Abstract
The highly pathogenic H5N1 (HK483) viral infection causes a depressed hypercapnic ventilatory response (dHCVR, 20%↓) at 2 days postinfection (dpi) and death at 7 dpi in mice, but the relevant mechanisms are not fully understood. Glomus cells in the carotid body and catecholaminergic neurons in locus coeruleus (LC), neurokinin 1 receptor (NK1R)-expressing neurons in the retrotrapezoid nucleus (RTN), and serotonergic neurons in the raphe are chemosensitive and responsible for HCVR. We asked whether the dHCVR became worse over the infection period with viral replication in these cells/neurons. Mice intranasally inoculated with saline or the HK483 virus were exposed to hypercapnia for 5 min at 0, 2, 4, or 6 dpi, followed by immunohistochemistry to determine the expression of nucleoprotein of H5N1 influenza A (NP) alone and coupled with 1) tyrosine hydroxylase (TH) in the carotid body and LC, 2) NK1R in the RTN, and 3) tryptophan hydroxylase (TPH) in the raphe. HK483 viral infection blunted HCVR by ∼20, 50, and 65% at 2, 4, and 6 dpi. The NP was observed in the pontomedullary respiratory-related nuclei (but not in the carotid body) at 4 and 6 dpi, especially in 20% of RTN NK1R, 35% of LC TH, and ∼10% raphe TPH neurons. The infection significantly reduced the local NK1R or TPH immunoreactivity and population of neurons expressing NK1R or TPH. We conclude that the HK483 virus infects the pontomedullary respiratory nuclei, particularly chemosensitive neurons in the RTN, LC, and raphe, contributing to the severe depression of HCVR and respiratory failure at 6 dpi.
Keywords: CO2 chemoreflex, respiratory failure, viral infection
INTRODUCTION
Patients infected by the highly pathogenic avian influenza H5N1 viruses have a spectrum of clinical abnormalities ranging from mild symptoms to respiratory failure and death (61, 71). In all fatal cases (60% mortality), patients present with cough, dyspnea, pulmonary inflammation, and cell infiltration during the first 6 days postinfection (dpi) and subsequently develop respiratory failure (hypoxemia) and death several days later (8, 57, 68a, 71). Results from studies in animals indicate that the lethal H5N1 virus isolated from patients, such as A/Hong Kong/483/97 (HK483), is neurotropic and induces death 7–8 days postinfection (dpi) in mice (15, 29), ferrets (50), and cats (31). Although respiratory failure is responsible for the lethality of H5N1 viral infection, its development over the infection period and the relevant mechanisms are not fully understood.
Hypercapnic ventilatory response (HCVR) is essential for maintaining arterial blood CO2 homeostasis and normal breathing in mammals. Depression of HCVR (dHCVR) is involved in the respiratory failure in many diseases, such as sudden infant death syndrome (55), chronic obstructive pulmonary diseases (27, 69), congenital central hypoventilation syndrome (19), and Leigh syndrome (51). Our previous studies have observed dHCVR (20%↓) in mice at the early stage (2 dpi) of HK483 viral infection (75). However, whether this ventilatory disorder becomes worse over the infection period, contributing to the respiratory failure, is undetermined.
HCVR is triggered by peripheral and central CO2-sensitive neurons. Peripherally, glomus cells in the carotid body are chemosensitive to CO2/H+ (7, 16, 59). Although full expression of HCVR requires the carotid body, HCVR is triggered predominantly by activation of putative CO2/H+-chemosensitive neurons located in the brainstem (21, 43, 52). These chemosensitive neurons are not limited to but located mainly in three pontomedullary nuclei with specific biomarkers. First, the retrotrapezoid nucleus (RTN) contains neurons expressing high levels of neurokinin 1 receptors (NK1R), and selective lesions of these neurons by 44% in rats had a 30% decrease in HCVR in wakefulness and non-rapid eye movement sleep (22, 43). Second, the majority of locus coeruleus (LC) neurons are catecholaminergic and local acidosis by injection of acetazolamide increases ventilation (4), whereas focal deletion of these neurons by 80% decreased HCVR by 64% in unanesthetized rats (4). Third, the medullary raphe contains a prominent population of serotonergic neurons (3). Focal acidification of the midline raphe by microinjection of acetazolamide also increased respiratory output in anesthetized rats (3). This is consistent with an elevation of ventilation immediately after focal acidification in conscious rats (42) and goats (25, 26) by reverse microdialysis of artificial cerebrospinal fluid equilibrated with increased CO2. Importantly, these NK1R-expressing catecholaminergic and serotonergic neurons are sensitive to extracellular CO2/H+ change in the studies conducted in vitro (22, 30, 33). The ability of lethal H5N1 virus to infect neurons of the brain has been reported in acutely infected mice, ferrets, and humans (29, 50, 74, 79), which is undetectable until 4 dpi in mice (29, 79). Our pilot study has shown a gradually worsening HCVR over a 6-day course of H5N1 viral infection in mice. These lines of evidence together lead to our hypothesis that dHCVR and respiratory failure induced by lethal H5N1 viral infection result in part from viral replication in the carotid body and pontomedullary respiratory-related nuclei, especially the neurons responsible for CO2 chemoreception.
MATERIALS AND METHODS
Animals.
The present study was approved by the Institutional Animal Care and Use Committee and Institutional Biosafety Committee of the Lovelace Respiratory Research Institute. All facilities were accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. BALB/c female mice experiments were conducted in the Animal Biosafety Level 3 enhanced (ABSL-3+) facility. Guidelines for mice housing, environment, and comfort described in the Guide for the Care and Use of Laboratory Animals (7th ed., National Research Council) were strictly followed. Euthanasia was performed following the recommendations of the American Veterinary Medical Association Guidelines for the Euthanasia of Animals.
Mice handling/care, identification, and adaptation.
A total of 64 pathogen-free female BALB/c mice were purchased from Charles River Laboratories (Wilmington, MA) and quarantined for 2 wk before the experiments. Female mice were chosen here because we (79) and other investigators (15, 28, 35) have demonstrated the neurotropic impacts of H5N1 virus (particularly HK483 virus) in female mice. Mice had access to food and water ad libitum. They were 4–5 wk of age on the day of infection, which is similar to our previous studies (75). Temperature and humidity in the animal facility ranged at 24–26°C and 30–65%, respectively, and the light cycle was 12 h on and 12 h off. Ventilation in the study room was 0.15 air exchanges per hour. Five days before the intranasal instillation of saline or HK483 virus, the animals were briefly anesthetized with 2–5% isoflurane delivered via an isoflurane anesthesia machine (model 100 vaporizer; SurgiVet, Dublin, OH). After the local hair between the shoulder blades was clipped under adequate anesthesia, iodine and a topical anesthetic (lidocaine jelly 2%) were applied to the skin. Subsequently, an IPTT-300 implantable, programmable temperature and identification transponder [Bio Medic Data Systems (BMDS), Seaford, DE] was inserted subcutaneously via a needle between the shoulder blades. The animal identification number and subcutaneous body temperature were read with a BMDS electronic proximity reader wand (WRS-6007). Mice were individually placed in a whole body, unrestrained plethysmograph chamber (PLY3211; Buxco Electronics, Troy, NY) with a bias flow (0.5 l/min, room air) for ∼25 min. The same habituation was applied once/day for 3 continuous days before infection. All mice handlers were vaccinated for circulating seasonal influenza strains and were not permitted to enter mice quarters if they exhibited any symptoms of upper or lower respiratory infection.
Experimental protocols.
Series I was designed to compare the impacts of H5N1 viral infection on body weight, body temperature, baseline ventilation, and HCVR at 0, 2, 4, and 6 dpi (n = 7, 7, 8, and 10, respectively, for each time point) in control and HK483 mice. On the experimental day, the animals were individually placed into the plethysmograph chamber to record baseline ventilation. Subsequently, the ventilatory response to hypercapnia (7% CO2) was measured for 5 min. After completion of the experiment at each time point, the mice were removed from the chamber and adequately anesthetized with urethane (1,200 mg/kg ip for the initial dose and 300 mg/kg ip for the supplemental one). The right femoral artery was isolated, and a PE50 catheter with the tip heated and pulled to a smaller size (OD = 0.5–0.6 mm) was inserted rostrally into the artery. Arterial blood was sampled for measurements of pH and blood gases, and the mice were then euthanized. Five infected mice at 0 (no viral inoculation), 2, 4, and 6 dpi were prepared for the following experiments.
Series II was performed to identify whether the HK483 virus was able to replicate in the carotid body at 0, 2, 4, and 6 dpi. After the fixation via intracardiac perfusion of 4% paraformaldehyde in PBS, the carotid body was isolated and harvested bilaterally. Paraffin-embedded tissues of the carotid body were sectioned (10 µm thick), and TH (TH-IR) was utilized to mark glomus cells in the carotid body (78). Costaining with H5N1 viral nucleoprotein (NP) was employed to detect viral replication.
Series III aimed to first identify which pontomedullary nuclei were infected by the HK483 virus over the infection period. After collection of the carotid body, the brainstem was harvested for immunohistochemistry analysis. Coronal sections were sequentially cut (10 µm thick) from the caudal medulla (obex level) to the pontine LC level and evenly divided into four sets in sequence. One set was used for detecting NP alone to confirm HK483 viral infection in the brainstem, whereas the remaining three sets were prepared for double-labeling of NP and one of the following immunoreactivities (IRs): 1) NK1R, 2) tyrosine hydroxylase (TH, a marker of catecholaminergic neuron), or 3) tryptophan hydroxylase (TPH, a marker of serotonergic neuron). We focused only on NP + NK1R in the RTN, NP + TH in the LC, and NP + TPH in the medullary raphe here because these neurons are well known as CO2/H+-chemosensitive neurons and responsible for generating HCVR (21, 43, 52).
