Abstract
Although obesity negatively influences the metabolic homeostasis of cells within a broad range of tissues, its impact on oocyte metabolism is not fully understood. Prior evidence suggests that obesity increases expression of oocyte genes associated with inflammation, oxidative stress, and lipid metabolism; however, the metabolic impact of these genetic differences is not known. To address this gap, we conducted an exploratory assessment of the follicular fluid (FF) metabolome in eight overweight/obese (OW) and nine normal-weight (NW) women undergoing in vitro fertilization. FF and serum were collected and analyzed by untargeted metabolomics using gas chromatography-quadrupole time-of-flight mass spectrometry and charged-surface hybrid column-electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Untargeted metabolomics identified obesity-associated changes in FF metabolites related to oxidative stress/antioxidant capacity, xenometabolism/amino acid biosynthesis, and lipid metabolism. Discriminant FF metabolites included elevated uric acid, isothreonic acid, one unknown primary metabolite, and six unknown complex lipids in OW compared with NW women. Conversely, 2-ketoglucose dimethylacetal, aminomalonate, two unknown primary metabolites, and two unknown complex lipids were decreased in FF of OW relative to NW women. Indole-3-propionic acid (IPA), a bacteria-derived metabolite, was also decreased in both FF and serum of OW women (P < 0.05). The significant correlation between antioxidant IPA in serum and FF (R = 0.95, P < 0.0001) suggests a potential serum biomarker of FF antioxidant status or reflection of the gut metabolism interaction with the follicle. These results suggest that obesity has important consequences for the follicular environment during the preconception period, a window of time that may be important for lifestyle interventions to ameliorate obesity-associated risk factors.
Keywords: follicular fluid, infertility, metabolomics, obesity
INTRODUCTION
In the United States, 25% of women of reproductive age are obese [body mass index (BMI) >30 kg/m2], which predisposes them to infertility and pregnancy complications (57). Obese women are 2.7 times more likely to suffer from infertility compared with normal-weight (NW) women, which has led to an increased need for reproductive assistance (43). Obesity is associated with menstrual irregularities, impaired oocyte quality, low rates of implantation, and delayed embryo development (6, 49). Clinical studies in women undergoing in vitro fertilization (IVF) procedures showed that obese women have reduced quantity and quality of oocytes compared with NW women (32). Recent work from our group demonstrated differential gene expression in oocytes of overweight/obese (OW) compared with NW women. Genes related to inflammation, oxidative stress, lipid metabolism, and transcription factors were differentially expressed (50). These results indicate that obesity leads to changes in the oocyte environment during the preconception period.
The ovarian follicular environment, which is composed of follicular fluid (FF) and granulosa cells, is thought to provide nutrients and growth factors that promote oocyte growth, development, and viability (40). Analysis of FF content provides a unique opportunity to evaluate the oocyte environment at the time of ovulation as well as information about the metabolism of the oocyte and surrounding granulosa cells. Clinical studies have shown that obese women undergoing fertility treatments have elevated FF triglyceride (TG), leptin, and C-reactive protein (CRP) levels, which were found to be positively associated with BMI, and poorer oocyte quality and pregnancy outcomes (50, 58). Together, these studies have demonstrated the importance of the FF in providing an optimal follicular environment for successful fertility outcomes. Imbalances in nutritional status and hormonal levels may transform the ovarian environment to induce intracellular stress in the oocyte, which could influence oocyte development and quality.
Recently, there has been an increased interest in using broader hypothesis-neutral tools, such as metabolomics, to examine FF metabolites. This technology can enhance our understanding of the metabolites that surround the oocyte and might affect its development (46). To date, few studies have investigated FF metabolites with metabolomics analyses with the primary focus of examining fertility biomarkers that would predict oocyte quality and IVF success rates. To our knowledge, no studies have examined the impact of obesity on human FF metabolites with comprehensive metabolomics profiling. To address this research gap, we designed a clinical study of women undergoing fertility treatments to 1) investigate the impact of obesity on the metabolome of human FF with untargeted metabolomics for primary metabolism and lipidomics and 2) to determine whether serum metabolites are reflective of FF profiles in OW compared with NW women.
MATERIALS AND METHODS
Participants.
For this study, we analyzed FF obtained from women undergoing IVF treatments as a subset from our previously published study looking at oocyte gene expression (50). Participants were healthy women between 18 and 36 yr of age who were recruited by BMI: NW (18.5–24.9 kg/m2, n = 9) and OW (≥25 kg/m2, n = 8). Exclusion criteria included patients with polycystic ovarian syndrome, cardiovascular disease, or diabetes. Medical history and medication information were obtained from all participants to evaluate eligibility. No indications of gastrointestinal disorders were reported, nor was antibiotic use reported by any participant enrolled in the study. Written informed consent was obtained from all participants, and the study protocol was reviewed and approved by the Institutional Review Board at the University of Arkansas for Medical Sciences (https://clinicaltrials.gov; NCT01480024). Since adiposity is a more acceptable measure in the field of obesity, we choose to examine our metabolomics data with both BMI cutoffs that divided women into NW (BMI 18.5–24.9 kg/m2; n = 9) and OW (BMI ≥25 kg/m2; n = 8) and percent body fat (BF). For percent BF, we choose to use a cutoff of 35% BF to discriminate between high body fat (HBF: n = 9) and low body fat (LBF: n = 8) (26, 47, 53).
