Abstract
Our laboratory has previously shown in an ovine model of pregnancy that abnormal elevations in maternal cortisol during late gestation lead to increased fetal cardiac arrhythmias and mortality during peripartum. Furthermore, transcriptomic analysis of the fetal heart suggested alterations in TCA cycle intermediates and lipid metabolites in animals exposed to excess cortisol in utero. Therefore, we utilized a sheep model of pregnancy to determine how chronic increases in maternal cortisol alter maternal and fetal serum before birth and neonatal cardiac metabolites and lipids at term. Ewes were either infused with 1 mg·kg−1·day−1 of cortisol starting at gestational day 115 (n = 9) or untreated (n = 6). Serum was collected from the mother and fetus (gestational day 125), and hearts were collected following birth. Proton nuclear magnetic resonance (1H-NMR) spectroscopy was conducted to measure metabolic profiles of newborn heart specimens as well as fetal and maternal serum specimens. Mass spectrometry was conducted to measure lipid profiles of newborn heart specimens. We observed alterations in amino acid and TCA cycle metabolism as well as lipid and glycerophospholipid metabolism in newborn hearts after excess maternal cortisol in late gestation. In addition, we observed alterations in amino acid and TCA cycle metabolites in fetal but not in maternal serum during late gestation. These results suggest that fetal exposure to excess maternal cortisol alters placental and fetal metabolism before birth and limits normal cardiac metabolic maturation, which may contribute to increased risk of peripartum cardiac arrhythmias observed in these animals or later life cardiomyopathies.
Keywords: cortisol, fetus, heart, metabolomics, newborn
INTRODUCTION
The heart is undergoing many metabolic alterations during the peripartum period to prepare for the metabolic switch from primarily utilizing lactate and glucose in utero to preferential utilization of fatty acids following birth (2, 32, 33). The last 30 days of pregnancy are a crucial developmental period for the heart, as cardiomyocytes undergo the transition from cardiomyocyte proliferation to terminal maturation (21). Cortisol is a natural glucocorticoid produced in the adrenal glands that plays an important role in the maturation of fetal organs during late gestation, including the heart (16, 43). Prenatal glucocorticoid administration has been linked to cardiac hypertrophy both in humans and sheep, suggesting disruption of these normal structural changes during late gestation (5, 34, 55). However, the effect of excess maternal glucocorticoids in late gestation on metabolic alterations in the newborn heart is yet to be determined.
Previous work in our laboratory showed that chronic elevations in maternal cortisol in an ovine model of pregnancy during a 10- to 14-day period in late gestation (to gestational day 130) lead to enlargement of the fetal heart (41). Furthermore, we found that this cardiac proliferation is attenuated with mineralocorticoid antagonist infusion into the heart (12, 41). If the chronic maternal infusion of cortisol was maintained from 115 days of gestation until birth, we observed an increased incidence of stillbirth during the peripartum period (22). This incidence of stillbirth was associated with fetal bradycardia and increased incidence of arrhythmias in the heart during the late stages of labor and delivery (1). Transcriptomic analysis of the term fetal heart suggested alterations in lipid and small molecule metabolism due to excess maternal cortisol exposure in a similarly treated cohort of sheep (42). Therefore, we utilized metabolomic and lipidomic techniques to test whether metabolic alterations in the newborn heart at birth would be evident in animals exposed to excess maternal cortisol during late gestation. We hypothesized that chronic cortisol exposure in late gestation leads to metabolic alterations of the newborn heart and that use of metabolomics techniques would identify pathways altered in this model. We also hypothesized that the pattern of changes in the newborn heart metabolome would reflect changes in fetal and/or maternal serum metabolomes. These changes in metabolic pathways could be used to better understand the role of excess maternal cortisol on metabolic development of the newborn heart and to design further studies to better understand the role of cardiac metabolism in fetal distress during labor, which may lead to the development of biomarkers for fetal distress in high-stress pregnancies.
METHODS
Animals.
Fifteen Ramboullet cross ewes and their lambs were studied. All animal use was approved by the University of Florida Institutional Animal Care and Use Committee. To control for metabolic variation in the blood, ewes were fed a standardized diet based on the National Research Council calculations of energy requirements in the late gestation ewe. Obese or very lean ewes (judged on the basis of body condition score, the ovine equivalent of body mass index) were excluded from the study. Animals were housed in a temperature and light-controlled environment and feed was provided each morning. Ewes with multiple gestations were excluded from the study. Ewes were assigned to one of two groups at gestational day 115: a control group (n = 9) with no treatment and a cortisol group (CORT; n = 6) with infusion of 1 mg·kg−1·day−1 of hydrocortisone sodium succinate in sodium phosphate (Solu-Cortef; Pfizer, New York, NY). This amount of cortisol is enough to roughly double maternal plasma cortisol levels (1). Cortisol infusion and surgery were conducted at gestational day 118 (±1 day) to insert maternal and fetal catheters, fetal telemetry device, and a flow probe as previously described (1). Blood pressure, heart rate, ECG, and plasma cortisol concentrations from these ewes and fetuses have been previously reported (1).
Blood samples were collected from ewe and fetus on gestational days 125, 130, 135, 138, and 140 and every subsequent day until birth (control: 144 ± 1; CORT: 142 ± 2 days gestational age); samples for metabolomics analysis were collected in glass tubes on ice with no anticoagulant and spun for 20 min at 4°C; the resulting serum was stored at −20°C until metabolomic analysis. For two fetuses in the cortisol treatment group, serum was not collected, and, therefore, these animals were excluded from serum metabolomic analysis. Immediately following birth, the lambs were euthanized with an overdose of Euthasol (pentobarbital sodium and phenytoin sodium; Virbac AH), and heart tissue was collected from the right ventricle (RV), left ventricle (LV), and intraventricular septum and immediately frozen in liquid nitrogen. Tissue samples were stored at −80°C until data collection. Three control animals were excluded from high-resolution magic angle spinning (HR-MAS) analysis; two were excluded due to inadequate tissues available for analysis, while one was excluded due to timing of heart collection (>3 h following birth). Therefore, heart tissue was collected from 12 total animals (n = 6 controls; n = 6 CORT), while serum specimens were collected from 13 total animals (n = 9 controls; n = 4 CORT).
Nuclear magnetic resonance metabolomics analysis.
HR-MAS proton nuclear magnetic resonance (1H-NMR) was used to determine metabolites present in RV and LV free walls and intraventricular septum as previously described (51). Metabolites identified with this technique were not found to be significantly altered between different areas of the heart and were therefore treated as biological replicates, effectively defining cardiac tissue as our experimental unit (29) (Supplemental Table S1; Supplemental Material for this article is available online at the Journal website). Briefly, 30 μl of deuterium oxide (D2O) were added to 17.2–56.6 mg (mean: 29.15 mg) of intact heart tissue before being placed into a 4-mm HR-MAS rotor (Bruker Biospin, Billerica, MA) and spun at 6 kHz with a spectral width of 10 ppm. Data were acquired using a one-dimensional nuclear Overhauser effect pulse sequence with presaturation of water resonance (NOESYPR1D) on an Avance III 600 MHz Bruker NMR spectrometer equipped with a 4-mm HR-MAS probe at the University of Florida Advanced Magnetic Resonance Imaging and Spectroscopy Facility.
To further identify the source of altered metabolites in the newborn hearts after in utero exposure to excess cortisol, we used untargeted NMR metabolomics on serum samples collected from the ewe and fetus. One-dimensional (1D) 1H-NMR was used to determine metabolites present in maternal and fetal serum as previously described (3). Briefly, samples were thawed on ice and centrifuged at 3,000 g for 20 min at 4°C. Two-hundred microliters of serum were mixed with 400 μl of saline buffer (0.9% NaCl) in 10% D2O, and the mixture was centrifuged for 10 min at 3,000 g at 4°C. For each sample, 500 μl of resulting supernatant were transferred into a 5-mm SampleJet NMR tube (Bruker Biospin). Samples were analyzed on an Avance II 600 MHz Bruker NMR spectrometer equipped with a 5-mm cryoprobe and Bruker SampleJet cooled to 5.6°C at the University of Florida Advanced Magnetic Resonance Imaging and Spectroscopy Facility. Data were acquired using a one-dimensional experiment with T2 filter using Carr-Purcell-Meiboom-Gill pulse sequence with water presaturation.
