Abstract
We previously showed that mice deficient in apoptosis signal-regulating kinase-1 (ASK1) were partially protected against ventilator-induced lung injury. Because ASK1 can promote both cell death and inflammation, we hypothesized that ASK1 activation regulates inflammasome-mediated inflammation. Mice deficient in ASK1 expression (ASK1−/−) exhibited significantly less inflammation and lung injury (as measured by neutrophil infiltration, IL-6, and IL-1β) in response to treatment with inhaled lipopolysaccharide (LPS) compared with wild-type (WT) mice. To determine whether this proinflammatory response was mediated by ASK1, we investigated inflammasome-mediated responses to LPS in primary macrophages and bone marrow-derived macrophages (BMDMs) from WT and ASK1−/− mice, as well as the mouse alveolar macrophage cell line MH-S. Cells were treated with LPS alone for priming or LPS followed by ATP for activation. When macrophages were stimulated with LPS followed by ATP to activate the inflammasome, we found a significant increase in secreted IL-1β from WT cells compared with ASK1-deficient cells. LPS priming stimulated an increase in NOD-like receptor 3 (NLRP3) and pro-IL-1β in WT BMDMs, but expression of NLRP3 was significantly decreased in ASK1−/− BMDMs. Subsequent ATP treatment stimulated an increase in cleaved caspase-1 and IL-1β in WT BMDMs compared with ASK1−/− BMDMs. Similarly, treatment of MH-S cells with LPS + ATP caused an increase in both cleaved caspase-1 and IL-1β that was diminished by the ASK-1 inhibitor NQDI1. These results demonstrate, for the first time, that ASK1 promotes inflammasome priming.
Keywords: acute respiratory distress syndrome, apoptosis signal regulating kinase-1, LPS priming, lung inflammation
INTRODUCTION
Acute respiratory distress syndrome (ARDS) remains a tragic illness with high morbidity and mortality (6, 11, 24, 32, 42, 46). Although mortality associated with ARDS has decreased, it still remains high at ~40% (32). Interleukin-1β (IL-1β) is an inflammatory mediator produced by macrophages and known to be chemotactic for neutrophils and to stimulate proliferation of fibroblasts (16, 45). Previous studies have shown a correlation between increased IL-1β in the bronchoalveolar lavage fluid (BALF) of patients with ARDS and those at high risk of developing ARDS compared with BALF from healthy individuals (2, 17, 24, 42, 45). The increase in IL-1β, IL-6, and other inflammatory mediators has been shown to occur early in the progression of ARDS, with concomitant IL-1β receptor antagonists implicating it as important early in the inflammatory insult (39).
Activation of pattern recognition receptors (PRRs) results in gene expression of numerous inflammatory mediators in alveolar macrophages and other immune cells. Recent studies have focused on the role of an intracellular PRR, NOD-like receptor 3 (NLRP3), in acute lung injury (ALI) and ARDS (5, 12, 18, 27, 28). NLRP3 is one component of the inflammasome, which also consists of the adaptor molecular apoptosis-associated speck-like protein containing a CARD (ASC) and caspase-1 (3, 5, 23, 30). Following recognition of its ligand, NLRP3 associates with ASC and procaspase-1, resulting in activation of caspase-1. Caspase-1 has many substrates, including pro-IL-1β, which when cleaved to its biologically active form plays a critical role in ARDS. Because of the potential for proinflammatory-associated tissue damage, IL-1β production is highly regulated. The canonical pathway of IL-1β production requires the following two steps: 1) priming [production of the nonactive precursor (pro-IL-1β)], followed by 2) activation (NLRP3 inflammasome scaffold formation and subsequent cleavage of caspase-1, resulting in active caspase-1 and proteolytic cleavage of pro-IL-1β in its bioactive form) (22, 40, 44, 49, 52). The expression of NLRP3 and pro-IL-1β can be induced by Toll-like receptors (TLRs) via the NF-κB signaling pathway. Although some progress has been made in understanding how the NLRP3 inflammasome is regulated, there are still large gaps in our understanding of this.
Previous studies have suggested a role for apoptosis signal-regulating kinase-1 (ASK1) in ventilator-induced lung injury (VILI) (34), hyperoxia-induced lung injury, production of IL-1β and TNF-α (8), and lipopolysaccharide (LPS)-induced septic shock in mice (31). Other studies have suggested that ASK1 is an intermediate signal in TLR-associated innate immune responses (53, 54). However, there have been no studies investigating the role of ASK1 in NLRP3 inflammasome activation. We hypothesized that the ASK1 pathway promotes assembly and activation of the NLRP3 inflammasome in alveolar macrophages in ALI, leading to an increase in IL-1β secretion. Using an LPS model in both mice and cultured cells, we found that deficiency or inhibition of ASK1 significantly reduced markers of lung injury and NLRP3-mediated inflammation.
MATERIALS AND METHODS
Mice.
The Institutional Animal Care and Use Committee for both the University of Tennessee Health Science Center and the University of Kentucky approved all animal procedures. ASK1 knockout mice (ASK1−/−, kindly provided by H. Ichijo, University of Tokyo) and C57BL/6N mice (Harlan, Indianapolis, IN) were used to isolate bone marrow cells and bronchoalveolar lavage fluid cells. The ASK1 knockout mice were derived from the C57BL/6J strain. Mice (male and female) ages 10–16 wk were used in all experiments.
In vivo LPS stimulation.
We exposed mice to normal saline nebulization (vehicle) ± LPS (5 mg/ml for 15 min). After 3, 6, or 24 h, the mice were anesthetized with intraperitoneal ketamine (80 mg/kg) and xylazine (15 mg/kg), and a tracheostomy was performed using an 18-gauge catheter. Quasistatic lung compliance was measured from pressure-volume loops using the flexiVent (SCIREQ, Montreal, QC, Canada), and BALF was collected using 2 × 0.7 ml PBS. One aliquot of cells was counted using a hemocytometer, and a separate aliquot was cytospun on a glass slide. The hemocytometer counts were used to quantify macrophages and neutrophils based on size and morphology. In addition, the cytospun slides were stained, and then a differential cell count was performed based on the size, morphology, and staining of the cells to confirm the percentage of macrophages and neutrophils. Negligible lymphocytes and eosinophils were counted. At least 100 were counted for each differential measurement. The lungs were then retrograde perfused with 10–20 ml PBS, and lung tissue was collected for histology.
Isolation of macrophages.
Macrophages were isolated by consecutive bronchoalveolar lavage as previously described (7, 43, 52, 55) with some minor modifications indicated below. Mice were anesthetized by an intraperitoneal injection of ketamine-xylazine (80 mg/kg-15 mg/kg). A tracheostomy was performed as described above. Eight consecutive BALF of sterile Mg2+- and Ca2+-free PBS with 2 mM EDTA with 0.7 ml/lavage were performed per animal. BALF was centrifuged at 400 g for 5 min. Cell pellets were suspended in 5 ml of R10-DMEM. The total number of cells in the suspension was counted using a hemocytometer, and aliquots of 0.4–0.8 × 106 cells were seeded in 12-well plates and allowed to adhere for 2 h. We examined aliquots of cells before adhesion by anti-F4/80 immunofluorescence and found that >98% of the cells were macrophages (data not shown).
Isolation of bone marrow-derived macrophages.