FF retrieval procedures.
All participants underwent fertility treatments and hormonal superovulation that included an oocyte retrieval procedure 36 h after human chorionic gonadotrophin injection by ultrasound-guided transvaginal aspiration (6). FF was collected from multiple mature follicles and pooled together for each participant. Samples contaminated with blood were not retained for analysis. The pooled FF supernatant (~1–2 ml) obtained after centrifugation at 4,000 rpm (1,792 g) for 10 min was divided into aliquots and stored at −70°C.
Anthropometrics and body composition.
Body weight and height were collected to the nearest 0.1 kg and 0.1 cm, respectively, with standardized methods. Body composition (fat mass and fat-free mass) was assessed by air displacement plethysmography (BOD POD; COSMED) according to manufacturer’s recommendations from overnight-fasted participants.
Dietary intake.
Nutrient Data System for Research software (NDSR, Nutrition Coordinating Center; University of Minnesota) was used to estimate average daily dietary intakes from 3-day food records obtained in a subset of women (NW: n = 7, OW: n = 6). Percentages of calories from fat (% fat kcal), carbohydrates, protein, polyunsaturated fat, saturated fat, and monounsaturated fat were calculated. It is important to note that one major limitation with 3-day food records is underreporting. Numerous studies have demonstrated that among all individuals, especially obese participants, there is a tendency to report lower food intake than actually consumed (21, 30, 31, 35). However, 3-day food records are the best methodology available to minimize this bias compared with food frequency questionnaires or food recalls (67).
Serum and FF content of a subset of metabolites.
Serum samples were collected from overnight-fasted participants after venipuncture of an antecubital vein. A RX Daytona clinical analyzer (Randox Laboratories, Keaneysville, WV) was used to measure high-density lipoprotein and low-density lipoprotein. Total cholesterol (Synermed, Westfield, IN), TG (Caymen Chemical, Ann Arbor, MI), and glucose (Synermed) were measured by colorimetric assays. Levels of leptin, insulin, CRP, chemokine (C-C motif) ligand 2, and tumor necrosis factor-α were assessed with ELISA assays (Millipore, Billerica, MA).
Metabolomics analyses.
FF and serum samples were used for metabolomics analyses for untargeted primary metabolism and complex lipids. Samples were analyzed by the West Coast Metabolomics Center at the University of California-Davis with previously published methods (8, 18). Briefly, FF (100 µl) and serum (20 µl) were extracted and derivatized by silylation/methyloximation (18). Samples were injected into an Agilent 6890 gas chromatograph and separated with a 30-m-long, 0.25-mm-ID Rtx5Sil-MS column. Mass spectrometry was conducted on a Leco Pegasus IV time-of-flight mass spectrometer. Resulting data were annotated with the BinBase (BB) algorithm with an automated database at the West Coast Metabolomics Center. The BinBase database matches mass spectrum information and retention times to the Fiehn laboratory mass spectral library of >1,200 authentic standards in addition to the NIST05 commercial library. Each metabolite’s peak heights of quantifier ions were measured and normalized by the sum of intensities of all known metabolites. All metabolites that were not structurally identified (i.e., unknown metabolites) were given a BB number and included in the analyses (e.g., BB11303).
Metabolomics assessment of complex lipids for both FF (100 µl) and serum (20 µl) samples were assayed by charged-surface hybrid column-electrospray ionization quadrupole time-of-flight tandem mass spectrometry (CSH-ESI QTOF MS/MS) with previously established methods for both positively and negatively charged modes (8). Both serum and FF samples were extracted according to the Matyash protocol (8). Mass spectrometry was conducted on both negatively charged ions (free fatty acids and phosphatidylinositols) with QTOF MS (Agilent 6550, resolution 20,000) and positively charged ions [phosphatidylcholine (PC), lysophosphatidylcholine (LPC), phosphatidylserine, and phosphatidylethanolamine (PE)] with QTOF MS (Agilent 6530, resolution 10,000). Peaks were then processed and identified with MS1 and MS2 information in MS Dial and the Fiehn laboratory LipidBlast spectral library (8). Peak alignment was conducted in MassProfilerProfessional. Data are quantifier ion peak heights and normalized to the sum of all peak heights for all detected metabolites for each sample. Metabolites with known chemical classes were annotated with their lipid species followed by their carbon chain number and level of saturation [e.g., TG (49:1)] Unknown metabolites were identified with a LipidBlast (LB) prefix followed by their retention time and mass-to-charge ratio (e.g., LB10.56_932.86).
Quantitative assessment of indole-3-propionic acid by ultrahigh-performance liquid chromatography-high-resolution accurate mass analysis for both serum and follicular fluid.
Indole-3-propionic acid (IPA) and lorazepam compounds at stock concentrations of 1 mg/ml were prepared in ethanol and methanol, respectively (Sigma-Aldrich, St. Louis, MO). Working IPA calibration standards (0–1,000 ng/ml) were diluted in acetonitrile-water (50:50) with 0.1% formic acid and spiked with lorazepam (10 ng/ml) as an internal standard. Methanol (400 µl) was added to FF or serum (100 µl) and then vortexed 20 s. Samples were allowed to precipitate overnight (4°C) and then centrifuged for 10 min at 10,000 g and 4°C. Supernatant was collected and dried down under a nitrogen stream. Extracts were reconstituted in 100 µl of acetonitrile-water (50:50) with 0.1% formic acid spiked with internal standard.