Two-dimensional (2D) 1H-NMR was used to aid in annotation of metabolites identified in 1D NMR data of cardiac and serum specimens and was not used for any statistical analyses. For cardiac tissue, one pooled control (n = 2) and one pooled cortisol (n = 2) specimen were created by pooling 100 mg of cardiac tissue. The two tissue samples were homogenized and prepared for NMR metabolomics analysis as previously described (51). For serum, one pooled maternal (from 4 ewes) and one pooled fetal (from 4 fetuses) specimen were each used for 2D NMR data acquisition. Serum samples were prepared for extraction as previously described (17) and all solvents used were high-performance liquid chromatography (HPLC) grade. Briefly, serum samples were thawed at 4°C and 1.2 ml of pooled serum and saline buffer specimens was mixed with 2.4 ml of cold methanol (HPLC-grade), vortexed briefly to mix, and incubated in 20 °C for 20 min. Samples were then centrifuged at 16,000 rcf for 30 min to pellet proteins and the resulting supernatant was transferred into a 1.5-ml Eppendorf tube and dried using CentriVap Vacuum Concentrator (Labconco, Kanas City, MO). Concentrated serum specimens were reconstituted in 100 µM DSS-D6 in D2O as a reference standard and vortexed until the pellets dissolved. The samples were then centrifuged at 14,000 rcf for 15 min at 4°C before the transfer of 180 μl into 3-mm NMR tubes (Bruker Biospin). Extracted tissue and serum specimens were run on an Avance III HD 600 MHz Bruker NMR spectrometer equipped with a 5-mm cryoprobe and Bruker SampleJet cooled to 5.6°C at the University of Georgia Complex Carbohydrate Research Center. Data were acquired using 2D 1H-13C heteronuclear single quantum correlation (HSQC), 1H-13C HSQC-TOCSY (HSQC-total correlation spectroscopy), and 1H-1H homonuclear Harman-Hahn (TOCSY) experiments. A total of 27 metabolites were identified in cardiac tissue, 42 metabolites in fetal serum, and 30 metabolites in maternal serum using Bruker AssureNMR software (Bruker Biospin) with BBiorefcode metabolite database and COLMARm (4). The metabolites were assigned a confidence level ranging from 1 to 5 as previously described (50). The spectra were processed using Bruker Topspin 3.6 software and in-house MATLAB scripts. The exact spectral areas for integration and confidence values for each metabolite or feature included in statistical analysis are listed in Supplemental Table S2.
Lipidomic analysis of cardiac specimens.
Tissue specimens were prepared for lipidomic analysis as previously described (51). Internal and injections standard mixtures are listed in Supplemental Table S3. Lipid analysis was performed on a Thermo Q-Exactive Orbitrap with Dionex Ultimate 3000 UHPLC and autosampler. The mass spectrometer was operated in the positive and negative ionization mode, using a heated electrospray ionization source. Spectra were collected from m/z 200–2200 at with a mass resolution setting of 70,000 (defined at m/z 200), and tandem mass spectra were collected using data-dependent scanning (top 10) and all-ion fragmentation. Feature finding and alignment were performed with MZmine 2.26 (40) as previously described (25). LipidMatch was used to identify lipids (26), and identified lipids were normalized to their representative internal standard using an in-house R script, LipidMatch Normalizer, which can be accessed at (http://secim.ufl.edu/secim-tools/). UHPLC-tandem mass spectrometry (MS/MS) identified 284 lipids across 26 different lipid classes in control and cortisol right ventricle tissues (Supplemental Table S4).
Statistics.
All raw and processed metabolomics and lipidomics data are available on the Metabolomics workbench (http://www.metabolomicsworkbench.org/), along with detailed experimental NMR and mass spectrometry methods. Metabolomic and lipidomic data were normalized using probabilistic quotient normalization and then batch corrected using an empirical Bayes method in Metaboanalyst 4.0, an online webserver for metabolomic data analysis (8) to correct for batch-effects over multiple sheep seasons (8, 9, 20). Following batch correction, data were range scaled before multivariate statistical analysis (9, 11). Multivariate analyses of processed spectra were performed using in-house MATLAB scripts (https://github.com/artedison/Edison_Lab_Shared_Metabolomics_UGA) and the PLS Toolbox for MATLAB (Eigenvector Research, Manson, WA) (49). Orthogonal signal correction partial least squares discriminant analysis (OSC-PLSDA) was conducted with a venetian blinds cross validation including 20 latent variables and 10 data splits. A heatmap of the top 30 significant lipids was generated in Metaboanalyst 4.0 using a Pearson correlation coefficients of autoscaled features, and clustering was determined using the Ward algorithm (52).
Univariate statistics were performed on metabolites identified in serum and heart tissue using 1D 1H-NMR data as well as lipids identified in heart tissue after probabilistic quotient normalization. Initially, a one-way ANOVA was used to determine the metabolites that differed between areas of the heart for HR-MAS metabolomics data in different cardiac regions; a Tukey-Kramer post hoc analysis determined that there are no significant metabolite variations between different regions of the cardiac walls for metabolites identified by HR-MAS (Supplemental Table S1). As values of metabolites in all three regions were not significantly different (P > 0.05), values in all three regions were used in further analyses as independent replicates, defining cardiac tissue as our experimental unit (29). We utilized a Student’s t-test to determine significant metabolites, as well as lipids in cardiac tissue to perform further analyses to identify significant pathways altered by treatment.
For analysis of maternal and fetal serum specimens, metabolite concentrations at five different ranges of time relative to birth were determined: 1) 0–2 days, 2) 3–6 days, 3) 7–10 days, 4) 11–15 days, and 5) >15 days before birth. For animals that had greater than one sample in each time period, the average value of the metabolites in those samples were used in the analysis. Differences in metabolites in maternal and fetal serum were analyzed using a linear mixed-effects model, taking into account a random effect (animal), random error (within animals), fixed effect (cortisol treatment), and a covariate (time leading to delivery). A Student’s t-test was used to determine metabolites altered due to treatment at all time points, as well as the three time points within 10 days of delivery. Due to low sample size, individual metabolites and lipids were considered significantly changed if they had a raw P < 0.05. This broad criteria for inclusion of metabolites and lipids was used to assess significant pathways [false discovery rate (FDR)-corrected P < 0.10] that included these compounds (see section below).
Finally, to identify pathways that showed enrichment for significant compounds in heart tissue (metabolites and lipid) and metabolites in serum specimens, we utilized an overrepresentation analysis (ORA) based on a hypergeometric test (54). Significant pathways were defined as pathways with FDR-corrected P < 0.10. Pathways with d-amino acids were removed from results as we were not able to distinguish between l- and d-configurations with our NMR metabolomic technique, and we therefore could not determine the relevance of these pathways in our model.
RESULTS
Excess maternal cortisol exposure in late gestation alters the metabolic profile of newborn cardiac tissue.
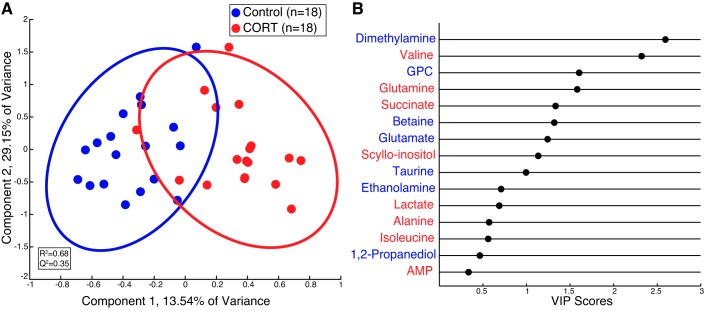
HR-MAS 1H-NMR revealed changes in the metabolite profile of cardiac tissue in the newborn heart exposed to excess maternal cortisol in utero compared with control animals (Fig. 1). Although principal component analysis scores plot did not yield group separation (data not shown), an OSC-PLSDA scores plot (R2 = 0.68; Q2 = 0.35) revealed separation of control and CORT tissues (Fig. 1A). A variable importance projection (VIP) plot was used to identify the first 15 metabolites that contribute to the OSC-PLSDA separation in the first component. The VIP plot revealed that valine, glutamine, succinate, and scyllo-inositol, which were elevated in CORT cardiac tissue, and dimethylamine, glycerophosphocholine (GPC), betaine, and glutamate, which were diminished in CORT cardiac tissue, were driving OSC-PLSDA separation with VIP scores greater than one (Fig. 1B). Furthermore, comparison of metabolites by Student’s t-test indicated that valine, glutamine, and succinate were significantly elevated in CORT cardiac tissue, whereas dimethylamine, GPC, and betaine, and glutamate were diminished (P < 0.05, Table 1).
Fig. 1.