Viruses and cells.
Avian influenza A H5N1 viruses were obtained from the Centers for Disease Control and Prevention (CDC; Atlanta, GA). They were propagated from the CDC stock in eggs twice to produce working stocks, aliquoted, titrated by plaque assay on Madin-Darby canine kidney (MDCK) cells, and stored at −80°C (75).
Viral infection of mice and behavioral observation.
After anesthesia with isoflurane, 50 μl of vehicle alone or coupled with HK483, the virus was intranasally given [100 plaque-forming units (PFU)] via dropwise application to the nares, as previously reported (75), and the mice were named as either control or HK483 mice. Behavioral observations were conducted twice daily from 0 to ≤6 dpi. They included body temperature and recording of the onset, severity, and duration of all visible changes, such as abnormal respiration (cough and sneezing), excretions, behavioral characteristics, and neurological signs (i.e., paresis, torticollis, seizures, and paralysis).
Measurements of V̇e and HCVR.
Following stabilization, minute ventilation (V̇e), respiratory frequency (fR), and tidal volume (VT), were recorded for 10 min by using the plethysmography while room air was supplied. Subsequently, the mice were exposed to 40% O2 + 60% N2 for 10 min before hypercapnia (7% CO2 + 40% O2 balance with N2) for 5 min, with the above variables monitored and recorded continuously. The temperature inside the chamber was maintained at ∼30.0°C, as previously reported (75, 79). All studies were performed at 0900 and 1700 to avoid any influence from the circadian rhythm.
Measurements of arterial blood pH and gases.
Arterial blood was sampled (100 µl) to measure pH and blood gases (pH, PaO2, PaCO2, and SaO2) using a blood gas analyzer (GEM Premier 3000; Instrumentation Laboratory, Lexington, MA).
Virus titer in the lungs.
Plaque assay was performed in MDCK cells to quantify the multiplicity of viral infection in the right lungs. In brief, after euthanasia, the right lungs from each mouse were harvested and homogenized in 1.0 ml of PBS with one 5-mm stainless-steel bead and homogenized with a Qiagen TissueLyser (Qiagen, Valencia, CA) for 2 min at 30 Hz. Homogenized material was spun down, and 100 μl of supernatant with 10-fold series dilution was applied to preseeded 12-well plates of MDCK cells (95% confluent) and then overlaid with agar containing 3 µg/ml of trypsin (Sigma-Aldrich, St. Louis, MO). Three days later, the PFU was counted after fixation, removal of the agar, and staining with 1.6% wt/vol crystal violet.
Immunofluorescence and digital image acquisition.
The paraformaldehyde-fixed carotid bodies and brainstems were dehydrated, paraffin embedded, and coronally sectioned (10 μm thick). Brainstem sections were evenly and sequentially divided into four sets, depending on the primary antibody used for only NP and for NP coupled with NK1R, TH, or TPH. In each animal, all sections of the carotid body and selective sections from the brainstem were collected. As mentioned above, coronal sections of the brainstem were sequentially cut from the caudal medulla (obex level) to the pontine LC level and evenly divided into four sets in sequence for detecting different IRs. We focused on four levels of the brainstem, including 0 (obex level), 0.5, 1.3, and 2.2 mm rostral to the obex, which contain the NTS, PBC/BC, raphe, RTN, and LC (48). Eight sections were collected at each level in each mouse so that two sections from each set were used for detecting NP-IR alone and coupled with HK1R, TH, or TPH-IR. The sections were deparaffinized, rehydrated, and washed for immunohistological processing. Antigen recovery was performed by using preheated sodium citrate buffer (10 mM, pH 6.0) for 10 min in the microwave. They were permeabilized and nonspecific antibody binding sites were blocked using a blocking buffer (3% BSA, 2% normal goat serum, 0.3% Triton X-100 in PBS) at room temperature for 1 h. The sections were then incubated at 4°C with the solution containing the primary antibody or antibody mixture to specifically target the nucleoprotein of H5N1 influenza A (NP, mouse anti-influenza A monoclonal IgG 1:1,000; EMD Millipore, Billerica, MA), NK1R (guinea pig anti-NK1R, AB15810, EMD Millipore), TH (rabbit anti-TH polyclonal IgG 1:1000, EMD Millipore), and TPH (rabbit anti-TPH2 polyclonal IgG 1:1,000, AB111828; Abcam) for 36 h. The antibody concentrations and incubation durations were determined and optimized before the experiment. The immunoreactivities were revealed by incubating the sections with solution containing one or two of the secondary antibodies raised in goat and conjugated with Alexa Fluor 488 (green) and 594 (red) alone or in combination (1:200, Life Technologies). For multilabeling, antibodies raised from different animal species were selected. The immunoreactivity was visualized using an epifluorescent microscope (Axioskop FS 2 plus; Zeiss) equipped with a charge-coupled device digital camera (Zeiss Axiocam), and images were captured using Axiovision 4 software (Zeiss).
Data acquisition and statistical analysis.
Raw data of the airflow was digitized, monitored, and recorded by PowerLab/8sp (model ML 785; ADInstruments, Colorado Springs, CO) and a computer with the LabChart Pro 7 software. Respiratory variables, including V̇e, VT, and fR, were derived from the online calculations of the airflow signals. Body temperatures, body weight, arterial blood pH, and gases were also measured. All variables were expressed as absolute values, with the exception that HCVR was presented as Δ% change from the baseline values. The baseline values were determined by measuring the variables for 1 min immediately before hypercapnia, whereas HCVR was defined by measuring the peak V̇e response. In this study, the morphological data were bilaterally analyzed as summarized here. 1) The boundary of each nucleus in the brainstem was defined according to the mouse brain atlas (48) and the RTN (including parapyramidal region) was defined based on previous reports in rats and mice (43, 67). 2) The viral replication in the brainstem was localized by marking NP-staining cells against corresponding coronal brainstem section drawings with the neural numbers in each nucleus and further normalized with the nucleus area as expressed by NP-cell density (no./mm2). 3) The population of neurons expressing the given biomarker (NK1R, TPH, or TH) in the RTN, LC, or medullary raphe was counted and presented as the biomarker-IR cell no. to reflect the viral impact on the neural population in these nuclei. 4) Every neuron expressing NK1R-IR in the RTN, TPH-IR in the medullary raphe, and TH-IR in the LC was selected. The biomarker-IR grayscale values of all selected neurons were analyzed, averaged, and presented as NK1R-, TPH-, and TH-IR density, respectively. This analysis allowed us to determine the viral impact on the ability of the remaining neurons to express the given biomarker in the three nuclei. 5) To reveal the degree of viral replication in a given type of neurons, the numbers of neurons double-labeled by NP + biomarker-IR over those single-labeled by the biomarker-IR were calculated and expressed as percentages, such as NP + NK1R-IR/NK1R-IR in the RTN. Group data were reported as means ± SE. One-way analysis of variance (ANOVA) was employed to analyze the significant differences in the above variables. Two-way ANOVA with repeated measures was used to analyze the significant differences of other variables at 0, 2, 4, and 6 dpi between the two groups. Tukey’s test was utilized for specific comparisons between individual groups. P values of <0.05 were considered significant.
RESULTS
HK483 replicated in the lungs and induced abnormal behaviors.
Viral titers in the lungs were undetectable in control mice but were observed in HK483 mice at 2, 4, and 6 dpi (5.9 ± 0.4, 5.7 ± 0.3, and 5.5 ± 0.6 log PFU/g, respectively). HK483 mice showed no discernible behavior abnormalities compared with control mice until 4 dpi. The abnormalities were characterized by less movement, loss of appetite, torticollis, and neurological signs, including ataxia and tremor, which are in agreement with the ability of lethal H5N1 viruses to induce neuroinflammation and neurodegeneration with neurological signs (15, 29, 50). As illustrated in Table 1, HK483 mice showed body weight loss, as previously reported in mice, cats, ferrets, and macaques (32, 58, 64), and hypothermia as observed in mice (75, 76, 79). No coughing and/or sneezing were observed throughout the experiment in all of the mice tested.
Table 1.
Comparisons of BW, BT, and resting ventilatory changes in control and HK483 mice over the infection period
| Days Postinfection | ||||
|---|---|---|---|---|
| Variable (Group) | 0 | 2 | 4 | 6 |
| BW, Δ% | ||||
| Control | 0.0 ± 0.0 | −0.8 ± 0.7 | 0.2 ± 1.2 | −1.9 ± 0.7 |
| HK483 | 0.0 ± 0.0 | −3.7 ± 1.6 | −13.8 ± 1.5*†# | −23.6 ± 1.8*†# |
| BT, °C | ||||
| Control | 37.2 ± 0.1 | 36.8 ± 0.1 | 37.1 ± 0.2 | 37.0 ± 0.2 |
| HK483 | 37.5 ± 0.1 | 37.0 ± 0.1 | 36.1 ± 0.3*†# | 31.1 ± 1.1*†# |
| V̇e, ml/min | ||||
| Control | 44.7 ± 2.5 | 43.8 ± 2.2 | 46.0 ± 2.1 | 44.7 ± 2.1 |
| HK483 | 45.5 ± 3.3 | 53.0 ± 4.2*# | 62.5 ± 5.5*†# | 49.3 ± 4.9# |
| fR, breaths/min | ||||
| Control | 245.9 ± 4.5 | 247.1 ± 4.3 | 249.8 ± 6.0 | 250.2 ± 7.4 |
| HK483 | 243.3 ± 6.1 | 279.7 ± 6.8*†# | 287.8 ± 6.9*† | 256.8 ± 9.9# |
| VT, ml | ||||
| Control | 0.18 ± 0.01 | 0.18 ± 0.00 | 0.18 ± 0.00 | 0.18 ± 0.00 |
| HK483 | 0.19 ± 0.00 | 0.20 ± 0.01 | 0.22 ± 0.01*†# | 0.20 ± 0.01# |
Values are means ± SE; n = 7, 7, 8, and 10 on 0, 2, 4, and 6 dpi for both control and HK483 groups, which are the same for Fig. 1 and Table 2. BT, body temperature; BW, body weight; dpi, days postinfection; fR, respiratory frequency; V̇e, minute ventilation; VT, tidal volume.
