Abstract
Endometriosis is a gynecologic disease common among reproductive-aged women caused by the growth of endometrial tissue outside the uterus. Altered expression of numerous genes and microRNAs has been reported in endometriosis. Steroidogenic factor 1 (SF-1), an essential transcriptional regulator of multiple genes involved in estrogen biosynthesis, is aberrantly increased and plays an important role in the pathogenesis of endometriosis. Here, we show the expression of SF-1 in endometriosis is regulated by miR-370-3p. Sera and tissue were collected from 20 women surgically diagnosed with endometriosis and 26 women without endometriosis. We found that miR-370-3p levels were decreased in the serum of patients with endometriosis while SF-1 mRNA levels were inversely upregulated in endometriotic lesions compared with respective controls. Transfection of primary endometriotic cells with miR-370-3p mimic or inhibitor resulted in the altered expression of SF-1 and SF-1 downstream target genes steroidogenic acute regulatory protein (StAR) and CYP19A1. Overexpression of miR-370-3p inhibited cell proliferation and induced apoptosis in endometriotic cells. This study reveals that miR-370-3p functions as a negative regulator of SF-1 and cell proliferation in endometriotic cells. We suggest a novel therapeutic strategy for controlling SF-1 in endometriosis.
Keywords: apoptosis, cell proliferation, endometriosis, miR-370-3p, SF-1
INTRODUCTION
Endometriosis is an estrogen-dependent disorder characterized by the presence of endometrial glands and stroma outside the uterus, most commonly in the ovaries and peritoneal cavity (6). This disease affects ~10% of reproductive-aged women and 20–50% of infertile women (17, 47). It can result in substantial morbidity, including chronic pelvic pain, multiple surgeries, and infertility (18, 37). The high prevalence and severe consequences associated with this disease have made endometriosis a major public health concern. Although the mechanism responsible for the initiation of this disease remains unclear, aberrant production of estrogen by ectopic endometriotic implants has been demonstrated to be a critical factor associated with endometriosis (24, 25).
Expression of the steroidogenic genes is regulated by the nuclear receptor subfamily 5, group A, member 1 (NR5A1), commonly known as steroidogenic factor 1 (SF-1). SF-1 is an essential transcriptional regulator coordinately regulating multiple genes involved in estrogen biosynthesis. Estrogen is produced from cholesterol through six serial enzymatic conversions. SF-1 can activate genes involved in both cholesterol transfer and steroidogenesis. In the biosynthesis of estrogen, the two rate-limiting steps include the entry of cholesterol into the mitochondria facilitated by steroidogenic acute regulatory protein (StAR) and the conversion of androstenedione to estrogen by aromatase (CYP19A1). StAR and aromatase are directly regulated by SF-1 in endometriotic stromal cells (9, 52). SF-1 binds and activates the promoters of steroidogenic genes including StAR, side-chain cleavage enzyme (SCC), 3b-hydroxysteroid dehydrogenase type 2 (HSD3B2), 17-hydroxylase/17 to 20-lyase (CYP17A1), and aromatase (P450arom or CYP19A1) (8, 35, 39). SF-1 plays an extraordinarily important role in the pathogenesis of endometriosis. Previous studies have reported aberrant SF-1 expression in endometriotic tissues and stromal cells compared with eutopic endometrial tissues and stromal cells (3, 5, 7, 9, 49, 53). The mechanisms that regulate SF-1 expression in endometriosis are not fully understood. Precise regulation of SF-1 is achieved by the activity of positive and negative transcription factors, posttranslational modifications, phospholipid ligand availability, tissue-specific and epigenetic gene expression regulation, and gene dosage (21, 27, 43).
MicroRNAs (miRNAs) are small single-stranded noncoding RNAs that can play important regulatory roles in animals and plants by targeting mRNAs for cleavage or translational repression (4, 45). Aberrant miRNA expression profiles have recently been identified in endometriosis and may therefore play important roles in the pathogenesis of this disease (12–14, 20, 36, 41, 44). Thus a full understanding of the relation between miRNAs and SF-1 may provide a new therapeutic target for endometriosis treatment.
Recently, miR-370 was reported to function as a tumor suppressor by targeting genes in various cancer cells and as a repressor of gene expression in several benign diseases (10, 11, 15, 22, 38, 40, 54). Previous studies indicated that miR-370 was decreased in the serum of patients with endometriosis (12, 14, 51). However, the functions and mechanisms of miR-370 in endometriosis remain unclear. Here, we report the identification of SF-1 as a target of miR-370-3p using bioinformatics prediction. In this study, we aimed to determine whether miR-370-3p controlled the growth of endometriotic cells by regulating SF-1. Here, we report the regulation of SF-1 and its downstream target genes by overexpressing miR-370-3p in primary cells cultured from endometriotic tissue. These alterations led to changes in cell proliferation and apoptosis.
EXPERIMENTAL PROCEDURES
Sample collection from patients with and without endometriosis.
Institutional Review Board approval was obtained from Yale School of Medicine (New Haven, Connecticut) for using human samples (Human Investigations Committee Protocol No. 1004006657). Subjects were recruited from the population of women undergoing benign surgery in the reproductive endocrinology practice of the Yale School of Medicine. Written informed consent was obtained from subjects admitted to the hospital and undergoing laparoscopy or laparotomy for suspected benign indications such as pelvic masses, pelvic pain, infertility, or endometriosis. Inclusion criteria included women who were aged 18–49 yr, had regular menstrual cycles, and used no hormonal therapy for at least 3 mo preceding surgery. Exclusion criteria included postmenopausal patients, previous hormone use within 3 mo of surgery, hyperplasia, polyps, malignancy, autoimmune disease, cardiovascular disease, or use of anti-inflammatory medications. Forty-six (46) subjects were included in the study and divided into two groups as follows: the endometriosis group comprised 20 women with surgically diagnosed and histologically verified endometriosis, and the control group consisted of 26 women who were visually verified to be free of endometriosis during the surgery. The phase of the menstrual cycle was determined based on the patient’s menstrual history and last menstrual period.
Serum collection.
Whole blood samples were collected under sterile conditions immediately before surgery from patients who had regular menstrual cycles and not used hormonal therapy for at least 3 mo preceding surgery. Serum was obtained by centrifuging the blood clot at 2,000 g for 15 min at 4°C within 30 min of collection. The serum was immediately aliquoted for storage at −80°C until use.
Human endometrial tissue collection.
A separate set of endometrial tissue samples were collected from 12 patients who underwent surgery for diagnosis or treatment of endometriosis. All patients were between 24 and 42 yr old and had regular menstrual cycles. None had received steroid hormone medication for at least 6 mo before surgery. Ovarian endometriotic cyst wall and eutopic endometrial tissues in proliferative phase were obtained from women with a laparoscopic and histological diagnosis of stages III and IV endometriosis, according to the revised classification system (2). Control subjects consist of women with no evidence of endometriosis or other endometrial diseases (n = 10). Endometrial biopsies were performed using a Pipelle catheter (Cooper Surgical, Trumbull, CT). Approval for the collection of the specimens was obtained from Yale University Human Investigations Committee.
