SMA mRNA expression were measured as described above. Data are means ± SE with n = 4 per group. *P < 0.05 compared with sham group. F: liver histology was assessed by H and E staining. Original magnification is ×100. There were no differences in the development of liver injury between mice undergoing previous sham or I/R injury.
DISCUSSION
In this study, we investigated the development of liver fibrosis after I/R injury. Previous reports have documented liver fibrosis after I/R, but they have not provided any detail about the genesis of the pathology (1, 4). We showed that myofibroblasts expanded along the border of necrotic areas in the postischemic liver, resulting in liver parenchymal fibrosis. We also documented that liver fibrosis after I/R injury extends beyond the period of liver repair and regeneration and that the fibrosis was reversible and resolved many weeks after I/R. Finally, we showed that previous I/R injury and resultant fibrosis do not make the liver more susceptible to subsequent liver injury.
After hepatic I/R, functional liver mass is greatly reduced, yet total liver mass remains relatively unchanged. This is very different from models of hepatectomy, in which there are concomitant reductions in both functional mass and total mass. After I/R, the liver undergoes a coordinated process of clearance of dead cells/tissue, tissue remodeling, and cell proliferation to restore functional mass. Here we show that the severity of liver fibrosis is dependent on the duration of ischemia and that the fibrotic response appears to be a component of the reparative process after I/R injury. We previously reported that liver regeneration after I/R involves hepatocyte proliferation near the interface of viable and necrotic liver parenchyma, where there is expansion of HSCs in close association with Kupffer cells/infiltrating macrophages (14). Studies by others have also detected activated HSCs surrounding macrophages and a ductular reaction in human liver samples after acute liver failure with evidence that liver fibrosis is a reparative process of injured liver (5). Macrophages are heterogeneous and have diverse functions from initiation to resolution of liver injury. Specifically, the Ly-6chi macrophage is a proinflammatory phenotype and activates HSCs to transdifferentiate into myofibroblasts via mitogenic and profibrotic cytokines (10, 28). Together with our previous findings, the present data suggest that fibrotic changes following marked myofibroblast expansion are associated with macrophages and increased expression of TGF-β, PDGF-BB, and CTGF.
The nature of the development of liver fibrosis is highly dependent on the injury and/or pathology. Arterial liver ischemia results in fibrosis around ductular structures, as well as cholestatic liver injury (2). Models of donation after cardiac death liver transplantation display biliary epithelial injury and biliary fibrosis in the portal area (3, 22). In the present case, liver I/R injury causes hepatocyte damage by oxidative stress, which leads to parenchymal necrosis. We found collagen deposition in the liver parenchyma at the active site of tissue repair, the interface of viable and necrotic tissues. Our results suggested that activated α-SMA-positive myofibroblasts along the necrotic border likely produce TIMP-1 for collagen type 1 deposition during liver repair after I/R. We also detected fibrosis in periportal areas and found that myofibroblasts expanded in these areas of the injured liver as well. Although there was collagen deposition around bile ducts as previously reported (4), we found few myofibroblasts around these structures in the postischemic liver. We did, however, observe portal fibroblasts in these areas that may have contributed to the fibrosis around bile ducts. However, although the proliferation of cholangiocytes is induced after I/R injury (18, 29), our present data do not support biliary injury as a driving force for liver fibrosis after I/R. Other studies have linked the nature of liver fibrosis to the origin of the myofibroblasts. HSCs are a predominant source of myofibroblasts after hepatotoxic injury (19), whereas portal fibroblasts are the primary source of myofibroblasts in cholestatic injury (9). We found that after I/R injury HSCs were the primary source of myofibroblasts.
Upon restoration of normal liver architecture, we observed reduced levels of profibrotic gene, such as TIMP-1 and collagen type 1, which was followed by resolution of liver fibrosis in histology. Liver fibrosis has been reported to be reversible when the causative agents of the liver injury are removed (24). We found reductions in the numbers of myofibroblasts, which was characterized by decreases in α-SMA staining in fibrotic areas. This is consistent with other reports that have shown that myofibroblasts apoptose or revert back to a quiescent, HSC-like phenotype (12). The disappearance of myofibroblasts would result in decreased TIMP-1 production and subsequent upregulation of MMP activities that degrade excessive extracellular matrix (7). In addition, MMPs may also be released by tissue macrophages (21, 25). Although we assessed several MMPs, we found that only the expression of MMP-13 was increased during the time period that fibrosis regressed. This finding is consistent with a previous report that implicated MMP-13 as a central mediator in the resolution of liver fibrosis (6). Collectively, it appears that liver fibrosis after I/R reverses because of the disappearance of myofibroblasts and their production of factors, such as TIMP-1 and collagen degradation by MMP-13.
As to the question of whether the injury and/or fibrosis induced by I/R might make the liver more susceptible or more sensitive to injury or fibrosis from subsequent insults, this appears not to be the case. Using CCl4 as a “second hit” that was administered at a time when liver fibrosis is maximum and at a time when liver fibrosis had largely resolved, we found no evidence of any exaggerated injury or fibrotic response. A previous study reported that myofibroblasts can convert to an inactivated HSC phenotype that is more rapidly and robustly reactivated by a subsequent stimulus (12). We found no evidence of any different injury phenotype after CCl4 administration in our experimental groups, suggesting that the previously postischemic liver had similar cellular responses as their normal counterparts regarding subsequent liver injury.