P < 0.01 compared with 0 dpi;
P < 0.01 vs. control on the given day;
P < 0.05 compared with the previous day in HK483 mice.
HK483 virus initially caused tachypnea and subsequently respiratory failure.
We compared the eupneic breathing and HCVR at 0, 2, 4, and 6 dpi between control and HK483 mice. V̇e, fR, and VT in HK483 mice were initially enhanced at 2 and 4 dpi and then went back to the levels before the infection at 6 dpi (Table 1). We also compared arterial blood gases/pH at the four time points between the two groups (Table 2). The tachypnea at 2 dpi in HK483 mice without a difference in blood gases/pH when compared with control mice might be due to the tachypnea that masked changes in blood gases/pH. In sharp contrast, HK483 viral infection induced a significant decrease in PaO2 and SaO2 and a remarkable increase in PaCO2 at 4 and 6 dpi, with acidosis occurring at 6 dpi. Moreover, a significant elevation of HCO−3 occurred at 4 dpi and worsened at 6 dpi in HK483 mice. Taken together, these data suggest that there is a renal compensation occurring at 4 and 6 dpi that fails to maintain the pH homeostasis, thereby leading to the acidosis. The values of arterial blood pH are reportedly dependent on temperature (1), and the pH values listed in Table 2 were measured at 37°C. Owing to a remarkable drop of BT in HK483 mice (31°C) compared with control ones (37°C) at 6 dpi, we corrected the pH values to those at the actual hypothermia point in HK483 rats (1). We found a more severe acidosis after the correction (pH = 7.20) in these infected mice at 6 dpi.
Table 2.
Comparisons of pH and arterial blood gases changes in control and HK483 mice over the infection period
| Days Postinfection | ||||
|---|---|---|---|---|
| Variable (Group) | 0 | 2 | 4 | 6 |
| pH (units) | ||||
| Control | 7.39 ± 0.02 | 7.38 ± 0.02 | 7.37 ± 0.03 | 7.38 ± 0.03 |
| HK483 | 7.40 ± 0.03 | 7.42 ± 0.02 | 7.38 ± 0.03 | 7.29 ± 0.04*†# |
| PaCO2, Torr | ||||
| Control | 39.0 ± 1.2 | 39.5 ± 1.4 | 39.1 ± 1.7 | 39.9 ± 3.1 |
| HK483 | 40.4 ± 2.9 | 40.9 ± 1.7 | 50.5 ± 3.0*†# | 62.5 ± 5.2*†# |
| HCO−3, mM | ||||
| Control | 23.6 ± 0.4 | 23.3 ± 0.4 | 22.6 ± 0.5 | 23.6 ± 0.6 |
| HK483 | 25.0 ± 1.5 | 26.5 ± 1.1† | 29.8 ± 1.6*†# | 30.1 ± 2.1*† |
| PaO2, Torr | ||||
| Control | 84.3 ± 2.8 | 85.3 ± 3.7 | 85.6 ± 2.8 | 86.1 ± 1.9 |
| HK483 | 80.8 ± 3.7 | 80.1 ± 3.9 | 71.8 ± 2.9*†# | 54.1 ± 4.7*†# |
| SaO2, % | ||||
| Control | 91.8 ± 1.7 | 91.4 ± 1.1 | 92.4 ± 1.2 | 92.3 ± 1.3 |
| HK483 | 90.7 ± 2.2 | 90.4 ± 1.5 | 83.7 ± 1.9*†# | 68.0 ± 6.5*†# |
Values are means ± SE. dpi, days postinfection.
P < 0.01 compared with 0 dpi;
P < 0.01 compared with between control and HK483 mice on the given day;
P < 0.05 vs. the previous day in HK483.
There was an aggravated dHCVR over the viral infection period.
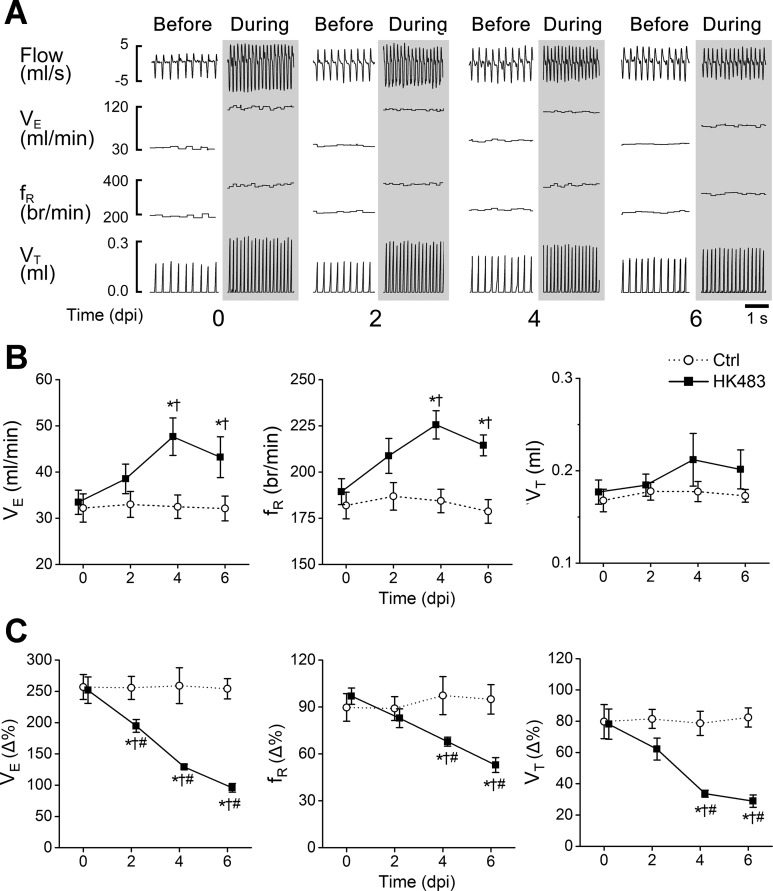
The typical recordings of V̇e, VT, and fR before and during hypercapnia at 0, 2, 4, and 6 dpi in HK483 mice are presented in Fig. 1A, whereas the corresponding group data from control and HK483 mice are compared in Fig. 1B and 1C. Compared with control mice, there was a significant increase in V̇e and fR, with little change in VT during hyperoxia (40% O2 before CO2 exposure) in HK483 mice at 4 and 6 dpi (Fig. 1, A and B). These changes likely resulted from an alleviation of central hypoxemia-induced ventilatory depression (62). HK483 viral infection markedly and gradually attenuated HCVR, with the degree of depression aggravated over the infection period. As depicted in Fig. 1C, HCVR (∼250% increase compared with bassline V̇e) did not change over the 6 days in control mice. However, compared with HK483 mice at 0 dpi (∼250%, before viral infection), HCVR in HK483 mice gradually worsened over the infection period, i.e., 195, 129, and 96% at 2, 4, and 6 dpi, respectively. This dHCVR resulted from lowering both fR and VT responses.
Fig. 1.
Development of depressed hypercapnic ventilatory response (dHCVR) over the viral infection period in mice. A: typical recordings of airflow, minute ventilation (V̇e), respiratory frequency (fR), and tidal volume (VT) before and during exposure to hypercapnia in HK483 mice at 0, 2, 4, 6, days postinfection (dpi). Columns showing each day without and with shade represent before (40% O2) and during hypercapnia (40% O2 + 7% CO2 for 5 min). Owing to the similarity of the baseline V̇e and hypercapnic ventilatory response (HCVR) between control (Ctrl) mice (at 0, 2, 4, and 6 days post-intranasal administration of vehicle) and HK483 mice at 0 dpi (see B and C), the typical recordings from the Ctrl mice are omitted here. B: group data of VE, fR, and VT during 40% O2 (before hypercapnia) in Ctrl and HK483 mice at 0, 2, 4, 6, dpi. Values are means ± SE. *P < 0.05 compared with 0 dpi; †P < 0.05 compared with Ctrl on the corresponding day. C: group data show that HCVR is progressively and significantly depressed after viral inoculation in HK483 mice due to both decreases in fR and VT response. *P < 0.01 compared with 0 dpi; †P < 0.01 compared with Ctrl on the corresponding day; #P < 0.05 vs. the previous day in HK483.
HK483 virus failed to replicate in the carotid body (glomus cells).
Considering the important role of the carotid body in control of HCVR, we tested whether lethal H5N1 virus was able to infect the carotid body, particularly in glomus cells marked by TH-IR. NP-IR was not denoted over the infection period in the glomus cells of the carotid body of HK483 mice (Fig. 2).
Fig. 2.
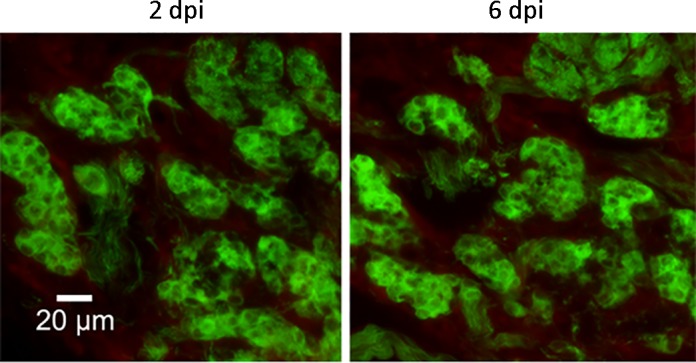
The lack of nucleoprotein (NP) expression in the carotid body and glomus cells marked by tyrosine hydroxylase immunoreactivities (TH-IR; green) at 2 and 6 days postinfection (dpi). No viral replication in the carotid body and glomus cells of HK483 mice are observed at 2, 4, and 6 dpi.