Isolation of ectopic and eutopic endometrial stromal cells.
Cyst walls of ovarian endometriomas and eutopic endometrial tissues were processed to collect and culture stromal cells using a protocol previously described by Ryan et al. (42) with minor modifications (34). Briefly, endometriotic or endometrial tissues were minced finely and digested with collagenase B (1 mg/ml) and deoxyribonuclease I (0.1 mg/ml) at 37°C for 30 min. The tissues were pipetted gently to disperse the cells every 15 min. Stromal cells were filtrated through a 40-μm cell strainer, and cultured in DMEM/F-12 containing 10% fetal bovine serum and 1% antibiotic and antimycotic. The fibroblast-like appearance of endometriosis derived stromal cells in culture under phase contrast microscopy appeared identical to that of endometrial stromal cells. Primary ectopic and eutopic endometrial stromal cells at two to three passages were used for all experiments.
Transfection of miRNA miR-370 mimic and inhibitor.
Once the cells reached 80% confluency, they were digested, counted, and seeded into six-well plates in growth medium without antibiotics at a density of 2.0 × 105 cells/well. After culturing for 18 to 24 h when 50% confluency was reached, the cells were transfected with 50 nM of hsa-miR-370-3p mimic or hsa-miR-370-3p inhibitor or the respective hsa-miR negative controls (scrambled sequence) (Bioneer, Alameda, CA) using Lipofectamine RNAiMAX reagent (Invitrogen, Carlsbad, CA), according to the manufacturer’s protocol. Forty-eight hours posttransfection, cells were harvested for flow cytometry, RNA isolation, or protein extraction. Transfection efficiency was measured by quantitative real-time polymerase chain reaction (qRT-PCR) for miR-370-3p. All transfection experiments were performed in duplicate wells and repeated three times.
Overexpression and knockdown of SF-1 in endometriotic cyst stromal cells.
Briefly, cells were transfected at 65% confluence in a six-well tissue culture plates with either SF-1 plasmid (no. sc-402334-ACT, 10 µg/ml) and hsa-miR-370-3p mimic together or siRNA plasmid (no. sc-402334, 1 µg/ml) later with hsa-miR-370-3p mimic using Lipofectamine RNAiMAX reagent according to the manufacturer’s protocol, with respective controls (no. sc-437275 control for overexpression of mimic; no. sc-418922 for the siRNA negative control) purchased from Santa Cruz Biotechnology (Dallas TX). The same cells were then transfected with hsa-miR-370-3p mimic or hsa-miR-370-3p inhibitor or the respective hsa-miR negative controls. Total RNA was extracted after 48 h posttransfection from cells in which SF-1 overexpressed and knockdown followed by cDNA preparation and qRT-PCR as described below. All experiments were performed in duplicate wells and repeated three times.
RNA extraction and qRT-PCR.
Total RNA was extracted from cultured endometriotic and endometrial stromal cells using the TRIzol reagent method (Invitrogen). Briefly, cells were treated with 1.0 ml TRIzol for 10 min at room temperature (RT) on ice, and then 0.2 ml of chloroform were added to each, and samples were mixed very vigorously for 20 s, incubated at RT for 3 more min, and then centrifuged at 12,000 rpm at 4°C for 15 min. After centrifugation, the aqueous layer was transferred to a fresh tube, and then, RNA was precipitated by addition of 0.5 ml of isopropyl alcohol, incubated at RT for 10 min, and centrifuged at 12,000 rpm for 10 min; then, the supernatant was removed and the RNA pellet was washed two times with 75% ethanol and dissolved in 30 µl of RNase-free water. The total RNA was purified using the RNeasy Cleanup Kit (Qiagen, Valencia, CA), according to the manufacturer’s protocol, treated using recombinant shrimp DNase (USB, Cleveland, OH) to eliminate DNA contamination and quantified by a NanoDrop spectrophotometer (ThermoFicher Scientific, MA). Purified RNA was immediately used for cDNA synthesis or stored at −80°C for later use. RNA was reverse-transcribed into complementary DNA (cDNA) using iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA). qRT-PCR was performed on CFX Connect Real-Time PCR Detection System (Bio-Rad) using SYBR Green (Bio-Rad) according to the manufacturer’s instructions. Gene Expression levels were normalized to endogenous β-actin mRNA. The following specific primers were used: SF-1: forward, GACGAGGACCTGGACGAGCTGTG and reverse, GATCTTGCAGCTCTGGCTCTCGGTG; StAR: forward, CGTGGCTACTCAGCATCGA and reverse, TGGGCACAGTTGGGAACA; CYP19A1: forward, TGTCTCTTTGTTCTTCATGCTATTTCTC and reverse, TCACCAATAACAGTCTGGATTTCC; Ki67: forward, CTTTGGGTGCGACTTGACG and reverse, GTCGACCCCGCTCCTTTT; and β-actin: forward, GGACTTCGAGCAAGAGATGG and reverse, AGCACTGTGTTGGCGTACAG. The specificity of the amplified transcript and the absence of primer-dimers were confirmed by a melting curve analysis. Relative expression of each gene was calculated using the comparative cycle threshold (Ct) method (2−ΔΔCt). All experiments were carried out three times each in duplicate. For miRNA assays, RNA was extracted using the miRNeasy Kit (Qiagen, Hilden, Germany) and reverse-transcribed into cDNA using TaqMan Advanced miRNA cDNA Synthesis Kit (Applied Biosystems, Foster City, CA) following the manufacturer’s protocol. The following specific primers were used: hsa-miR-370-3p forward, GCCTGCTGGGGTGGAACCTGGT; and hsa-miR-uni-reverse, CCAGTGCAGGGTCCGAGG. U6 small nuclear RNA was used as an internal control. All experiments were performed in duplicate and repeated three times.
Analysis of apoptosis by flow cytometry.
Single cell suspensions were prepared from cells transfected with miRNA miR-370-3p in a FACS buffer. The percentage of cells undergoing apoptosis was measured using annexin V-FITC Apoptosis Detection Kit according to the manufacturer’s procedure (Sigma, St. Louis, Missouri). All cells were stained with annexin V-FITC conjugate and propidium lodide (PI). Apoptotic cells were detected and quantified on a single-cell basis by flow cytometry. The CellQuest software was used to quantify and analyze the cells in three groups: live, apoptotic, and necrotic. Live cells show no or only weak annexin V-FITC staining of the cellular membrane (FITC−PI−), and apoptotic cells show a significantly higher degree of membrane labeling (FITC+PI−), while necrotic cells show both membrane staining by annexin V-FITC and strong nuclear staining by PI (FITC+PI+). All experiments were performed in duplicate and repeated three times.
Protein extraction and Western blotting.