In summary, the present study characterizes the nature of liver fibrosis after I/R injury. We found that fibrosis is initiated during the reparative period and appears to be the result of proliferation of HSCs and their differentiation into myofibroblasts. The fibrosis is self-limiting but not for several weeks after the liver has repaired itself and restored functional mass. Finally, I/R injury and the resulting fibrosis do not increase susceptibility of the liver to subsequent fibrotic insults.
GRANTS
This work was supported in part by National Institutes of Health Grant DK-56029.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.K. and A.B.L. conceived and designed research; T.K. and R.M.S. performed experiments; T.K. analyzed data; T.K. and A.B.L. interpreted results of experiments; T.K. prepared figures; T.K., R.M.S., and A.B.L. drafted manuscript; T.K. and A.B.L. edited and revised manuscript; T.K., R.M.S., and A.B.L. approved final version of manuscript.
REFERENCES
- 1.Amer AO, Probert PM, Dunn M, Knight M, Vallance AE, Flecknell PA, Oakley F, Cameron I, White SA, Blain PG, Wright MC. Sustained isoprostane E2 elevation, inflammation and fibrosis after acute ischaemia-reperfusion injury are reduced by pregnane X receptor activation. PLoS One 10: e0136173, 2015. doi: 10.1371/journal.pone.0136173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beaussier M, Wendum D, Schiffer E, Dumont S, Rey C, Lienhart A, Housset C. Prominent contribution of portal mesenchymal cells to liver fibrosis in ischemic and obstructive cholestatic injuries. Lab Invest 87: 292–303, 2007. doi: 10.1038/labinvest.3700513. [DOI] [PubMed] [Google Scholar]
- 3.Chen L, Chen G, Guo Y, Liu L, Xiao L, Fan W, Shi B, Qian Y. Ketanserin, a serotonin 2A receptor antagonist, alleviates ischemia-related biliary fibrosis following donation after cardiac death liver transplantation in rats. Liver Transpl 20: 1317–1326, 2014. doi: 10.1002/lt.23947. [DOI] [PubMed] [Google Scholar]
- 4.Cheng F, Li Y, Feng L, Li S. Hepatic stellate cell activation and hepatic fibrosis induced by ischemia/reperfusion injury. Transplant Proc 40: 2167–2170, 2008. doi: 10.1016/j.transproceed.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 5.Dechêne A, Sowa JP, Gieseler RK, Jochum C, Bechmann LP, El Fouly A, Schlattjan M, Saner F, Baba HA, Paul A, Dries V, Odenthal M, Gerken G, Friedman SL, Canbay A. Acute liver failure is associated with elevated liver stiffness and hepatic stellate cell activation. Hepatology 52: 1008–1016, 2010. doi: 10.1002/hep.23754. [DOI] [PubMed] [Google Scholar]
- 6.Fallowfield JA, Mizuno M, Kendall TJ, Constandinou CM, Benyon RC, Duffield JS, Iredale JP. Scar-associated macrophages are a major source of hepatic matrix metalloproteinase-13 and facilitate the resolution of murine hepatic fibrosis. J Immunol 178: 5288–5295, 2007. doi: 10.4049/jimmunol.178.8.5288. [DOI] [PubMed] [Google Scholar]
- 7.Friedman SL, Bansal MB. Reversal of hepatic fibrosis — fact or fantasy? Hepatology 43, Suppl 1: S82–S88, 2006. doi: 10.1002/hep.20974. [DOI] [PubMed] [Google Scholar]
- 8.Huguet C, Gavelli A, Bona S. Hepatic resection with ischemia of the liver exceeding one hour. J Am Coll Surg 178: 454–458, 1994. [PubMed] [Google Scholar]
- 9.Iwaisako K, Jiang C, Zhang M, Cong M, Moore-Morris TJ, Park TJ, Liu X, Xu J, Wang P, Paik YH, Meng F, Asagiri M, Murray LA, Hofmann AF, Iida T, Glass CK, Brenner DA, Kisseleva T. Origin of myofibroblasts in the fibrotic liver in mice. Proc Natl Acad Sci USA 111: E3297–E3305, 2014. doi: 10.1073/pnas.1400062111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karlmark KR, Weiskirchen R, Zimmermann HW, Gassler N, Ginhoux F, Weber C, Merad M, Luedde T, Trautwein C, Tacke F. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology 50: 261–274, 2009. doi: 10.1002/hep.22950. [DOI] [PubMed] [Google Scholar]
- 11.Kisseleva T, Brenner DA. Role of hepatic stellate cells in fibrogenesis and the reversal of fibrosis. J Gastroenterol Hepatol 22, Suppl 1: S73–S78, 2007. doi: 10.1111/j.1440-1746.2006.04658.x. [DOI] [PubMed] [Google Scholar]