HK483 virus replicated in multiple regions of the brainstem.
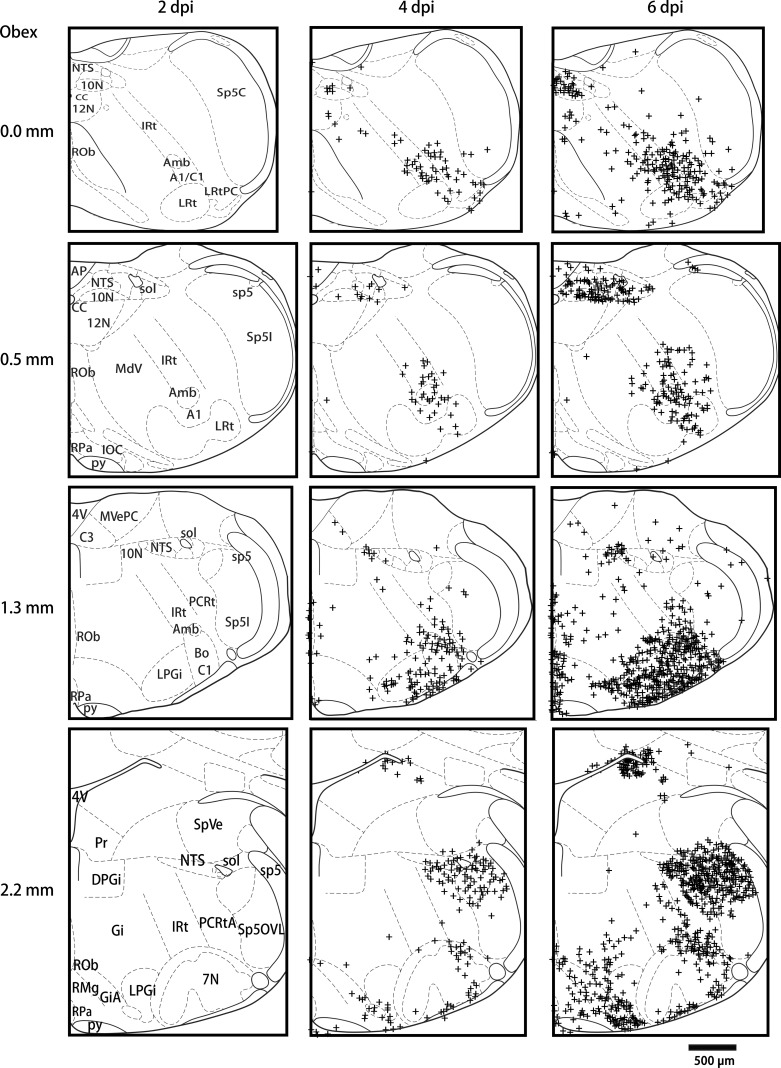
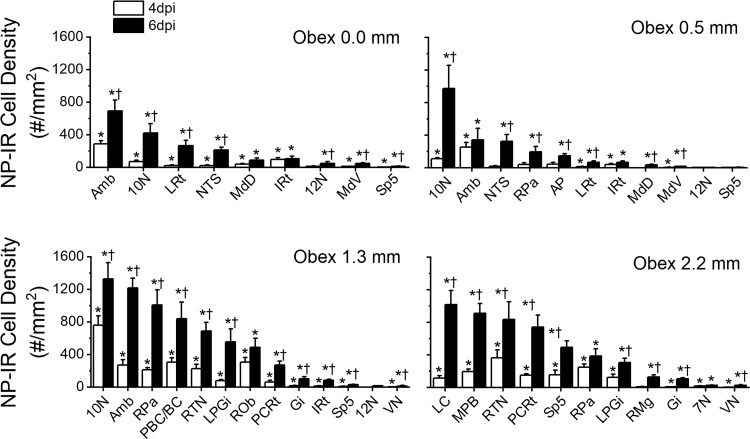
We examined pontomedullary NP-IR expression at 0, 2, 4, and 6 dpi in HK483 mice. NP expression was not observed in the brainstem until 4 dpi. Figure 3 shows the typical examples from three infected mice at 2, 4, and 6 dpi. The NP expression was not detectable in the pontomedullary regions until 4 dpi and was more spread out at 6 dpi. There were several major findings in identifying the density of NP-IR positive neurons in each pontomedullary nucleus at 4 and 6 dpi (Fig. 4). First, the densities of NP-IR at both 4 and 6 dpi were higher at the rostral medulla and pone (1.3 and 2.2 mm rostral to the obex) than at the caudal medulla (0.0 and 0.5 mm rostral to the obex). Second, the order of NP expression density (from high to low) at 6 dpi was the 10N, ambiguous nucleus (Amb), pallidus nuclei of the medullary raphe (RPa), LC, medial parabrachial nucleus (MPB), retrotrapezoid nucleus (RTN), pre-Botzinger complex (PBC)/Botzinger complex (BC), parvicellular reticular nucleus (PCRt), obscurus nuclei of the medullary raphe (ROb), lateral paragigantocellular nucleus (LPGi), spinal trigeminal nucleus (Sp5), nuclei of the solitary tract (NTS), magnus nuclei of the medullary raphe (RMg), area postrema (AP), lateral reticular nuclei (LRt), intermediate reticular nuclei (IRt), 12N, gigantocellular reticular nucleus (Gi), MdD, MdV, VN, and 7N. Third, the spreadout from 4 to 6 dpi appeared to be relatively faster in the LRt, 10N, LC, RPa, Amb, and LPGi.
Fig. 3.
Schematic drawings showing typical nucleoprotein immunoreactivity (NP-IR) locations in coronal medullary sections at 0, 0.5, 1.3, 2.2 mm rostral to the obex found in 3 HK483 mice at 2, 4, and 6 days postinfection (dpi). NP-IR is not detectable until 4 dpi. NP-IR is detected in all of those levels, but it is relatively higher in the rostral than the caudal regions. This is more widespread in the LRt, 10N, LC, RPa, Amb, and LPGi from 4 to 6 dpi compared with the other nuclei.
Fig. 4.
Group data of the density of nucleoprotein immunoreactivity (NP-IR; positive neurons/mm2) in different pontomedullary nuclei at 0, 0.5, 1.3, and 2.2 mm rostral to the obex at 4 and 6 days postinfection (dpi). Among these nuclei, the higher expression of NP-IR at 6 dpi was found in the 10N, ambiguous nucleus (Amb), pallidus nuclei of the medullary raphe (RPa), locus coeruleus (LC), medial parabrachial nucleus (MPB), retrotrapezoid nucleus (RTN), pre-Botzinger complex (PBC)/Botzinger complex (BC), parvicellular reticular nucleus (PCRt), obscurus nuclei of the medullary raphe (ROb), lateral paragigantocellular nucleus (LPGi), spinal trigeminal nucleus (Sp5), and nuclei of the solitary tract (NTS), and the spreadout from 4 to 6 dpi is relatively faster in the lateral reticular nuclei (LRt), 10N, LC, RPa, Amb, and LPGi. *P < 0.05 compared with 2 dpi; †P < 0.05 compared between 6 and 4 dpi.
HK483 virus replicated in RTN neurons expressing NK1R, LC neurons expressing TH, and medullary raphe neurons expressing TPH.
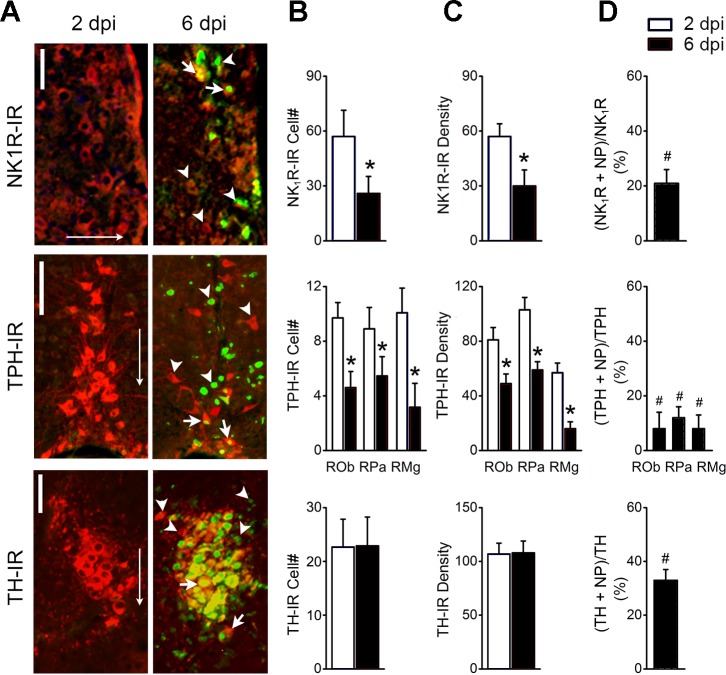
Figure 5 exhibits the impacts of the viral infection at 6 dpi on the neurons expressing NK1R-IR in the RTN, TPH-IR in the medullary raphe (ROb, RPa, and RMg nuclei), and TH-IR in the LC. The results could be summarized with the following four points. 1) NP was presented in the neurons expressing NK1R-IR in the RTN, TPH-IR in the medullary raphe, and TH-IR in the LC (Fig. 5A). 2) As shown in Fig. 5, B and C, HK483 viral infection significantly diminished not only populations of NK1R neurons in the RTN and TPH neurons in the raphe by 50% but also the density of NK1R-IR and TPH-IR expression of the local remaining neuron by ∼50%. In contrast, such changes were not observed in LC neurons expressing TH-IR. 3) PN was co-labeled with 20% of NK1R-neurons in the RTN, 35% of TH-neurons in the LC, and ∼10% of TPH-neurons in the raphe (Fig. 5D), indicating that TH-neurons in the LC are most susceptible among the three types of chemosensitive neurons. 4) There were many more neurons expressing only NP-IR (green alone) in the RTN and raphe than the LC (Fig. 5A), pointing to a preservation of LC neurons after viral infection consistent with no significant changes in the TH-IR cell no. and the TH-IR density in the LC (Fig. 5, B and C).