Protein was extracted from the transfected as well as respective control cells with RIPA lysis buffer containing protease inhibitors (Bio-Rad Laboratories). Total protein concentration was determined by BCA Protein Assay Kit (Pierce, Rockford, IL). Equal amounts of protein (25 µg) from lysates were separated on 4–20% SDS-PAGE (Bio-Rad) and transferred on to a polyvinylidene difluoride membranes (Bio-Rad). The membranes were blocked in 5% nonfat milk at room temperature for 1 h and incubated overnight at 4°C with specific primary antibodies purchased from Abcam (Cambridge, MA). On the next day, the membranes were washed three times each for 5 min in 1% TBS-Tween followed by incubation with a goat anti-rabbit IgG conjugated to horseradish peroxidase secondary antibody (Abcam) in 5% milk. The protein bands on the membrane were visualized while using enhanced chemiluminescence solution A and B mix for 3 min (PerkinElmer, Waltham, MA). The density of the bands was assessed by the ImageJ software, and values were normalized to the densitometric values of GAPDH. Western blots were run twice with duplicate samples on each of three repeats.
Statistical analysis.
GraphPad Prism 7.0 (GraphPad Software for Science, San Diego, CA) was used for statistical analysis. All data are presented as means ± SE. The differences between groups were determined using unpaired t-test or one-way ANOVA test for more than two subgroups. P < 0.05 was considered statistically significant.
RESULTS
Expression of SF-1 in endometriotic lesions and miR-370-3p levels in the serum of patients with endometriosis.
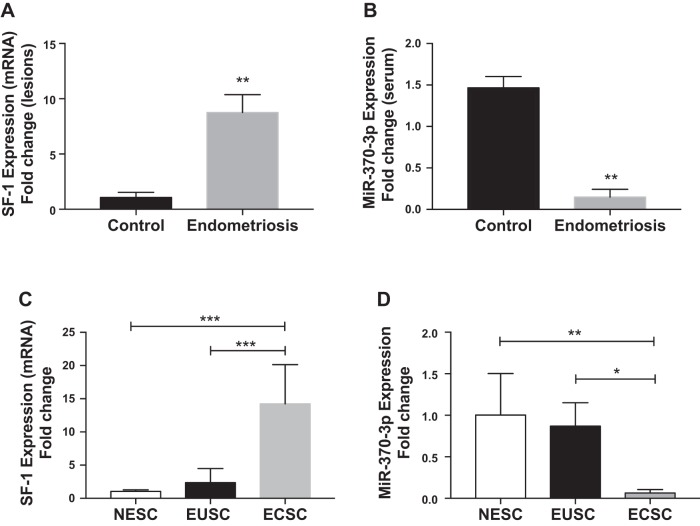
We verified the increased SF-1 expression in endometriotic lesions from women with histologically confirmed endometriosis; here we compared the SF-1 mRNA levels between endometriosis and eutopic endometrium from women without endometriosis as shown in Fig. 1A. There was a ninefold (means 8.76; SE 0.92) increase in SF-1 mRNA levels in the lesions obtained from subjects with endometriosis compared with controls.
Fig. 1.
Inverse expression of steroidogenic factor 1 (SF-1) and miR-370-3p in endometriosis determined by quantitative RT-PCR. A and B: SF-1 mRNA levels were upregulated (A) in the tissue of endometriotic lesions and miR-370-3p was downregulated (B) in serum from patients with endometriosis compared with controls. C and D: for primary cells SF-1 mRNA levels were upregulated (C) while miR-370-3p was downregulated (D) in endometriotic cyst stromal cells (ECSCs) compared with eutopic endometrial stromal cells (EUSCs) and normal endometrial stromal cells (NESC)s. Each bar represents the means ± SE for data from 3 individual experiments, and each experiment was performed in duplicate. *P < 0.05, statistical significance, control vs, endometriosis ECSCs vs EUSCs. **P < 0.01, statistical significance, control vs, endometriosis, NESCs vs. EUSCs. ***P < 0.001, statistical significance compared with NESCs and EUSCs.
Our previous miRNA microarray expression profiling analyzed a total of 36,354 miRNAs in the pooled plasma of women with endometriosis compared with controls free of endometriosis. Of these miRNAs, 11,653 were downregulated (12, 14). miR-370-3p was one of the significantly downregulated circulating miRNAs. To confirm the microarray data, quantitative real-time PCR was performed to analyze miR-370-3p expression in serum samples obtained from women with and without endometriosis. As shown in Fig. 1B, qRT-PCR results validated the microarray analysis that indicated miR-370-3p expression levels in the serum of patients with endometriosis were significantly lower than those of the controls (P = 0.01; means 0.15; SE 0.05). These findings show that expression of SF-1 and miR-370-3p is inversely proportional to each other in women with endometriosis. To confirm these results, we then tested the expression levels of SF-1 as well as miR-370-3p in primary cells isolated and cultured from the tissue collected from women with and without endometriosis. As shown in Fig. 1C, there was a significant increase of SF-1 mRNA expression in endometriotic cyst stromal cells (ECSCs; (means 14.24; SE 3.40) that was ~14- and 12-fold higher than SF-1 mRNA expression in normal endometrial stromal cells (NESCs; means 1.04; SE 0.13) from subjects without endometriosis (P = 0.0002) and eutopic endometrial stromal cells (EUSCs; means 2.37; SE 0.71) from subjects with endometriosis (P = 0.0004), respectively. SF-1 mRNA expression in EUSCs was 1.3-fold higher than in NESCs, a difference that was not significant. Expression of miR-370-3p in ECSCs, (means 0.06; SE 0.02) was decreased by 10- and 8.5-fold compared with NESCs (P = 0.002; means 1.00; SE 0.25) and EUSCs (P = 0.04; means 0.87; SE 0.14), respectively, as shown in Fig. 1D. No significant change in expression was observed between EUSCs and NESCs. Based on the similarity between normal eutopic endometrium and the eutopic endometrium of subjects with endometriosis, subsequent studies were conducted comparing ectopic endometriosis to eutopic control endometrium.
miR-370-3p suppresses SF-1 expression in primary endometriotic cells.
