Abstract
The purpose of this study was to explore the role of microRNA-451a (miR-451a) in diabetic retinopathy through activating transcription factor 2 (ATF2). The epiretinal membrane samples from patients with proliferative diabetic retinopathy (PDR) were immunolabeled with an antibody for Ki-67 to identify the proliferative cells. The expression of miR-451a was measured by qRT-PCR in the retina of Akita mice and in RPE cells under diabetic conditions. The potential downstream targets of miR-451a were predicted by bioinformatics and confirmed by dual luciferase assay, qRT-PCR, and Western blotting. Mitochondrial function, cell proliferation, and migration assays were used to detect the functional change after transfection of miR-451a mimic and inhibitor. Proliferative RPE cells were identified in the epiretinal membrane from PDR patients. The expression of miR-451a was downregulated both in the retina of Akita mice and 4-hydroxynonenal (4-HNE)-treated RPE cells. Bioinformatic analysis and luciferase assay identified ATF2 as a potential target of miR-451a. miR-451a inhibited proliferation and migration of RPE cells. The mitochondrial function was enhanced by miR-451a mimic, but suppressed by miR-451a inhibitor. In diabetic conditions, miR-451a showed a protective effect on mitochondrial function. The results of qRT-PCR and Western blotting revealed that overexpression of miR-451a downregulated the expression of ATF2 and its downstream target genes CyclinA1, CyclinD1, and MMP2. In conclusion, miR-451a/ATF2 plays a vital role in the regulation of proliferation and migration in RPE cells through regulation of mitochondrial function, which may provide new perspectives for developing effective therapies for PDR.
Keywords: ATF2, cell proliferation, diabetic retinopathy, miRNA-451a, mitochondrial function
INTRODUCTION
Diabetic retinopathy (DR) is a common diabetic complication and the major cause of acquired blindness in working-age adults. Population-based studies suggest that approximately one-third of the diabetic population have signs of DR and approximately one-tenth of diabetic patients have vision-threatening stages of retinopathy such as diabetic macular edema (DME) and proliferative diabetic retinopathy (PDR) (6, 24).
The most severe stage of PDR is epiretinal membrane formation, because of retinal traction or even tractional retinal detachment secondary to epiretinal membrane contraction. To clarify the mechanism of epiretinal membrane formation, a series of studies have been conducted using human patient samples, which were collected during surgery (5, 46). These studies demonstrated that multiple cell types, such as retinal pigment epithelium (RPE), were present in the epiretinal membrane (33). The RPE layer is composed of a monolayer of hexagonal cells that are quiescent without proliferation and migration under physiological conditions (17). Previously, RPE cells were believed unrelated to DR. Recently, however, the role of RPE cells in DR has been generally recognized. Documented clinical studies showed that proliferation and migration of RPE were involved in pathophysiology of DR, especially epiretinal membrane formation (33). The present study was aimed at elucidating the mechanism for proliferation and migration of RPE cells in DR.
miR-451a is considered a promising miRNA which might be a possible prognostic biomarker and treatment target (26, 36), because its functions range from regulating the proliferation and migration of cells to inducing epithelial-mesenchymal transition in many diseases (20, 44). It is also known that miR-451a is a metabolism-related miRNA, as it can inhibit hepatic gluconeogenesis and alleviate hyperglycemia (47); meanwhile, its expression is regulated by glucose (14), lipids (16, 43), and hypoxia (13). As we know, hyperglycemia (32), dyslipidemia (23), and hypoxia (32) are basic pathophysiological factors contributing to diabetes. However, the role of miR-451a in PDR has not been studied yet.
Activating transcription factor 2 (AFT2) is a transcription factor in the leucine zipper family of DNA-binding proteins. Several studies demonstrated that ATF2 is overexpressed in diabetes-related diseases (11); furthermore, abnormal activation of ATF2 promoted cell growth and migration in several aggressive cancers (4).
The aim of this study was to identify RPE cells in the proliferative membrane from PDR patients, which reflected RPE cell proliferation and migration. In this study, we demonstrated that miR-451a had a regulatory role in cellular energy production as well as cell proliferation and migration through targeting ATF2. Thus our findings highlighted a novel connection between the miR-451a/ATF2 signal pathway and mitochondrial function, proliferation, and migration of RPE cells in PDR.
MATERIALS AND METHODS
Patients.
The study adhered to the tenets of the Declaration of Helsinki (human subjects) and was approved by the Tianjin Medical University Eye Hospital. Written informed consent was obtained from each patient to collect samples. Inclusion criteria included: 1) younger than 75 years old, 2) absence of renal or hematological diseases or uremia, 3) no administration of chemotherapy or life-support measures, and 4) no obvious vitreous hemorrhage. Exclusion criteria included 1) ocular treatment history with anti-VEGF or steroid therapy, 2) previous ocular surgery, and 3) other possible chronic pathologies. Epiretinal membranes (ERMs) and vitreous fluid were surgically removed during the pars plana vitrectomy. Patient characteristics are shown in Table 1. This study comprised 14 patients, including 5 men (35.7%) and 9 women (64.3%), with a median age of 53.36 ± 11.78 yr (range 30–75 yr). All patients had been diagnosed with PDR.
Table 1.
Demographic information of DR patients
| Men (n = 5) | Women (n = 9) | Total (n = 14) | |
|---|---|---|---|
| Age, yr | 57.8 ± 13.6 | 52.4 ± 11.0 | 53.36 ± 11.78 |
| DR stage, V/VI | 2/3 | 4/5 | 6/8 |
| Duration, yr | 6.4 ± 3.3 | 4.5 ± 3.3 | 5.2 ± 3.2 |
Data are presented as means ± standard error of the mean (SE). DR, diabetic retinopathy.
Immunostaining.
PDR membranes were removed from patients during routine surgical procedures. The samples were fixed with neutral buffered 4% formalin (NBF) for at least 48 h and routinely processed to paraffin blocks. Sections (4 μm) were cut and mounted on coated slides and dried overnight at 37°C. Then, the sections were double-labeled by Ki-67 [anti-Ki-67 antibody (ZA-0502), ZSGB-BIO, Beijing, China] and RPE65 [anti-RPE65 antibody (401.8B11.3D9), Abcam, Cambridge, MA]. The red-brown color combination is composed of HRP activity in brown (SP-9000, SPlink Detection Kits, ZSGB-BIO) and AP activity in red (SAP-9012, Histonstain-SAP Kit, ZSGB-BIO). The Ki-67 proliferation indexes (PI) of proliferative epiretinal membrane were reported as the percentage of cell nuclei labeled with the Ki-67 monoclonal antibody in formalin-fixed paraffin-embedded tissue sections. In each case, three random areas were selected for counting by ImageJ at a magnification power of ×40.
Animals.
Male homozygous C57BL/6J-Ins2Akita (Akita) and C57BL/6J (nondiabetic control) (Jackson Laboratories, Bar Harbor, ME) mice were housed and raised at the animal facility of the University of Oklahoma Health Sciences Center (OUHSC). Blood glucose concentrations were measured monthly. Care, use, and experiments on mice were approved by the Institutional Animal Care and Use Committees (IACUC) at OUHSC.
Bioinformatic analysis for possible microRNA target genes.