Fig. 5.
Double labeling of nucleoprotein immunoreactivity (NP-IR), with the neurons expressing neurokinin 1 receptor immunoreactivity (NK1R-IR) in the retrotrapezoid nucleus (RTN), tryptophan hydroxylase immunoreactivity(TPH-IR) in the medullary raphe (ROb, RPa, and RMg nuclei), and tyrosine hydroxylase immunoreactivity (TH-IR) in the locus coeruleus (LC) at 2 and 6 postinfection (dpi); n = 5. A: typical micrographs showing nucleoprotein (NP) co-labeled with NK1R-IR in the retrotrapezoid nucleus (RTN; top), TPH-IR in the medullary raphe (ROb, RPa, and RMg nuclei; middle), and TH-IR in the LC (bottom) at 6 but not 2 dpi. Arrowheads indicate single labeling of NP (green) or NK1R/TPH/TH-IR alone (red), and arrows indicate the co-labeling of NP-IR with the cell marker NK1R-, TPH-, or TH-IR. Scale bars, 50 µm. Arrows point toward ventral. B: %double-labeled neurons relative to the single-labeled neurons in the corresponding nucleus. C: averaged neuronal populations double labeled with NP + NK1R-IR in the RTN, NP + TPH-IR in the raphe, and NP + TH-IR in the LC at 2 and 6 dpi. D: averaged grayscale values of neurons expressing NK1R-IR in the RTN, TPH-IR in the medullary raphe, and TH-IR in the LC. #P < 0.05 compared with NK1R-, TH- or TPH-IR neurons without NP-IR couple-labeling (“0”); *P < 0.05 compared with 2 dpi.
DISCUSSION
Our previous study has shown that HK483 viral infection causes dHCVR (20%↓) in mice at 2 dpi (75) and death usually at 7 dpi (79), which raises a fundamental question as to whether HCVR worsens over the infection period, leading to death. One of the major findings in this study is that HK483 viral infection gradually worsens HCVR (20%↓ at 2 dpi and 62%↓ at 6 dpi). In addition, HK483 viral infection induced hypoxemia and hypercapnia associated with respiratory acidosis at 6 dpi. It is well documented that hypoxia and hypercapnia synergistically stimulate ventilation (60, 68). However, although severe hypercapnic hypoxemia occurred at 6 dpi, the ventilation was not different from the baseline level (compared with 0 dpi). These changes described above clearly point to the appearance of respiratory failure at the late phase of HK483 viral infection. Besides the respiratory failure, H5N1 viral infection-induced pulmonary injury may also participate in the genesis of the hypoxemia and hypercapnia noted in this study (20, 46). Collectively, our data provide for the first time convincing evidence showing a process of dysfunction of HCVR over the lethal H5N1 viral infection, contributing to respiratory failure and death in mice.
HCVR is triggered by CO2/H+ activation of glomus cells in the carotid body (7, 16, 59) and mainly by chemosensitive neurons expressing NK1R in the RTN, TH in the LC, and TPH in the medullary raphe (21, 43, 52). We examined HK483 viral replication in these cells/neurons. Interestingly, NP expression was not detectable in the glomus cells of the carotid body over the infection period (Fig. 2), suggesting that the carotid body is not the major player responsible for generating dHCVR. In sharp contrast, NP was co-stained with 20% of NK1R neurons in the RTN, 35% of TH neurons in the LC, and 10% of TPH neurons in the medullary raphe at 6 dpi. More importantly, HK483 viral infection significantly diminished not only populations of NK1R neurons in the RTN and TPH neurons in the raphe by 50% but also the density of local remaining neurons’ NK1R-IR and TPH-IR expression by ∼50%. This diminution in the population of NK1R neurons in the RTN and TPH neurons in the raphe is likely due to the apoptosis of these neurons induced by HK483 viral infection. We have reported that HK483 viral infection produces neural apoptosis in the pre-Botzinger complex at 6 dpi (79). In fact, apoptosis in brain neurons has been observed at 4 dpi in lethal H5N1 virus infected mice, with the underlying mechanisms remaining unknown (5, 15). However, we are aware that our data cannot exclude the possibility that the diminution is partially due to failure of these neurons to express the detectable biomarker (NK1R, TPH, or TH) by the IR. Although the underlying mechanisms of the neural apoptosis in vivo by HK483 viral infection remain unclear, a recent study addressed this issue in differentiated human astrocytic and neuronal cell lines (44). It was found that H5N1 virus receptors (sialic acid-2,3-galactose and sialic acid-2,6-galactose) were expressed on these neurons, and the tumor necrosis factor mRNA was upregulated in both cells at 6 and 24 h postinfection, contributing to neural apoptosis by the viral infection. Nevertheless, both reductions of the neural population and the ability to sufficiently express NK1R and TPH-IR likely contribute to the dHCVR observed at 6 dpi. On the other hand, the viral replication in LC neuron expression TH-IR may also be involved in the pathogenesis of the dHCVR, albeit with no change in neural population and TH-IR density by the viral infection. In this study, LC neurons expressing TH-IR present the highest viral replication (35%) compared with RTN neurons expressing NK1R (20%) and raphe neurons expressing TPH-IR (10%), but they show little change in the neural population and the TH-IR density. The mechanisms by which these LC neurons are preserved after HK483 viral infection are unclear. However, LC neurons are the major source of noradrenaline that exerts the anti-inflammatory and neuroprotective actions during stress (14), such as exposure to glutamate or hypoxia (13, 36). This, along with abundant expression of noradrenaline receptors in these neurons (63), may account for the preservation of these LC neurons after HK483 viral infection. It is noteworthy that HK483 viral infection was greatly attenuated (62%↓ at 6 dpi), but it did not abolish HCVR. This is consistent with our morphological finding that the viral replication is observed in some but not all chemosensitive neurons that we examined at 6 dpi.
The chemoreflex of HCVR depends not only on CO2/H+ chemosensitive cells/neurons but also multiple pontomedullary nuclei on the pathways of these chemosensitive neurons. Numerous studies have demonstrated that multiple pontomedullary nuclei such as the paragigantocellular nucleus, nuclei of solitary tract, pre-Botzinger complex, Botzinger complex, and medial parabrachial nucleus are involved in the chemoreflex (10, 17, 21, 34, 43, 52–54, 65, 81). The characteristics of NP expressions in the pontomedullary nuclei in the present study can be summarized as follows. First, NP staining was not observed in the brainstem until 4 dpi. Second, the densities of NP-IR-positive neurons in each pontomedullary nucleus at both 4 and 6 dpi were higher at the rostral medulla and in the pons than in the caudal medulla. Third, the higher expression of NP at 6 dpi was found in the 10N, Amb, RPa, LC, MPB, RTN, PBC/BC, PCRt, ROb, LPGi, Sp5, and NTS compared with other nuclei, with the spreadout from 4 to 6 dpi relatively faster in the LRt, 10N, LC, RPa, Amb, and LPGi. Such PN expressions in the pontomedullary respiratory-related nuclei should be also attributed to dHCVR and respiratory failure induced by HK483 viral infection. Investigators believe that lethal H5N1 virus, such as HK483 virus, enters into the brain via routes that include the olfactory system, trigeminal and sympathetic nerves (47), vagal efferents (29) and vagal sensory C-fibers (75). Our morphological data cannot determine the route by which HK483 virus enters the central nervous system (CNS).
Why does dHCVR occur at 2 dpi when no viral replication is observed in the carotid body and the brainstem? It is reported that activation of bronchopulmonary C-fibers (PCFs) abolishes CO2 chemoreception of the RTN, presumably via PCF projections to the commissural subnucleus of the NTS and then to the RTN (39). This is in agreement with the results showing that overexpression of vagal C-fibers and PCF sensitization depress HCVR (6, 69, 77). Our previous study has indicated that HK483 viral replication appears in the vagal pulmonary C-neurons (cell bodies of PCFs) associated with an overexpression of substance P in these neurons at 2 dpi (75). The overexpression of substance P in PCFs by respiratory syncytial virus infection or prenatal nicotinic exposure has been reported to result in sensitization and excitation of these fibers in rats (49, 80), thus supporting a stimulatory effect of HK483 viral replication found on PCFs. In addition, PCF stimulation is the major player for genesis of tachypnea (9, 72, 73) when they are moderately stimulated, and tachypnea is the major respiratory change in HK483 mice at 2–4 dpi (Table 1). When PCFs are intensively stimulated, the PCF-mediated tachypnea shifts to apnea (9, 12), and the latter has been reported in our previous study (79). Consequently, it is possible that although lethal H5N1 virus does not infect the carotid body and the CNS at 2 dpi, its PCF infection stimulates these fibers to attenuate HCVR through indirectly inhibiting RTN chemoreception. Certainly, further investigations are required to examine this assumption.