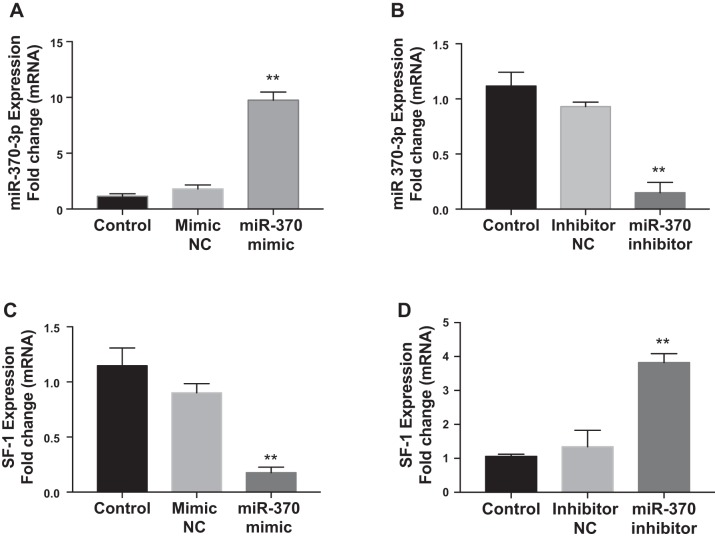
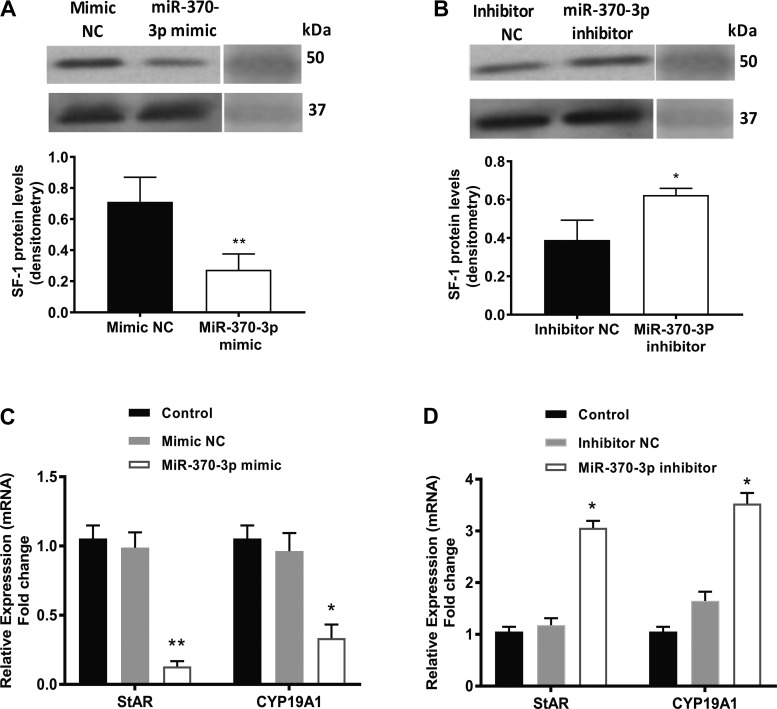
On the basis of two computational algorithms, TargetScan (29) and an online tool (microRNA.org) (23), miR-370 was predicted to regulate SF-1 gene expression and the putative miR-370-3p targeting site located in SF-1 gene sequence. Analyzed data show the mirSVR score −0.5214 and phastScan score 0.5384. We analyzed the effect of miR-370-3p on the mRNA and protein expression of the predicted target, SF-1 by transfecting primary endometriotic cyst stromal cells with miR-370-3p mimic or inhibitor. The results of real-time PCR confirmed the validity of miR-370-3p transfection in ECSCs. Transfection with miR-370-3p mimic led to a greater than ninefold increase in miRNA expression levels (means 8.76; SE 0.42) compared with mimic negative control (Fig. 2A); in contrast, the miR-370-3p inhibitor decreased mature miR-370-3p expression by 10-fold (means 0.11; SE 0.04) compared with inhibitor negative control group (Fig. 2B). Then, we examined SF-1 expression after miR-370-3p mimic or inhibitor transfection in ECSCs. As shown in Fig. 2, C and D, SF-1 mRNA expression was repressed sevenfold (means 0.15; SE 0.05) (P = 0.0004) by miR-370-3p mimic, while it was increased fourfold (means 3.82; SE 0.15) (P = 0.001) by miR-370-3p inhibitor. Similarly, overexpression of miR-370-3p via transfection markedly reduced SF-1 protein level (Fig. 3A), whereas, inhibition of miR-370-3p significantly increased SF-1 protein expression, compared with the respective control group (Fig. 3B). Quantification of protein bands performed by densitometry measurements and normalized to GAPDH is shown in Fig. 3A (means 0.27; SE 0.06) and B (means 0.62; SE 0.02).
Fig. 2.
Effects of miR-370-3p on steroidogenic factor 1 (SF-1) mRNA expression in endometriotic cyst stromal cells (ECSCs). A and B: expression of miR-370-3p is upregulated (A) in ECSCs transfected with miR-370-3p mimic compared with untreated controls or a scrambled miR370-3p control; conversely, miR-370-3p inhibitor decreased (B) miR370-3p compared with controls. C: SF-1 expression was downregulated in ECSCs after overexpression of miR-370-3p mimic, while there is no change after treatment with mimic negative control (NC) compared with the untreated control. D: SF-1 expression is upregulated in ECSCs after suppression of endogenous miR-370-3p by its inhibitor. Data represent the means ± SE from 3 individual experiments, and each experiment was carried out in duplicate. **P < 0.01, statistical significance compared with control (untreated cells) and NC for mimic and inhibitor.
Fig. 3.
Effect of miR-370-3p on steroidogenic factor 1 (SF-1) expression. A: overexpression of miR-370-3p suppressed SF-1 protein expression. B: knockdown of miR-370-3p enhanced SF-1 protein expression level in endometriotic cyst stromal cells (ECSCs). Quantification of protein bands measured by densitometry and normalized to respective GAPDH protein bands is shown in bar graphs. Bar graphs represent the average values of 3 individual experiments (blots). *P < 0.05, statistical significance compared with inhibitor negative control (NC). **P < 0.01, statistical significance compared with mimic NC. C and D: effect of miR-370-3p on SF-1 downstream genes steroidogenic acute regulatory protein (StAR) and CYP19A1 expression and cell proliferation in ECSCs. C: quantitative RT-PCR data showing significant decrease in the mRNA levels of StAR and CYP19A1 in cells transfected with miR-370-3p mimic compared with either treatment with the scrambled control or untreated controls. D: StAR and CYP19A1 mRNA levels were significantly upregulated by overexpression of miR-370-3p inhibitor compared with the controls. Each bar represents the means ± SE for data from 3 individual experiments, and each experiment was performed in duplicate. *P < 0.05, statistical significance compared with control. **P < 0.01, statistical significance compared with control.
miR-370-3p regulates expression of SF-1 downstream genes StAR and CYP19A1.