To predict the target genes of the miRNA(s) of interest, bioinformatic analyses were performed with available internet databases: miRbase (http://www.mirbase.org), TargetScan (http://www.targetscan.org), and PicTar (https://pictar.mdc-berlin.de/) (https://pictar.mdc-berlin.de/). Further, the potential targets were narrowed down by examination of genes that are known to be involved in cell proliferation and migration.
Validations of potential miRNA targets.
The method of luciferase assay has been described in detail previously (30). In brief, the 3′-UTR of ATF2 containing the predicted miR-451a binding sites was amplified by PCR using the following primers: sense 5′-GGCCCTCGAGCTTTTGACCACCCCTTAACC-3′; antisense 5′-CCCGGGGCGGCCGCACAATGAATTATAATACAAT-3′. Fragments were cloned into the XhoI and NotI sites in the 3′-UTR of Renilla luciferase of the psiCHECK-2 reporter vector (psiCHECK-2; Promega, Madison, WI). The psiCHECK-2/ ATF2 3′-UTR or empty psiCHECK-2 vector (2 μg each) and miR-451a mimic or control miR-mimic (2.25 µg each, GenePharma, Shanghai, China) were cotransfected into 293T cells (ATCC, Manassas, VA) at 75% confluence in 12-well plates using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA). At 24 h posttransfection, the cells were lysed, and reporter activity was measured using the Dual-Luciferase Reporter Assay System (Promega) in accordance with the manufacturer’s protocols.
Cell culture.
To identify the downstream target of miR-451a, ARPE-19 (ATCC), primary human RPE cells (Lonza, Basel, Switzerland), and 293T cell lines were used to evaluate the effects of miR-451a in this study. RPE cells were cultured in DMEM/F12 medium, supplemented with 5% fetal bovine serum. To compare the cellular response in HNE conditions, the cells were treated with 10 μM 4-hydroxynonenal (4-HNE) for 24 h and equal volume ethanol as control. HEK-293T cells were cultured in low-glucose (5 mM glucose) DMEM, supplemented with 10% FBS in a 5% CO2 humidified atmosphere at 37°C. Cells were transfected at ~75% confluence.
Transfection.
The cell transfection was performed using Lipofectamine 2000 according to manufacturer’s protocol (Invitrogen). To analyze the effects of miRNA in the cultured cells, the miR-451a mimic (50 nM), miR-451a inhibitor (100 nM), and its respective controls were transfected separately using Lipofectamine 2000 reagent (ThermoFisher, Waltham, MA). At 6 h posttransfection, the culture medium was removed, and fresh medium was added. At 48 h posttransfection, the cells were harvested for further analysis.
Cell proliferation assay.
The cell proliferation assay was performed using a cell counting kit-8 (CCK8, Beyotime, Shanghai, China) according to the manufacturer’s protocol. In brief, the optical density was measured at 450 nm using a microplate reader (ThermoFisher).
In vitro wound-healing assay.
In brief, ARPE-19 cells were seeded in a six-well plate (3 × 106 cells/well). The cells were transfected with miR-451a mimic and microRNA control mimic, miR-451a inhibitor, and microRNA inhibitor control when they reached ~90% confluence, and then the monolayers of the ARPE-19 cells were scratched using a sterile, 1-ml pipette tip. Wounded monolayers were washed with PBS to remove detached cells and debris, and fresh medium was added to each well. The wounds in each well were photographed at 0, 12, 24, and 36 h. The width of the remaining wound in each image was measured three times at 24 h. The data were quantified based on the percentage of average gap (AG); the wound at 0 h was considered 100% AG. The results were analyzed in triplicate.
Mitochondrial functionality measurement.
Evaluation of ARPE-19 cells respiration was performed using Seahorse XFe96 Flux Analyzer (Agilent, Santa Clara, CA) by measuring the oxygen consumption rate (OCR). Cells were seeded in a 96-well microplate at a density of 2 × 104 cells/well. miRNA mimic and inhibitor were separately transfected with Fast-forward method using HiPerFect transfection reagent (Qiagen, Germany). The compounds, oligomycin (1 μM), carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP, 2 μM), and a mix of rotenone and antimycin A (RAA, 1 μM), are serially injected to measure basal OCR, ATP generation, and maximal OCR. Data were normalized by protein level.
Quantitative RT-PCR (qRT-PCR).
The primers of miR-451a and U6 were obtained from Genecopoeia (Rockville, MD). The sequences of other primers are as follows: ATF2 sense, 5′-CATGGTAGCGGATTGGTTAGG-3′; ATF2 antisense, 5′-AGTGGATGTGGCTGGCTGTT-3′; CyclinA1 sense, 5′-TGGCATTTGAGGATGTGTATGA-3′; CyclinA1 antisense, 5′-TGGCATTTGAGGATGTGTATGA-3′; MMP2 sense, 5′-ACTGTGACGCCACGTGACA-3′; MMP2 antisense, 5′-CGTATACCGCATCAATCTTTTCC-3′; CyclinD1 sense, 5′-GCATGTTCGTGGCCTCTAAGA-3′; CyclinD1 antisense, 5′-CGGTGTAGATGCACAGCTTCTC-3′.
Western blot analysis.
The miRNA mimic or inhibitor-transfected RPE cells were lysed in RIPA buffer containing protease inhibitor cocktail (Roche). Total cellular protein concentrations were measured by BCA protein Quantitation Kit (ThermoFisher). Equal amounts of protein (20 μg) were resolved by SDS-PAGE and electrotransferred onto a nitrocellulose membrane. The membrane was blocked with 5% (wt/vol) nonfat dry milk in Tris-buffered saline with 0.1% Tween-20 (TBST) for 30 min and subsequently incubated overnight at 4°C with a 1:1,000 dilution of an anti-human ATF2, CyclinA1, CyclinD1, and MMP2 monoclonal antibody (Abcam, Cambridge, MA). After three washes with TBST, the membrane was incubated for 2 h with a 1:5,000 dilutions of an HRP-conjugated secondary IgG antibody in TBST containing 1% nonfat dry milk. After three washes with TBST, the bands were detected using ECL solution (Pierce Biotech, Waltham, MA). As needed, the membrane was stripped in stripping buffer (ThermoFisher) and reblotted with an antibody specific for GAPDH for loading control. The band intensities were semiquantified by densitometry using Fluorochem-Q software (Proteinsimple, San Jose, CA); values are an average of at least three independent experiments.
Statistical analysis.
All experiments were performed at least three times. Quantitative data are presented as means ± standard error of the mean (SE) and were analyzed by Student’s t-test when two groups are compared. Data were analyzed by ANOVA when more than two groups are compared. P value of <0.05 was considered statistically significant.
RESULTS
RPE proliferation and migration in PDR patients.
To test the rate of retinal cell proliferation and migration in PDR, 14 epiretinal membrane samples were collected from patients with diagnosed PDR. The demographic information of patients is listed in Table 1.