Respiratory failure may also result from other factors in addition to dHCVR. First, our previous study has demonstrated that HK483 viral infection at 6 dpi induces the apneas, especially with the prolonged apneas and ataxic breathing associated with viral replication in the pre-Botzinger complex (a critical region for respiratory rhythmogenesis) (79). We also found that hypoxic ventilatory response was eliminated in HK483 mice at 6 dpi (J. Zhuang and F. Xu, unpublished observations). This lack of hypoxic ventilatory response after HK483 viral infection and the remarkable reduction of HCVR (62%) noted in this study support a difference in the viral adverse impacts on pontomedullary respiratory-related neural loops responsible for hypoxic and hypercapnic ventilatory responses. Second, different from the fever observed in ferrets, cats, and macaques (32, 58, 64), hypothermia is observed in mice at 6 dpi in both our previous (79) and current studies. The discrepancy of body temperature response to the lethal H5N1 virus seems to be species dependent. In the present study, NP expresses in the raphe pallidus (RPa) serotonergic neurons that are known to be involved in thermoregulation (37, 40). This may promote the HK483 virus-induced hypothermia. Additionally, hypoxia could induce hypothermia (11, 45); therefore, the hypothermia may be related to the viral infection-induced hypoxemia. Hypothermia reportedly lowers HCVR in dogs (56), and more interestingly, hypothermia could interact with hypoxia to depress respiration in rats (38), which can participate in generation of the dHCVR after HK483 viral infection. Third, HK483 viral infection leads to a pulmonary edema and pulmonary inflammation in lethal H5N1-infected mice (79). This is consistent with pulmonary infiltration and immune changes at 6 dpi in patients infected by lethal H5N1 viruses (8, 57, 68a, 70, 71). Taken together, besides dHCVR, the ataxic breathing with apneas and the presence of decreased body temperature, pulmonary inflammation, and edema at the late stage of the infection would also be involved in the respiratory failure induced by lethal H5N1 viral infection.
There are two concerns in the present study. On the one hand, recent studies demonstrate that RTN-chemosensitive neurons exert a chemoreception function by activating different ion channels. For example, KCNQ (Kv7) channels of these neurons can regulate chemoreception (23, 24), whereas TASK-2 (TWIK-related acid-sensitive K+; K2p5.1) channels function as a key component of CO2/H+ sensing (67). These dynamics question whether the viral infection observed in this study affects the regulatory and/or the CO2/H+-sensing function of these neurons. On the other hand, some astrocytes and microglia in the central nervous system are also CO2/H+ chemosensitive and involved in respiratory modulation (41). For example, astrocytes within the RTN can function as chemoreceptors by sensing CO2/H+ (18). They are highly chemosensitive to decreases in pH and capable of releasing adenosine triphosphate that propagates astrocytic Ca2+ excitation and activates chemoreceptor neurons, contributing to HCVR. Interestingly, lethal H5N1 viral infection has been reported to infect astrocytes and microglia in the central nervous system (66). Therefore, further clarification of HK483 viral replication in these glial cells, especially in RTN astrocytes, and determination of the changes in the local ATP release and the sensitivity of these neurons/cells to local CO2/H+ are necessary to mechanistically define the functional impacts of HK483 viral infection on local glial cells’ and neurons’ chemoreception. Nevertheless, our results support that lethal H5N1 viral replication in the pontomedullary respiratory-related nuclei, especially the chemosensitive neurons in the RTN, raphe, and LC, at least partially, contributes to dHCVR observed in HK483 mice.
Perspectives
Although the lethality of H5N1 virus has been linked to respiratory failure (8, 57, 61, 68a, 70, 71), the relevant mechanisms remain unclear. The current unavailability of an effective vaccine coupled with the continuous zoonotic transmission of endemic H5N1 viruses in domesticated birds makes widespread epidemics highly plausible, particularly if viral mutations lead to more sustainable human-to-human transmission. Therefore, it is critical to elucidate the pathogenesis of the lethality, such as respiratory failure, and develop the relevant therapeutic countermeasure to prevent and alleviate the lethality. Our results in this study suggest that replication of lethal H5N1 virus in the pontomedullary respiratory-related nuclei, especially the chemosensitive neurons in the RTN, raphe, and LC, likely contributes to dHCVR observed in HK483 virus- infected mice, leading to respiratory failure and subsequent death. These findings not only provide insight into the mechanisms by which lethal H5N1 viral infection induces respiratory failure but also benefit targeting the therapeutic intervention with respiratory failure. Further study is warranted to determine the impacts of viral infection on the chemosensitivity of these neurons (glial cells) in the RTN, raphe, and LC in vitro.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-119683.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.Z. and F.X. conceived and designed research; J.Z., N.Z., and C.Y. performed experiments; J.Z. and N.Z. analyzed data; J.Z., N.Z., and F.X. interpreted results of experiments; J.Z. and F.X. prepared figures; J.Z. and F.X. drafted manuscript; J.Z., N.Z., and F.X. edited and revised manuscript; F.X. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Ellen Blake for editing and Jennifer Tipper for intranasal instillation of H5N1 viruses. Parts of the results were presented in poster format at Experimental Biology 2015 in Boston and Experimental Biology 2016 in San Diego.
REFERENCES
- 1.Ashwood ER, Kost G, Kenny M. Temperature correction of blood-gas and pH measurements. Clin Chem 29: 1877–1885, 1983. [PubMed] [Google Scholar]
- 3.Bernard DG, Li A, Nattie EE. Evidence for central chemoreception in the midline raphé. J Appl Physiol (1985) 80: 108–115, 1996. doi: 10.1152/jappl.1996.80.1.108. [DOI] [PubMed] [Google Scholar]
- 4.Biancardi V, Bícego KC, Almeida MC, Gargaglioni LH. Locus coeruleus noradrenergic neurons and CO2 drive to breathing. Pflugers Arch 455: 1119–1128, 2008. doi: 10.1007/s00424-007-0338-8. [DOI] [PubMed] [Google Scholar]
- 5.Bissel SJ, Giles BM, Wang G, Olevian DC, Ross TM, Wiley CA. Acute murine H5N1 influenza A encephalitis. Brain Pathol 22: 150–158, 2012. doi: 10.1111/j.1750-3639.2011.00514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brennan M, Palaniswami M, Kamen P. Do existing measures of Poincaré plot geometry reflect nonlinear features of heart rate variability? IEEE Trans Biomed Eng 48: 1342–1347, 2001. doi: 10.1109/10.959330. [DOI] [PubMed] [Google Scholar]
- 7.Carroll JL. Developmental plasticity in respiratory control. J Appl Physiol (1985) 94: 375–389, 2003. doi: 10.1152/japplphysiol.00809.2002. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC) Update: isolation of avian influenza A (H5N1) viruses from humans—Hong Kong, 1997–1998. MMWR Mortal Wkly Rep 46: 1245–1247, 1998. . [PubMed] [Google Scholar]
- 9.Coleridge HM, Coleridge JC. Pulmonary reflexes: neural mechanisms of pulmonary defense. Annu Rev Physiol 56: 69–91, 1994. doi: 10.1146/annurev.ph.56.030194.000441. [DOI] [PubMed] [Google Scholar]
- 10.Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol 347: 64–86, 1994. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- 11.Donatti AF, Soriano RN, Sabino JP, Branco LG. Involvement of endogenous hydrogen sulfide (H2S) in the rostral ventrolateral medulla (RVLM) in hypoxia-induced hypothermia. Brain Res Bull 108: 94–99, 2014. doi: 10.1016/j.brainresbull.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Dutta A, Deshpande SB. Cardio-respiratory reflexes evoked by phenylbiguanide in rats involve vagal afferents which are not sensitive to capsaicin. Acta Physiol (Oxf) 200: 87–95, 2010. doi: 10.1111/j.1748-1716.2010.02105.x. [DOI] [PubMed] [Google Scholar]
- 13.Facci L, Stevens DA, Pangallo M, Franceschini D, Skaper SD, Strijbos PJ. Corticotropin-releasing factor (CRF) and related peptides confer neuroprotection via type 1 CRF receptors. Neuropharmacology 45: 623–636, 2003. doi: 10.1016/S0028-3908(03)00211-9. [DOI] [PubMed] [Google Scholar]
- 14.Feinstein DL, Kalinin S, Braun D. Causes, consequences, and cures for neuroinflammation mediated via the locus coeruleus: noradrenergic signaling system. J Neurochem 139, Suppl 2: 154–178, 2016. doi: 10.1111/jnc.13447. [DOI] [PubMed] [Google Scholar]
- 15.Gao P, Watanabe S, Ito T, Goto H, Wells K, McGregor M, Cooley AJ, Kawaoka Y. Biological heterogeneity, including systemic replication in mice, of H5N1 influenza A virus isolates from humans in Hong Kong. J Virol 73: 3184–3189, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gauda EB, Lawson EE. Developmental influences on carotid body responses to hypoxia. Respir Physiol 121: 199–208, 2000. doi: 10.1016/S0034-5687(00)00128-6. [DOI] [PubMed] [Google Scholar]