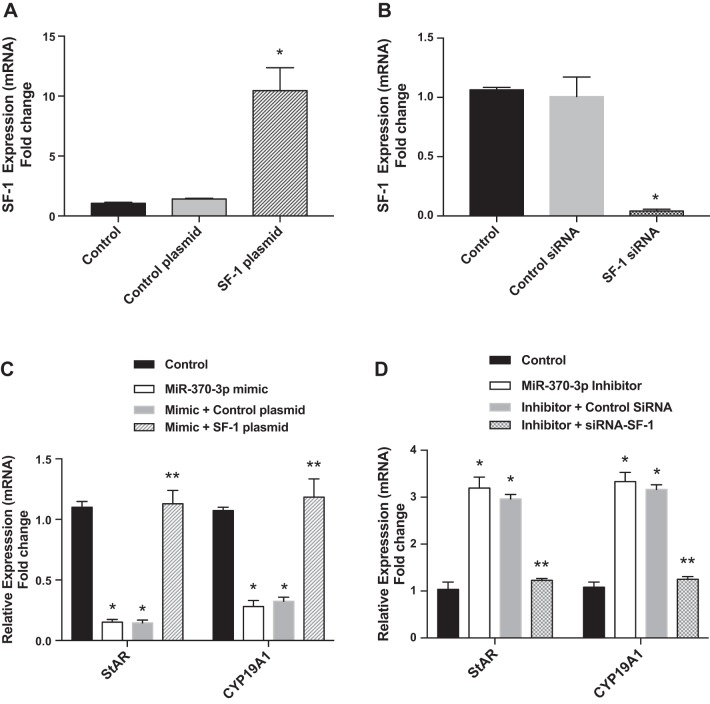
To determine whether miR-370-3p can regulate SF-1 downstream gene expression, StAR and CYP19A1 mRNA expressions were measured by qRT-PCR in ECSCs transfected with miR-370-3p mimic/control or miR-370-3p inhibitor/control. Both StAR and CYP19A1 mRNA expressions were significantly decreased in miR-370-3p mimic transfected ECSCs relative to control cells (Fig. 3C, StAR: means 0.15; SE 0.04; CYP19A1: means 0.44; SE 0.13), whereas knockdown of miR-370-3p increased StAR and CYP19A1 mRNA expression (Fig. 3D, StAR: means 3.14; SE 0.05; CYP19A1: means 3.87; SE 0.13). We next examined whether SF-1 overexpression could rescue the inhibitory effect of miR-370-3p expression on STAR and CYP19A1 expression and if SF-1 knockdown could block the stimulatory effect of the miR-370-3p inhibitor. SF-1 expression levels increased by 11-fold (means 10.5; SE 1.10) in SF-1 transfected cells while SF-1 successfully knocked down by > 90% (means 0.04; SE 0.007) by its siRNA as shown in Fig. 4, A and B, respectively. As shown in Fig. 4C, overexpression of SF-1 rescued the inhibitory effect of miR-370-3p expression on StAR (means 1.157; SE 0.03) and CYP19A1 expression (means 1.205; SE 0.05). Knockdown of SF-1 blocks the stimulatory effect of the miR-370-3p inhibitor on STAR (means 1.24; SE 0.01) and CYP19A1 expression as shown in Fig. 4D (means 1.32; SE 0.02).
Fig. 4.
Steroidogenic factor 1 (SF-1) overexpression or knockdown rescued or blocked, respectively, the effect of miR-370-3p on steroidogenic acute regulatory protein StAR and CYP19A1 in endometriotic cyst stromal cells. A and B: SF-1 overexpression (A) and SF-1 knockdown (B). *P < 0.01, statistical significance compared with control and control plasmid. C: overexpression of SF-1 rescued the inhibitory effect of miR-370-3p expression on StAR and CYP19A1 expression. D: knockdown of SF-1 by its siRNA blocks the stimulatory effect of the miR-370-3p inhibitor. Each bar represents the means ± SE for data from 3 individual experiments and each experiment was performed in duplicate. *P < 0.01, statistical significance compared with control. **No statistical significance compared with control.
miR-370-3p inhibits cell proliferation and induces apoptosis in ECSCs.
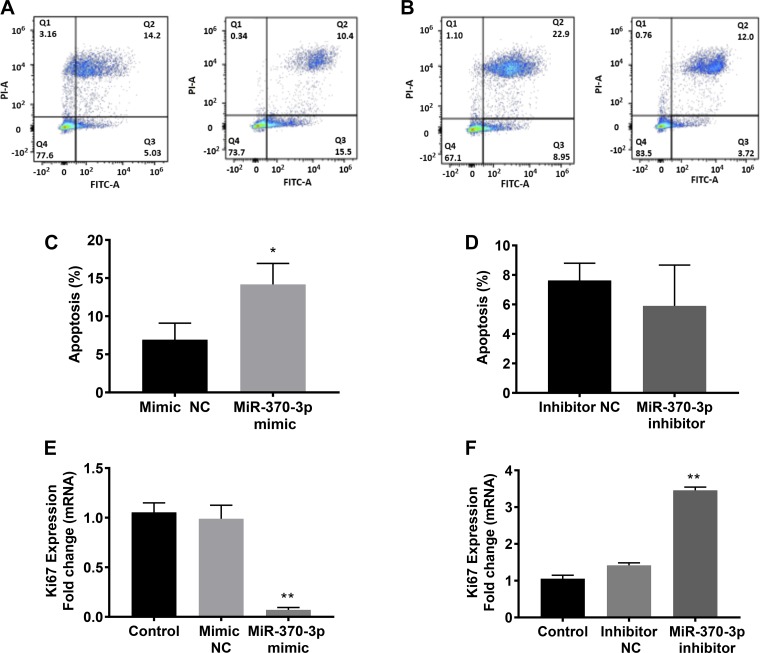
We next investigated the role of miR-370-3p in the cell apoptotic process of ECSCs. In miR-370-3p mimic or inhibitor transfected cells, annexin V-FITC and PI were used to identify apoptotic cells. Apoptotic cells stain with the annexin V FITC conjugate alone. Live cells will show no staining by either PI or annexin V FITC vonjugate. Necrotic cells are stained by both PI and annexin V FITC conjugate. Figure 5, A and B, shows the respective histograms for miR-370-3p mimic and inhibitor. Flow cytometry analysis showed that overexpression of miR-370-3p increased the percentage of cells in the apoptotic phase (Fig. 5C; means 14.17; SE 1.59). However, inhibition of miR-370-3p had no effect on the apoptosis rate of ECSCs, compared with the negative control cells (Fig. 5D; (means 5.91; SE 1.59). Endometriotic cyst stromal cell proliferation was evaluated by Ki67, a sensitive and specific marker for cell proliferation (26). As shown in Fig. 5E, there is a remarkable decrease in Ki67 mRNA expression levels following miR-370-3p overexpression (P = 0.003; means 0.064; SE 0.02). Conversely, inhibition of miR-370-3p significantly enhanced the expression levels of Ki67 mRNA (Fig. 5F, P = 0.005; means 3.13; SE 0.34). The results indicated that cell proliferation of ECSCs was influenced by miR-370-3p. As miR-370-3p is already dramatically decreased in endometriosis, additional decreases were likely below the threshold required for an effect on apoptosis.
Fig. 5.
miR-370-3p induced apoptosis determined by flowcytometric analysis. A and B: histograms of FACS analysis of cells transfected with of miR-370-3p mimic (A) and inhibitor (B). The percentage of apoptosis was shown in the bar graphs below. C: number of apoptotic cells increased significantly in cells in which the miR-370-3p mimic was overexpressed compared with controls. Bars represent the means ± SE for data from 3 individual experiments. *P < 0.05, statistical significance compared with the negative controls. D: no significant changes in the number of apoptotic cells between cells in which miR-370-3p was inhibited compared with the negative control. E and F: overexpression of miR-370-3p mimic inhibited cell proliferation showing the decreased mRNA levels of Ki67 (E) while shows the increased Ki67 mRNA levels (F) in ECSCs in which miR-370-3p inhibitor is overexpressed compared with the control cells that are not treated either with mimic or inhibitor. Each bar represents the means ± SE for data from 3 individual experiments, and each experiment was performed in duplicate. *P < 0.05, statistical significance compared with controls. **P < 0.01, statistical significance compared with controls.