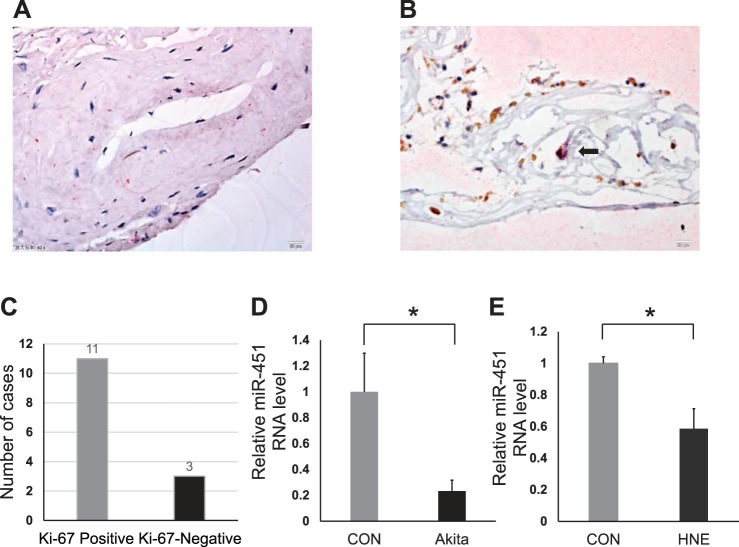
The membranes were processed for immunochemistry of Ki-67 and RPE65 as described in materials and methods (Fig. 1). Among the 14 epiretinal membrane samples, 11 cases were Ki-67 positive (78.6%), and 3 cases were Ki-67 negative (21.4%) (Fig. 1, A–C). The average of proliferative index (PI) was 4.18 ± 4.58% (0–16.67%). According to RPE65 staining, RPE cells account for 2.57 ± 3.15% (1.61–11.11%) of total cells in the membrane. In some membranes, cells with Ki-67 and RPE65 costaining were observed (Fig. 1B). This result suggested that cell proliferation and migration did contribute to the development of PDR in human, and targeting cell migration in the retina may have therapeutic potential for PDR.
Fig. 1.
Immunostaining of proliferative membrane and miR-451a expression change in diabetic conditions. Formalin-fixed paraffin-embedded tissue sections were stained with antibodies against Ki-67 (brown) and RPE65 (red) with nuclei stained with DAPI (blue). A: Ki-67 and RPE65 double negative staining (40×, scale bar = 20 μm). B: arrow points to the double positive staining of Ki-67 and RPE65 which were colocalized (40×, scale bar = 20 μm). C: numbers of samples with Ki-67 positive and Ki-67 negative in 14 cases. miR-451a levels were measured by quantitative reverse transcription PCR (qRT-PCR) assay. D: retinal RNA was isolated from 3-mo-old Akita mice (n = 5) and age- and genetic background-matched nondiabetic controls (CON; n = 8). The retinal levels of miR-451a in Akita mice were decreased compared with that in the age-matched nondiabetic controls [means ± standard error of the mean (SE), n > 5, *P < 0.05]. E: effect of 4-hydroxynonenal (4-HNE) treatment on miR-451a expression. ARPE-19 cells were exposed to media containing HNE (10 μM) or its vehicle (EtOH, CON) for 24 h. Expression of miR-451a was compared within two groups by Student’s t test (mean ± SE, n = 3, *P < 0.05).
Expression of miR-451a was decreased under diabetic conditions in vivo and in vitro.
miR-451a is known to regulate the proliferation and migration of cells in some diseases. Indeed, in the retinas of Akita mice, a type 1 diabetic animal model, using qPCR, we found that levels of miR-451a were significantly decreased (3 mo of age) compared with age- and genetic background-matched nondiabetic control mice (Fig. 1D). Further, we exposed ARPE-19 cells and primary human RPE cells to 4-HNE (10 μM) for 24 h to mimic diabetic conditions in vitro. qRT-PCR results also showed decreased levels of miR-451a in 4-HNE-treated ARPE-19 cells and human RPE cells (Fig. 1E; see Supplemental Fig. S1; all Supplemental Materials are available online at the Journal website).
miR-451a regulated RPE proliferation and migration.
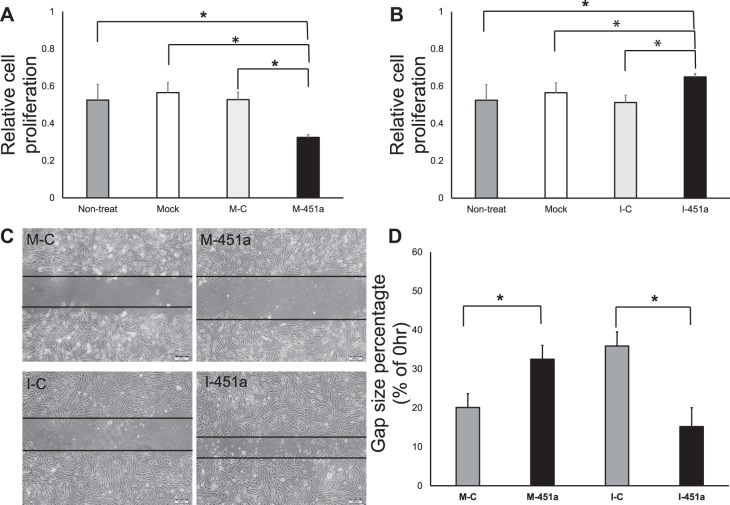
To examine whether the miR-451a level change was related to RPE cell proliferation and migration, ARPE-19 cells were transfected with miR-451a mimic and inhibitor. Forty-eight hours after transfection, cell proliferation was detected by CCK8 Kit. As expected, miR-451a mimic decreased cell proliferation (Fig. 2A), whereas inhibition of miR-451a induced the cell proliferation (Fig. 2B). The scratch assay also showed that miR-451a inhibited the migration of RPE cells (Fig. 2, C and D). These results demonstrated that miR-451a directly regulated the cell proliferation and migration of RPE.
Fig. 2.
Regulation of RPE cell proliferation and migration by miR-451a and its inhibitor. A: ARPE-19 cells were transfected with miR-451a mimic (M-451a), mimic-control (M-C), blank transfection (Mock), and untreated control without RNA oligos (Nontreated) for 48 h, and then cell proliferation was measured using CCK-8 kit (mean ± SE, n = 3, *P < 0.05). B: ARPE-19 cells were transfected with miR-451a inhibitor (I-451a), Inhibitor-control (I-C), blank transfection (Mock), and untreated control (Nontreated) 48 h, and cell proliferation assay was measured by CCK-8 (mean ± SE, n = 3, *P < 0.05). C: ARPE-19 cells transfected with miR-451a mimic (M-451a), mimic-control (M-C), miR-451a inhibitor (I-451a), and inhibitor-control (I-C) 48 h; cell migration analysis was performed by scratch assay. D: data are presented as the percentage of gap size (48 h) of the control (0 h) (mean ± SE, n = 3, *P < 0.05).
miR-451a regulated RPE mitochondrial function.