Orthogonal signal corrected partial least squares discriminant analysis (OSC-PLSDA) reveals separation of high-resolution magic angle spinning (HR-MAS) cardiac tissue specimens from control and cortisol (CORT) newborn sheep immediately following birth. A: OSC-PLSDA scores plot reveals separation of control (n = 18 from 6 animals, blue) and CORT (n = 18 from 6 animals, red) cardiac tissue in the first principal component. B: variable importance of projection (VIP) plot for the first 15 metabolites that contribute to separation in OSC-PLSDA component 1. The more a metabolite contributes to the separation in the scores plot, the greater the VIP score (x-axis). Metabolites colored in blue are elevated in control cardiac tissue specimens, while those colored in red are elevated in CORT specimens relative to control. GPC, glycerophosphocholine.
Table 1.
Metabolites altered in the newborn cardiac tissue due to chronic maternal cortisol exposure in late gestation
| Relative Means ± SE |
||||
|---|---|---|---|---|
| Metabolite | P Value | Control | Cortisol | Log2(FC) (Cortisol/Control) |
| Dimethylamine | 1.20E−04 | 0.305 ± 0.018 | 0.196 ± 0.009 | −0.64 |
| Valine | 3.11E−04 | 0.078 ± 0.005 | 0.189 ± 0.022 | 1.27 |
| GPC | 6.48E−03 | 11.356 ± 0.333 | 9.014 ± 0.569 | −0.33 |
| Glutamine | 0.01 | 1.847 ± 0.097 | 2.331 ± 0.114 | 0.34 |
| Succinate | 0.01 | 0.234 ± 0.016 | 0.340 ± 0.028 | 0.54 |
| Betaine | 0.03 | 2.600 ± 0.130 | 2.085 ± 0.138 | −0.32 |
| Glutamate | 0.05 | 2.915 ± 0.115 | 2.445 ± 0.145 | −0.25 |
| Scyllo-inositol | 0.06 | 0.732 ± 0.039 | 0.851 ± 0.033 | 0.22 |
| Taurine | 0.07 | 9.844 ± 0.646 | 7.945 ± 0.496 | −0.31 |
| Lactate | 0.22 | 5.931 ± 0.236 | 6.612 ± 0.375 | 0.16 |
| Ethanolamine | 0.28 | 0.645 ± 0.018 | 0.615 ± 0.013 | −0.07 |
| Isoleucine | 0.32 | 0.352 ± 0.032 | 0.423 ± 0.047 | 0.26 |
| Alanine | 0.35 | 2.491 ± 0.131 | 2.792 ± 0.220 | 0.16 |
| 1,2-Propanediol | 0.44 | 4.104 ± 0.463 | 3.511 ± 0.418 | −0.23 |
| AMP | 0.59 | 0.238 ± 0.016 | 0.254 ± 0.018 | 0.10 |
| 2-Hydroxybutyrate | 0.60 | 0.469 ± 0.058 | 0.526 ± 0.067 | 0.16 |
| Myo-inositol | 0.61 | 8.404 ± 0.443 | 8.755 ± 0.331 | 0.06 |
| Lipid (-CH = CH-) | 0.64 | 5.072 ± 0.616 | 5.581 ± 0.634 | 0.14 |
| Lipid (β-CH2) | 0.69 | 4.936 ± 0.657 | 5.371 ± 0.608 | 0.12 |
| Inosine | 0.81 | 0.497 ± 0.033 | 0.485 ± 0.023 | −0.03 |
| Lipid (α-CH2) | 0.84 | 5.023 ± 0.719 | 5.276 ± 0.739 | 0.07 |
| Creatine | 0.85 | 13.463 ± 0.291 | 13.360 ± 0.317 | −0.01 |
| Ethanol | 0.87 | 1.004 ± 0.115 | 1.041 ± 0.136 | 0.05 |
| Choline | 0.88 | 2.072 ± 0.129 | 2.043 ± 0.089 | −0.02 |
| Glutathione | 0.91 | 0.547 ± 0.022 | 0.543 ± 0.014 | −0.01 |
| Lipid (CO2-CH) | 0.93 | 1.051 ± 0.091 | 1.064 ± 0.081 | 0.02 |
| Lipid (-CH3) | 0.95 | 19.128 ± 1.352 | 19.273 ± 1.488 | 0.01 |
For control and cortisol groups, n = 18 from 6 animals. GPC, glycerophosphocholine; AMP: adenosine 5′-monophosphate; GPC, glycerophosphocholine; FC, fold change.
Excess maternal cortisol exposure in late gestation alters the lipid profile of newborn cardiac tissue.
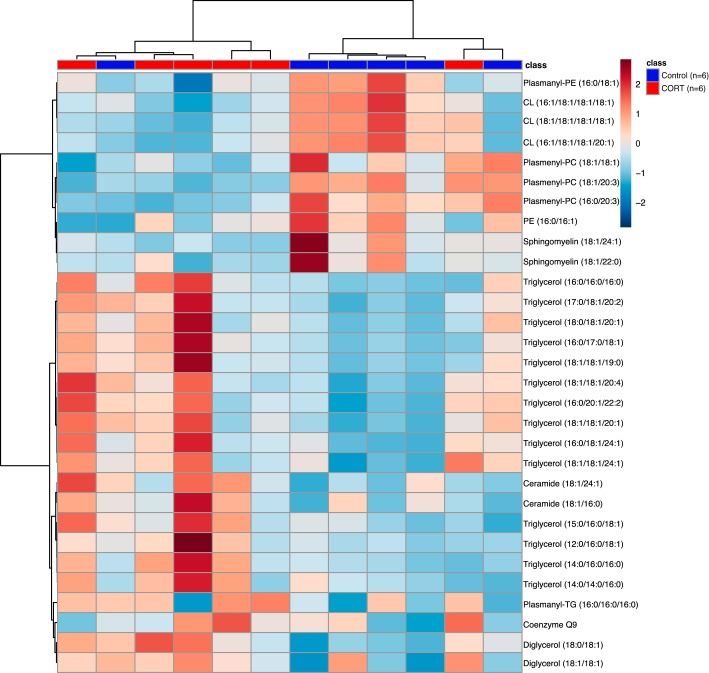
A Pearson correlation heatmap revealed clustering of specimens based on group (Fig. 2). However, a principal component analysis scores plot did not yield group separation of lipids between control and CORT cardiac tissue (data not shown). Relative means of each lipid following normalization determined eight lipid species that were significant by Student’s t-test in CORT cardiac tissue compared with control (Table 2). We observed decreases in plasmenyl-phosphatidylcholines (plasmenyl-PCs), plasmanyl-phosphatidylethanolamines, and cardiolipins (CLs) in CORT cardiac tissue compared with controls, whereas triglycerides (TGs) and diglycerides (DGs) were elevated in CORT cardiac tissue (P < 0.05, Table 2).
Fig. 2.
Pearson correlation heatmap of top 30 significant features reveals clustering of control (n = 6) and cortisol (CORT; n = 6) newborn cardiac tissue samples based on lipid class. Ether-linked lipids as well as cardiolipins and sphingomyelins were elevated in control cardiac tissue, while triglycerides were the main class upregulated in CORT cardiac tissue. PC, phosphatidylcholine; PE, phosphatidylethanolamine; CL, cardiolipin; TG, triglyceride.
Table 2.
Lipids significantly altered in the newborn cardiac tissue due to chronic maternal cortisol exposure in late gestation
| Relative Means ± SE |
||||
|---|---|---|---|---|
| Lipids | P Value | Control | Cortisol | Log2(FC) (Cortisol/Control) |
| DG (18:0/18:1) | 0.02 | 0.092 ± 0.002 | 0.141 ± 0.002 | 0.61 |
| TG (16:0/18:1/24:1) | 0.03 | 0.006 ± 0.000 | 0.009 ± 0.000 | 0.58 |
| Plasmenyl-PC (18:1/20:3) | 0.03 | 1.019 ± 0.011 | 0.728 ± 0.013 | −0.49 |
| Plasmenyl-PC (16:0/20:3) | 0.03 | 0.822 ± 0.014 | 0.544 ± 0.009 | −0.60 |
| CL (16:1/18:1/18:1/18:1) | 0.03 | 0.112 ± 0.003 | 0.063 ± 0.001 | −0.82 |
| TG (14:0/16:0/16:0) | 0.04 | 0.047 ± 0.000 | 0.076 ± 0.002 | 0.69 |
| Plasmanyl-PE (16:0/18:1) | 0.05 | 0.011 ± 0.000 | 0.009 ± 0.000 | −0.31 |
| TG (18:1/18:1/24:1) | 0.05 | 0.003 ± 0.000 | 0.004 ± 0.000 | 0.66 |
For control and cortisol groups, n = 18 from 6 animals. DG, diglyceride; TG, triglyceride; PC, phosphatidylcholine; CL, cardiolipin; PE, phosphatidylethanolamine; FC, fold change.