- 12.Kisseleva T, Cong M, Paik Y, Scholten D, Jiang C, Benner C, Iwaisako K, Moore-Morris T, Scott B, Tsukamoto H, Evans SM, Dillmann W, Glass CK, Brenner DA. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci USA 109: 9448–9453, 2012. doi: 10.1073/pnas.1201840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konishi T, Lentsch AB. Hepatic ischemia/reperfusion: mechanisms of tissue injury, repair, and regeneration. Gene Expr 17: 277–287, 2017. doi: 10.3727/105221617X15042750874156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konishi T, Schuster RM, Lentsch AB. Proliferation of hepatic stellate cells, mediated by YAP and TAZ, contributes to liver repair and regeneration after liver ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol 314: G471–G482, 2018. doi: 10.1152/ajpgi.00153.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuboki S, Shin T, Huber N, Eismann T, Galloway E, Schuster R, Blanchard J, Edwards MJ, Lentsch AB. Hepatocyte signaling through CXC chemokine receptor-2 is detrimental to liver recovery after ischemia/reperfusion in mice. Hepatology 48: 1213–1223, 2008. doi: 10.1002/hep.22471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lemasters JJ, Thurman RG. Reperfusion injury after liver preservation for transplantation. Annu Rev Pharmacol Toxicol 37: 327–338, 1997. doi: 10.1146/annurev.pharmtox.37.1.327. [DOI] [PubMed] [Google Scholar]
- 17.Lentsch AB, Yoshidome H, Cheadle WG, Miller FN, Edwards MJ. Chemokine involvement in hepatic ischemia/reperfusion injury in mice: roles for macrophage inflammatory protein-2 and KC. Hepatology 27: 507–512, 1998. doi: 10.1002/hep.510270226. [DOI] [PubMed] [Google Scholar]
- 18.Mancinelli R, Glaser S, Francis H, Carpino G, Franchitto A, Vetuschi A, Sferra R, Pannarale L, Venter J, Meng F, Alpini G, Onori P, Gaudio E. Ischemia reperfusion of the hepatic artery induces the functional damage of large bile ducts by changes in the expression of angiogenic factors. Am J Physiol Gastrointest Liver Physiol 309: G865–G873, 2015. doi: 10.1152/ajpgi.00015.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mederacke I, Hsu CC, Troeger JS, Huebener P, Mu X, Dapito DH, Pradere JP, Schwabe RF. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun 4: 2823, 2013. doi: 10.1038/ncomms3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nejak-Bowen KN, Orr AV, Bowen WC Jr., Michalopoulos GK. Gliotoxin-induced changes in rat liver regeneration after partial hepatectomy. Liver Int 33: 1044–1055, 2013. doi: 10.1111/liv.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramachandran P, Pellicoro A, Vernon MA, Boulter L, Aucott RL, Ali A, Hartland SN, Snowdon VK, Cappon A, Gordon-Walker TT, Williams MJ, Dunbar DR, Manning JR, van Rooijen N, Fallowfield JA, Forbes SJ, Iredale JP. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci USA 109: E3186–E3195, 2012. doi: 10.1073/pnas.1119964109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlegel A, Graf R, Clavien PA, Dutkowski P. Hypothermic oxygenated perfusion (HOPE) protects from biliary injury in a rodent model of DCD liver transplantation. J Hepatol 59: 984–991, 2013. doi: 10.1016/j.jhep.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 23.Stewart RK, Dangi A, Huang C, Murase N, Kimura S, Stolz DB, Wilson GC, Lentsch AB, Gandhi CR. A novel mouse model of depletion of stellate cells clarifies their role in ischemia/reperfusion- and endotoxin-induced acute liver injury. J Hepatol 60: 298–305, 2014. doi: 10.1016/j.jhep.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun M, Kisseleva T. Reversibility of liver fibrosis. Clin Res Hepatol Gastroenterol 39, Suppl 1: S60–S63, 2015. doi: 10.1016/j.clinre.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tacke F, Zimmermann HW. Macrophage heterogeneity in liver injury and fibrosis. J Hepatol 60: 1090–1096, 2014. doi: 10.1016/j.jhep.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 26.Wells RG, Schwabe RF. Origin and function of myofibroblasts in the liver. Semin Liver Dis 35: 97–106, 2015. doi: 10.1055/s-0035-1550061. [DOI] [PubMed] [Google Scholar]
- 27.Wright MC, Issa R, Smart DE, Trim N, Murray GI, Primrose JN, Arthur MJ, Iredale JP, Mann DA. Gliotoxin stimulates the apoptosis of human and rat hepatic stellate cells and enhances the resolution of liver fibrosis in rats. Gastroenterology 121: 685–698, 2001. doi: 10.1053/gast.2001.27188. [DOI] [PubMed] [Google Scholar]
- 28.Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis 30: 245–257, 2010. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu WH, Ye QF, Xia SS. Apoptosis and proliferation of intrahepatic bile duct after ischemia-reperfusion injury. Hepatobiliary Pancreat Dis Int 3: 428–432, 2004. [PubMed] [Google Scholar]