Bone marrow-derived macrophages (BMDMs) were isolated and differentiated as previously described (55), with some minor modifications indicated below. Postmortem, bilateral femurs and tibias were harvested, and bone marrow was isolated by centrifugation at 10,000 g for 15 s, resuspended in fetal bovine serum containing DMSO, and frozen at −80°C until used. At the start of each experiment, bone marrow cells were thawed quickly at 37°C and placed in 10 ml of BMDM growth medium (R10 medium supplemented with 20% L-929-conditioned media, as a source of macrophage-colony-stimulating factor) in 100-mm petri dishes. On day 4, 8 ml of fresh BMDM growth media were added, and the cells were allowed to differentiate for 3 additional days, at which time essentially 100% of the cells were macrophages (55). Following 7 days in culture, nonadherent cells were removed, and the remaining cells were washed with 1× PBS before being lifted from the petri dishes with cell stripper (Corning CellStripper Dissociation reagent). Cells were counted, spun down at 1,500 rpm for 10 min, and resuspended in BMDM growth media before being placed in 12-well culture dishes at a cell density of 4.0 × 105 to 5.0 × 105. Cells were given 24 h to adhere before each experiment. We examined cell morphology at this stage using a Diff-Quik stain following a cytospin, and we separately examined anti-F4/80 immunofluorescence and found that >98% of the cells were macrophages (data not shown).
ASK1 inhibitor, NQDI1, in BMDMs and MH-S cells.
MH-S cells (Sigma-Aldrich) were pretreated for 1 h with or without the ASK1 inhibitor NQDI1 (2 μM; Sigma-Aldrich) before LPS ± ATP treatment. The Ki for NQDI1 was reported to be 500 nM (50). BMDMs were plated in 12-well culture dishes at a cell density of 5.0 × 105 and allowed to adhere for 24 h. The next day, medium was removed, and the following conditions were applied to the cells: 1) media + vehicle for 1 h and then media + vehicle for 30 min; 2) LPS (1 μg/ml) + vehicle for 1 h and then ATP (5 mM) + vehicle for 30 min; 3) NQDI1 (2 μM) for 1 h and then LPS + NQDI1 for 1 h followed by ATP + NQDI1 for 30 min; 4) LPS + vehicle for 1 h and then ATP + NQDI1 for 30 min; and 5) NQDI1 for 1 h and then LPS + vehicle for 1 h followed by ATP + vehicle for 30 min. Supernatants were collected at each time point, centrifuged at 3,000 g for 5 min to remove any cellular debris, and then stored at −80°C. Cell lysates were collected after the last time point for each condition, sonicated for 30 s, and then stored at −80°C.
In vitro LPS stimulation.
Cells were treated either with LPS (1 μg/ml, 055:B5; Sigma) for 2, 4, or 6 h for priming studies, or for 1 h of LPS (1 μg/ml), followed by 30 min of ATP (5 mM) for activation studies. Nigericin-stimulated MH-S cells and BMDMs were used as a positive control for caspase-1 activation. Supernatant was collected, centrifuged at 1,000 g for 10 min at 4°C, separated into aliquots in 450 µl, and stored at −80°C. Cell lysates were collected with Laemmli 1× SDS sample buffer (Bio-Rad), sonicated for 30 s, and stored at −20°C.
Western blot analysis.
After being boiled at 80°C, cell lysates were separated using SDS gel electrophoresis and then transferred to 0.2 µm polyvinylidene difluoride membranes. These membranes were blocked in 1% TBS-casein with 0.1% Tween or 5% milk and then incubated with anti-IL-1β (1:1,000, no. 46812507; Cell Signaling), anti-NLRP3 [1:1,000, D4D8T/46815101 (Cell Signaling) and 1:250, 25N10E9 (Thermo Scientific)], and anti-caspase-1p20 (1:1,000, 1055042/AG-20B-0042-C100; Adipogen) antibodies. The membranes were washed with Tris-buffered saline-Tween 20, incubated with species-specific horseradish peroxidase-conjugated secondary antibody (1:2,000), and visualized with Luminatea Forte Western horseradish peroxidase substrate. Band densitometry measurements to determine relative quantities of protein were performed using ImageJ 1.42 software for Windows (https://imagej.nih.gov/ij).
ELISA.
IL-1β was measured in the cell supernatant and BALF using species-specific ELISA kits as per the manufacturer’s instructions [BD559603 (BD Biosciences) and DY401 (R&D Systems)]. Additionally, IL-6 was measured in BALF using a species-specific ELISA kit as per the manufacturer’s instructions (BD555240; BD Biosciences). For data analysis, a curve fit was applied to the standards, and the sample concentrations were extrapolated from the standard curve using four-parameter logistic software.
Statistical analysis.
Statistical analysis was performed with SigmaPlot (Jandel Scientific) and GraphPad Prism (GraphPad Software, La Jolla, CA). Comparison of variables between two groups was performed using a two-way ANOVA, normality and equal variance tests with Shapiro-Wilk, and pairwise multiple comparison using Holm-Sidak methods. P values <0.05 were considered significant. Unless otherwise stated, data were plotted as means ± SE, and significant differences are indicated.