Excess maternal cortisol exposure in late gestation alters the metabolic profile of fetal serum before birth.
We identified 42 metabolites in fetal serum using 1H-NMR. A linear mixed effects model of samples from all time points revealed a significant decrease of methionine and creatine in serum from CORT animals compared with control, while very low-density lipoproteins, branched-chain amino acids (BCAAs; valine, leucine, and isoleucine), choline, and glycine were significantly elevated in serum from CORT fetuses (Table 3). In addition, a Student’s t-test of samples within 10 days of delivery revealed a significant increase in 3-hydroxyisobutyrate at 7–10 days before delivery in serum specimens from CORT fetuses (Table 3). In addition, citrate and anserine were both significantly lower 0–2 days and 7–10 days, respectively, before delivery in CORT fetal serum specimens compared with control. In contrast, myo-inositol was significantly elevated in CORT fetal serum specimens at 0–2 days before delivery along with citrulline at 3–6 days (Table 3).
Table 3.
Significant metabolites in fetal serum due to chronic maternal cortisol exposure in late gestation
| Metabolite | Intercept | SE | P Value | Trend |
|---|---|---|---|---|
| All time points (n = 41 control from 9 animals, n = 14 cortisol from 4 animals) | ||||
| Creatine | −0.11 | 0.04 | 0.01 | Control>Cort |
| Glycine | 0.43 | 0.19 | 0.03 | Cort>Control |
| VLDL | 0.67 | 0.31 | 0.04 | Cort>Control |
| BCAA | 1.43 | 0.69 | 0.04 | Cort>Control |
| Choline | 0.17 | 0.08 | 0.05 | Cort>Control |
| Methionine | −0.07 | 0.04 | 0.05 | Control>Cort |
| Relative Means ± SE | ||||
|---|---|---|---|---|
| Control | Cortisol | P Value | Log2(FC) (Cortisol/Control) | |
| 0–2 Days until delivery (n = 8 control, n = 3 cortisol) | ||||
| Citrate | 0.708 ± 0.022 | 0.429 ± 0.032 | 0.03 | −0.72 |
| Myo-inositol | 1.4 11 ± 0.067 | 2.067 ± 0.059 | 0.05 | 0.55 |
| 3–6 Days until delivery (n = 9 control, n = 3 cortisol) | ||||
| Citrulline | 0.188 ± 0.005 | 0.257 ± 0.012 | 0.05 | 0.45 |
| 7–10 Days until delivery (n = 8 control, n = 4 cortisol) | ||||
| 3-Hydroxyisobutyrate | 0.298 ± 0.005 | 0.391 ± 0.004 | 7.43E−04 | 0.39 |
| Anserine | 0.178 ± 0.005 | 0.135 ± 0.004 | 0.04 | −0.40 |
VLDL, very-low density lipoprotein; BCAA, branched chain amino acids; FC, fold change.
Excess maternal cortisol exposure in late gestation alters the metabolic profile of maternal serum before birth.
We identified 30 metabolites in maternal serum specimens using 1H-NMR. A linear mixed effects model from all time points revealed a significant decrease of 1-methylhistidine and betaine in serum from CORT ewes compared with control while scyllo-inositol was significantly elevated in serum from CORT ewes (Table 4). A Student’s t-test of samples at the three closest time points until delivery revealed significant decreases in 1-methylhistidine and betaine at 0–2 and 3–6 days until delivery in serum from CORT compared with serum from control ewes (Table 4). Anserine and scyllo-inositol were significantly elevated in CORT specimens at 3–6 days until delivery, while lactate and 3-hydroxyisobutyrate were significantly elevated in serum from CORT ewes at 7–10 days until delivery (Table 4).
Table 4.
Significant metabolites in maternal serum due to chronic maternal cortisol exposure in late gestation
| Metabolite | Intercept | SE | P Value | Trend |
|---|---|---|---|---|
| All time points (n = 42 control from 9 animals, n = 16 cortisol from 4 animals) | ||||
| Betaine | −1.95 | 0.69 | 0.01 | Control > Cort |
| Scyllo-inositol | 0.35 | 0.10 | 1.28E−03 | Cort > control |
| 1-Methylhistidine | −0.07 | 0.02 | 1.20E−03 | Control > Cort |
| Relative Means ± SE | ||||
|---|---|---|---|---|
| Control | Cortisol | P Value | Log2(FC) (Cortisol/Control) | |
| 0–2 Days until delivery (n = 9 control, n = 3 cortisol) | ||||
| 1-Methylhistidine | 0.172 ± 0.007 | 0.079 ± 0.004 | 2.68E−03 | −1.13 |
| Betaine | 9.880 ± 0.311 | 6.670 ± 0.174 | 0.01 | −0.57 |
| 3–6 Days until delivery (n = 9 control, n = 4 cortisol) | ||||
| Betaine | 9.791 ± 0.238 | 6.647 ± 0.117 | 8.78E−04 | −0.56 |
| Anserine | 0.291 ± 0.011 | 0.398 ± 0.008 | 9.53E−03 | 0.45 |
| 1-Methylhistidine | 0.173 ± 0.010 | 0.081 ± 0.007 | 0.01 | −1.11 |
| Scyllo-inositol | 0.216 ± 0.030 | 0.804 ± 0.106 | 0.01 | 1.90 |
| 7–10 Days until delivery (n = 8 control, n = 4 cortisol) | ||||
| Lactate | 6.432 ± 0.220 | 11.754 ± 1.060 | 0.03 | 0.87 |
| 3-Hydroxyisobutyrate | 0.110 ± 0.005 | 0.165 ± 0.010 | 0.04 | 0.59 |
| 1-Methylhistidine | 0.149 ± 0.009 | 0.093 ± 0.003 | 0.05 | −0.68 |
FC, fold change.
Pathways altered in newborn cardiac tissue and fetal and maternal serum due to excess cortisol treatment during late gestation.
ORA of significantly altered metabolites and lipids (P < 0.05) identified in fetal heart tissue revealed significant enrichment in six pathways (FDR-corrected P < 0.10). This included one pathway involved in amino acid metabolism: alanine, aspartate, and glutamate metabolism. In addition, two pathways involved in carbohydrate metabolism, propanoate, and butanoate metabolism, as well as aminoacyl-tRNA biosynthesis, glycerophospholipid metabolism, and nitrogen metabolism, were significantly enriched (Table 5).
Table 5.
Pathways significantly altered due to chronic maternal cortisol exposure in late gestation as identified by overrepresentation analysis
| Pathway | No. Metabolite Hits/No. Total Metabolites | P Value | FDR-Corrected P Value | Significant Metabolite(s) Increased in Controls | Significant Metabolite(s) Increased in Cortisol |
|---|---|---|---|---|---|
| Newborn heart | |||||
| Alanine, aspartate, and glutamate metabolism | 3/24 | 7.04E−05 | 5.63E−03 | Glutamate | Glutamine, succinate |
| Aminoacyl-tRNA biosynthesis | 3/75 | 2.13E−03 | 0.06 | Glutamate | Glutamine, valine |
| Propanoate metabolism | 2/35 | 6.94E−03 | 0.10 | — | Succinate, valine |
| Nitrogen metabolism | 2/39 | 8.57E−03 | 0.10 | Glutamate | Glutamine |
| Glycerophospholipid metabolism | 2/39 | 8.57E−03 | 0.10 | GPC, cardiolipin | — |
| Butanoate metabolism | 2/40 | 9.01E−03 | 0.10 | Glutamate | Succinate |
| Fetal serum | |||||
| Aminoacyl-tRNA biosynthesis | 5/75 | 1.72E−05 | 1.18E−03 | Methionine | Valine, leucine, isoleucine, glycine |
| BCAA degradation | 4/40 | 2.94E−05 | 1.18E−03 | — | Valine, leucine, isoleucine, 3-hydroxyisobutyrate |
| BCAA biosynthesis | 3/27 | 2.59E−04 | 6.91E−03 | — | Valine, leucine, isoleucine |
| Glycine, serine, and threonine metabolism | 3/48 | 1.44E−03 | 0.03 | Creatine | Choline, glycine |
FDR, false discovery rate; BCAA, branched-chain amino acid; GPC, glycerophosphocholine.