Chromatographic separation was performed on an UltiMate 3000 ultrahigh-performance liquid chromatography (UHPLC) system (Thermo Scientific; Sunnyvale, CA) fitted with an XSelect CSH C18 XP column (100 × 2.1 mm, 2.5 μm; Waters, Milford, MA) kept at 49°C. A flow rate of 0.4 ml/min and an injection volume of 5 µl were used. Mobile phases consisted of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B) with an elution gradient as follows: 0–1% B from 0 to 2 min; 1–20% B from 2 to 6.5 min; 20–95% B from 6.5 to 11.5 min; 95–99% B from 11.5 to 13.5 min; 99–1% B from 13.5 to 16.5 min; hold at 1% B from 16.5 to 20 min; 1–0% B from 20 to 21 min; hold at 0% B from 21 to 22 min. Identification was carried out on a Q Exactive high-resolution accurate mass (HRAM) spectrometer (Thermo Scientific) with data acquisition and analysis performed with Xcalibur 4.0 and TraceFinder 3.3 software. Data were acquired by positive ESI targeted-single ion monitoring scan mode. Nitrogen as sheath, auxiliary, and sweep gas was set at 50, 13, and 3 units, respectively. Spray voltage, capillary temperature, and source heater were 3.5 kV, 320°C, and 425°C, respectively. targeted-single ion monitoring conditions included resolution 35,000 FWHM; automatic gain control target 5e4 ions; maximum injection time 100 ms; targeted mass 190.08626 m/z. Mass accuracy within 5 ppm plus a retention time within 30 s of working calibration standards were used for peak confirmation. Peak area measurements normalized to the internal standard were used to quantitate IPA. The calibration curve showed linearity, with r2 = 0.9999. The limit of detection and quantitation were 0.25 and 1.37 ng/ml, respectively. Spiked serum samples had an average recovery of 99.4% of expected concentration.
Statistical analyses.
Group differences for clinical variables were assessed by Mann-Whitney nonparametric U-test with GraphPad Prism v. 7.3 (La Jolla, CA). All other statistical analyses were conducted in the R statistical language (v. 3.3.2). Partial least squares-discriminant analysis (PLS-DA) was used to identify metabolites discriminating OW and NW women. Before modeling, data were screened for missing data and were removed if >3 measurements (>17.6% of samples within a metabolite) were missing. No metabolites from the primary assay met this criterion, whereas 17 and 42 complex lipids were removed from FF and serum data, respectively. Metabolites were then screened for misaligned or artificial peaks (outliers) with an iterative Grubbs’ test for outliers at α < 0.001. Detected outliers were removed, and all missing data were imputed with the k-nearest neighbors algorithm (54). Imputed data represented the following data used in multivariate analyses: 0.18% and 0.25% in complex lipid data for FF and serum, respectively; 0.19% and 0.22% in primary metabolism data for FF and serum, respectively. Metabolomics data were log transformed and scaled to unit variance before PLS-DA modeling. Separate PLS-DA models were fit with metabolites within a fluid and platform (e.g., serum complex lipid data) with the mvr() function from the pls package (37). Performance of PLS-DA models, i.e., validation, was assessed with sixfold cross-validation (CV) of true classification rates of out-of-bag samples combined with permutation. A total of 100 permutations were run to assess whether the mean CV results were greater than the distribution of permuted results. Discriminant metabolites in the PLS-DA models were identified by an iterative backward selection strategy using variable importance in projection (VIP) calculations. Initially, a VIP was calculated from PLS-DA models for each metabolite, and metabolites with a VIP calculation ≥ 1 were selected for the selection strategy. A cutoff of VIP > 1 has previously been noted as an accepted threshold to identify important metabolites contributing to PLS-DA classifier separation (34). Metabolites with a VIP ≥ 1 were ranked in order, a PLS-DA model was then fit with these metabolites, and then the classification accuracy of sixfold CV was assessed. This process was repeated but with the highest remaining ranked metabolite removed at each iteration. Metabolites were selected as important discriminant features if they were dropped from the iteration before the last iteration that resulted in 100% CV accuracy. All metabolites meeting this criteria were fit into a final PLS-DA model and assessed by CV and permutation. All final models had at least 95% of held-out samples correctly by sixfold CV and <0.05% of permutations below their respective cross-validated statistics. Models were fit with FF and serum data from primary metabolism and complex lipids assays separately, but each followed the same workflow. Discriminant metabolites selected in the iterative backward selection strategy were correlated to clinical variables (Spearmen’s correlations; α ≤ 0.01) and presented as a correlation network (Cytoscape v3.2.1). Group comparisons in metabolomics data were assessed with Mann-Whitney U-tests and adjusted for multiple comparisons with methods described by Benjamini and Hochberg (3).
RESULTS
Participant characteristics.
Women in this study did not differ in age between groups (Table 1). Fertility diagnosis of participants included 47% male factor, 24% tubal factor, 12% ovulatory disorder, 5% endometriosis, and 12% unexplained. There were no differences in pregnancy rates or live births between groups. By design, OW women had higher BMI and BF percentages compared with NW women (P ≤ 0.05; Table 1). As anticipated, OW women also consumed more total calories on average than NW women. Their diets were also composed of significantly more calories from total fat, fewer calories from carbohydrates, and similar calories from protein compared with NW women (Table 2).
Table 1.