RESULTS
ASK1 deficiency abrogated LPS-induced lung injury.
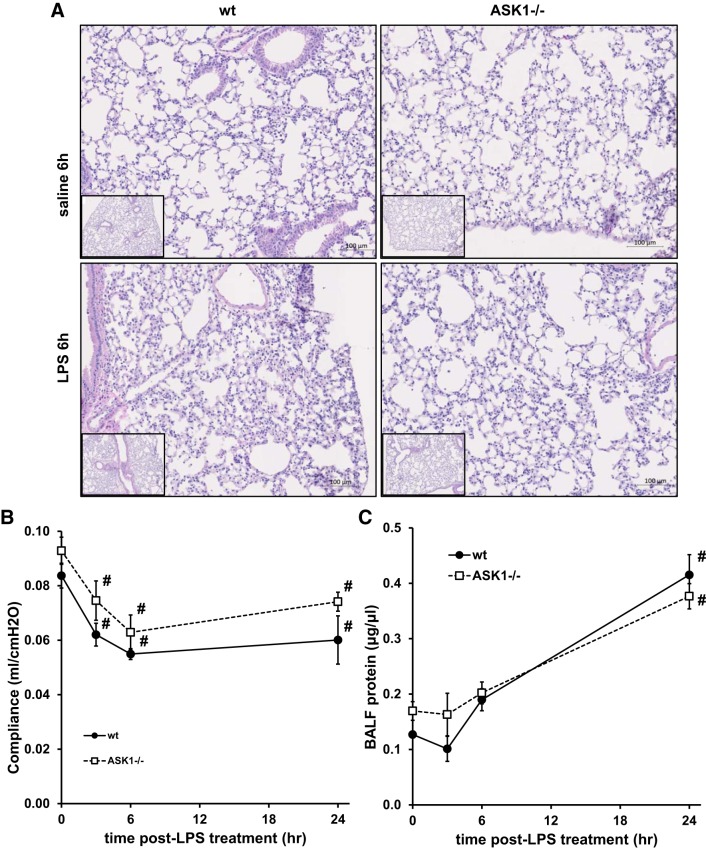
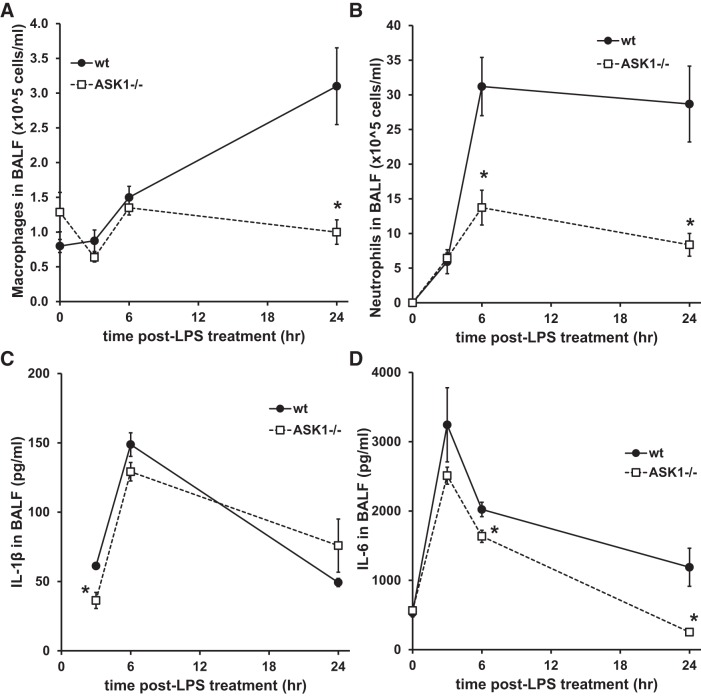
To investigate the role of ASK1 in lung inflammation, mice were treated with aerosolized LPS for 15 min, and responses were measured 3, 6, and 24 h later. As shown in Fig. 1, LPS treatment caused increased inflammation, neutrophil accumulation, and protein in the BALF of wild-type (WT) mice as indicated by histology and BALF protein measurements. In addition, lung compliance was significantly decreased 3, 6, and 24 h after LPS treatment in WT mice (Fig. 1B). ASK1−/− mice exhibited decreased infiltration of immune cells in lung tissue, but the change in compliance was not significantly different from WT mice. Protein leak in the BALF did not appear to be abrogated by ASK1 deficiency, since there were no significant differences between WT and ASK1−/− mice (Fig. 1C). LPS treatment also stimulated an increase in macrophages and neutrophils in the BALF in WT mice, but these changes were significantly reduced in ASK1−/− mice (Fig. 2). Macrophage accumulation in BALF was reduced at 24 h in ASK1−/− mice, whereas neutrophil infiltration was decreased at 6 and 24 h. LPS treatment stimulated a rapid increase in both IL-1β and IL-6 in BALF in WT and ASK1−/− mice, but cytokine levels decreased by 24 h. There was a significant decrease in IL-1β in the BALF of ASK1−/− mice at the earliest measurement time after LPS (3 h), whereas IL-6 was reduced at 6 and 24 h.
Fig. 1.
Apoptosis signal-regulating kinase-1 (ASK1) deficiency abrogated lipopolysaccharide (LPS)-induced lung injury. Wild-type (WT) and ASK1-deficient (ASK1−/−) mice were either unexposed [time (t) = 0 h] or exposed to nebulized LPS in normal saline for 15 min, and measurements of lung compliance were made at the indicated times, followed by collection of bronchoalveolar lavage (BAL) fluid and tissue for histology. A: representative hematoxylin- and eosin-stained lung sections 6 h after exposure to saline or LPS indicating increased inflammation following LPS treatment in WT mice. B: quasistatic lung compliance was measured in WT and ASK1−/− mice using the flexiVent, demonstrating a significant reduction in WT mice following LPS exposure (n = 4–7 mice/group). #Significant difference from untreated (t = 0 h) mice (P < 0.05). C: LPS treatment caused increased protein in the BAL fluid (n = 4–6). #Significant difference from t = 0 h mice (P < 0.05). There were no significant differences in compliance or protein between WT and ASK1−/− mice at any of the time points.
Fig. 2.
Lipopolysaccharide (LPS)-induced bronchoalveolar lavage (BAL) fluid cell counts and cytokines were reduced in apoptosis signal-regulating kinase-1 (ASK1)-deficient (ASK1−/−) mice. Aerosolized LPS induced inflammatory cytokine secretion and cell recruitment in wild-type (WT) mice, but these effects were decreased in ASK1−/− mice. BAL fluid was collected from mice at the indicated times following exposure to LPS, and macrophages (A) and neutrophils (B) were quantified via microscopy. C and D: IL-1β (C) and IL-6 (D) were measured in the BAL fluid using ELISAs (BD Biosciences). *Significant difference from WT (P < 0.05; n = 4–5 in A, 4–6 in B, 3–6 in C, and 4–6 in D).
ASK1 deficiency decreased inflammasome activation in primary macrophages.
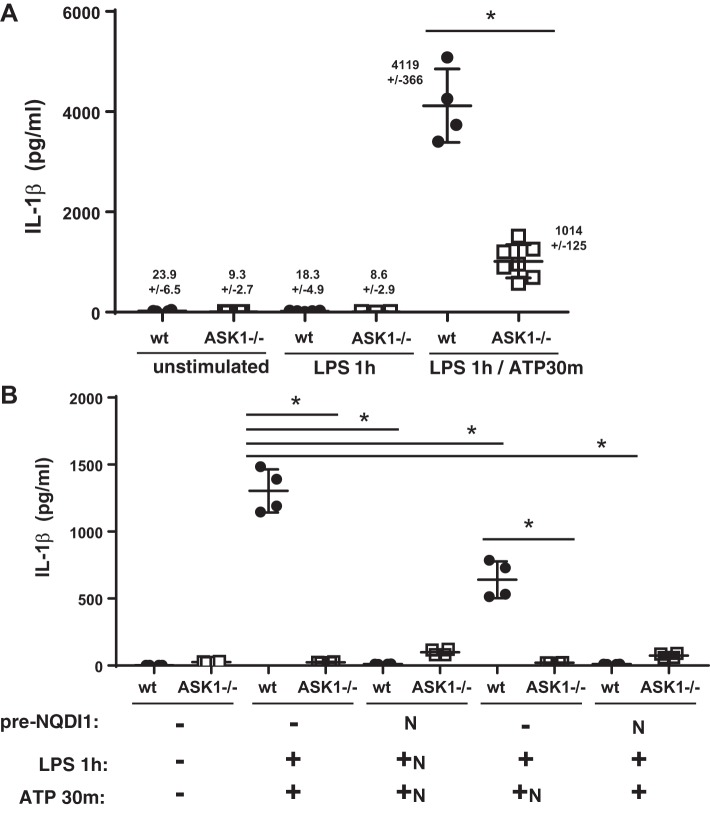
Because we observed reduced IL-1β, IL-6, and neutrophil recruitment in the ASK1−/− mice similar to what was previously reported by Kuipers et al. (20) using NLRP3 knockout mice in a mechanical ventilation-induced lung injury model, we investigated whether ASK1 deficiency affected NLRP3 inflammasome activation. Stimulation of primary macrophages from WT mice with LPS followed by ATP (to activate the inflammasome) resulted in a significant increase in secreted IL-1β compared with unstimulated macrophages or macrophages treated with LPS alone (Fig. 3A). Consistent with the 3-h results from mice exposed to LPS, the amount of IL-1β secreted from ASK1−/− macrophages in response to LPS + ATP was significantly less than that released by WT macrophages. Because the number of macrophages recovered from BALF is limited, we continued experiments examining the response to LPS + ATP in BMDMs. As shown in Fig. 3B, unstimulated BMDMs secreted negligible IL-1β, but LPS + ATP stimulated significant IL-1β release from WT cells. ASK1-deficient BMDMs secreted significantly less IL-1β. To determine whether pharmacological inhibition of ASK1 would diminish IL-1β secretion, we treated BMDMs with NQDI1 (2 μM). When cells were pretreated with NQDI1, and the inhibitor was present during priming and activation, secretion of IL-1β was almost completely abolished in WT cells. If NQDI1 was present only during the activation step (+ATP), IL-1β secretion from WT cells was diminished compared with untreated cells, but ASK1-deficient cells secreted significantly less IL-1β.