Four pathways were significantly enriched in fetal serum (FDR-corrected P < 0.10; Table 5) including two involved in amino acid metabolism, specifically glycine, serine, and threonine metabolism and BCAA degradation. In addition, BCAA biosynthesis was significantly over-represented. Finally, aminoacyl-tRNA biosynthesis, also significantly enriched in heart tissue, was found to be overrepresented in fetal serum. ORA of maternal serum did not reveal any significantly altered pathways (FDR-corrected P < 0.10; data not shown).
DISCUSSION
In this study, we used metabolomics to identify changes in metabolic pathways in the newborn heart as a result of excess cortisol exposure in utero. The metabolomics analysis indicates changes in TCA cycle and lipid metabolism as a consequence of chronically elevated maternal cortisol levels. These findings are consistent with transcriptomic modeling of the gene expression in a previous cohort of similarly treated fetuses at ~142 days gestation (42). More importantly, the metabolic profiling suggests a metabolic defect may occur, as we have previously reported bradycardia and arrhythmias in these same fetuses (1). In the most severe cases (2 of the 6 CORT hearts in this study), this resulted in cardiac failure at birth.
Chronic exposure to excess maternal cortisol in late gestation leads to altered amino acid and TCA cycle intermediate profiles in the fetus and newborn cardiac tissue.
Metabolomic analysis revealed upregulation in some amino acids (valine and glutamine) as well as succinate, a TCA cycle intermediate, in the newborn cardiac tissue that developed in an environment of excess maternal cortisol during late gestation (Table 1). Valine is a BCAA and is involved in aminoacyl-tRNA biosynthesis as well as propanoate metabolism. Following birth, the heart gradually transitions from utilizing lactate and glucose as primary energy substrates in utero to fatty acids, which is sustained in the healthy heart into adult life (2, 32, 33). Previous work in our laboratory has found that transcripts for BCAA degradation are enriched in fetal hearts in healthy sheep compared with newborn hearts on the first day of life (51). Additionally, studies in adult human hearts has suggested that BCAA degradation is diminished in the failing heart (27, 48). Therefore, elevated valine in cardiac tissue exposed to excess cortisol in utero may reflect an inability of this tissue to undergo normal metabolic transitions during birth, in turn leading to activation of other pathways for energy, including propanoate metabolism, which was significantly enriched in both the newborn cardiac tissue and fetal serum before delivery (Table 5). Furthermore, BCAAs have been shown to be upregulated in serum from humans with insulin resistance (19, 36, 37). While insulin resistance was not found in ewes or fetuses in these sheep (1), fetal serum did show an upregulation of BCAA across all time points and pathway analysis modeled significant enrichment in BCAA synthesis and degradation (Table 3). However, these elevations in BCAA were not observed in maternal serum (Table 4), suggesting that alterations in fetal BCAA levels are not maternal in origin. Interestingly, ongoing research in our laboratory has found a significant decrease in expression of transcripts involved in BCAA degradation in the placenta at gestational day 142 in a cohort of animals that received the same cortisol treatment (data not published), indicating that these BCAA alterations observed in fetal serum before birth may be due to altered placental metabolism. Although the source of these elevations in BCAAs in the fetal serum and newborn cardiac tissue from animals exposed to excess maternal cortisol in utero are yet to be determined, their alterations suggest that newborn cardiac tissue may be altered in BCAA degradation at birth.
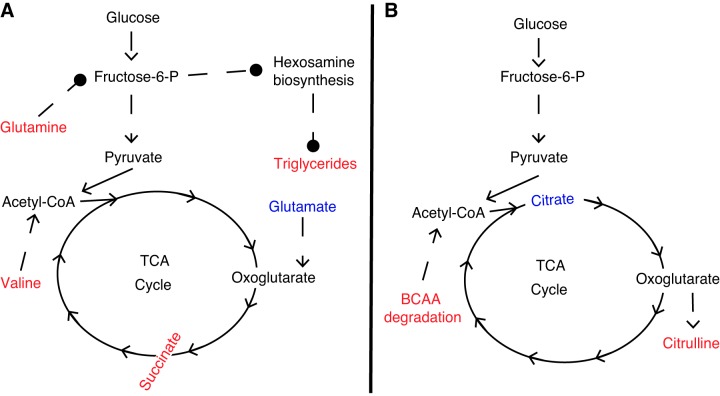
In addition to valine, glutamine was significantly elevated in newborn cardiac tissue from CORT animals (Table 1). Glutamine is involved in numerous significantly enriched metabolic pathways identified by ORA, including alanine, aspartate and glutamate metabolism, aminoacyl-tRNA biosynthesis, and nitrogen metabolism. Glutamine has previously been shown to be an important metabolite in maintaining normal cardiac function in the adult heart (23, 24, 31). Furthermore, elevations in cardiac glutamine activate the hexosamine biosynthetic pathway (HBP) leading to elevations in β-oxidation and cardiac TGs due to increased translocation of CD36, a fatty acid translocase protein, to the cellular membrane (28). However, the exact mechanism leading to this CD36 translocation by HBP is not known. In our model, three TG species [TG (16:0/18:1/24:1); TG (14:0/16:0/16:0); and TG (18:1/18:1/24:1)] were significantly upregulated in the CORT newborn cardiac tissue (Table 2), as well as TGs being upregulated as a class, suggesting increased activation of HBP in the cardiac tissue of CORT newborns. However, glutamine was not found to be significantly altered in CORT fetal or maternal serum before birth (Tables 3 and 4). Finally, glutamate was significantly diminished in cardiac tissue from CORT animals (Table 1). Glutamate is a major substrate in the heart important in the synthesis of TCA cycle intermediates, including succinate, which was upregulated in cardiac tissue from CORT animals (39, 53). Therefore, decreases in glutamate in the newborn cardiac tissue due to chronic maternal cortisol exposure in utero may be due to upregulation in TCA cycle anaplerosis, as evident through elevations in succinate upon cortisol exposure. Although glutamate and succinate were not significantly altered in fetal serum before birth, citrate, a TCA cycle intermediate, and citrulline, a urea cycle intermediate, were significantly increased at 0–2 days and 3–6 days before delivery (Table 3). However, TCA or urea cycle intermediates were not altered in maternal serum before delivery (Table 4), suggesting that these metabolic changes occur in the placental-fetal unit. Furthermore, on-going research in our laboratory has found a significant decrease in expression of transcripts involved in placental glycolysis at gestational day 142 (data not published). Therefore, alterations in glycolysis in the placenta due to excess cortisol exposure may lead to alterations in TCA cycle intermediates in the fetal serum before delivery, resulting in altered substrates for the newborn cardiac tissue at term. Figure 3 shows the proposed mechanism of altered glycolysis and TCA cycle flux in the newborn CORT cardiac tissue (Fig. 3A) and CORT fetal serum before birth (Fig. 3B). Further studies with labeled isotopes are needed to confirm altered TCA cycle flux in the placental-fetal unit and newborn cardiac tissue. However, our study provides novel information of alterations in amino acid and TCA cycle intermediates that occur in the fetus before birth and the newborn cardiac tissue due to altered chronic increase in maternal cortisol.
Fig. 3.
Altered amino acid and TCA cycle metabolism present in newborn cardiac tissue immediately following birth (A) and fetal serum before birth (B) due to chronic maternal cortisol exposure in late gestation. Citrate was significantly diminished in cortisol (CORT) serum at 0–2 days before delivery, while citrulline was significantly elevated in CORT serum at 3–6 days before delivery. Branched-chain amino acids (BCAA) degradation was significantly enriched in fetal serum following overrepresentation analysis (ORA), with BCAA and 3-hydroxyisobutyrate (both elevated in CORT serum) contributing to this pathway. Metabolites in red are significantly elevated in CORT compared with control specimens, while metabolites in blue are significantly elevated in control compared with CORT specimens. Solid arrows (→) reflect single enzymatic steps, while dashed arrows (- ->) reflect multiple enzymatic steps. Dashed lines with circles (- -•) reflect associations observed in the literature with no direct relationship.
Exposure to excess maternal cortisol in late gestation leads to altered lipid profiles in newborn cardiac tissue.
Lipidomic analysis of the CORT newborn cardiac tissue revealed significant elevations in DG [DG (18:0/18:1)] and three TG species [TG (16:0/18:1/24:1); TG (14:0/16:0/16:0); and TG (18:1/18:1/24:1)] due to excess cortisol exposure in utero. Previous work in our laboratory found significant elevations of TGs immediately following birth in the newborn cardiac tissue, with evidence that these elevations in TGs are due to synthesis from DGs (51). In humans, elevations in circulating TG levels are linked to heart failure (35), but the relation of elevated TGs in the heart to cardiac failure is yet to be determined. TGs in the heart account for 10–50% of fatty acids for energy when circulating levels of fatty acids are low (44). Therefore, abnormal elevations of TGs and DGs following birth may be indicative of an altered ability to efficiently utilize DGs and TGs in the heart for energy and may lead to metabolic failure of the newborn heart.