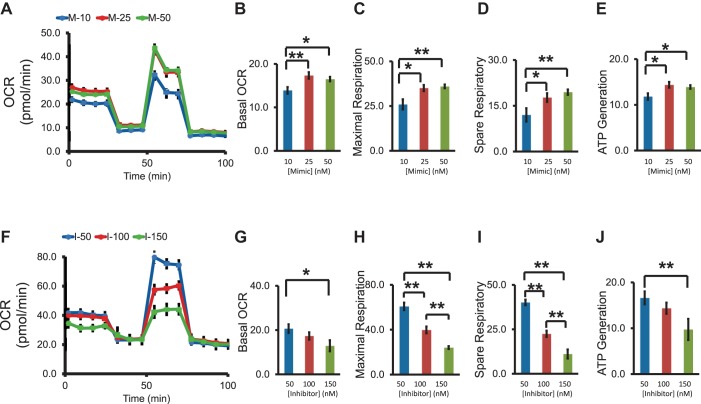
Mitochondrial respiration plays an important role in cell proliferation and migration. We evaluated the role of miR-451a in the regulation of mitochondrial function in RPE cells. After transfection of miR-451a mimic and inhibitor, the mitochondria bioenergetic profile was recorded by Seahorse oxygen consumption rate (OCR) assay. miR-451a overexpression increased basal OCR, ATP generation, and maximal and spare respiration, whereas miR-451a inhibitor reduced the basal OCR, ATP generation, and maximal and spare respiration in ARPE-19 cells (Fig. 3 and Fig. 4, A–C). Moreover, the regulation of miR-451a on mitochondrial function occurs in a concentration-dependent manner (Fig. 3).
Fig. 3.
The dose-dependent effects of miR-451a on mitochondria bioenergetic profile in nondiabetic conditions. A: the injection of reagents (oligomycin, FCCP, RAA) over time was presented with the indicated lines; the representative trace identified the dose-dependent pattern of oxygen consumption rate (OCR) when ARPE-19 cells were treated with miR-451a. Basal levels of OCR (B), maximal respiration (C), spare respiratory capacity (D), and ATP production (E) in miR-451 overexpressed condition were calculated and compared. F: representative trace identified the dose-dependent pattern of OCR when ARPE-19 cells were treated with miR-451a inhibitor. Basal levels of OCR (G), maximal respiration (H), spare respiratory capacity (I), and ATP production (J) were calculated and compared in miR-451 knockdown condition. (mean ± SE, n = 4–7, *P < 0.05, **P < 0.01).
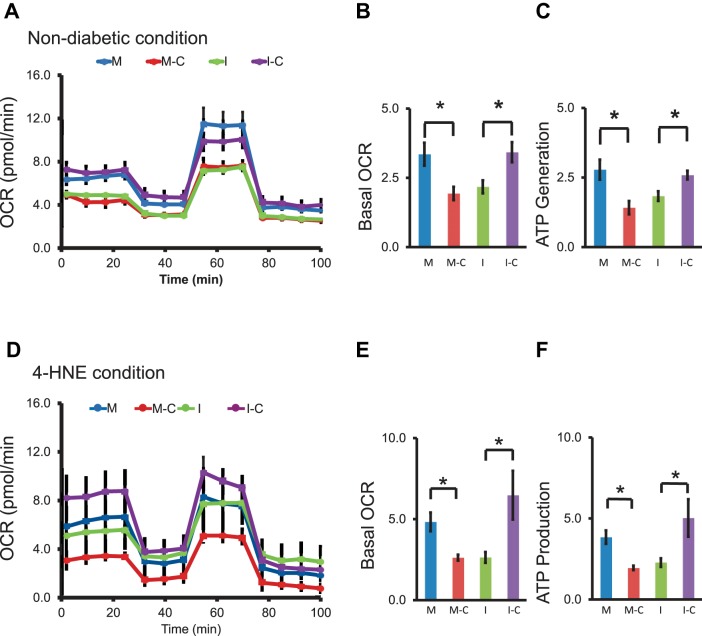
Fig. 4.
The effects of miR-451a on mitochondria bioenergetic profile in diabetic conditions. A: the injection of reagents (oligomycin, FCCP, RAA) during the Seahorse analysis was represented with the indicated lines, and their effects on the average OCR was measured in the bioenergetic profile in normal culture (nondiabetic) conditions. Basal levels of OCR (B) and ATP production (C) were calculated and compared. D: representative trace identifying the OCR after 4-HNE treated (10 μM, 24 h). Basal levels of OCR (E) and ATP production (F) were calculated and compared in 4-HNE conditions (mean ± SE, n = 4–7, *P < 0.05).
To further determine the role of miR-451a in the regulation of RPE cell metabolism, ARPE-19 cells were treated with 4-HNE to mimic diabetic conditions. miR-451a mimic upregulated the basal OCR and ATP generation in cells treated with 4-HNE (Fig. 4, D–F). On the other hand, miR-451a inhibitor reduced the basal OCR and ATP generation in HNE-treated cells (Fig. 4, D–F). These data suggested that miR-451a was involved in the regulation of mitochondrial function in RPE cells.
miR-451a targeted ATF2 gene expression.
It is well known that microRNAs regulate numerous physiological events through translational repression. To evaluate the functional significance of the downregulated miR-451a expression in DR, the putative target genes of miR-451a were predicted by bioinformatic analysis, using internet databases including miRbase, miRanda, TargetScan, and PicTar. The identified putative target genes with potential function in epithelial cell proliferation and kinase activity regulation were focused on in this study (7). Among them, ATF2 (27), a potential regulator in cell proliferation, was selected for further studies, as ATF2 is a transcription factor and implicated in transcriptional regulation of cell cycle-related genes (21). Further, ATF2 is located at the mitochondria outer membrane and can reduce mitochondrial membrane potential (25).
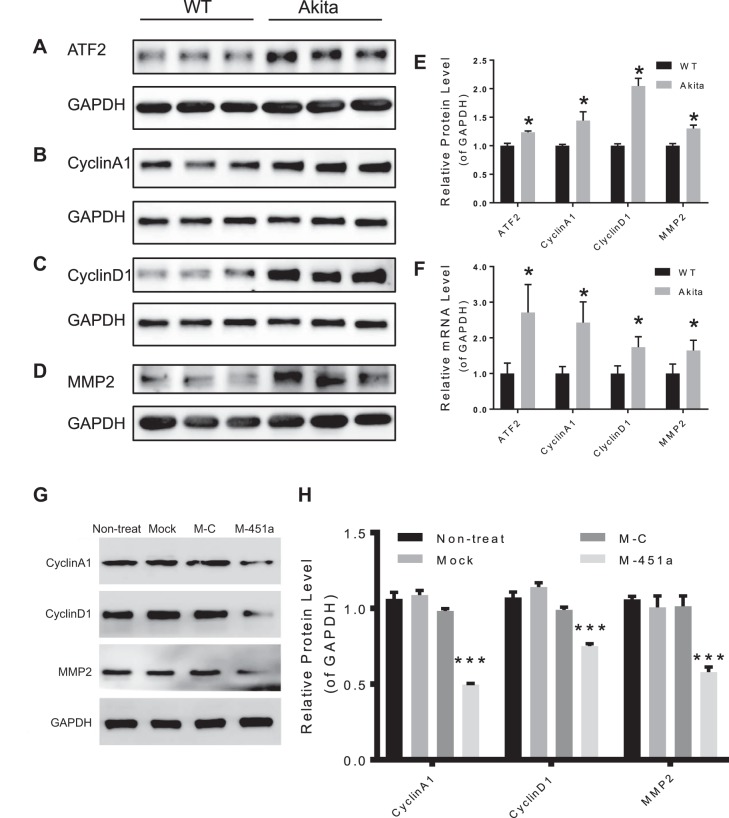
To validate that ATF2 is a target gene of miR-451a, the miR-451a binding sites in the 3′-UTR of ATF2 was cloned into the psiCHECK-2 dual luciferase report vector (Fig. 5A) and cotransfected into 293T cells together with miR-451a mimic. Forty-eight hours after cotransfection, luciferase reporter activity was significantly reduced by miR-451a mimic, indicating that ATF2 is a downstream target gene of miR-451a (Fig. 5B). Furthermore, transfection of miR-451a mimic into ARPE-19 and primary human RPE cells downregulated both ATF2 mRNA and protein levels as shown by real-time PCR and Western blot analysis (Fig. 5, C–E, and Supplemental Fig. S2). Meanwhile, transfection of miR-451a inhibitor resulted in upregulation of mRNA and protein levels of ATF2 (Fig. 5, C–E, and Supplemental Fig. S2). Also, the protein and mRNA levels of ATF2 were increased in the retina of Akita mice compared with WT (Fig. 6, A, E, and F). Taken together, these results confirmed that ATF2 is a direct downstream target of miR-451a.