Fig. 3.
Apoptosis signal-regulating kinase-1 (ASK1)-deficient (ASK1−/−) alveolar macrophages and bone marrow-derived macrophages (BMDM) secreted less IL-1β compared with cells from wild-type (WT) mice in response to lipopolysaccharide (LPS) priming and ATP activation. A: primary macrophages were obtained from bronchoalveolar lavage (BAL) fluid of WT and ASK1−/− mice and placed in culture. Cells were treated with LPS for 1 h followed by ATP for 30 min. Supernatants were collected, and IL-1β was measured by ELISA (BD Biosciences kit, n = 3–7). B: BMDMs were exposed to vehicle control ± the ASK1 inhibitor NQDI1 (N) for 1 h, and then exposed to LPS for 1 h ± NQDI1, followed by ATP for 30 min ± NQDI1. Supernatants were collected, and IL-1β was measured by ELISA (n = 4; R&D Systems kit). *Significant difference from WT (P < 0.05).
Inflammasome priming and activation were decreased in bone marrow-derived macrophages from ASK1−/− mice.
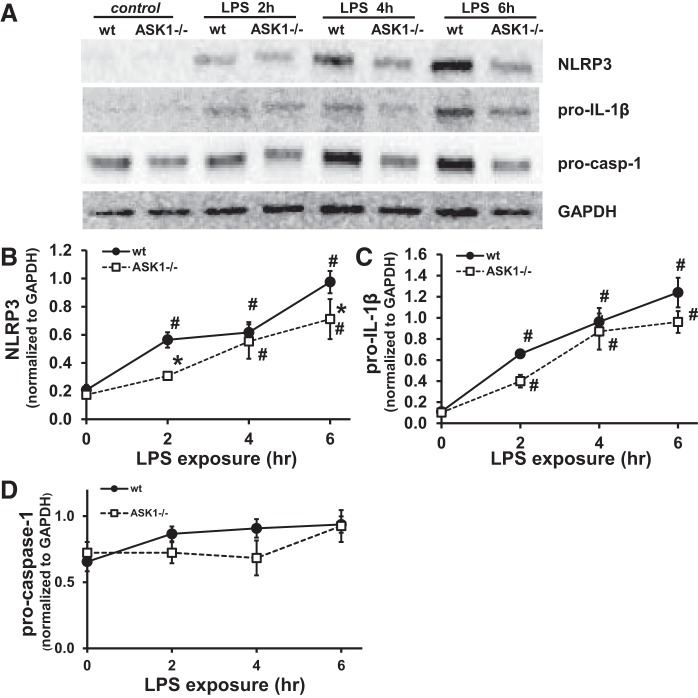
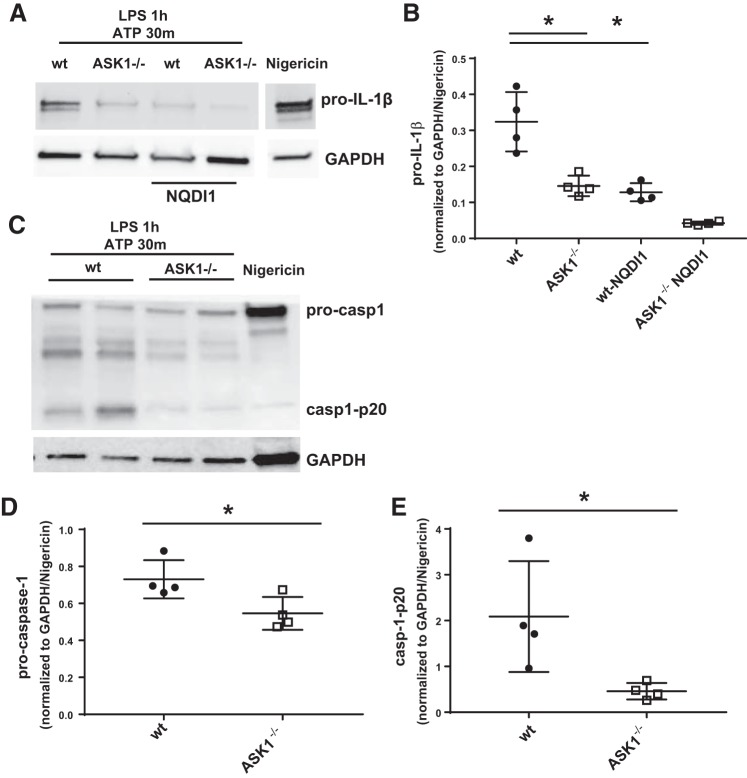
To determine whether ASK1 deficiency decreased LPS-induced priming of the inflammasome, we examined the expression of NLRP3, pro-IL-1β, and procaspase-1 by Western blot following treatment of BMDMs with LPS. As expected, expression of NLRP3 and pro-IL-1β increased in WT BMDMs following LPS treatment (Fig. 4), with the significant increase in NLRP3 and pro-IL-1β occurring within 2 h. In contrast, NLRP3 expression in the ASK1−/− BMDMs following LPS stimulation increased after only 4 h. Pro-IL-1β was significantly increased after 2 h in both WT and ASK1−/− cells. Procaspase-1 did not significantly increase in WT or ASK1-deficient cells as shown in Fig. 4D. To investigate NLRP3 inflammasome activation, cells were treated with LPS (1 h) followed by ATP. Nigericin treatment was used as a positive control and for normalization (Fig. 5). The results show that there was a significant reduction in pro-IL-1β, cleaved caspase-1-p20, and procaspase-1 in ASK1−/− BMDMs compared with WT. When WT BMDMs were treated with NQDI1 during the priming and activation steps, there was a significant decrease in pro-IL-1β (Fig. 5B) compared with untreated WT cells.
Fig. 4.
Lipopolysaccharide (LPS)-induced priming of bone marrow-derived macrophages (BMDMs) was decreased in ASK-1-deficient cells. BMDMs were isolated and treated with LPS for 2, 4, or 6 h. A: representative Western blots of NOD-like receptor 3 (NLRP3), pro-IL-1β, and procaspase-1 after LPS treatment of BMDMs. GAPDH was used as a loading control, and densitometry values were normalized to GAPDH. Each protein indicated was immunoblotted separately from independent gels, and each set of bands for a given protein was cropped from the same blot. Densitometry analysis is shown for NLRP3 (B), pro-IL-1β (C), and procaspase-1 (D) expression following LPS stimulation in wild-type and apoptosis signal-regulating kinase-1-deficient (ASK1−/−) BMDMs. P < 0.05, significant difference from wild type (*) and untreated (#) (n = 8–9 for B, 5–7 for C, and 6–7 for D).
Fig. 5.