In addition, two ether-linked lipid species, plasmenyl-PCs and plasmanyl-phosphatidylethanolamines, were diminished in the CORT newborn cardiac tissue. While plasmenyl-PCs have been previously reported in the bovine heart (45), the function of these plasmalogens in the heart is less clear. In the porcine heart, oxidized plasmalogen products were elevated following cardiac infarction, adding to evidence of their protective role in free radical consumption and prevention of oxidative damage following stress in the heart (6, 10). Furthermore, oxidative products of plasmalogens undergo lipid peroxidation in human brain tissue, which may help prevent oxidative damage (46). While the function of ether-linked lipids in the heart remains unclear, decreased levels in CORT cardiac tissue may leave these animals more vulnerable to altered cell membrane signaling and oxidative damage, ultimately leading to altered β-oxidation in cardiac tissue following birth.
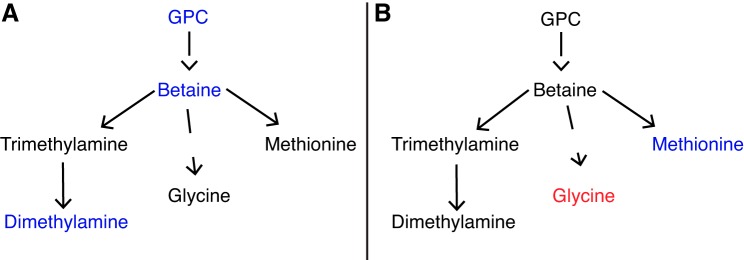
Pathway analysis indicated that glycerophospholipid metabolism was altered in newborn cardiac tissue of CORT ewes (Table 5). GPC and betaine were significantly diminished in newborn cardiac tissue from CORT animals. GPC is an abundant storage form of choline, whereas betaine, a breakdown product of choline, is important in maintaining osmotic homeostasis in cells. Betaine has been shown to significantly reduce oxidative damage and maintain mitochondrial function in the heart following isoprenaline-induced myocardial infarction (13–15). Therefore, altered GPC and betaine in CORT cardiac tissue could lead to an inability of the heart to adapt to oxidative stress at birth, leading to metabolic failure of the heart later in life. In addition, betaine is an important methyl-group donor, capable of forming methionine, which was found to be significantly downregulated in CORT fetal serum samples across all time points (Table 3). Dimethylamine, another breakdown product of choline, was diminished in cardiac tissue from CORT newborns, adding to the evidence of increased choline and betaine metabolism in tissue from CORT fetuses. While betaine was significantly diminished in CORT ewe serum at 0–2 to 3–6 days leading to labor (Table 4), no significant alterations were observed in fetal serum at these time points (Table 3), suggesting that alterations in glycerophospholipid metabolism occur in the fetal-placental unit and are not from maternal metabolic alterations. Figure 4 shows a summary of proposed glycerophospholipid metabolic alterations occurring in the CORT newborn cardiac tissue (Fig. 4A) and CORT fetal serum (Fig. 4B).
Fig. 4.
Altered glycerophosphocholine (GPC) metabolism present in newborn cardiac tissue immediately following birth (A) and fetal serum before birth (B) due to chronic maternal cortisol exposure in late gestation. Cardiolipin was also found to be significantly elevated in CORT cardiac tissue but is not pictured in this pathway. Metabolites in red are significantly elevated in CORT compared with control specimens, while metabolites in blue are significantly elevated in control compared with CORT specimens.
In addition to GPC, one CL species [CL (16:0/18:1/18:1/18:1)] was diminished in CORT newborn cardiac tissue. CLs are localized in the mitochondrial inner membrane and act not only as structural proteins but also play an important role in energy metabolism in the heart (18, 38). CLs in both humans and rats have been linked to the development of heart failure, showing its importance in maintaining normal cardiac function (7, 30, 47). This may be due to the role of CLs in maintaining mitochondrial function by supporting electron transport chain function. Ongoing transcriptomic analysis of LV tissue from these animals revealed that three genes (CDS2, LPCAT3, and MBOAT1) involved in cytidine diphosphate-diacylglycerol biosynthesis, a molecule important in biosynthesis of cardiolipin, were significantly downregulated (P < 0.05) in CORT cardiac tissue compared with control tissue. These results add to evidence of decreased synthesis of cardiolipin in newborn cardiac tissue due to chronic maternal cortisol exposure in late gestation (data not published). In addition, previous transcriptomic modeling of the effect of this cortisol treatment on the term fetal heart in our laboratory revealed that 20% of the differentially regulated genes encoded for mitochondrial proteins and that mitochondrial number was reduced (42). Our laboratory has also found that subunit 4 of cytochrome c mRNA and protein was reduced in the septum of CORT animals relative to controls (data not published). Therefore, diminished levels of CLs in the cardiac tissue may lead to these changes in mitochondrial structure and/or function. However, our metabolomic techniques were not able to determine the presence of these lipids in fetal or maternal serum before delivery, and future studies are needed to determine if these lipid alterations in cardiac tissue are also present in the fetus before delivery.
Perspectives.
Birth is a metabolically challenging event as the heart undergoes the transition from a low-oxygen to a high-oxygen environment and from preferential utilization of lactate and glucose to fatty acids for energy (2, 32, 33). Our laboratory has previously shown that chronic maternal cortisol elevations in late gestation lead to transcriptomic alterations in cardiac metabolism in the term fetal heart (42). In the cohort of animals studied in this article, we also found evidence of impaired cardiac function, including bradycardia at birth, and increased incidence of arrhythmias, including atrioventricular block and ectopic beats. The results presented here suggest that the amino acid and TCA cycle metabolism as well as lipid and glycerophospholipid metabolism are altered in the newborn cardiac tissue after excess maternal cortisol in late gestation. In addition, we observed alterations in amino acid and TCA cycle metabolites in fetal serum, but not in maternal serum, during late gestation, suggesting that placental metabolism and/or transport is altered as a consequence of excess maternal cortisol before birth. Future targeted studies, including flux with stable isotopes, would better determine altered metabolism in the newborn cardiac tissue due to chronic maternal cortisol exposure during late gestation. Our results suggest that excess maternal cortisol alters the normal cardiac metabolic maturation that occurs; this perturbation may contribute to the peripartum cardiac arrhythmias and stillbirth observed previously in our laboratory (1, 42). Therefore, metabolic alterations in the mother, either from high stress or disease, may lead to metabolic changes in the fetal heart before birth. These metabolic alterations may predispose the heart to abnormal metabolic adaptations during the stress of labor and delivery, leading to metabolic changes in the newborn heart and ultimately later life cardiomyopathies. A better understanding of how metabolic alterations in the mother affect the fetal and newborn cardiac tissue will lead to better treatments for neonates with cardiac failure following birth.
GRANTS
This study was supported by NIH Training Grants TL1-TR-001428, and T32-HL-083810; NIH Grants U24-DK-097209, HD-087306, and HD-057871; and American Heart Association Grant 14GRNT20420048. A. S. Edison was partially supported by the Georgia Research Alliance.
A portion of this work was performed in the McKnight Brain Institute at the National High Magnetic Field Laboratory’s AMRIS Facility, which is supported by National Science Foundation Cooperative Agreement No. DMR-1157490 and the State of Florida.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.M.W., A.S.E., and M.K.-W. conceived and designed experiments; A.A. and M.K.-W. collected the specimens; J.M.W. prepared samples and conducted metabolomic analyses; J.M.W., J.P.K., and T.J.G. conducted lipidomic analyses; J.M.W. conducted all statistical analyses; J.M.W., A.S.E., and M.K-W. interpreted results of experiments; J.M.W. prepared figures; J.M.W. drafted manuscript; J.M.W., A.A., J.P.K., T.J.G., A.S.E., and M.K.-W. edited and revised manuscript; J.M.W., A.A., J.P.K., T.J.G., A.S.E., and M.K.-W. approved final version of manuscript.