Participant characteristics
| NW (BMI 18.5–24.9 kg/m2) (n = 9) | OW (BMI ≥25 kg/m2) (n = 8) | P Value | |
|---|---|---|---|
| Age, yr | 31.4 ± 1.2 | 30.3 ± 1.8 | 0.744 |
| Pregnancy, N | 4 | 5 | 0.637 |
| Live births, N | 4 | 4 | 0.999 |
| Fertility diagnosis, N | |||
| Male factor | 5 | 3 | |
| Ovulatory disorder | 1 | 1 | |
| Tubal factor | 1 | 3 | |
| Endometriosis | 0 | 1 | |
| Unexplained | 2 | 0 | |
| BMI, kg/m2 | 21.5 ± 0.7 | 34.8 ± 1.2 | <0.001 |
| Body fat, % | 28.9 ± 3.2 | 47.0 ± 1.7 | 0.001 |
Data are expressed as means ± SE for continuous data from n women. Differences between groups were determined by Mann-Whitney nonparametric tests with a statistical significance level of P ≤ 0.05. Categorical data are summarized as counts, and comparisons between normal-weight (NW) and overweight/obese (OW) women were performed with Fisher’s exact test. BMI, body mass index.
Table 2.
Dietary intake calculated from 3-day food records with NDSR
| NW Women (n = 7) | OW Women (n = 6) | P Value | |
|---|---|---|---|
| Total energy intake, kcal | 1,313 ± 94.8 | 2,453 ± 149.9 | 0.001 |
| Carbohydrates, g | 183.2 ± 14.8 | 290.1 ± 20.0 | 0.004 |
| Carbohydrates, % kcal | 54.5 ± 1.6 | 47.02 ± 2.0 | 0.008 |
| Protein, g | 51.6 ± 3.9 | 96.4 ± 11.1 | 0.004 |
| Protein, % kcal | 16.4 ± 2.5 | 16.2 ± 1.9 | 0.946 |
| Fat, g | 44.4 ± 6.0 | 103.3 ± 10.3 | 0.001 |
| Fat, % kcal | 30.8 ± 1.8 | 36.8 ± 2.1 | 0.065 |
| Saturated fatty acid, % kcal | 10.2 ± 1.7 | 12.8 ± 1.3 | 0.180 |
| MUFA, % kcal | 9.9 ± 0.6 | 12.6 ± 0.7 | 0.061 |
| PUFA, % kcal | 6.8 ± 0.4 | 8.0 ± 0.9 | 0.234 |
| Cholesterol, mg | 152.9 ± 38.9 | 316.0 ± 49.2 | 0.035 |
Data are expressed as means ± SE for n women. Differences between groups were determined by Mann-Whitney nonparametric tests with a statistical significance level of P ≤ 0.05. MUFA, monounsaturated fatty acids; NDSR, Nutrient Data System for Research software; NW, normal weight; OW, overweight/obese; PUFA, polyunsaturated fatty acids.
Greater follicular fluid and serum leptin, inflammatory marker, and triglyceride content in OW women.
OW women had higher serum leptin levels and elevated serum inflammatory markers [CRP and chemokine (C-C motif) ligand 2] compared with NW women (Table 3). Additionally, OW women displayed a trending decrease in serum high-density lipoprotein and increase in serum TG levels (P = 0.09). There were no significant differences in other markers measured. FF leptin, TG, and CRP levels were significantly increased in OW compared with NW women (Table 3).
Table 3.
Serum and follicular fluid concentrations
| NW (BMI 18.5–24.9 kg/m2) (n = 9) | OW (BMI ≥25 kg/m2) (n = 8) | P Value | |
|---|---|---|---|
| Serum | |||
| Glucose, mg/dl | 108.1 ± 4.9 | 104.2 ± 6.4 | 0.627 |
| Insulin, ng/ml | 6.1 ± 1.2 | 8.2 ± 0.7 | 0.228 |
| HOMA-IR | 0.8 ± 0.2 | 1.1 ± 0.1 | 0.200 |
| Total cholesterol, mg/dl | 225.5 ± 9.0 | 223.4 ± 15.8 | 0.837 |
| LDL cholesterol, mg/dl | 74.4 ± 11.5 | 91.1 ± 9.6 | 0.365 |
| HDL cholesterol, mg/dl | 49.9 ± 3.1 | 41.53 ± 2.7 | 0.090 |
| Triglyceride, mg/dl | 58.7 ± 8.5 | 81.0 ± 7.1 | 0.091 |
| Leptin, ng/ml | 3.4 ± 0.6 | 33.0 ± 9.4 | 0.001 |
| TNF-α, pg/ml | 2.3 ± 0.3 | 3.1 ± 0.5 | 0.337 |
| CCL2, pg/ml | 259.8 ± 28.4 | 461.4 ± 61.6 | 0.011 |
| C-reactive protein, µg/ml | 2.7 ± 0.8 | 6.1 ± 1.2 | 0.040 |
| Follicular fluid | |||
| Leptin, ng/ml | 8.9 ± 3.2 | 32.0 ± 7.7 | 0.005 |
| Triglyceride, mg/dl | 9.1 ± 0.9 | 17.3 ± 3.4 | 0.029 |
| C-reactive protein, µg/ml | 2.2 ± 0.8 | 7.3 ± 2.2 | 0.030 |
Data are expressed as means ± SE for n women. Differences between groups were determined by Mann-Whitney nonparametric tests with a statistical significance level of P ≤ 0.05. BMI, body mass index; CCL2, chemokine (C-C motif) ligand; HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment-insulin resistance index; LDL, low-density lipoprotein; NW, normal weight; OW, overweight/obese.