- 17.Gaytán SP, Calero F, Núñez-Abades PA, Morillo AM, Pásaro R. Pontomedullary efferent projections of the ventral respiratory neuronal subsets of the rat. Brain Res Bull 42: 323–334, 1997. doi: 10.1016/S0361-9230(96)00292-4. [DOI] [PubMed] [Google Scholar]
- 18.Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science 329: 571–575, 2010. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gozal D, Marcus CL, Ward SL, Keens TG. Ventilatory responses to passive leg motion in children with congenital central hypoventilation syndrome. Am J Respir Crit Care Med 153: 761–768, 1996. doi: 10.1164/ajrccm.153.2.8564130. [DOI] [PubMed] [Google Scholar]
- 20.Gregory DJ, Kobzik L. Influenza lung injury: mechanisms and therapeutic opportunities. Am J Physiol Lung Cell Mol Physiol 309: L1041–L1046, 2015. doi: 10.1152/ajplung.00283.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guyenet PG, Bayliss DA. Neural Control of Breathing and CO2 Homeostasis. Neuron 87: 946–961, 2015. doi: 10.1016/j.neuron.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guyenet PG, Stornetta RL, Bayliss DA. Retrotrapezoid nucleus and central chemoreception. J Physiol 586: 2043–2048, 2008. doi: 10.1113/jphysiol.2008.150870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hawkins VE, Hawryluk JM, Takakura AC, Tzingounis AV, Moreira TS, Mulkey DK. HCN channels contribute to serotonergic modulation of ventral surface chemosensitive neurons and respiratory activity. J Neurophysiol 113: 1195–1205, 2015. doi: 10.1152/jn.00487.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hawryluk JM, Moreira TS, Takakura AC, Wenker IC, Tzingounis AV, Mulkey DK. KCNQ channels determine serotonergic modulation of ventral surface chemoreceptors and respiratory drive. J Neurosci 32: 16943–16952, 2012. doi: 10.1523/JNEUROSCI.3043-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hodges MR, Klum L, Leekley T, Brozoski DT, Bastasic J, Davis S, Wenninger JM, Feroah TR, Pan LG, Forster HV. Effects on breathing in awake and sleeping goats of focal acidosis in the medullary raphe. J Appl Physiol (1985) 96: 1815–1824, 2004. doi: 10.1152/japplphysiol.00992.2003. [DOI] [PubMed] [Google Scholar]
- 26.Hodges MR, Opansky C, Qian B, Davis S, Bonis J, Bastasic J, Leekley T, Pan LG, Forster HV. Transient attenuation of CO2 sensitivity after neurotoxic lesions in the medullary raphe area of awake goats. J Appl Physiol (1985) 97: 2236–2247, 2004. doi: 10.1152/japplphysiol.00584.2004. [DOI] [PubMed] [Google Scholar]
- 27.Horie Y, Kimura H, Niijima M, Hamaoka T, Hasako K, Uruma T, Yoshida Y. Appropriate respiratory chemosensitivities to hypoxia and hypercapnia play crucial roles in protecting against a near fatal episode (Abstract) Am J Respir Crit Care Med 159: A786, 1999. [Google Scholar]
- 28.Iwasaki T, Itamura S, Nishimura H, Sato Y, Tashiro M, Hashikawa T, Kurata T. Productive infection in the murine central nervous system with avian influenza virus A (H5N1) after intranasal inoculation. Acta Neuropathol 108: 485–492, 2004. doi: 10.1007/s00401-004-0909-0. [DOI] [PubMed] [Google Scholar]
- 29.Jang H, Boltz D, Sturm-Ramirez K, Shepherd KR, Jiao Y, Webster R, Smeyne RJ. Highly pathogenic H5N1 influenza virus can enter the central nervous system and induce neuroinflammation and neurodegeneration. Proc Natl Acad Sci USA 106: 14063–14068, 2009. doi: 10.1073/pnas.0900096106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jensen P, Farago AF, Awatramani RB, Scott MM, Deneris ES, Dymecki SM. Redefining the serotonergic system by genetic lineage. Nat Neurosci 11: 417–419, 2008. doi: 10.1038/nn2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim HM, Park EH, Yum J, Kim HS, Seo SH. Greater virulence of highly pathogenic H5N1 influenza virus in cats than in dogs. Arch Virol 160: 305–313, 2015. doi: 10.1007/s00705-014-2284-z. [DOI] [PubMed] [Google Scholar]
- 32.Kitano M, Itoh Y, Ishigaki H, Nakayama M, Ishida H, Pham VL, Arikata M, Shichinohe S, Tsuchiya H, Kitagawa N, Kobayashi M, Yoshida R, Sato A, Le QM, Kawaoka Y, Ogasawara K. Efficacy of repeated intravenous administration of peramivir against highly pathogenic avian influenza A (H5N1) virus in cynomolgus macaques. Antimicrob Agents Chemother 58: 4795–4803, 2014. doi: 10.1128/AAC.02817-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kogo N, Arita H. In vivo study on medullary H(+)-sensitive neurons. J Appl Physiol (1985) 69: 1408–1412, 1990. doi: 10.1152/jappl.1990.69.4.1408. [DOI] [PubMed] [Google Scholar]
- 34.Li A, Emond L, Nattie E. Brainstem catecholaminergic neurons modulate both respiratory and cardiovascular function. Adv Exp Med Biol 605: 371–376, 2008. doi: 10.1007/978-0-387-73693-8_65. [DOI] [PubMed] [Google Scholar]
- 35.London NR, Zhu W, Bozza FA, Smith MC, Greif DM, Sorensen LK, Chen L, Kaminoh Y, Chan AC, Passi SF, Day CW, Barnard DL, Zimmerman GA, Krasnow MA, Li DY. Targeting Robo4-dependent Slit signaling to survive the cytokine storm in sepsis and influenza. Sci Transl Med 2: 23ra19, 2010. doi: 10.1126/scitranslmed.3000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madtes P Jr, Lee KH, King JS, Burry RW. Corticotropin releasing factor enhances survival of cultured GABAergic cerebellar neurons after exposure to a neurotoxin. Brain Res Dev Brain Res 151: 119–128, 2004. doi: 10.1016/j.devbrainres.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 37.Martín-Cora FJ, Fornal CA, Metzler CW, Jacobs BL. Single-unit responses of serotonergic medullary and pontine raphe neurons to environmental cooling in freely moving cats. Neuroscience 98: 301–309, 2000. doi: 10.1016/S0306-4522(00)00133-0. [DOI] [PubMed] [Google Scholar]
- 38.Maskrey M. Influence of body temperature on responses to hypoxia and hypercapnia: implications for SIDS. Clin Exp Pharmacol Physiol 22: 527–532, 1995. doi: 10.1111/j.1440-1681.1995.tb02061.x. [DOI] [PubMed] [Google Scholar]
- 39.Moreira TS, Takakura AC, Colombari E, Guyenet PG. Activation of 5-hydroxytryptamine type 3 receptor-expressing C-fiber vagal afferents inhibits retrotrapezoid nucleus chemoreceptors in rats. J Neurophysiol 98: 3627–3637, 2007. doi: 10.1152/jn.00675.2007. [DOI] [PubMed] [Google Scholar]
- 40.Morrison SF, Nakamura K, Madden CJ. Central control of thermogenesis in mammals. Exp Physiol 93: 773–797, 2008. doi: 10.1113/expphysiol.2007.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mulkey DK, Wenker IC, Kréneisz O. Current ideas on central chemoreception by neurons and glial cells in the retrotrapezoid nucleus. J Appl Physiol (1985) 108: 1433–1439, 2010. doi: 10.1152/japplphysiol.01240.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nattie EE, Li A. Central chemoreception in the region of the ventral respiratory group in the rat. J Appl Physiol (1985) 81: 1987–1995, 1996. doi: 10.1152/jappl.1996.81.5.1987. [DOI] [PubMed] [Google Scholar]
- 43.Nattie EE, Li A. Substance P-saporin lesion of neurons with NK1 receptors in one chemoreceptor site in rats decreases ventilation and chemosensitivity. J Physiol 544: 603–616, 2002. doi: 10.1113/jphysiol.2002.020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ng YP, Lee SM, Cheung TK, Nicholls JM, Peiris JS, Ip NY. Avian influenza H5N1 virus induces cytopathy and proinflammatory cytokine responses in human astrocytic and neuronal cell lines. Neuroscience 168: 613–623, 2010. doi: 10.1016/j.neuroscience.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 45.Osaka T. Hypoxia-induced hypothermia mediated by GABA in the rostral parapyramidal area of the medulla oblongata. Neuroscience 267: 46–56, 2014. doi: 10.1016/j.neuroscience.2014.02.035. [DOI] [PubMed] [Google Scholar]
- 46.Pan H, Zhang Y, Luo Z, Li P, Liu L, Wang C, Wang H, Li H, Ma Y. Autophagy mediates avian influenza H5N1 pseudotyped particle-induced lung inflammation through NF-κB and p38 MAPK signaling pathways. Am J Physiol Lung Cell Mol Physiol 306: L183–L195, 2014. doi: 10.1152/ajplung.00147.2013. [DOI] [PubMed] [Google Scholar]
- 47.Park CH, Ishinaka M, Takada A, Kida H, Kimura T, Ochiai K, Umemura T. The invasion routes of neurovirulent A/Hong Kong/483/97 (H5N1) influenza virus into the central nervous system after respiratory infection in mice. Arch Virol 147: 1425–1436, 2002. doi: 10.1007/s00705-001-0750-x. [DOI] [PubMed] [Google Scholar]
- 48.Paxinos G, Franklin KB. The Mouse Brain in Stereotaxic Coordinates, Ed2. San Diego, CA: Academic Press, 2004. [Google Scholar]
- 49.Peng W, Zhuang J, Harrod KS, Xu F. Respiratory syncytial virus infection in anesthetized weanling rather than adult rats prolongs the apneic responses to right atrial injection of capsaicin. J Appl Physiol (1985) 102: 2201–2206, 2007. doi: 10.1152/japplphysiol.01436.2006. [DOI] [PubMed] [Google Scholar]