Fig. 5.
miR-451a interacts with the 3′-untranslated region (UTR) of the ATF2 mRNA and regulates ATF2 expression. A: the binding sites of miR-451a on ATF2 were predicted by DIANA. The region in the box shows the potential binding sequences (position 1880–1879). B: miR-451a mimic was cotransfected with the blank vector or ATF2–3′-UTR-vector into 293T cells. Luciferase activity was normalized by the ratio of Firefly/Renilla (mean ± SE, n = 3, *P < 0.05). C: ARPE-19 cells were transfected with miR-451a mimic and inhibitor or the corresponsive control. ATF2 mRNA levels at 48 h after transfection were measured by qRT-PCR. The ATF2 mRNA level was normalized to GAPDH (mean ± SE, n = 3, *P < 0.05). D: ARPE-19 cells were transfected with miR-451a mimic and inhibitor or their respective control, blank transfection (Mock), the nontreated control (Nontreated). ATF2 protein expression at 48 h after transfection was detected by Western blot analysis. E: the expression level was normalized to GAPDH levels (mean ± SE, n = 3, *P < 0.05, ****P < 0.0001).
Fig. 6.
Altered expression of cell cycle proteins in the retina of Akita mice and RPE cells treated with miR-451a. A–D: Western blots of ATF2, CyclinA1, CyclinD1 and MMP2 in the retina of Akita mice compared with age-matched wild-type (WT) mouse; GAPDH was used as loading control. E: quantification of the Western blots (A–D) normalized by WT (mean ± SE, n = 3, *P < 0.05). F: the mRNA levels of ATF2, CyclinA1, CyclinD1, and MMP2 in the retina of Akita mice were measured by qRT-PCR compared with WT control (mean ± SE, n = 3, *P < 0.05). G: ARPE-19 cells were transfected with miR-451a mimic (M-451a) and mimic-control (M-C) for 48 h, and protein levels of CyclinA1, CyclinD1, MMP-2, and GAPDH were measured by Western blot analysis. H: CyclinA1, CyclinD1, and MMP-2 protein levels were quantified by densitometry (mean ± SE; n = 3, ***P < 0.001).
Downstream genes regulated by miR-451a via ATF2.
ATF2 is a transcription factor and regulates expression of multiple target genes related to cell migration and proliferation (21). The expression of ATF2 target genes CyclinA1, CyclinD1, and MMP2, was analyzed by Western blotting and qRT-PCR, after transfection with miR-451a mimic. Western blot and qRT-PCR data revealed that protein and mRNA levels of CyclinA1, CyclinD1, and MMP2 were significantly reduced by transfection of miR-451a mimic in ARPE-19 cells (Fig. 6, G and H) and primary human RPE cells (Supplemental Figs. S2 and S3). On the other hand, transfection of miR-451a inhibitor into human RPE cells increased ATF2 and CyclinA1, CyclinD1, and MMP2 expression (Supplemental Figs. S2 and S3). Furthermore, the protein and mRNA levels of CyclinA1, CyclinD1, and MMP2 were also elevated in the retina of Akita mice (Fig. 6, B–F). In conclusion, all these results indicated that miR-451a regulated CyclinA1, CyclinD1, and MMP2 through directly targeting ATF2.
DISCUSSION
Abnormal cell proliferation and migration are the basic features of epimembrane formation in PDR. The present study identified RPE cells in the epiretinal membranes from patients with PDR, suggesting proliferation and migration of RPE cells under PDR conditions. Furthermore, we demonstrated that diabetes-induced miR-451a expression change in DR and subsequent regulation of ATF2 may play an important role in metabolism, migration, and proliferation of RPE cells in DR, suggesting that miR-451 may represent a promising therapeutic target for PDR.
It is known that Ki-67 can bind to a nuclear antigen present in all cells that are in the G1, S, G2, and M phases of the cell cycle, but not those in the G0 phase (18, 38). Therefore, Ki-67 is a commonly accepted marker to detect the proliferative stage of the epiretinal membrane. In this study, we have identified that some epiretinal membranes in DR patients are in the proliferative stage based on Ki-67 staining.
miR-451a was first reported to have a potential role in the differentiation of erythroid cells (35). Furthermore, later studies identified that miR-451a was related to cell proliferation and migration (1, 13). miR-451a is consistently detected as the most highly enriched miRNA in the extracellular vesicles (EV) from a number of different cell types (15). Recent EV-associated studies were conducted due to its role in cell-to-cell communications (37), which makes it a possible disease biomarker and therapeutic target in diabetic complications (22). Clinical research showed that the expression of miR-451a in patients’ urine was correlated to the severity of diabetic complications (36). Our study demonstrated that miR-451a expression was downregulated in the retina of Akita mice, and in RPE cells exposed to 4-HNE, suggesting that it may play a crucial role in RPE cell proliferation and migration in DR. Besides the function of regulating proliferation and migration, miR-451a also regulates metabolism (14, 16, 43, 47). Previous studies showed that metabolic responses are closely related to proliferative stimuli, and metabolism and proliferation share common regulatory pathways (12). Taken together, miR-451a expression was changed in diabetic conditions, resulting in dysregulation of cell metabolic state, which consequently altered cell proliferation and migration.
Further, we have identified ATF2 as a target gene of miR-451a. First, bioinformatic analysis showed that the 3′-UTR of ATF2 contains possible binding sites of miR-451a. Second, following transfection of ATF2 3′-UTR in dual-luciferase vector and miR-451a mimic into HEK-293T cells, luciferase reporter assay was performed to confirm the interaction of miR-451a to the binding site in the ATF2 3′-UTR. Third, the level of miR-451a has a negative correlation to ATF2 mRNA and protein levels in primary RPE cells. Therefore, we concluded that overexpression of miR-451a can lead to downregulated ATF2 expression at both mRNA and protein levels.
In addition, it is known that ATF2, a cAMP-dependent transcription factor, can form homodimers or heterodimers with transcription factor families, such as Jun, Fos, or Maf, to regulate cellular functions (21). Intriguingly, ATF2 has different functions based on its tissue distribution (4) and subcellular localization (25).