PLS-DA showed good model performance and discrimination of samples and metabolites.
We fit PLS-DA models to discriminate metabolomics data using both BMI and BF classifications and only observed poor CV performance in complex lipid data derived from FF samples (Table 4; full model); however, CV performance improved after identification of a set of discriminant features with the backward selection strategy (Table 4; reduced model). Comparison of PLS-DA models fit with differing obesity classifiers (BMI vs. BF) showed CV accuracy rates > 94% across all reduced models, providing evidence that all models were resistant to overfitting (Tables 4 and 5). Relative to the BMI classification, BF classification identified approximately three times as many featured metabolites in all models except models fit from the primary metabolism data derived from FF (Tables 4 and 5).
Table 4.
PLS-DA model prediction of overweight and normal weight women using untargeted metabolomics of complex lipids
| Full Model |
Reduced Model |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fluid | n | Metabolites* | LV† | CV Acc.‡ | P Value§ | Metabolites | LV | CV Acc. | P Value |
| BMI classifiers (NW vs. OW) | |||||||||
| Follicular fluid | 17 | 984 | 2 | 66.7% | 0.13 | 17 | 1 | 100% | <0.01 |
| Serum | 17 | 1,032 | 1 | 94.4% | <0.01 | 59 | 2 | 100% | <0.01 |
| % Body fat classifiers (NW vs. OW) | |||||||||
| Follicular fluid | 17 | 984 | 4 | 69.4% | 0.17 | 19 | 1 | 100% | <0.01 |
| Serum | 17 | 1,032 | 3 | 100% | <0.01 | 183 | 1 | 94.4% | <0.01 |
Samples assessed by charged-surface hybrid column-electrospray ionization quadrupole time of flight tandem mass spectrometry. Normal weight (NW): n = 9 and overweight (OW): n = 8 for both fluids. Acc, accuracy; CV, cross-validation; LV, latent variable; PLS-DA, partial least squares-discriminant analysis.
No. of metabolites used to fit PLS-DA model. Metabolites from negative and positive modes were included together in PLS-DA models.
No. of LVs needed to reach highest CV accuracy.
Mean out-of-bag classification accuracy of 6-fold CV.
Proportion of permuted (100 permutations) mean CV accuracy less than actual mean CV accuracy.
Table 5.
PLS-DA model performance for 2-class models fit with untargeted metabolomics of primary metabolism
| Full Model |
Reduced Model |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fluid | n | Metabolites* | LV† | CV Acc.‡ | P Value§ | Metabolites | LV | CV Acc. | P Value |
| BMI classifiers (NW vs. OW) | |||||||||
| Follicular fluid | 16 | 295 | 2 | 100% | <0.01 | 10 | 3 | 100% | <0.01 |
| Serum | 17 | 295 | 3 | 100% | <0.01 | 11 | 2 | 100% | <0.01 |
| % Body fat classifiers (NW vs. OW) | |||||||||
| Follicular fluid | 16 | 295 | 1 | 100% | <0.01 | 26 | 1 | 100% | <0.01 |
| Serum | 17 | 295 | 1 | 76.4% | 0.05 | 29 | 1 | 94.4% | <0.01 |
Samples assessed by gas chromatography-quadrupole time-of-flight mass spectrometry. Normal weight (NW) and overweight (OW): n = 8 for follicular fluid; NW: n = 9 and OW: n = 8 for serum. Acc, accuracy; CV, cross-validation; LV, latent variable; PLS-DA, partial least squares-discriminant analysis.
No. of metabolites used to fit PLS-DA model.
No. of LVs needed to reach highest CV accuracy.
Mean out-of-bag classification accuracy of 6-fold CV.
Proportion of permuted (100 permutations) mean CV accuracy less than actual mean CV accuracy.
PLS-DA score and loading plots, which graphically represent the model’s clustering and discrimination of samples and metabolites, are provided for primary metabolite (Fig. 1) and complex lipid (Fig. 2) data filtered by the backward selection strategy. All models show tight within-group clustering, regardless of classifying type (i.e., BMI or BF) or fluid type, and discriminate classifiers along latent variable 1.
Fig. 1.