- 50.Plourde JR, Pyles JA, Layton RC, Vaughan SE, Tipper JL, Harrod KS. Neurovirulence of H5N1 infection in ferrets is mediated by multifocal replication in distinct permissive neuronal cell regions. PLoS One 7: e46605, 2012. doi: 10.1371/journal.pone.0046605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quintana A, Zanella S, Koch H, Kruse SE, Lee D, Ramirez JM, Palmiter RD. Fatal breathing dysfunction in a mouse model of Leigh syndrome. J Clin Invest 122: 2359–2368, 2012. doi: 10.1172/JCI62923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quintero MC, Putnam RW, Cordovez JM. Theoretical perspectives on central chemosensitivity: CO2/H+-sensitive neurons in the locus coeruleus. PLOS Comput Biol 13: e1005853, 2017. doi: 10.1371/journal.pcbi.1005853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rekling JC, Feldman JL. PreBötzinger complex and pacemaker neurons: hypothesized site and kernel for respiratory rhythm generation. Annu Rev Physiol 60: 385–405, 1998. doi: 10.1146/annurev.physiol.60.1.385. [DOI] [PubMed] [Google Scholar]
- 54.Ribas-Salgueiro JL, Gaytán SP, Ribas J, Pásaro R. Characterization of efferent projections of chemosensitive neurons in the caudal parapyramidal area of the rat brain. Brain Res Bull 66: 235–248, 2005. doi: 10.1016/j.brainresbull.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 55.Robinson DM, Peebles KC, Kwok H, Adams BM, Clarke LL, Woollard GA, Funk GD. Prenatal nicotine exposure increases apnoea and reduces nicotinic potentiation of hypoglossal inspiratory output in mice. J Physiol 538: 957–973, 2002. doi: 10.1113/jphysiol.2001.012705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ruiz AV. Carbon dioxide response curves during hypothermia. Pflugers Arch 358: 125–133, 1975. doi: 10.1007/BF00583923. [DOI] [PubMed] [Google Scholar]
- 57.Soepandi PZ, Burhan E, Mangunnegoro H, Nawas A, Aditama TY, Partakusuma L, Isbaniah F, Ikhsan M, Swidarmoko B, Sutiyoso A, Malik S, Benamore R, Baird JK, Taylor WR. Clinical course of avian influenza A(H5N1) in patients at the Persahabatan Hospital, Jakarta, Indonesia, 2005-2008. Chest 138: 665–673, 2010. doi: 10.1378/chest.09-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stark GV, Long JP, Ortiz DI, Gainey M, Carper BA, Feng J, Miller SM, Bigger JE, Vela EM. Clinical profiles associated with influenza disease in the ferret model. PLoS One 8: e58337, 2013. doi: 10.1371/journal.pone.0058337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol 572: 503–523, 2006. doi: 10.1113/jphysiol.2005.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tankersley CG, Broman KW. Interactions in hypoxic and hypercapnic breathing are genetically linked to mouse chromosomes 1 and 5. J Appl Physiol (1985) 97: 77–84, 2004. doi: 10.1152/japplphysiol.01102.2003. [DOI] [PubMed] [Google Scholar]
- 61.Tumpey TM, Lu X, Morken T, Zaki SR, Katz JM. Depletion of lymphocytes and diminished cytokine production in mice infected with a highly virulent influenza A (H5N1) virus isolated from humans. J Virol 74: 6105–6116, 2000. doi: 10.1128/JVI.74.13.6105-6116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van Beek JH, Berkenbosch A, de Goede J, Olievier CN. Effects of brain stem hypoxaemia on the regulation of breathing. Respir Physiol 57: 171–188, 1984. doi: 10.1016/0034-5687(84)90091-4. [DOI] [PubMed] [Google Scholar]
- 63.Van Bockstaele EJ, Valentino RJ. Neuropeptide regulation of the locus coeruleus and opiate-induced plasticity of stress responses. Adv Pharmacol 68: 405–420, 2013. doi: 10.1016/B978-0-12-411512-5.00019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van den Brand JM, Stittelaar KJ, van Amerongen G, van de Bildt MW, Leijten LM, Kuiken T, Osterhaus AD. Experimental pandemic (H1N1) 2009 virus infection of cats. Emerg Infect Dis 16: 1745–1747, 2010. doi: 10.3201/eid1611.100845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Volgin D, Volgina A, Seredenko M. Involvement of nitric oxide in regulation of the medullary respiratory rhythm in neonatal rats. Acta Neurobiol Exp (Wars) 60: 175–186, 2000. [DOI] [PubMed] [Google Scholar]
- 66.Wang G, Zhang J, Li W, Xin G, Su Y, Gao Y, Zhang H, Lin G, Jiao X, Li K. Apoptosis and proinflammatory cytokine responses of primary mouse microglia and astrocytes induced by human H1N1 and avian H5N1 influenza viruses. Cell Mol Immunol 5: 113–120, 2008. doi: 10.1038/cmi.2008.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang S, Benamer N, Zanella S, Kumar NN, Shi Y, Bévengut M, Penton D, Guyenet PG, Lesage F, Gestreau C, Barhanin J, Bayliss DA. TASK-2 channels contribute to pH sensitivity of retrotrapezoid nucleus chemoreceptor neurons. J Neurosci 33: 16033–16044, 2013. doi: 10.1523/JNEUROSCI.2451-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wolsink JG, Berkenbosch A, DeGoede J, Olievier CN. The effects of hypoxia on the ventilatory response to sudden changes in CO2 in newborn piglets. J Physiol 456: 39–48, 1992. doi: 10.1113/jphysiol.1992.sp019325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68a.Writing Committee of the Second World Health Organization Consultation on Clinical Aspects of Human Infection with Avian Influenza A (H5N1) Virus; Abdel-Ghafar AN, Chotpitayasunondh T, Gao Z, Hayden FG, Nguyen DH, de Jong MD, Naghdaliyev A, Peiris JS, Shindo N, Soeroso S, Uyeki TM. Update on avian influenza A (H5N1) virus infection in humans. N Engl J Med 358: 261–273, 2008. doi: 10.1056/NEJMra0707279. [DOI] [PubMed] [Google Scholar]
- 69.Xu F, Zhuang J, Wang R, Seagrave JC, March TH. Blunted ventilatory response to hypoxia/hypercapnia in mice with cigarette smoke-induced emphysema. Respir Physiol Neurobiol 158: 5–13, 2007. doi: 10.1016/j.resp.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu H, Gao Z, Feng Z, Shu Y, Xiang N, Zhou L, Huai Y, Feng L, Peng Z, Li Z, Xu C, Li J, Hu C, Li Q, Xu X, Liu X, Liu Z, Xu L, Chen Y, Luo H, Wei L, Zhang X, Xin J, Guo J, Wang Q, Yuan Z, Zhou L, Zhang K, Zhang W, Yang J, Zhong X, Xia S, Li L, Cheng J, Ma E, He P, Lee SS, Wang Y, Uyeki TM, Yang W. Clinical characteristics of 26 human cases of highly pathogenic avian influenza A (H5N1) virus infection in China. PLoS One 3: e2985, 2008. doi: 10.1371/journal.pone.0002985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yuen KY, Chan PK, Peiris M, Tsang DN, Que TL, Shortridge KF, Cheung PT, To WK, Ho ET, Sung R, Cheng AF. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet 351: 467–471, 1998. doi: 10.1016/S0140-6736(98)01182-9. [DOI] [PubMed] [Google Scholar]
- 72.Zhang Z, Zhang C, Zhou M, Xu F. Activation of opioid μ-receptors, but not δ- or κ-receptors, switches pulmonary C-fiber-mediated rapid shallow breathing into an apnea in anesthetized rats. Respir Physiol Neurobiol 183: 211–217, 2012. doi: 10.1016/j.resp.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Z, Zhang C, Zhuang J, Xu F. Contribution of central μ-receptors to switching pulmonary C-fibers-mediated rapid shallow breathing into an apnea by fentanyl in anesthetized rats. Brain Res 1469: 73–81, 2012. doi: 10.1016/j.brainres.2012.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Z, Zhang J, Huang K, Li KS, Yuen KY, Guan Y, Chen H, Ng WF. Systemic infection of avian influenza A virus H5N1 subtype in humans. Hum Pathol 40: 735–739, 2009. doi: 10.1016/j.humpath.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhuang J, Gao P, Pollock Z, Harrod KS, Xu F. Depressed Hypoxic and Hypercapnic Ventilatory Responses at Early Stage of Lethal Avian Influenza A Virus Infection in Mice. PLoS One 11: e0147522, 2016. doi: 10.1371/journal.pone.0147522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhuang J, Gao X, Gao F, Xu F. Mu-opioid receptors in the caudomedial NTS are critical for respiratory responses to stimulation of bronchopulmonary C-fibers and carotid body in conscious rats. Respir Physiol Neurobiol 235: 71–78, 2017. doi: 10.1016/j.resp.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 77.Zhuang J, Xu F.. Aerosolized capsaicin (CAP) pretreatment prevents lipopolysaccharide (LPS)-induced inhibition of ventilatory responses to hypoxia and hypercapnia in rats. FASEB J 22: 1172.1114, 2008. [Google Scholar]
- 78.Zhuang J, Zang N, Pollock Z, Ye C, Tipper J, Xu F.. Depressed hypoxic and hypercapnic ventilatory responses (dHVR and dHCVR) occur in mice at the late stage of lethal avian influenza A (H5N1) virus infection. FASEB J 29: 860.863, 2015. [Google Scholar]
- 79.Zhuang J, Zang N, Ye C, Xu F. Lethal avian influenza A (H5N1) virus induces ataxic breathing in mice with apoptosis of pre-Botzinger complex neurons expressing neurokinin-1 receptor. Am J Physiol Lung Cell Mol Physiol 313: L772–L780, 2017. doi: 10.1152/ajplung.00145.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhuang J, Zhao L, Zang N, Xu F. Prenatal nicotinic exposure augments cardiorespiratory responses to activation of bronchopulmonary C-fibers. Am J Physiol Lung Cell Mol Physiol 308: L922–L930, 2015. doi: 10.1152/ajplung.00241.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zuperku EJ, Stucke AG, Hopp FA, Stuth EA. Characteristics of breathing rate control mediated by a subregion within the pontine parabrachial complex. J Neurophysiol 117: 1030–1042, 2017. doi: 10.1152/jn.00591.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]