DISCUSSION
The exact mechanism of induction or development of endometriosis is still incompletely characterized. Important and consistent features of endometriosis are its high sensitivity to estrogens and increased local estrogen production. As a master regulator of steroidogenic enzymes, SF-1 plays a key role in sustained survival of endometrial tissue at the ectopic sites by promoting a hyperestrogenic state in endometriosis (9). In this study, we evaluated the SF-1 expression in cultured primary ECSCs, EUSCs from patients with endometriosis, as well as NESCs from women without endometriosis. In accordance with previous studies, our results showed that SF-1 expression in ECSCs was remarkably higher than that in EUSCs or NESCs. These results further support the role of nuclear receptor SF-1, which was highly expressed in endometriotic cells and low in normal endometrial cells, in the pathogenesis of endometriosis.
Furthermore, in addition to the role of SF-1 in steroidogenesis, recent studies have revealed that SF-1 has an important role in a variety of biological processes including angiogenesis, apoptosis, cell proliferation, adhesion to the extracellular matrix, cytoskeleton dynamics, and key signaling pathways in the adrenal cortex (28). Similarly, in endometriosis, the role of SF-1 likely goes beyond contributing to local steroidogenesis and also contributes to inflammation and growth of ectopic endometrial tissue. SF-1 also directly promotes the abnormal uterine gland morphogenesis, inhibits the ability of the endometrium to undergo decidualization resulting in infertility, alters endometrial immune homeostasis, and triggers a physiological inflammatory response (50). Considering the pivotal role of this transcription factor in endometriosis, investigating the regulators of SF-1 may open new insights for therapeutic intervention.
Until now, accumulated data demonstrated that miRNAs contribute to the pathogenesis of endometriosis by regulating abnormal cell differentiation, invasion, and inflammation (32, 41, 48). Dysregulation of miR-370 has been described in a variety of human diseases (43). In the present study, we found that miR-370-3p was downregulated in endometriosis, consistent with our previous miRNA microarray data (12).
To investigate the effect of miR-370-3p on SF-1 and cell growth in endometriosis, we changed miR-370-3p levels in endometriotic cells by transfecting them with miR-370-3p mimic or inhibitor. Our findings showed that miR-370-3p regulated the expression of SF-1 at both the mRNA and protein levels. Since the aberrant presence of SF-1 in endometriosis and its absence in endometrium is the key event for the differential expression of StAR and CYP19A1 (9), we further analyzed the effect of miR-370-3p on the expression these two downstream genes. Consistent with SF-1, the expression of StAR and CYP19A1 genes was indirectly regulated by miR-370-3p. Therefore, our results indicated that miR-370-3p may function as a negative regulator in the estrogen synthesis pathway. Upregulation of SF-1 through a posttranscriptional mechanism in response to miR-370-3p deregulation in endometriosis led to overexpression of components of the estrogen synthesis pathway and is expected to result in increased local estrogen formation.
In addition to steroidogenesis, SF-1 is also involved in other physiological behaviors of cells such as cell proliferation and apoptosis. Recent studies demonstrated that miR-370-3p is implicated in the progression of various cancers and appears to be involved in inhibiting cell proliferation-related signal pathways. miR-370 inhibits tumor growth in cancers or tumors, including breast, colon, gastric, hepatocellular carcinoma cancers (46), and cancer cell lines (10, 15) by either arresting cell proliferation, inducing apoptosis, or both. In glioma tumors miR-370 expression was reduced in glioma tissues and negatively correlated with the malignancy of many tumors (16, 31). Upregulation of miRNA-370 promotes cell apoptosis and inhibits proliferation by targeting phosphatase and tensin homolog deleted on chromosome 10 in human gastric cancer (54). For instance, miR-370-3p suppresses cell growth and induces cell cycle arrest by directly targeting β-catenin in human glioma cells (40). We evaluated the effects of miR-370-3p on cell proliferation and apoptosis in ECSCs. We found that overexpression of miR-370-3p inhibited cell proliferation and induced apoptosis in endometriotic cells in vitro. Taken together, our data indicated that miR-370-3p suppresses SF-1, which plays an important role in estrogen production, cell proliferation, and survival in endometriosis. Lower miR-370-3p levels, as are seen in endometriosis, are expected to increase estrogen production and cell proliferation while decreasing apoptosis.
Importantly, here we also detected decreased miR-370-3p in the circulation of women with endometriosis, indicating the potential for remote effects far removed from the areas affected by endometriosis. We previously reported that circulating miRNAs can effect inflammatory cytokine production from macrophages (33). Similarly, we identified multiple systemic effects of endometriosis in tissues outside of the pelvis; endometriosis effects the brain (30), body composition (19), inflammation, and multiple other organ systems (1). miR-370-3p may affect steroidogenesis in multiple organs, altering steroid production in several tissues and effecting the local estrogen effect throughout the body.
In summary, miR-370-3p acts to suppress proliferation by regulating SF-1 in endometriotic cells. Circulating miRNA-370-3p may have similar effects on other organs indirectly influenced by miRNAs in women with endometriosis. These findings provide new insights into the role of miRNAs in endometriosis. Use of miR-370-3p to regulate SF-1 may provide a new therapeutic strategy for endometriosis.
GRANTS
This work was supported by National Institute of Child Health and Human Development Grant HD-052668 (to H. S. Taylor).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.M. and H.S.T. conceived and designed research; Z.H. performed experiments; Z.H., R.M., and H.S.T. analyzed data; R.M. interpreted results of experiments; R.M. prepared figures; Z.H. drafted manuscript; R.M. and H.S.T. edited and revised manuscript; R.M. and H.S.T. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address of Z. Hu: Dept. of Obstetrics and Gynecology, The First Affiliated Hospital of Chongqing Medical Univ., Chongqing, 400016, China.