Inflammasome activation was decreased in apoptosis signal-regulating kinase-1 (ASK1)-deficient (ASK1−/−) bone marrow-derived macrophages (BMDMs). Cell lysates were collected from BMDMs after lipopolysaccharide (LPS) stimulation for 1 h followed by ATP for 30 min. Pro-IL-1β, procaspase-1, and cleaved caspase-1-p20 were decreased in ASK1−/− BMDMs compared with wild type (WT). A and C: representative Western blots of pro-IL-1β, procaspase-1, and cleaved caspase-1-p20 from independent experiments. A includes results from BMDMs pretreated with NQDI1, with the inhibitor present throughout the experiment. GAPDH was used as a loading control. Each protein indicated was immunoblotted separately, and each set of bands for a given protein was cropped from the same blot. Unrelated lanes were removed for clarity in A (break between nigericin-treated and other samples). Nigericin treatment was used as a positive control, and all densitometry values were normalized to GAPDH and then to nigericin/GAPDH. Densitometry analysis of pro-IL-1β (B), procaspase-1 (D), and cleaved caspase-1-p20 (E) expression is shown. *Significant difference compared with WT (P < 0.05; n = 4).
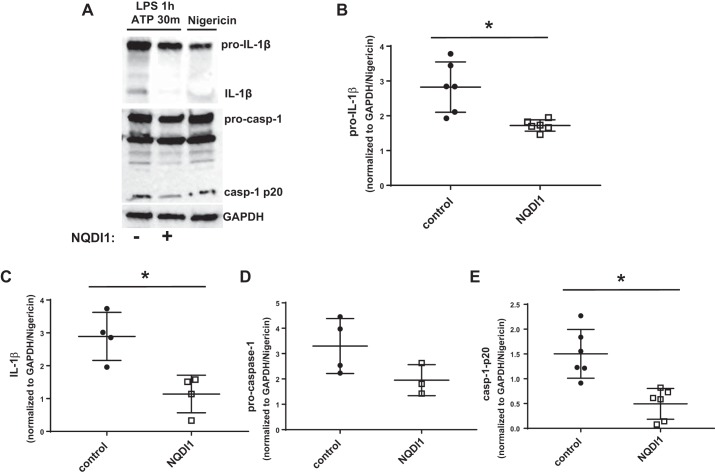
Pharmacological inhibition of ASK1 reduced inflammasome activation in an alveolar macrophage cell line.
To confirm our results demonstrating that ASK1 promotes inflammasome activation, MH-S cells were treated with LPS + ATP in the presence or absence of the ASK1 inhibitor NQDI1, and pro-IL-1β, IL-1β, procaspase-1, and cleaved caspase-1-p20 were measured by Western blot. ASK1 inhibition caused significantly decreased pro-IL-1β, cleaved IL-1β, and cleaved caspase-1-p20 (Fig. 6).
Fig. 6.
Pharmacological inhibition of apoptosis signal-regulating kinase-1 (ASK1) reduced inflammasome activity in MH-S cells. MH-S cells were treated with lipopolysaccharide (LPS) for 1 h followed by ATP for 30 min in the presence or absence of the ASK1 inhibitor NQDI1, and cell lysates were collected. A: representative Western blots of pro-IL-1β, IL-1β, procaspase-1, and cleaved caspase-1-p20. GAPDH was used as a loading control. Nigericin treatment was used as a positive control. Each protein indicated was immunoblotted separately, and each set of bands for a given protein was cropped from the same blot. Unrelated lanes were removed for clarity. Densitometry analysis of pro-IL-1β (B), IL-1β (C), procaspase-1 (D), and cleaved caspase-1-p20 (E) expression is shown. Densitometry values were first normalized to GAPDH and then to the level of the corresponding protein for nigericin-treated cells. *Significant difference compared with wild type (P < 0.05; n = 3–6).
DISCUSSION
The NLRP3 inflammasome is an important component in the progression of ALI (4, 9, 12, 16, 18, 19, 21, 52). Some studies have suggested that this is because of its role in IL-1β production (19, 21, 23, 25, 27, 28, 52); however, it has also been demonstrated to play a role in IL-6 production and neutrophil recruitment independent of IL-1β in murine lung injury models (36). Both IL-1β and IL-6 are increased in BALF of patients at risk for or with established ARDS/ALI (1, 2, 17, 34, 35, 39, 41, 45). Additionally, high concentrations of IL-6 may be a prognosticator of poor outcome (1, 24, 26, 33, 34, 48). In the current study, we demonstrated that ASK1 deficiency reduced the inflammatory response to LPS in mice, as indicated by histology, macrophage migration, neutrophil recruitment, and IL-1β and IL-6 in the BALF. Furthermore, inflammasome priming and activation were significantly decreased in ASK1-deficient macrophages and BMDMs and a macrophage cell line (MH-S) treated with an ASK1 inhibitor. ASK1 has not previously been identified in inflammasome-mediated signaling or lung injury.
Alveolar macrophages are the sentinels of innate immunity in the lung, constantly encountering infectious agents and particles from the environment (16). Alveolar macrophages express high levels of PRRs that recognize both pathogen-associated and damage-associated molecular patterns to alert the immune system to the presence of a pathogen or stressor (47). Activation of PRRs results in gene expression of numerous inflammatory mediators. Grailer et al. (12) demonstrated that activation of NLRP3 is required for development of ALI using the murine LPS-induced ALI model. In the absence of NLRP3, there was reduced neutrophil recruitment in the lung, reduced IL-1β production, and reduced albumin leakage compared with WT LPS-exposed mice. Similarly, other studies have demonstrated the critical role of the NLRP3 inflammasome in LPS-induced and VILI models (12, 18, 19, 21, 27, 28, 52). These studies all point to the important contribution that NLRP3 makes toward the inflammation characteristic of ARDS and highlight the need for increased understanding of the regulation of NLRP3 activation.
We previously demonstrated that mice deficient in ASK1 were protected against VILI in a model with hyperoxia pretreatment and subsequent high tidal volume mechanical ventilation (34). Fukumoto et al. recently demonstrated that ASK1 was necessary for IL-1β and TNF-α production, macrophage apoptosis, and recruitment of immune cells in the lung following exposure to hyperoxia (8). Using systemic administration of LPS, Matsuzawa et al. (31) showed that ASK1-deficient mice were resistant to septic shock and that splenocyte production of proinflammatory cytokines (including IL-1β, TNF-α, and IL-6) was significantly reduced. Other studies have suggested that ASK1 is an intermediate signal in TLR-associated innate immune responses (53, 54). However, none of these studies had linked ASK1 to NLRP3 inflammasome priming or activation.
Because of the important role of macrophages in host defense, we focused on the role of ASK1 in inflammasome activity in these cells. Alveolar macrophages are known to be involved in early inflammatory responses in the lung, including IL-1β production, and a previous study demonstrated that exposure of hydrogen peroxide and ATP promoted ASK1/p38-mediated apoptosis of RAW 264.7 macrophage cells (38). However, inflammasome activity was not investigated in that study. Although many studies identify macrophage-induced IL-1β as the delineating step for LPS-induced inflammation (12, 18, 27, 28), other studies have suggested that LPS-induced lung injury was dependent on caspase-1 in alveolar macrophages (15, 51). In the current study, we demonstrated a decrease in lung injury in ASK1-deficient mice (Figs. 1 and 2), and we specifically demonstrated decreased LPS-induced priming of the NLRP3 inflammasome before the activation step in ASK1-deficient and ASK1-inhibited cells. LPS-induced priming of BMDMs was decreased in ASK1-deficient cells compared with WT cells as indicated by expression of NLRP3 (Fig. 4). Similarly, after stimulation with LPS + ATP to activate the NLRP3 inflammasome, we found a significant decrease in secreted IL-1β from ASK1−/− macrophages compared with WT cells (Fig. 3). Similarly, LPS + ATP-induced inflammasome activation stimulated cleavage of caspase-1 and IL-1β in WT BMDMs, but this was significantly inhibited in ASK1−/− BMDMs (Fig. 5). These results suggest that ASK1 promotes both priming and activation of the NLRP3 inflammasome in response to LPS. To further investigate ASK1-mediated inflammasome signaling, we used the alveolar macrophage cell line MH-S and BMDMs in the presence and absence of an ASK1 inhibitor, NQDI1. Similar to our results with ASK1-deficient BMDMs, inhibition of ASK1 caused decreased cleavage of procaspase-1 and IL-1β in response to LPS + ATP (Figs. 5 and 6). When NQDI1 was added at the same time as the ATP (without pretreatment), IL-1β release from BMDMs was significantly reduced. However, it is possible that NQDI1 was not present long enough in these experiments to more effectively inhibit ASK1.