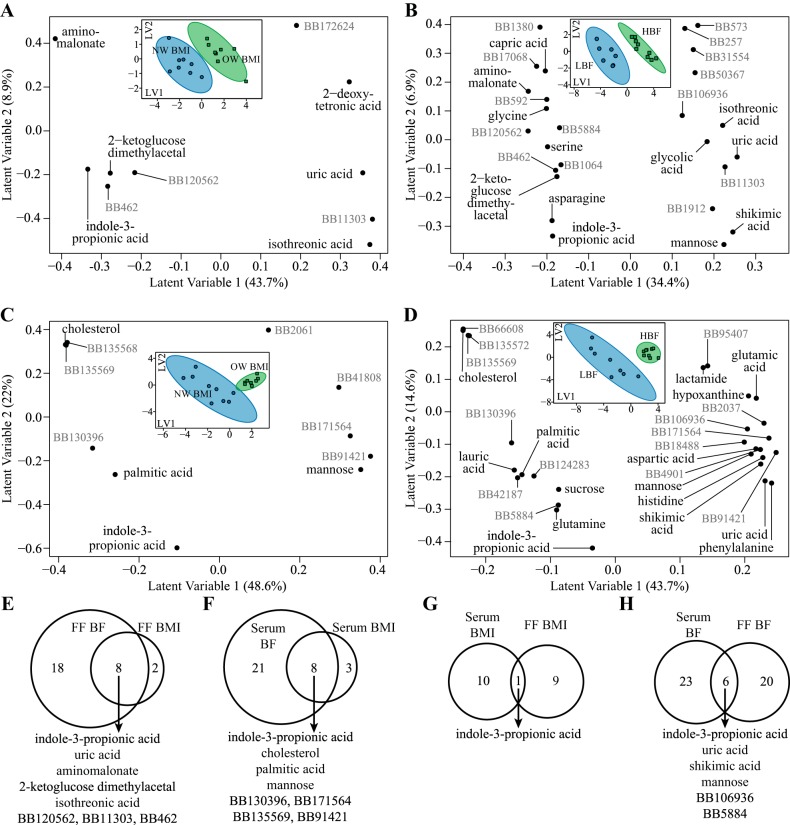
Untargeted metabolomics of primary metabolism discriminates obesity in follicular fluid (FF) and serum samples from 17 women undergoing in vitro fertilization. A and B: partial least squares-discriminant analysis (PLS-DA) loading plot of discriminant metabolites in FF using body mass index (BMI; A) and % body fat (BF; B) classifiers. C and D: PLS-DA loading plots of discriminant metabolites in serum using BMI (C) and BF (D) classifiers. The loading plots are composed of the first 2 latent variables (LV1, LV2) of each PLS-DA model, and each point represents a single metabolite. Known metabolites are labeled in black, and unknown metabolites are labeled in gray. Corresponding PLS-DA score plots are shown in insets in each loading plot (A–D). Each point in the PLS-DA score plot represents a single participant. Women classified as either normal-weight (NW) BMI and/or low body fat (LBF) are shown in blue (n = 8 or 9), whereas women classified as overweight (OW) BMI and/or high body fat (HBF) are shown in green (n = 8 or 9). Ellipses surrounding each class cluster are 95% confidence ellipses based on Hotelling’s T2 statistic. The score plots suggest good discrimination of groups due to lower variance among within-group scores combined with minimal overlap of confidence ellipses. The majority of variance explaining the binary classification is along LV1. Thus scores with positive values along LV1 (e.g., OW and/or HBF participants) have higher abundances of metabolites that also have positive loadings along LV1. Conversely, metabolites with negative loadings along LV1 are lower in participants with positive scores along LV1. E–H: Venn diagrams describe metabolites that intersect between PLS-DA models as follows: PLS-DA categorizing BF and BMI within FF (E), categorizing BF and BMI within serum (F), categorizing BMI in serum and FF (G), and categorizing BF in serum and FF (H). Intersecting metabolites found within each comparison are listed below each Venn diagram. BB, BinBase.
Fig. 2.
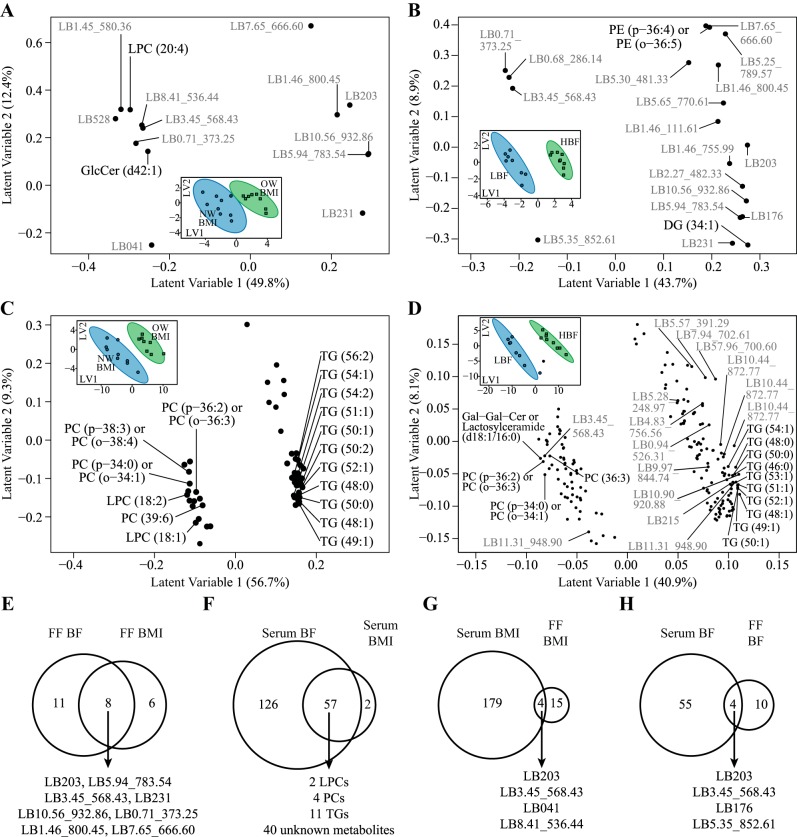
Untargeted metabolomics of complex lipids discriminates obesity in follicular fluid (FF) and serum samples from 17 women undergoing in vitro fertilization. A and B: partial least squares-discriminant analysis (PLS-DA) loading plots of discriminant metabolites in FF using body mass index (BMI; A) and % body fat (BF; B) classifiers. C and D: PLS-DA loading plots of discriminant metabolites in serum using BMI (C) and BF (D) classifiers. The loading plots are composed of the first 2 latent variables (LV1, LV2) of each PLS-DA model, and each point represents a single metabolite. Known metabolites are labeled in black, and unknown metabolites are labeled in gray. Corresponding PLS-DA score plots are shown in insets in each loading plot (A–D). Each point in the PLS-DA score plots represents a single participant. Women classified as either normal weight (NW) BMI and/or low body fat (LBF) are shown in blue (n = 8 or 9), whereas women classified as overweight (OW) BMI and/or high body fat (HBF) are shown in green (n = 8 or 9). Ellipses surrounding each class cluster are 95% confidence ellipses based on Hotelling’s T2 