Supplemental Tables
Table S1: Tukey-Kramer Post-Hoc Results for Metabolites Identified by HR-MAS Between Areas of Newborn Heart Tissue
Table S2: Metabolites Identified by HR-MAS in Newborn Heart Tissue and 1H-NMR in Maternal and Fetal Serum and Confidence Levels
Table S3: Internal and Injection Standards for Lipidomics
Table S4: Lipids identified by UHPLC-MS in Newborn Cardiac Tissue
ACKNOWLEDGMENTS
We thank the members of the Keller-Wood, Edison, and Garrett Laboratories for resources and expertise and Steven Robinette for the MATLAB Metabolomics Toolbox. We also thank staff at AMRIS, including Jim Rocca for assistance with NMR.
REFERENCES
- 1.Antolic A, Wood CE, Keller-Wood M. Chronic maternal hypercortisolemia in late gestation alters fetal cardiac function at birth. Am J Physiol Regul Integr Comp Physiol 314: R342–R352, 2018. doi: 10.1152/ajpregu.00296.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartelds B, Gratama JW, Knoester H, Takens J, Smid GB, Aarnoudse JG, Heymans HS, Kuipers JR. Perinatal changes in myocardial supply and flux of fatty acids, carbohydrates, and ketone bodies in lambs. Am J Physiol Heart Circ Physiol 274: H1962–H1969, 1998. doi: 10.1152/ajpheart.1998.274.6.H1962. [DOI] [PubMed] [Google Scholar]
- 3.Beckonert O, Keun HC, Ebbels TM, Bundy J, Holmes E, Lindon JC, Nicholson JK. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat Protoc 2: 2692–2703, 2007. doi: 10.1038/nprot.2007.376. [DOI] [PubMed] [Google Scholar]
- 4.Bingol K, Li DW, Zhang B, Brüschweiler R. Comprehensive metabolite identification strategy using multiple two-dimensional NMR spectra of a complex mixture implemented in the COLMARm web server. Anal Chem 88: 12411–12418, 2016. doi: 10.1021/acs.analchem.6b03724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boeuf B, Maragnes P, Belzic I, Lacotte J, Bonte JB, Guillois B. [Glucocorticoid-induced hypertrophic cardiomyopathy in premature infants: apropos of 4 cases]. Arch Pediatr 4: 152–157, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Brosche T, Platt D. The biological significance of plasmalogens in defense against oxidative damage. Exp Gerontol 33: 363–369, 1998. doi: 10.1016/S0531-5565(98)00014-X. [DOI] [PubMed] [Google Scholar]
- 7.Chatfield KC, Sparagna GC, Sucharov CC, Miyamoto SD, Grudis JE, Sobus RD, Hijmans J, Stauffer BL. Dysregulation of cardiolipin biosynthesis in pediatric heart failure. J Mol Cell Cardiol 74: 251–259, 2014. doi: 10.1016/j.yjmcc.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chong J, Soufan O, Li C, Caraus I, Li S, Bourque G, Wishart DS, Xia J. MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res 46: W486–W494, 2018. doi: 10.1093/nar/gky310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dieterle F, Ross A, Schlotterbeck G, Senn H. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in 1H NMR metabonomics. Anal Chem 78: 4281–4290, 2006. doi: 10.1021/ac051632c. [DOI] [PubMed] [Google Scholar]
- 10.Dudda A, Spiteller G, Kobelt F. Lipid oxidation products in ischemic porcine heart tissue. Chem Phys Lipids 82: 39–51, 1996. doi: 10.1016/0009-3084(96)02557-1. [DOI] [PubMed] [Google Scholar]
- 11.Eriksson L, Johansson E, Kettaneh-Wold N, Wold S. Multi- and Megavariate Data Analysis. Principles and Applications. Umea, Sweden: Umetrics, 2001. [Google Scholar]
- 12.Feng X, Reini SA, Richards E, Wood CE, Keller-Wood M. Cortisol stimulates proliferation and apoptosis in the late gestation fetal heart: differential effects of mineralocorticoid and glucocorticoid receptors. Am J Physiol Regul Integr Comp Physiol 305: R343–R350, 2013. doi: 10.1152/ajpregu.00112.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganesan B, Anandan R, Lakshmanan PT. Studies on the protective effects of betaine against oxidative damage during experimentally induced restraint stress in Wistar albino rats. Cell Stress Chaperones 16: 641–652, 2011. doi: 10.1007/s12192-011-0276-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ganesan B, Buddhan S, Anandan R, Sivakumar R, AnbinEzhilan R. Antioxidant defense of betaine against isoprenaline-induced myocardial infarction in rats. Mol Biol Rep 37: 1319–1327, 2010. doi: 10.1007/s11033-009-9508-4. [DOI] [PubMed] [Google Scholar]
- 15.Ganesan B, Rajesh R, Anandan R, Dhandapani N. Biochemical studies on the protective effect of betaine on mitochondrial function in experimentally induced myocardial infarction in rats. J Health Sci 53: 671–681, 2007. doi: 10.1248/jhs.53.671. [DOI] [Google Scholar]
- 16.Giraud GD, Louey S, Jonker S, Schultz J, Thornburg KL. Cortisol stimulates cell cycle activity in the cardiomyocyte of the sheep fetus. Endocrinology 147: 3643–3649, 2006. doi: 10.1210/en.2006-0061. [DOI] [PubMed] [Google Scholar]
- 17.Nagana Gowda GA, Raftery D. Quantitating metabolites in protein precipitated serum using NMR spectroscopy. Anal Chem 86: 5433–5440, 2014. doi: 10.1021/ac5005103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houtkooper RH, Vaz FM. Cardiolipin, the heart of mitochondrial metabolism. Cell Mol Life Sci 65: 2493–2506, 2008. doi: 10.1007/s00018-008-8030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huffman KM, Shah SH, Stevens RD, Bain JR, Muehlbauer M, Slentz CA, Tanner CJ, Kuchibhatla M, Houmard JA, Newgard CB, Kraus WE. Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care 32: 1678–1683, 2009. doi: 10.2337/dc08-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 8: 118–127, 2007. doi: 10.1093/biostatistics/kxj037. [DOI] [PubMed] [Google Scholar]
- 21.Jonker SS, Zhang L, Louey S, Giraud GD, Thornburg KL, Faber JJ. Myocyte enlargement, differentiation, and proliferation kinetics in the fetal sheep heart. J Appl Physiol (1985) 102: 1130–1142, 2007. doi: 10.1152/japplphysiol.00937.2006. [DOI] [PubMed] [Google Scholar]
- 22.Keller-Wood M, Feng X, Wood CE, Richards E, Anthony RV, Dahl GE, Tao S. Elevated maternal cortisol leads to relative maternal hyperglycemia and increased stillbirth in ovine pregnancy. Am J Physiol Regul Integr Comp Physiol 307: R405–R413, 2014. doi: 10.1152/ajpregu.00530.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khogali SE, Harper AA, Lyall JA, Rennie MJ. Effects of L-glutamine on post-ischaemic cardiac function: protection and rescue. J Mol Cell Cardiol 30: 819–827, 1998. doi: 10.1006/jmcc.1998.0647. [DOI] [PubMed] [Google Scholar]
- 24.Khogali SEO, Pringle SD, Weryk BV, Rennie MJ. Is glutamine beneficial in ischemic heart disease? Nutrition 18: 123–126, 2002. doi: 10.1016/S0899-9007(01)00768-7. [DOI] [PubMed] [Google Scholar]
- 25.Koelmel JP, Kroeger NM, Gill EL, Ulmer CZ, Bowden JA, Patterson RE, Yost RA, Garrett TJ. Expanding lipidome coverage using LC-MS/MS data-dependent acquisition with automated exclusion list generation. J Am Soc Mass Spectrom 28: 908–917, 2017. doi: 10.1007/s13361-017-1608-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koelmel JP, Kroeger NM, Ulmer CZ, Bowden J, Garland RP, Cochran JA, Beecher CW, Garrett TJ, Yost RA. LipidMatch: an automated workflow for rule-based lipid identification using untargeted high-resolution tandem mass spectrometry data. BMC Bioinformatics, 18: 331, 2017. doi: 10.1186/s12859-017-1744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai L, Leone TC, Keller MP, Martin OJ, Broman AT, Nigro J, Kapoor K, Koves TR, Stevens R, Ilkayeva OR, Vega RB, Attie AD, Muoio DM, Kelly DP. Energy metabolic reprogramming in the hypertrophied and early stage failing heart: a multisystems approach. Circ Heart Fail 7: 1022–1031, 2014. doi: 10.1161/CIRCHEARTFAILURE.114.001469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lauzier B, Vaillant F, Merlen C, Gélinas R, Bouchard B, Rivard ME, Labarthe F, Dolinsky VW, Dyck JR, Allen BG, Chatham JC, Des Rosiers C. Metabolic effects of glutamine on the heart: anaplerosis versus the hexosamine biosynthetic pathway. J Mol Cell Cardiol 55: 92–100, 2013. doi: 10.1016/j.yjmcc.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lazic SE, Clarke-Williams CJ, Munafo MR. What exactly is ‘N’ in cell culture and animal experiments? PLoS Biol 16: e2005282, 2018. doi: 10.1371/journal.pbio.2005282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le CH, Mulligan CM, Routh MA, Bouma GJ, Frye MA, Jeckel KM, Sparagna GC, Lynch JM, Moore RL, McCune SA, Bristow M, Zarini S, Murphy RC, Chicco AJ. Delta-6-desaturase links polyunsaturated fatty acid metabolism with phospholipid remodeling and disease progression in heart failure. Circ Heart Fail 7: 172–183, 2014. [Erratum in: Circ Heart Fail 7: 382, 2014.] 10.1161/CIRCHEARTFAILURE.113.000744. [DOI] [PubMed] [Google Scholar]