There are several limitations in our study. Although we measured decreased caspase-1 and pro-IL-1β in ASK1-deficient macrophages after NLRP3 inflammasome activation, we cannot definitively conclude that decreased IL-1β release in our in vivo model was mediated exclusively through the NLRP3 inflammasome. Although significantly decreased 3 h after LPS in vivo, there were no significant decreases in IL-1β at later times. Secretion of IL-1β can occur through multiple pathways, but previous studies of LPS-induced lung injury have implicated the NLRP3 inflammasome proteolytic activity as the key player in IL-1β and caspase-1-associated inflammatory changes (12, 18, 27, 28, 51). The lack of a strong effect on IL-1β in BAL in our studies, despite significant effects on lung injury in vivo and on inflammasome signaling in vitro, suggests that IL-1β may also be produced from non-ASK1-dependent sources and that ASK1 impacts other pathways such as those involving recruitment of neutrophils and regulation of barrier function. We have not yet demonstrated increased ASK1 phosphorylation during inflammasome priming and activation, and we do not know which downstream mitogen-activated protein kinase mediators are necessary for regulation of the NLRP3 inflammasome. Previous studies have suggested that IL-1β production is dependent on p38 activation in macrophages and other tissues (13, 25, 37, 38), and JNK has been reported to be an upstream regulator of the phosphorylation of ASC (14). Recent evidence also suggests that ERK can potentiate NLRP3 priming (10). We previously demonstrated that a combined hyperoxia/VILI was dependent on JNK-mediated signaling (29), but we did not previously link that to ASK1 activation. We are currently investigating these pathways. Another limitation is that our isolation of alveolar macrophages may have included a small population of nonmacrophage cells that could potentially affect the response. When we used F4/80 staining to identify macrophages, we found that >98% of the cells were positive for this marker. Despite the limitations described here, the use of both ASK1-deficient cells and a pharmacological inhibitor strongly supports the role of ASK1 in inflammasome priming and activation.
In summary, we have identified ASK1 as a key regulator of NLRP3 inflammasome priming and activation in response to LPS-induced lung injury. Targeting an upstream regulator of inflammasome activation that has also been linked to apoptosis and fibrosis of the lung may provide a novel therapeutic strategy for the prevention of cellular injury during infectious and/or VILI. The role of ASK1 at the intersection between apoptosis and inflammation could lead to an approach for minimizing the prolonged inflammatory effect seen in ARDS.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-131526 (C. M. Waters), HL-123540 (C. M. Waters), and K08-HL118118 (A. Schwingshackl) and the Le Bonheur Children’s Medical Center Research Foundation (C. N. Immanuel). Research reported in this study used the COBRE Pathology Core at the University of Kentucky, supported by an Institutional Development Award from the National Institute of General Medical Sciences under Grant P20-GM-103527.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.N.I., A.S., S.A.C., E.A.F., and C.M.W. conceived and designed research; C.N.I., B.T., B.E.D., E.M.G., J.A.K., C.L.L., E.A.F., and C.M.W. performed experiments; C.N.I., B.E.D., E.M.G., S.A.C., E.A.F., and C.M.W. analyzed data; C.N.I., B.T., B.E.D., E.M.G., A.S., S.A.C., E.A.F., and C.M.W. interpreted results of experiments; C.N.I., B.E.D., and C.M.W. prepared figures; C.N.I. and C.M.W. drafted manuscript; C.N.I., B.T., B.E.D., E.M.G., A.S., S.A.C., E.A.F., and C.M.W. edited and revised manuscript; C.N.I., B.T., B.E.D., E.M.G., J.A.K., C.L.L., A.S., S.A.C., E.A.F., and C.M.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We gratefully acknowledge the technical assistance of James Tatum.
REFERENCES
- 1.Bouros D, Alexandrakis MG, Antoniou KM, Agouridakis P, Pneumatikos I, Anevlavis S, Pataka A, Patlakas G, Karkavitsas N, Kyriakou D. The clinical significance of serum and bronchoalveolar lavage inflammatory cytokines in patients at risk for Acute Respiratory Distress Syndrome. BMC Pulm Med 4: 6, 2004. doi: 10.1186/1471-2466-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conway Morris A, Kefala K, Wilkinson TS, Moncayo-Nieto OL, Dhaliwal K, Farrell L, Walsh TS, Mackenzie SJ, Swann DG, Andrews PJ, Anderson N, Govan JR, Laurenson IF, Reid H, Davidson DJ, Haslett C, Sallenave JM, Simpson AJ. Diagnostic importance of pulmonary interleukin-1beta and interleukin-8 in ventilator-associated pneumonia. Thorax 65: 201–207, 2010. doi: 10.1136/thx.2009.122291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 27: 519–550, 2009. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 4.Dolinay T, Kim YS, Howrylak J, Hunninghake GM, An CH, Fredenburgh L, Massaro AF, Rogers A, Gazourian L, Nakahira K, Haspel JA, Landazury R, Eppanapally S, Christie JD, Meyer NJ, Ware LB, Christiani DC, Ryter SW, Baron RM, Choi AM. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am J Respir Crit Care Med 185: 1225–1234, 2012. doi: 10.1164/rccm.201201-0003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.dos Santos G, Kutuzov MA, Ridge KM. The inflammasome in lung diseases. Am J Physiol Lung Cell Mol Physiol 303: L627–L633, 2012. doi: 10.1152/ajplung.00225.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dushianthan A, Grocott MP, Postle AD, Cusack R. Acute respiratory distress syndrome and acute lung injury. Postgrad Med J 87: 612–622, 2011. doi: 10.1136/pgmj.2011.118398. [DOI] [PubMed] [Google Scholar]
- 7.Franke-Ullmann G, Pförtner C, Walter P, Steinmüller C, Lohmann-Matthes ML, Kobzik L. Characterization of murine lung interstitial macrophages in comparison with alveolar macrophages in vitro. J Immunol 157: 3097–3104, 1996. [PubMed] [Google Scholar]