REFERENCES
- 1.Alderman MH 3rd, Yoder N, Taylor HS. The systemic effects of endometriosis. Semin Reprod Med 35: 263–270, 2017. doi: 10.1055/s-0037-1603582. [DOI] [PubMed] [Google Scholar]
- 2.American Society for Reproductive Medicine Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril 67: 817–821, 1997. doi: 10.1016/S0015-0282(97)81391-X. [DOI] [PubMed] [Google Scholar]
- 3.Attar E, Tokunaga H, Imir G, Yilmaz MB, Redwine D, Putman M, Gurates B, Attar R, Yaegashi N, Hales DB, Bulun SE. Prostaglandin E2 via steroidogenic factor-1 coordinately regulates transcription of steroidogenic genes necessary for estrogen synthesis in endometriosis. J Clin Endocrinol Metab 94: 623–631, 2009. doi: 10.1210/jc.2008-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281–297, 2004. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 5.Borghese B, Mondon F, Noël JC, Fayt I, Mignot TM, Vaiman D, Chapron C. Gene expression profile for ectopic versus eutopic endometrium provides new insights into endometriosis oncogenic potential. Mol Endocrinol 22: 2557–2562, 2008. doi: 10.1210/me.2008-0322. [DOI] [PubMed] [Google Scholar]
- 6.Bulun SE. Endometriosis. N Engl J Med 360: 268–279, 2009. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 7.Bulun SE, Lin Z, Imir G, Amin S, Demura M, Yilmaz B, Martin R, Utsunomiya H, Thung S, Gurates B, Tamura M, Langoi D, Deb S. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol Rev 57: 359–383, 2005. doi: 10.1124/pr.57.3.6. [DOI] [PubMed] [Google Scholar]
- 8.Bulun SE, Monsivais D, Kakinuma T, Furukawa Y, Bernardi L, Pavone ME, Dyson M. Molecular biology of endometriosis: from aromatase to genomic abnormalities. Semin Reprod Med 33: 220–224, 2015. doi: 10.1055/s-0035-1554053. [DOI] [PubMed] [Google Scholar]
- 9.Bulun SE, Utsunomiya H, Lin Z, Yin P, Cheng YH, Pavone ME, Tokunaga H, Trukhacheva E, Attar E, Gurates B, Milad MP, Confino E, Su E, Reierstad S, Xue Q. Steroidogenic factor-1 and endometriosis. Mol Cell Endocrinol 300: 104–108, 2009. doi: 10.1016/j.mce.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Chen T, Gao F, Feng S, Yang T, Chen M. MicroRNA-370 inhibits the progression of non-small cell lung cancer by downregulating oncogene TRAF4. Oncol Rep 34: 461–468, 2015. doi: 10.3892/or.2015.3978. [DOI] [PubMed] [Google Scholar]
- 11.Chen XP, Chen YG, Lan JY, Shen ZJ. MicroRNA-370 suppresses proliferation and promotes endometrioid ovarian cancer chemosensitivity to cDDP by negatively regulating ENG. Cancer Lett 353: 201–210, 2014. doi: 10.1016/j.canlet.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 12.Cho S, Mutlu L, Grechukhina O, Taylor HS. Circulating microRNAs as potential biomarkers for endometriosis. Fertil Steril 103: 1252–1260.e1, 2015. doi: 10.1016/j.fertnstert.2015.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho S, Mutlu L, Zhou Y, Taylor HS. Aromatase inhibitor regulates let-7 expression and let-7f-induced cell migration in endometrial cells from women with endometriosis. Fertil Steril 106: 673–680, 2016. doi: 10.1016/j.fertnstert.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cosar E, Mamillapalli R, Ersoy GS, Cho S, Seifer B, and Taylor HS. Serum microRNAs as diagnostic markers of endometriosis: a comprehensive array-based analysis. Fertil Steril 106: 402-409, 2016. doi: 10.1016/j.fertnstert.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Duan N, Hu X, Yang X, Cheng H, Zhang W. MicroRNA-370 directly targets FOXM1 to inhibit cell growth and metastasis in osteosarcoma cells. Int J Clin Exp Pathol 8: 10250–10260, 2015. [PMC free article] [PubMed] [Google Scholar]
- 16.Gao YT, Chen XB, Liu HL. Up-regulation of miR-370-3p restores glioblastoma multiforme sensitivity to temozolomide by influencing MGMT expression. Sci Rep 6: 32972, 2016. doi: 10.1038/srep32972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giudice LC. Clinical practice. Endometriosis. N Engl J Med 362: 2389–2398, 2010. doi: 10.1056/NEJMcp1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giudice LC, Kao LC. Endometriosis. Lancet 364: 1789–1799, 2004. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 19.Goetz LG, Mamillapalli R, Taylor HS. Low body mass index in endometriosis is promoted by hepatic metabolic gene dysregulation in mice. Biol Reprod 95: 115, 2016. doi: 10.1095/biolreprod.116.142877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grechukhina O, Petracco R, Popkhadze S, Massasa E, Paranjape T, Chan E, Flores I, Weidhaas JB, Taylor HS. A polymorphism in a let-7 microRNA binding site of KRAS in women with endometriosis. EMBO Mol Med 4: 206–217, 2012. doi: 10.1002/emmm.201100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoivik EA, Lewis AE, Aumo L, Bakke M. Molecular aspects of steroidogenic factor 1 (SF-1). Mol Cell Endocrinol 315: 27–39, 2010. doi: 10.1016/j.mce.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Hou WZ, Chen XL, Wu W, Hang CH. MicroRNA-370-3p inhibits human vascular smooth muscle cell proliferation via targeting KDR/AKT signaling pathway in cerebral aneurysm. Eur Rev Med Pharmacol Sci 21: 1080–1087, 2017. [PubMed] [Google Scholar]
- 23.John B, Enright AJ, Aravin A, Tuschl T, Sander C, Marks DS. Human MicroRNA targets. PLoS Biol 2: e363, 2004. doi: 10.1371/journal.pbio.0020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitawaki J, Kado N, Ishihara H, Koshiba H, Kitaoka Y, Honjo H. Endometriosis: the pathophysiology as an estrogen-dependent disease. J Steroid Biochem Mol Biol 83: 149–155, 2002. doi: 10.1016/S0960-0760(02)00260-1. [DOI] [PubMed] [Google Scholar]
- 25.Kitawaki J, Noguchi T, Amatsu T, Maeda K, Tsukamoto K, Yamamoto T, Fushiki S, Osawa Y, Honjo H. Expression of aromatase cytochrome P450 protein and messenger ribonucleic acid in human endometriotic and adenomyotic tissues but not in normal endometrium. Biol Reprod 57: 514–519, 1997. doi: 10.1095/biolreprod57.3.514. [DOI] [PubMed] [Google Scholar]
- 26.Kordek R, Biernat W, Alwasiak J, Liberski PP. Proliferating cell nuclear antigen (PCNA) and Ki-67 immunopositivity in human astrocytic tumours. Acta Neurochir (Wien) 138: 509–513, 1996. doi: 10.1007/BF01411168. [DOI] [PubMed] [Google Scholar]
- 27.Lalli E. Adrenocortical development and cancer: focus on SF-1. J Mol Endocrinol 44: 301–307, 2010. doi: 10.1677/JME-09-0143. [DOI] [PubMed] [Google Scholar]