When ATF2 is translocated into the nucleus, it can regulate the expression of downstream genes, such as Matrix Metalloproteinase 2 (MMP2) (41), CyclinA (39), and CyclinD1 (3). In this study, we found that miR-451a mimic downregulated ATF2 expression and inhibited proliferation and migration of ARPE-19 cells. Hence, the mechanism of miR-451a affecting the proliferation and migration of RPE cells may be through ATF2-induced downregulation of MMP2, CyclinA1, and CyclinD1. As shown by documented studies, MMP2 can facilitate RPE cell migration (19). CyclinA1 and CyclinD1 are also related to RPE proliferation and migration (10).
Notably, mitochondrial metabolism influences cell fate (2). Proliferating and nonproliferating cells display different cellular bioenergetics (12, 28). In nonproliferating cells, cell prefers to use energy from mitochondrial oxidative phosphorylation (OXPHOS). On the contrary, in highly proliferative cells, metabolism shifts toward to glycolysis (12). The phenomenon of proliferative cells enhancing aerobic glycolysis and reducing mitochondrial oxidation was known as Warburg metabolism and first described by Otto Warburg in 1956 (42). The energy phenotype shifting from oxidation to glycolysis is to facilitate biosynthetic processes to support cell growth and proliferation. Biosynthesis includes lipids, proteins, and nucleic acids. Increased fatty acid synthesis can modulate transcriptional regulation of cell growth receptors (29). Furthermore, some signaling lipids are recognized as important factors that trigger proliferative and survival pathways, such as the PI3K/AKT, Ras, or Wnt pathways (31). In conclusion, pathological proliferating cells rely on glycolysis, and decrease fatty acid oxidation for lipogenesis, which in turn activate the cell proliferative pathway. This phenomenon may explain the reason that fenofibrate, a fatty acid synthesis inhibitor, has a protective effect against the progression of DR (8).
Our data show that miR-451a mimic inhibited proliferation of ARPE-19 cells (Fig. 2, A and B), while it increased mitochondrial oxidation in a dose-dependent manner. Meanwhile, miR-451a inhibitor induced proliferation and decreased mitochondrial oxidation in a concentration-dependent manner. Under HNE-induced diabetic condition, miR-451a displayed a protective effect on the mitochondrial oxidation in ARPE-19 cells, whereas inhibition of miR-451a deteriorated the mitochondrial oxidation and ATP generation (Fig. 4, D–F). Based on the miR-451a mimic and inhibitor effects on mitochondrial function of ARPE-19 cells in nondiabetic and in diabetic conditions, miR-451a can modulate mitochondrial oxidation, corresponding to its antiproliferation effect.
The role of ATF2 in metabolism pathways can explain the effects of miR-451a on the bioenergetic pattern of ARPE-19. ATF2 interacts with other transcription factors. Under cobalt chloride (CoCl2)-induced hypoxia conditions, ATF2 upregulated the protein expression level and transcriptional activity of hypoxia inducible factor 1 alpha (HIF-1α) (9); stabilized HIF-1α then promotes ATP generation by enhancing anaerobic glycolysis (45) and suppressing mitochondria function (34). Besides glycolysis, ATF2 also directly affects mitochondrial function. Cytoplasm ATF2 downregulated the formation of complexes containing hexokinase 1 (HK1) and voltage-dependent anion channel (VDAC1) (25), and disrupted the outer-membrane permeability, initiating breakdown of mitochondrial membrane potential, which drives ATP generation (28, 40). In diabetic conditions, we observed downregulation of miR-451a (Fig. 1, D and E) and upregulation of ATF2 expression (Fig. 6A) which may stabilize HIF-1α, promote glycolysis, and, on the other hand, decrease mitochondrial membrane potential and promote the pathological cell proliferation and development of PDR.
In conclusion, our studies showed abnormal RPE proliferation and migration in the retinas of DR patients, and miR-451a expression was dysregulated in DR animal models. Through targeting ATF2, miR-451a inhibited RPE cell proliferation and migration via downregulating CyclinA1, CyclinD1, and MMP2, as well as regulating RPE mitochondrial oxidation (Fig. 7). PDR is a very severe vision-threatening complication of diabetes. RPE proliferation and migration is not the only pathological event in the progression of PDR; however, the beneficial effect of miR-451a on RPE delineated by the current study provides novel therapeutic insights into the treatment strategies for PDR. The clinical potential warrants further investigation.
Fig. 7.
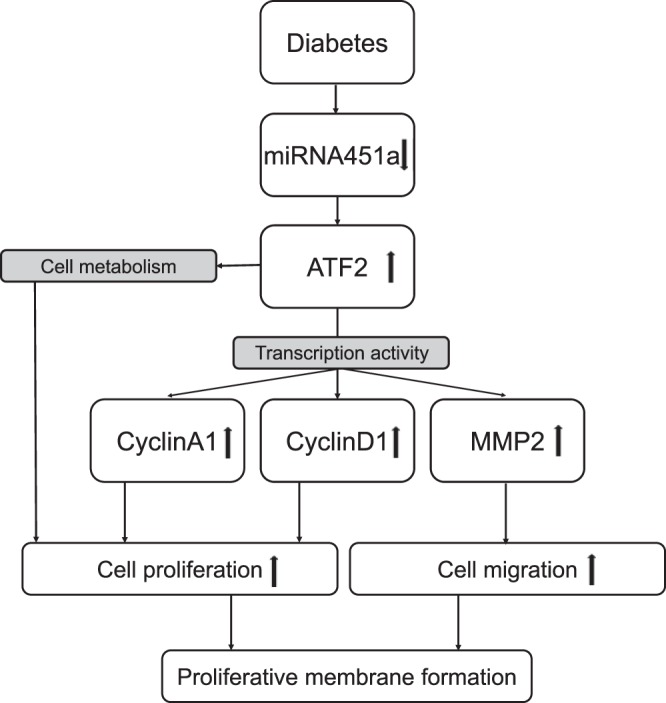
The role of miR-451a in the regulation of ATF2 signaling and its transcriptional activity. miR-451a levels are downregulated in diabetic conditions, which leads to upregulation of ATF2 and its downstream genes CyclinA1, CyclinD1, and MMP2. Meanwhile, ATF2 upregulation decreases mitochondrial membrane potential and facilitates cell proliferation.
GRANTS
Sources of funding for this study are NIH grants (EY-018659, EY-019309, EY-012231, and GM-122744), a Juvenile Diabetes Research Foundation (JDRF) grant (2-SRA-2014-147-Q-R), an Oklahoma Center for the Advancement of Science and Technology (OCAST) grant (HR16-041), a Natural Science Foundation of Tianjin, China grant (No. 15JCQNJ11400), and a National Natural Science Foundation of China grant (NSFC: 81570872).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.S. and X.-r.L. conceived and designed research; Y.S., L.-j.D., X.L., and Q.C. performed experiments; Y.S. analyzed data; Y.S. and Y.T. interpreted results of experiments; Y.S. and J.C. prepared figures; Y.S. and J.C. drafted manuscript; Y.S. and J.-x.M. edited and revised manuscript; Y.S. and X.-r.L. approved final version of manuscript.