- 31.Liu J, Marchase RB, Chatham JC. Glutamine-induced protection of isolated rat heart from ischemia/reperfusion injury is mediated via the hexosamine biosynthesis pathway and increased protein O-GlcNAc levels. J Mol Cell Cardiol 42: 177–185, 2007. doi: 10.1016/j.yjmcc.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopaschuk GD, Spafford MA. Energy substrate utilization by isolated working hearts from newborn rabbits. Am J Physiol 258: H1274–H1280, 1990. doi: 10.1152/ajpheart.1990.258.5.H1274. [DOI] [PubMed] [Google Scholar]
- 33.Lopaschuk GD, Spafford MA, Marsh DR. Glycolysis is predominant source of myocardial ATP production immediately after birth. Am J Physiol 261: H1698–H1705, 1991. doi: 10.1152/ajpheart.1991.261.6.H1698. [DOI] [PubMed] [Google Scholar]
- 34.Lumbers ER, Boyce AC, Joulianos G, Kumarasamy V, Barner E, Segar JL, Burrell JH. Effects of cortisol on cardiac myocytes and on expression of cardiac genes in fetal sheep. Am J Physiol Regul Integr Comp Physiol 288: R567–R574, 2005. doi: 10.1152/ajpregu.00556.2004. [DOI] [PubMed] [Google Scholar]
- 35.Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris-Etherton PM, Lennie TA, Levi M, Mazzone T, Pennathur S; American Heart Association Clinical Lipidology, Thrombosis, and Prevention Committee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease . Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 123: 2292–2333, 2011. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- 36.Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab 15: 606–614, 2012. doi: 10.1016/j.cmet.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, Rochon J, Gallup D, Ilkayeva O, Wenner BR, Yancy WS Jr, Eisenson H, Musante G, Surwit RS, Millington DS, Butler MD, Svetkey LP. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 9: 311–326, 2009. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paradies G, Paradies V, De Benedictis V, Ruggiero FM, Petrosillo G. Functional role of cardiolipin in mitochondrial bioenergetics. Biochim Biophys Acta 1837: 408–417, 2014. doi: 10.1016/j.bbabio.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 39.Pietersen HG, Langenberg CJ, Geskes G, Soeters PB, Wagenmakers AJ. Glutamate metabolism of the heart during coronary artery bypass grafting. Clin Nutr 17: 73–75, 1998. doi: 10.1016/S0261-5614(98)80308-7. [DOI] [PubMed] [Google Scholar]
- 40.Pluskal T, Castillo S, Villar-Briones A, Oresic M. MZmine 2: modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinformatics 11: 395, 2010. doi: 10.1186/1471-2105-11-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reini SA, Dutta G, Wood CE, Keller-Wood M. Cardiac corticosteroid receptors mediate the enlargement of the ovine fetal heart induced by chronic increases in maternal cortisol. J Endocrinol 198: 419–427, 2008. doi: 10.1677/JOE-08-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richards EM, Wood CE, Rabaglino MB, Antolic A, Keller-Wood M. Mechanisms for the adverse effects of late gestational increases in maternal cortisol on the heart revealed by transcriptomic analyses of the fetal septum. Physiol Genomics 46: 547–559, 2014. doi: 10.1152/physiolgenomics.00009.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rog-Zielinska EA, Richardson RV, Denvir MA, Chapman KE. Glucocorticoids and foetal heart maturation; implications for prematurity and foetal programming. J Mol Endocrinol 52: R125–R135, 2014. doi: 10.1530/JME-13-0204. [DOI] [PubMed] [Google Scholar]
- 44.Saddik M, Lopaschuk GD. Myocardial triglyceride turnover and contribution to energy substrate utilization in isolated working rat hearts. J Biol Chem 266: 8162–8170, 1991. [PubMed] [Google Scholar]
- 45.Schmid HH, Takahashi T. The alk-i-enyl ether and alkyl ether lipids of bovine heart muscle. Biochim Biophys Acta 164: 141–147, 1968. doi: 10.1016/0005-2760(68)90140-9. [DOI] [PubMed] [Google Scholar]
- 46.Sindelar PJ, Guan Z, Dallner G, Ernster L. The protective role of plasmalogens in iron-induced lipid peroxidation. Free Radic Biol Med 26: 318–324, 1999. doi: 10.1016/S0891-5849(98)00221-4. [DOI] [PubMed] [Google Scholar]
- 47.Sparagna GC, Chicco AJ, Murphy RC, Bristow MR, Johnson CA, Rees ML, Maxey ML, McCune SA, Moore RL. Loss of cardiac tetralinoleoyl cardiolipin in human and experimental heart failure. J Lipid Res 48: 1559–1570, 2007. doi: 10.1194/jlr.M600551-JLR200. [DOI] [PubMed] [Google Scholar]
- 48.Sun H, Olson KC, Gao C, Prosdocimo DA, Zhou M, Wang Z, Jeyaraj D, Youn JY, Ren S, Liu Y, Rau CD, Shah S, Ilkayeva O, Gui WJ, William NS, Wynn RM, Newgard CB, Cai H, Xiao X, Chuang DT, Schulze PC, Lynch C, Jain MK, Wang Y. Catabolic Defect of Branched-Chain Amino Acids Promotes Heart Failure. Circulation 133: 2038–2049, 2016. doi: 10.1161/CIRCULATIONAHA.115.020226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trygg J, Wold S. Orthogonal projections to latent structures (O-PLS). J Chemometr 16: 119–128, 2002. doi: 10.1002/cem.695. [DOI] [Google Scholar]
- 50.Walejko JM, Chelliah A, Keller-Wood M, Gregg A, Edison AS. Global metabolomics of the placenta reveals distinct metabolic profiles between maternal and fetal placental tissues following delivery in non-labored women. Metabolites 8: 10, 2018. doi: 10.3390/metabo8010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walejko JM, Koelmel JP, Garrett TJ, Edison AS, Keller-Wood M. Multiomics approach reveals metabolic changes in the heart at birth. Am J Physiol Endocrinol Metab 315: E1212–E1223, 2018. doi: 10.1152/ajpendo.00297.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ward JH., Jr Hierarchical grouping to optimize an objective function. J Am Stat Assoc 58: 236–244, 1963. doi: 10.1080/01621459.1963.10500845. [DOI] [Google Scholar]
- 53.Wiesner RJ, Rösen P, Grieshaber MK. Pathways of succinate formation and their contribution to improvement of cardiac function in the hypoxic rat heart. Biochem Med Metab Biol 40: 19–34, 1988. doi: 10.1016/0885-4505(88)90100-4. [DOI] [PubMed] [Google Scholar]
- 54.Xia J, Wishart DS. MetPA: a web-based metabolomics tool for pathway analysis and visualization. Bioinformatics 26: 2342–2344, 2010. doi: 10.1093/bioinformatics/btq418. [DOI] [PubMed] [Google Scholar]
- 55.Yunis KA, Bitar FF, Hayek P, Mroueh SM, Mikati M. Transient hypertrophic cardiomyopathy in the newborn following multiple doses of antenatal corticosteroids. Am J Perinatol 16: 17–21, 1999. doi: 10.1055/s-2007-993830. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Tukey-Kramer Post-Hoc Results for Metabolites Identified by HR-MAS Between Areas of Newborn Heart Tissue
Table S2: Metabolites Identified by HR-MAS in Newborn Heart Tissue and 1H-NMR in Maternal and Fetal Serum and Confidence Levels
Table S3: Internal and Injection Standards for Lipidomics
Table S4: Lipids identified by UHPLC-MS in Newborn Cardiac Tissue