- 28.Lalli E, Doghman M, Latre de Late P, El Wakil A, Mus-Veteau I. Beyond steroidogenesis: novel target genes for SF-1 discovered by genomics. Mol Cell Endocrinol 371: 154–159, 2013. doi: 10.1016/j.mce.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell 115: 787–798, 2003. doi: 10.1016/S0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 30.Li T, Mamillapalli R, Ding S, Chang H, Liu ZW, Gao XB, Taylor HS. Endometriosis alters brain electro-physiology, gene expression and increased pain sensitization, anxiety, and depression in female mice. Biol Reprod 99: 349–359, 2018. doi: 10.1093/biolre/ioy035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu M, Wang Y, Zhou S, Xu J, Li J, Tao R, Zhu Y. MicroRNA-370 suppresses the progression and proliferation of human astrocytoma and glioblastoma by negatively regulating β-catenin and causing activation of FOXO3a. Exp Ther Med 15: 1093–1098, 2018. doi: 10.3892/etm.2017.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naqvi H, Mamillapalli R, Krikun G, Taylor HS. Endometriosis located proximal to or remote from the uterus differentially affects uterine gene expression. Reprod Sci 23: 186–191, 2016. doi: 10.1177/1933719115613449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nematian SE, Mamillapalli R, Kadakia TS, Majidi Zolbin M, Moustafa S, Taylor HS. Systemic inflammation induced by microRNAs: endometriosis-derived alterations in circulating microRNA 125b-5p and Let-7b-5p regulate macrophage cytokine production. J Clin Endocrinol Metab 103: 64–74, 2018. doi: 10.1210/jc.2017-01199. [DOI] [PubMed] [Google Scholar]
- 34.Noble LS, Takayama K, Zeitoun KM, Putman JM, Johns DA, Hinshelwood MM, Agarwal VR, Zhao Y, Carr BR, Bulun SE. Prostaglandin E2 stimulates aromatase expression in endometriosis-derived stromal cells. J Clin Endocrinol Metab 82: 600–606, 1997. doi: 10.1210/jc.82.2.600. [DOI] [PubMed] [Google Scholar]
- 35.Noël JC, Borghese B, Vaiman D, Fayt I, Anaf V, Chapron C. Steroidogenic factor-1 expression in ovarian endometriosis. Appl Immunohistochem Mol Morphol 18: 258–261, 2010. doi: 10.1097/PAI.0b013e3181c06948. [DOI] [PubMed] [Google Scholar]
- 36.Ohlsson Teague EM, Van der Hoek KH, Van der Hoek MB, Perry N, Wagaarachchi P, Robertson SA, Print CG, Hull LM. MicroRNA-regulated pathways associated with endometriosis. Mol Endocrinol 23: 265–275, 2009. doi: 10.1210/me.2008-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olive DL, Pritts EA. Treatment of endometriosis. N Engl J Med 345: 266–275, 2001. doi: 10.1056/NEJM200107263450407. [DOI] [PubMed] [Google Scholar]
- 38.Pan XP, Wang HX, Tong DM, Li Y, Huang LH, Wang C. miRNA-370 acts as a tumor suppressor via the downregulation of PIM1 in hepatocellular carcinoma. Eur Rev Med Pharmacol Sci 21: 1254–1263, 2017. [PubMed] [Google Scholar]
- 39.Parker KL, Schimmer BP. Steroidogenic factor 1: a key determinant of endocrine development and function. Endocr Rev 18: 361–377, 1997. doi: 10.1210/edrv.18.3.0301. [DOI] [PubMed] [Google Scholar]
- 40.Peng Z, Wu T, Li Y, Xu Z, Zhang S, Liu B, Chen Q, Tian D. MicroRNA-370-3p inhibits human glioma cell proliferation and induces cell cycle arrest by directly targeting β-catenin. Brain Res 1644: 53–61, 2016. doi: 10.1016/j.brainres.2016.04.066. [DOI] [PubMed] [Google Scholar]
- 41.Petracco R, Grechukhina O, Popkhadze S, Massasa E, Zhou Y, Taylor HS. MicroRNA 135 regulates HOXA10 expression in endometriosis. J Clin Endocrinol Metab 96: E1925–E1933, 2011. doi: 10.1210/jc.2011-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ryan IP, Schriock ED, Taylor RN. Isolation, characterization, and comparison of human endometrial and endometriosis cells in vitro. J Clin Endocrinol Metab 78: 642–649, 1994. doi: 10.1210/jcem.78.3.8126136. [DOI] [PubMed] [Google Scholar]
- 43.Schimmer BP, White PC. Minireview: steroidogenic factor 1: its roles in differentiation, development, and disease. Mol Endocrinol 24: 1322–1337, 2010. doi: 10.1210/me.2009-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seifer BJ, Su D, Taylor HS. Circulating miRNAs in murine experimental endometriosis. Reprod Sci 24: 376–381, 2017. doi: 10.1177/1933719116667228. [DOI] [PubMed] [Google Scholar]
- 45.Shukla GC, Singh J, Barik S. MicroRNAs: processing, maturation, target recognition and regulatory functions. Mol Cell Pharmacol 3: 83–92, 2011. [PMC free article] [PubMed] [Google Scholar]
- 46.Sim J, Ahn H, Abdul R, Kim H, Yi KJ, Chung YM, Chung MS, Paik SS, Song YS, Jang K. High MicroRNA-370 expression correlates with tumor progression and poor prognosis in breast cancer. J Breast Cancer 18: 323–328, 2015. doi: 10.4048/jbc.2015.18.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor HS, Osteen KG, Bruner-Tran KL, Lockwood CJ, Krikun G, Sokalska A, Duleba AJ. Novel therapies targeting endometriosis. Reprod Sci 18: 814–823, 2011. doi: 10.1177/1933719111410713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teague EM, Print CG, Hull ML. The role of microRNAs in endometriosis and associated reproductive conditions. Hum Reprod Update 16: 142–165, 2010. doi: 10.1093/humupd/dmp034. [DOI] [PubMed] [Google Scholar]
- 49.Utsunomiya H, Cheng YH, Lin Z, Reierstad S, Yin P, Attar E, Xue Q, Imir G, Thung S, Trukhacheva E, Suzuki T, Sasano H, Kim JJ, Yaegashi N, Bulun SE. Upstream stimulatory factor-2 regulates steroidogenic factor-1 expression in endometriosis. Mol Endocrinol 22: 904–914, 2008. doi: 10.1210/me.2006-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vasquez YM, Wu SP, Anderson ML, Hawkins SM, Creighton CJ, Ray M, Tsai SY, Tsai MJ, Lydon JP, DeMayo FJ. Endometrial expression of steroidogenic factor 1 promotes cystic glandular morphogenesis. Mol Endocrinol 30: 518–532, 2016. doi: 10.1210/me.2015-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang L, Huang W, Ren C, Zhao M, Jiang X, Fang X, Xia X. Analysis of serum microRNA profile by solexa sequencing in women with endometriosis. Reprod Sci 23: 1359–1370, 2016. doi: 10.1177/1933719116641761. [DOI] [PubMed] [Google Scholar]
- 52.Xue Q, Xu Y, Yang H, Zhang L, Shang J, Zeng C, Yin P, Bulun SE. Methylation of a novel CpG island of intron 1 is associated with steroidogenic factor 1 expression in endometriotic stromal cells. Reprod Sci 21: 395–400, 2014. doi: 10.1177/1933719113497283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeitoun K, Takayama K, Michael MD, Bulun SE. Stimulation of aromatase P450 promoter (II) activity in endometriosis and its inhibition in endometrium are regulated by competitive binding of steroidogenic factor-1 and chicken ovalbumin upstream promoter transcription factor to the same cis-acting element. Mol Endocrinol 13: 239–253, 1999. doi: 10.1210/mend.13.2.0229. [DOI] [PubMed] [Google Scholar]
- 54.Zeng Y, Fu M, Wu GW, Zhang AZ, Chen JP, Lin HY, Fu YA, Jia J, Cai ZD, Wu XJ, Lan P. Upregulation of microRNA-370 promotes cell apoptosis and inhibits proliferation by targeting PTEN in human gastric cancer. Int J Oncol 49: 1589–1599, 2016. doi: 10.3892/ijo.2016.3642. [DOI] [PubMed] [Google Scholar]