Supplemental Figures
ACKNOWLEDGMENTS
We gratefully acknowledge Dr. Zhaohui Cheng and Dr. Longli Zhang for providing samples from patients.
REFERENCES
- 1.Bandres E, Bitarte N, Arias F, Agorreta J, Fortes P, Agirre X, Zarate R, Diaz-Gonzalez JA, Ramirez N, Sola JJ, Jimenez P, Rodriguez J, Garcia-Foncillas J. microRNA-451 regulates macrophage migration inhibitory factor production and proliferation of gastrointestinal cancer cells. Clin Cancer Res 15: 2281–2290, 2009. doi: 10.1158/1078-0432.CCR-08-1818. [DOI] [PubMed] [Google Scholar]
- 2.Bargiela D, Burr SP, Chinnery PF. Mitochondria and hypoxia: metabolic crosstalk in cell-fate decisions. Trends Endocrinol Metab 29: 249–259, 2018. doi: 10.1016/j.tem.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Beier F, Lee RJ, Taylor AC, Pestell RG, LuValle P. Identification of the cyclin D1 gene as a target of activating transcription factor 2 in chondrocytes. Proc Natl Acad Sci USA 96: 1433–1438, 1999. doi: 10.1073/pnas.96.4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger AJ, Kluger HM, Li N, Kielhorn E, Halaban R, Ronai Z, Rimm DL. Subcellular localization of activating transcription factor 2 in melanoma specimens predicts patient survival. Cancer Res 63: 8103–8107, 2003. [PubMed] [Google Scholar]
- 5.Chang W, Lajko M, Fawzi AA. Endothelin-1 is associated with fibrosis in proliferative diabetic retinopathy membranes. PLoS One 13: e0191285, 2018. doi: 10.1371/journal.pone.0191285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen E, Looman M, Laouri M, Gallagher M, Van Nuys K, Lakdawalla D, Fortuny J. Burden of illness of diabetic macular edema: literature review. Curr Med Res Opin 26: 1587–1597, 2010. doi: 10.1185/03007995.2010.482503. [DOI] [PubMed] [Google Scholar]
- 7.Chen ZN, Shao Y, Li XR. Prediction and bioinformatic analysis of hsa-miRNA-451 target genes. Chin J Ocul Fundus Dis 31: 597–599, 2015. doi: 10.3760/cma.j.issn.1005-1015.2015.06.022. [DOI] [Google Scholar]
- 8.Chew EY, Davis MD, Danis RP, Lovato JF, Perdue LH, Greven C, Genuth S, Goff DC, Leiter LA, Ismail-Beigi F, Ambrosius WT; Action to Control Cardiovascular Risk in Diabetes Eye Study Research Group . The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. Ophthalmology 121: 2443–2451, 2014. doi: 10.1016/j.ophtha.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi JH, Cho HK, Choi YH, Cheong J. Activating transcription factor 2 increases transactivation and protein stability of hypoxia-inducible factor 1alpha in hepatocytes. Biochem J 424: 285–296, 2009. doi: 10.1042/BJ20090371. [DOI] [PubMed] [Google Scholar]
- 10.Du ZD, Hu LT, Zhao GQ, Li Y, Ma ZZ. Protein tyrosine phosphatase 1B regulates the activity of retinal pigment epithelial cells. Mol Vis 21: 523–531, 2015. [PMC free article] [PubMed] [Google Scholar]
- 11.Faid I, Al-Hussaini H, Kilarkaje N. Resveratrol alleviates diabetes-induced testicular dysfunction by inhibiting oxidative stress and c-Jun N-terminal kinase signaling in rats. Toxicol Appl Pharmacol 289: 482–494, 2015. doi: 10.1016/j.taap.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 12.Fritz V, Fajas L. Metabolism and proliferation share common regulatory pathways in cancer cells. Oncogene 29: 4369–4377, 2010. doi: 10.1038/onc.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Godlewski J, Bronisz A, Nowicki MO, Chiocca EA, Lawler S. microRNA-451: A conditional switch controlling glioma cell proliferation and migration. Cell Cycle 9: 2742–2748, 2010. doi: 10.4161/cc.9.14.12248. [DOI] [PubMed] [Google Scholar]
- 14.Godlewski J, Nowicki MO, Bronisz A, Nuovo G, Palatini J, De Lay M, Van Brocklyn J, Ostrowski MC, Chiocca EA, Lawler SE. MicroRNA-451 regulates LKB1/AMPK signaling and allows adaptation to metabolic stress in glioma cells. Mol Cell 37: 620–632, 2010. doi: 10.1016/j.molcel.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guduric-Fuchs J, O’Connor A, Camp B, O’Neill CL, Medina RJ, Simpson DA. Selective extracellular vesicle-mediated export of an overlapping set of microRNAs from multiple cell types. BMC Genomics 13: 357, 2012. doi: 10.1186/1471-2164-13-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He J, Wang W, Lu L, Tian Y, Niu D, Ren J, Dong L, Sun S, Zhao Y, Chen L, Shen J, Li X. Analysis of miRNAs and their target genes associated with lipid metabolism in duck liver. Sci Rep 6: 27418, 2016. doi: 10.1038/srep27418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He S, Kumar SR, Zhou P, Krasnoperov V, Ryan SJ, Gill PS, Hinton DR. Soluble EphB4 inhibition of PDGF-induced RPE migration in vitro. Invest Ophthalmol Vis Sci 51: 543–552, 2010. doi: 10.1167/iovs.09-3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herault O, Gallay N, Domenech J, Colombat P, Binet C. Pharmacological doses of all-trans retinoic acid promote G0/G1 transition and G1 arrest of normal human marrow CD34+ cells. Cell Death Differ 11, Suppl 2: S207–S209, 2004. doi: 10.1038/sj.cdd.4401471. [DOI] [PubMed] [Google Scholar]
- 19.Hou X, Han QH, Hu D, Tian L, Guo CM, Du HJ, Zhang P, Wang YS, Hui YN. Mechanical force enhances MMP-2 activation via p38 signaling pathway in human retinal pigment epithelial cells. Graefes Arch Clin Exp Ophthalmol 247: 1477–1486, 2009. doi: 10.1007/s00417-009-1135-1. [DOI] [PubMed] [Google Scholar]
- 20.Huang JY, Zhang K, Chen DQ, Chen J, Feng B, Song H, Chen Y, Zhu Z, Lu L, De W, Wang R, Chen LB. MicroRNA-451: epithelial-mesenchymal transition inhibitor and prognostic biomarker of hepatocelluar carcinoma. Oncotarget 6: 18613–18630, 2015. doi: 10.18632/oncotarget.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huret JL. ATF2 (Activating Transcription Factor 2). Atlas of Genetics and Cytogenetics in Oncology and Haematology, 2013. http://atlasgeneticsoncology.org//Genes/ATF2ID718ch2q31.html.