statistic. The score plots suggest good discrimination of groups due to lower variance among within-group scores combined with minimal overlap of confidence ellipses. The majority of variance explaining the binary classification is along LV1. Thus scores with positive values along LV1 (e.g., OW and/or HBF participants) have higher abundances of metabolites that also have positive loadings along LV1. Conversely, metabolites with negative loadings along LV1 are lower in participants with positive scores along LV1. E–H: Venn diagrams describe metabolites that intersect between PLS-DA models as follows: PLS-DA categorizing BF and BMI within FF (E), categorizing BF and BMI within serum (F), categorizing BMI in serum and FF (G), and categorizing BF in serum and FF (H). Intersecting metabolites found within each comparison are listed below each Venn diagram. Metabolites with an elucidated chemical class are labeled by their lipid species abbreviation followed by the carbon chain number and level of saturation [e.g., LPC (20:2)]. Metabolites without chemical class identification are considered unknown and prefixed with LB, followed by their retention time and mass-to-charge ratio (e.g., LB 5.94_783.54). DG, diacylglycerol; GlcCer, glucosylceramide; LPC, lysophosphatidylcholine; PC, phosphatidylcholine; PE, phosphatidylethanolamines; PG, phosphatidylglycerol; TG, triglyceride.
Follicular fluid abundances of metabolites related to oxidative stress/antioxidant capacity, amino acid biosynthesis, and lipid metabolism were associated with obesity.
Strongest FF metabolite predictors of obesity (e.g., featured metabolites intersecting between BMI and BF models) from the primary metabolism assay included IPA, uric acid, aminomalonate, 2-ketoglucose dimethylacetal, isothreonic acid, and three unknowns (Fig. 1E, Fig. 3). With BMI classifiers, FF abundances of IPA, aminomalonate, 2-ketoglucose dimethylacetal, BB120562, and BB462 were lower in OB women relative to NW women, while isothreonic acid, uric acid, and BB11303 were greater in OB women. Only two metabolites were unique to BMI models in FF, 2-deoxytetronic acid and BB172624, which were both greater in OW women relative to NW women (Fig. 1A). Results specific to BF included elevated FF abundances of shikimic acid, mannose, and five unknowns and reduced FF abundances of serine, glycine, capric acid, asparagine, and five unknown metabolites in HBF compared with LBF women (Fig. 1B).
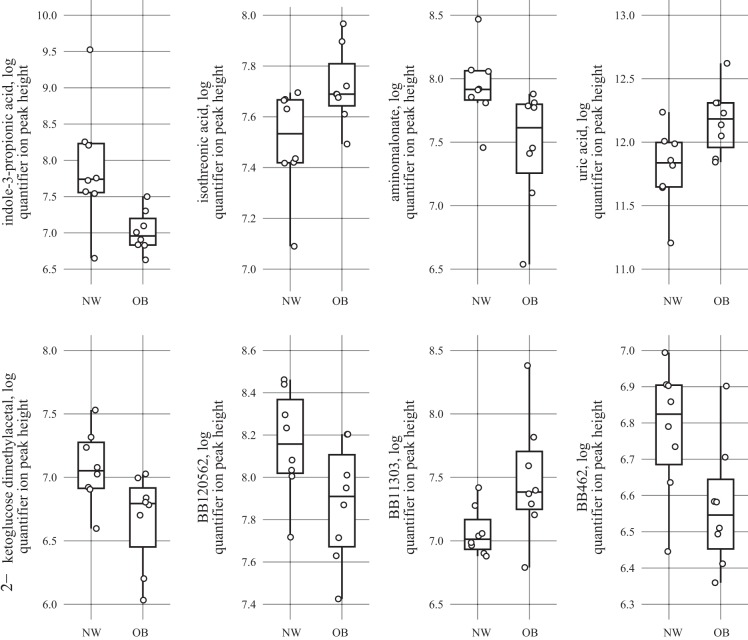
Fig. 3.
Mass spectrometry data of intersecting follicular fluid (FF) metabolites identified in both body fat and body mass index (BMI) analyses for primary metabolism are plotted. Log quantified ion peak height of FF metabolites identified by gas chromatography-time-of-flight mass spectrometry from normal-weight (NW) BMI women (n = 9) compared with overweight/obese (OB) BMI women (n = 8). Points in the plots represent individual women. Upper and lower hinges (box) represent data within the interquartile range and are values between the first and third quartiles, while the solid line represents the median value. All box plots were constructed with default settings in the R grDevices::boxplot.stats() function.