- 8.Fukumoto J, Cox R Jr, Fukumoto I, Cho Y, Parthasarathy PT, Galam L, Lockey RF, Kolliputi N. Deletion of ASK1 protects against hyperoxia-induced acute lung injury. PLoS One 11: e0147652, 2016. doi: 10.1371/journal.pone.0147652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukumoto J, Fukumoto I, Parthasarathy PT, Cox R, Huynh B, Ramanathan GK, Venugopal RB, Allen-Gipson DS, Lockey RF, Kolliputi N. NLRP3 deletion protects from hyperoxia-induced acute lung injury. Am J Physiol Cell Physiol 305: C182–C189, 2013. doi: 10.1152/ajpcell.00086.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghonime MG, Shamaa OR, Das S, Eldomany RA, Fernandes-Alnemri T, Alnemri ES, Gavrilin MA, Wewers MD. Inflammasome priming by lipopolysaccharide is dependent upon ERK signaling and proteasome function. J Immunol 192: 3881–3888, 2014. doi: 10.4049/jimmunol.1301974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzales JN, Lucas R, Verin AD. The acute respiratory distress syndrome: mechanisms and perspective therapeutic approaches. Austin J Vasc Med 2: 1009, 2015. [PMC free article] [PubMed] [Google Scholar]
- 12.Grailer JJ, Canning BA, Kalbitz M, Haggadone MD, Dhond RM, Andjelkovic AV, Zetoune FS, Ward PA. Critical role for the NLRP3 inflammasome during acute lung injury. J Immunol 192: 5974–5983, 2014. doi: 10.4049/jimmunol.1400368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haggadone MD, Grailer JJ, Fattahi F, Zetoune FS, Ward PA. Bidirectional crosstalk between C5a receptors and the NLRP3 inflammasome in macrophages and monocytes. Mediators Inflamm 2016: 1340156, 2016. doi: 10.1155/2016/1340156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hara H, Tsuchiya K, Kawamura I, Fang R, Hernandez-Cuellar E, Shen Y, Mizuguchi J, Schweighoffer E, Tybulewicz V, Mitsuyama M. Phosphorylation of the adaptor ASC acts as a molecular switch that controls the formation of speck-like aggregates and inflammasome activity. Nat Immunol 14: 1247–1255, 2013. doi: 10.1038/ni.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He X, Qian Y, Li Z, Fan EK, Li Y, Wu L, Billiar TR, Wilson MA, Shi X, Fan J. TLR4-Upregulated IL-1β and IL-1RI promote alveolar macrophage pyroptosis and lung inflammation through an autocrine mechanism. Sci Rep 6: 31663, 2016. doi: 10.1038/srep31663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herold S, Mayer K, Lohmeyer J. Acute lung injury: how macrophages orchestrate resolution of inflammation and tissue repair. Front Immunol 2: 65, 2011. doi: 10.3389/fimmu.2011.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobs RF, Tabor DR, Burks AW, Campbell GD. Elevated interleukin-1 release by human alveolar macrophages during the adult respiratory distress syndrome. Am Rev Respir Dis 140: 1686–1692, 1989. doi: 10.1164/ajrccm/140.6.1686. [DOI] [PubMed] [Google Scholar]
- 18.Jones HD, Crother TR, Gonzalez-Villalobos RA, Jupelli M, Chen S, Dagvadorj J, Arditi M, Shimada K. The NLRP3 inflammasome is required for the development of hypoxemia in LPS/mechanical ventilation acute lung injury. Am J Respir Cell Mol Biol 50: 270–280, 2014. doi: 10.1165/rcmb.2013-0087OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolliputi N, Shaik RS, Waxman AB. The inflammasome mediates hyperoxia-induced alveolar cell permeability. J Immunol 184: 5819–5826, 2010. doi: 10.4049/jimmunol.0902766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuipers MT, Aslami H, Janczy JR, van der Sluijs KF, Vlaar AP, Wolthuis EK, Choi G, Roelofs JJ, Flavell RA, Sutterwala FS, Bresser P, Leemans JC, van der Poll T, Schultz MJ, Wieland CW. Ventilator-induced lung injury is mediated by the NLRP3 inflammasome. Anesthesiology 116: 1104–1115, 2012. doi: 10.1097/ALN.0b013e3182518bc0. [DOI] [PubMed] [Google Scholar]
- 21.Kuipers MT, van der Poll T, Schultz MJ, Wieland CW. Bench-to-bedside review: Damage-associated molecular patterns in the onset of ventilator-induced lung injury. Crit Care 15: 235, 2011. doi: 10.1186/cc10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lang CJ, Barnett EK, Doyle IR. Stretch and CO2 modulate the inflammatory response of alveolar macrophages through independent changes in metabolic activity. Cytokine 33: 346–351, 2006. doi: 10.1016/j.cyto.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol 13: 397–411, 2013. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee YL, Chen W, Chen LY, Chen CH, Lin YC, Liang SJ, Shih CM. Systemic and bronchoalveolar cytokines as predictors of in-hospital mortality in severe community-acquired pneumonia. J Crit Care 25: 176.e7–13, 2010. doi: 10.1016/j.jcrc.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Liang L, Tan X, Zhou Q, Zhu Y, Tian Y, Yu H, Kijlstra A, Yang P. IL-1β triggered by peptidoglycan and lipopolysaccharide through TLR2/4 and ROS-NLRP3 inflammasome-dependent pathways is involved in ocular Behçet’s disease. Invest Ophthalmol Vis Sci 54: 402–414, 2013. doi: 10.1167/iovs.12-11047. [DOI] [PubMed] [Google Scholar]
- 26.Lin WC, Lin CF, Chen CL, Chen CW, Lin YS. Prediction of outcome in patients with acute respiratory distress syndrome by bronchoalveolar lavage inflammatory mediators. Exp Biol Med (Maywood) 235: 57–65, 2010. doi: 10.1258/ebm.2009.009256. [DOI] [PubMed] [Google Scholar]
- 27.Liu T, Zhou Y, Li P, Duan JX, Liu YP, Sun GY, Wan L, Dong L, Fang X, Jiang JX, Guan CX. Blocking triggering receptor expressed on myeloid cells-1 attenuates lipopolysaccharide-induced acute lung injury via inhibiting NLRP3 inflammasome activation. Sci Rep 6: 39473, 2016. doi: 10.1038/srep39473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo M, Hu L, Li D, Wang Y, He Y, Zhu L, Ren W. MD-2 regulates LPS-induced NLRP3 inflammasome activation and IL-1beta secretion by a MyD88/NF-κB-dependent pathway in alveolar macrophages cell line. Mol Immunol 90: 1–10, 2017. doi: 10.1016/j.molimm.2017.06.035. [DOI] [PubMed] [Google Scholar]
- 29.Makena PS, Gorantla VK, Ghosh MC, Bezawada L, Balazs L, Luellen C, Parthasarathi K, Waters CM, Sinclair SE. Lung injury caused by high tidal volume mechanical ventilation and hyperoxia is dependent on oxidant-mediated c-Jun NH2-terminal kinase activation. J Appl Physiol (1985) 111: 1467–1476, 2011. doi: 10.1152/japplphysiol.00539.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 10: 417–426, 2002. doi: 10.1016/S1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 31.Matsuzawa A, Saegusa K, Noguchi T, Sadamitsu C, Nishitoh H, Nagai S, Koyasu S, Matsumoto K, Takeda K, Ichijo H. ROS-dependent activation of the TRAF6-ASK1-p38 pathway is selectively required for TLR4-mediated innate immunity. Nat Immunol 6: 587–592, 2005. doi: 10.1038/ni1200. [DOI] [PubMed] [Google Scholar]
- 32.McNicholas BA, Rooney GM, Laffey JG. Lessons to learn from epidemiologic studies in ARDS. Curr Opin Crit Care 24: 41–48, 2018. doi: 10.1097/MCC.0000000000000473. [DOI] [PubMed] [Google Scholar]