- 22.Kato M, Castro NE, Natarajan R. MicroRNAs: potential mediators and biomarkers of diabetic complications. Free Radic Biol Med 64: 85–94, 2013. doi: 10.1016/j.freeradbiomed.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krauss RM. Lipids and lipoproteins in patients with type 2 diabetes. Diabetes Care 27: 1496–1504, 2004. doi: 10.2337/diacare.27.6.1496. [DOI] [PubMed] [Google Scholar]
- 24.Lamoureux EL, Wong TY. Diabetic retinopathy in 2011: further insights from new epidemiological studies and clinical trials. Diabetes Care 34: 1066–1067, 2011. doi: 10.2337/dc11-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lau E, Ronai ZA. ATF2 - at the crossroad of nuclear and cytosolic functions. J Cell Sci 125: 2815–2824, 2012. doi: 10.1242/jcs.095000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang Z, Gao KP, Wang YX, Liu ZC, Tian L, Yang XZ, Ding JY, Wu WT, Yang WH, Li YL, Zhang ZB, Zhai RH. RNA sequencing identified specific circulating miRNA biomarkers for early detection of diabetes retinopathy. Am J Physiol Endocrinol Metab 315: E374–E385, 2018. doi: 10.1152/ajpendo.00021.2018. [DOI] [PubMed] [Google Scholar]
- 27.Lv G, Hu Z, Tie Y, Du J, Fu H, Gao X, Zheng X. MicroRNA-451 regulates activating transcription factor 2 expression and inhibits liver cancer cell migration. Oncol Rep 32: 1021–1028, 2014. doi: 10.3892/or.2014.3296. [DOI] [PubMed] [Google Scholar]
- 28.Maldonado EN, Lemasters JJ. ATP/ADP ratio, the missed connection between mitochondria and the Warburg effect. Mitochondrion 19, Pt A: 78–84, 2014. doi: 10.1016/j.mito.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer 7: 763–777, 2007. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 30.Murray AR, Chen Q, Takahashi Y, Zhou KK, Park K, Ma JX. MicroRNA-200b downregulates oxidation resistance 1 (Oxr1) expression in the retina of type 1 diabetes model. Invest Ophthalmol Vis Sci 54: 1689–1697, 2013. doi: 10.1167/iovs.12-10921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nadolski MJ, Linder ME. Protein lipidation. FEBS J 274: 5202–5210, 2007. doi: 10.1111/j.1742-4658.2007.06056.x. [DOI] [PubMed] [Google Scholar]
- 32.Nyengaard JR, Ido Y, Kilo C, Williamson JR. Interactions between hyperglycemia and hypoxia: implications for diabetic retinopathy. Diabetes 53: 2931–2938, 2004. doi: 10.2337/diabetes.53.11.2931. [DOI] [PubMed] [Google Scholar]
- 33.Oberstein SY, Byun J, Herrera D, Chapin EA, Fisher SK, Lewis GP. Cell proliferation in human epiretinal membranes: characterization of cell types and correlation with disease condition and duration. Mol Vis 17: 1794–1805, 2011. [PMC free article] [PubMed] [Google Scholar]
- 34.Okamoto A, Sumi C, Tanaka H, Kusunoki M, Iwai T, Nishi K, Matsuo Y, Harada H, Takenaga K, Bono H, Hirota K. HIF-1-mediated suppression of mitochondria electron transport chain function confers resistance to lidocaine-induced cell death. Sci Rep 7: 3816, 2017. doi: 10.1038/s41598-017-03980-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rathjen T, Nicol C, McConkey G, Dalmay T. Analysis of short RNAs in the malaria parasite and its red blood cell host. FEBS Lett 580: 5185–5188, 2006. doi: 10.1016/j.febslet.2006.08.063. [DOI] [PubMed] [Google Scholar]
- 36.Sayilar EI, Gullulu M, Tuncel E, Peynirci H, Alemdar A, Tunca B, Egeli U, Cecener G, Bayindir M, Cosgun G. Biomarker potential of urine miR-451 at different stages of diabetic nephropathy. J Diabetes Metab 7: 650, 2016. doi: 10.4172/2155-6156.1000650. [DOI] [Google Scholar]
- 37.Schatz D, Vardi A. Extracellular vesicles - new players in cell-cell communication in aquatic environments. Curr Opin Microbiol 43: 148–154, 2018. doi: 10.1016/j.mib.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 38.Schwarting R, Gerdes J, Niehus J, Jaeschke L, Stein H. Determination of the growth fraction in cell suspensions by flow cytometry using the monoclonal antibody Ki-67. J Immunol Methods 90: 65–70, 1986. doi: 10.1016/0022-1759(86)90384-4. [DOI] [PubMed] [Google Scholar]
- 39.Shimizu M, Nomura Y, Suzuki H, Ichikawa E, Takeuchi A, Suzuki M, Nakamura T, Nakajima T, Oda K. Activation of the rat cyclin A promoter by ATF2 and Jun family members and its suppression by ATF4. Exp Cell Res 239: 93–103, 1998. doi: 10.1006/excr.1997.3884. [DOI] [PubMed] [Google Scholar]
- 40.Shoshan-Barmatz V, De Pinto V, Zweckstetter M, Raviv Z, Keinan N, Arbel N. VDAC, a multi-functional mitochondrial protein regulating cell life and death. Mol Aspects Med 31: 227–285, 2010. doi: 10.1016/j.mam.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Song H, Ki SH, Kim SG, Moon A. Activating transcription factor 2 mediates matrix metalloproteinase-2 transcriptional activation induced by p38 in breast epithelial cells. Cancer Res 66: 10487–10496, 2006. doi: 10.1158/0008-5472.CAN-06-1461. [DOI] [PubMed] [Google Scholar]
- 42.Warburg O. On the origin of cancer cells. Science 123: 309–314, 1956. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 43.Yamada H, Suzuki K, Ichino N, Ando Y, Sawada A, Osakabe K, Sugimoto K, Ohashi K, Teradaira R, Inoue T, Hamajima N, Hashimoto S. Associations between circulating microRNAs (miR-21, miR-34a, miR-122 and miR-451) and non-alcoholic fatty liver. Clin Chim Acta 424: 99–103, 2013. doi: 10.1016/j.cca.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 44.Yuan J, Lang J, Liu C, Zhou K, Chen L, Liu Y. The expression and function of miRNA-451 in osteosarcoma. Med Oncol 32: 324, 2015. doi: 10.1007/s12032-014-0324-x. [DOI] [PubMed] [Google Scholar]
- 45.Zhai P, Sadoshima J. Overcoming an energy crisis?: an adaptive role of glycogen synthase kinase-3 inhibition in ischemia/reperfusion. Circ Res 103: 910–913, 2008. doi: 10.1161/01.RES.0000338259.37472.b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang X, Barile G, Chang S, Hays A, Pachydaki S, Schiff W, Sparrow J. Apoptosis and cell proliferation in proliferative retinal disorders: PCNA, Ki-67, caspase-3, and PARP expression. Curr Eye Res 30: 395–403, 2005. doi: 10.1080/02713680590956306. [DOI] [PubMed] [Google Scholar]
- 47.Zhuo S, Yang M, Zhao Y, Chen X, Zhang F, Li N, Yao P, Zhu T, Mei H, Wang S, Li Y, Chen S, Le Y. MicroRNA-451 negatively regulates hepatic glucose production and glucose homeostasis by targeting glycerol kinase-mediated gluconeogenesis. Diabetes 65: 3276–3288, 2016. doi: 10.2337/db16-0166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.