- 33.Meduri GU, Eltorky M, Winer-Muram HT. The fibroproliferative phase of late adult respiratory distress syndrome. Semin Respir Infect 10: 154–175, 1995. [PubMed] [Google Scholar]
- 34.Meduri GU, Headley S, Kohler G, Stentz F, Tolley E, Umberger R, Leeper K. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest 107: 1062–1073, 1995. doi: 10.1378/chest.107.4.1062. [DOI] [PubMed] [Google Scholar]
- 35.Meduri GU, Kohler G, Headley S, Tolley E, Stentz F, Postlethwaite A. Inflammatory cytokines in the BAL of patients with ARDS. Persistent elevation over time predicts poor outcome. Chest 108: 1303–1314, 1995. doi: 10.1378/chest.108.5.1303. [DOI] [PubMed] [Google Scholar]
- 36.Mizushina Y, Shirasuna K, Usui F, Karasawa T, Kawashima A, Kimura H, Kobayashi M, Komada T, Inoue Y, Mato N, Yamasawa H, Latz E, Iwakura Y, Kasahara T, Bando M, Sugiyama Y, Takahashi M. NLRP3 protein deficiency exacerbates hyperoxia-induced lethality through Stat3 protein signaling independent of interleukin-1β. J Biol Chem 290: 5065–5077, 2015. doi: 10.1074/jbc.M114.603217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ng DC, Long CS, Bogoyevitch MA. A role for the extracellular signal-regulated kinase and p38 mitogen-activated protein kinases in interleukin-1 beta-stimulated delayed signal tranducer and activator of transcription 3 activation, atrial natriuretic factor expression, and cardiac myocyte morphology. J Biol Chem 276: 29490–29498, 2001. doi: 10.1074/jbc.M100699200. [DOI] [PubMed] [Google Scholar]
- 38.Noguchi T, Ishii K, Fukutomi H, Naguro I, Matsuzawa A, Takeda K, Ichijo H. Requirement of reactive oxygen species-dependent activation of ASK1-p38 MAPK pathway for extracellular ATP-induced apoptosis in macrophage. J Biol Chem 283: 7657–7665, 2008. doi: 10.1074/jbc.M708402200. [DOI] [PubMed] [Google Scholar]
- 39.Park WY, Goodman RB, Steinberg KP, Ruzinski JT, Radella F II, Park DR, Pugin J, Skerrett SJ, Hudson LD, Martin TR. Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 164: 1896–1903, 2001. doi: 10.1164/ajrccm.164.10.2104013. [DOI] [PubMed] [Google Scholar]
- 40.Patel NR, Bole M, Chen C, Hardin CC, Kho AT, Mih J, Deng L, Butler J, Tschumperlin D, Fredberg JJ, Krishnan R, Koziel H. Cell elasticity determines macrophage function. PLoS One 7: e41024, 2012. doi: 10.1371/journal.pone.0041024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pugin J, Ricou B, Steinberg KP, Suter PM, Martin TR. Proinflammatory activity in bronchoalveolar lavage fluids from patients with ARDS, a prominent role for interleukin-1. Am J Respir Crit Care Med 153: 1850–1856, 1996. doi: 10.1164/ajrccm.153.6.8665045. [DOI] [PubMed] [Google Scholar]
- 42.Reiss LK, Schuppert A, Uhlig S. Inflammatory processes during acute respiratory distress syndrome: a complex system. Curr Opin Crit Care 24: 1–9, 2018. doi: 10.1097/MCC.0000000000000472. [DOI] [PubMed] [Google Scholar]
- 43.Sasmono RT, Oceandy D, Pollard JW, Tong W, Pavli P, Wainwright BJ, Ostrowski MC, Himes SR, Hume DA. A macrophage colony-stimulating factor receptor-green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood 101: 1155–1163, 2003. doi: 10.1182/blood-2002-02-0569. [DOI] [PubMed] [Google Scholar]
- 44.Schroder K, Tschopp J. The inflammasomes. Cell 140: 821–832, 2010. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 45.Siler TM, Swierkosz JE, Hyers TM, Fowler AA, Webster RO. Immunoreactive interleukin-1 in bronchoalveolar lavage fluid of high-risk patients and patients with the adult respiratory distress syndrome. Exp Lung Res 15: 881–894, 1989. doi: 10.3109/01902148909069633. [DOI] [PubMed] [Google Scholar]
- 46.Smith LS, Zimmerman JJ, Martin TR. Mechanisms of acute respiratory distress syndrome in children and adults: a review and suggestions for future research. Pediatr Crit Care Med 14: 631–643, 2013. doi: 10.1097/PCC.0b013e318291753f. [DOI] [PubMed] [Google Scholar]
- 47.Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu Rev Immunol 23: 901–944, 2005. doi: 10.1146/annurev.immunol.23.021704.115816. [DOI] [PubMed] [Google Scholar]
- 48.Tsantes A, Tsangaris I, Kopterides P, Kapsimali V, Antonakos G, Zerva A, Kalamara E, Bonovas S, Tsaknis G, Vrigou E, Maniatis N, Dima K, Armaganidis A. The role of procalcitonin and IL-6 in discriminating between septic and non-septic causes of ALI/ARDS: a prospective observational study. Clin Chem Lab Med 51: 1535–1542, 2013. doi: 10.1515/cclm-2012-0562. [DOI] [PubMed] [Google Scholar]
- 49.Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat Rev Immunol 10: 210–215, 2010. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 50.Volynets GP, Chekanov MO, Synyugin AR, Golub AG, Kukharenko OP, Bdzhola VG, Yarmoluk SM. Identification of 3H-naphtho[1,2,3-de]quinoline-2,7-diones as inhibitors of apoptosis signal-regulating kinase 1 (ASK1). J Med Chem 54: 2680–2686, 2011. doi: 10.1021/jm200117h. [DOI] [PubMed] [Google Scholar]
- 51.Wu DD, Pan PH, Liu B, Su XL, Zhang LM, Tan HY, Cao Z, Zhou ZR, Li HT, Li HS, Huang L, Li YY. Inhibition of alveolar macrophage pyroptosis reduces lipopolysaccharide-induced acute lung injury in mice. Chin Med J (Engl) 128: 2638–2645, 2015. doi: 10.4103/0366-6999.166039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu J, Yan Z, Schwartz DE, Yu J, Malik AB, Hu G. Activation of NLRP3 inflammasome in alveolar macrophages contributes to mechanical stretch-induced lung inflammation and injury. J Immunol 190: 3590–3599, 2013. doi: 10.4049/jimmunol.1200860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang CS, Shin DM, Lee HM, Son JW, Lee SJ, Akira S, Gougerot-Pocidalo MA, El-Benna J, Ichijo H, Jo EK. ASK1-p38 MAPK-p47phox activation is essential for inflammatory responses during tuberculosis via TLR2-ROS signalling. Cell Microbiol 10: 741–754, 2008. doi: 10.1111/j.1462-5822.2007.01081.x. [DOI] [PubMed] [Google Scholar]
- 54.Yuk JM, Shin DM, Yang CS, Kim KH, An SJ, Rho J, Park JK, Jo EK. Role of apoptosis-regulating signal kinase 1 in innate immune responses by Mycobacterium bovis bacillus Calmette-Guérin. Immunol Cell Biol 87: 100–107, 2009. doi: 10.1038/icb.2008.74. [DOI] [PubMed] [Google Scholar]
- 55.Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Curr Protoc Immunol 14: 14.1, 2008. doi: 10.1002/0471142735.im1401s83. [DOI] [PMC free article] [PubMed] [Google Scholar]