There were eight unknown complex lipids in FF that intersected between BMI and BF models (Fig. 2E), which consisted of elevated levels of LB 5.94_783.54, LB 203, LB231, LB 10.56_932.86, and LB 7.65_666.60 and reduced levels of LB 0.71_373.25 and LB3.45_568.43 in OW women (by BMI and BF) compared with NW women (Fig. 4). The majority of metabolites selected from models using either BMI or BF classifiers in FF were unknown metabolites; however, abundances of LPC (LPC 20:4) and glucosylceramide (GlcCer d42:1) were found to be lower in FF of OW women by BMI classifiers (Fig. 2A), whereas FF abundances of diacylglycerol (DG 34:1) and PE (PE p-36:4 or o-36:5) were increased in OW women by BF classifiers (Fig. 2B).
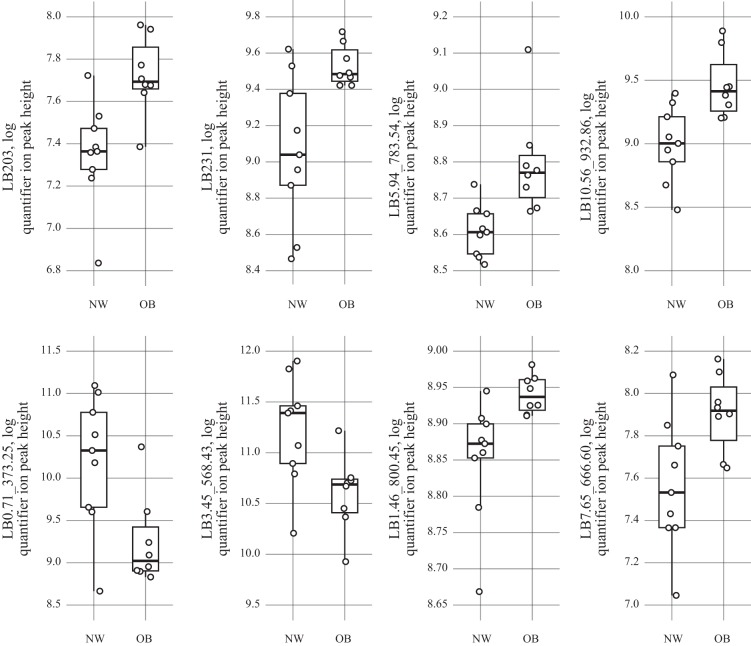
Fig. 4.
Mass spectrometry data of intersecting follicular fluid (FF) metabolites identified in both body fat and body mass index (BMI) analyses for complex lipids are plotted. Log quantified ion peak height of FF metabolites identified by gas chromatography-time-of-flight mass spectrometry from normal-weight (NW) BMI (n = 9) women compared with overweight/obese (OB) BMI women (n = 8). Points in the plots represent individual women. Upper and lower hinges (box) represent data within the interquartile range and are values between the first and third quartiles, while the solid line represents the median value. All box plots were constructed with default settings in the R grDevices::boxplot.stats() function.
Changes in the serum metabolome identified by both BMI and BF models.
Serum metabolites selected from the primary metabolism assay by BMI and BF classifiers are presented in Fig. 1, C and D. Similar to observations in FF, serum abundance of IPA was selected in both models and was lower in OW women relative to NW women (Fig. 1F). Cholesterol, palmitic acid, BB130396, and BB135569 also intersected between BMI and BF models and were also lower in OW women relative to NW women, whereas mannose, BB171564, and BB91421 intersected between models and were greater in OW women relative to NW women.
The total of serum metabolites discriminating OW and NW women was substantially greater in the complex lipid analysis relative to all other fluids and metabolomics outputs (Fig. 2, C and D). There were 57 metabolites that intersected between BMI and BF models, consisting of LPCs, PCs, TG, and unknown lipid species (Fig. 2F). In general, a pattern emerged in both models where LPCs and PCs were lower in OW women relative to NW women and TGs were higher in OW women relative to NW women.
Serum IPA concentrations correlate with FF IPA concentrations.
We then compared metabolites selected in FF models to those identified in serum models to identify potential serum biomarkers of FF metabolism. Only IPA intersected between FF and serum models using BMI classifiers (Fig. 1G), whereas IPA, uric acid, shikimic acid, mannose, and two unknown metabolites intersected between FF and serum models using BF classifiers (Fig. 1H). Four unknown complex lipid metabolites intersected between FF and serum models using BMI classifiers, including LB203, LB041, LB3.45_56.43, and LB8.41_536.44 (Fig. 2G). LB203, LB3.45_56.43, LB5.35_852.61, and LB176 also intersected between FF and serum models using BF classifiers (Fig. 2H).
We focused further validation efforts on IPA since it was the only metabolite that was identified across fluids and obesity classifications. We employed liquid chromatography-mass spectrometry to quantitatively measure IPA and observed significantly lower concentrations of IPA in both biological fluids in OW compared with NW women (Fig. 5A), confirming the semiquantitative data generated in the untargeted metabolomics data. There was a positive correlation between IPA concentration levels and peak ion height data (FF: R = 0.78, P = 0.001; serum: R = 0.75, P = 0.0008) in addition to a positive correlation between serum and FF levels of IPA (R = 0.95, P < 0.0001; Fig. 5B).
Fig. 5.
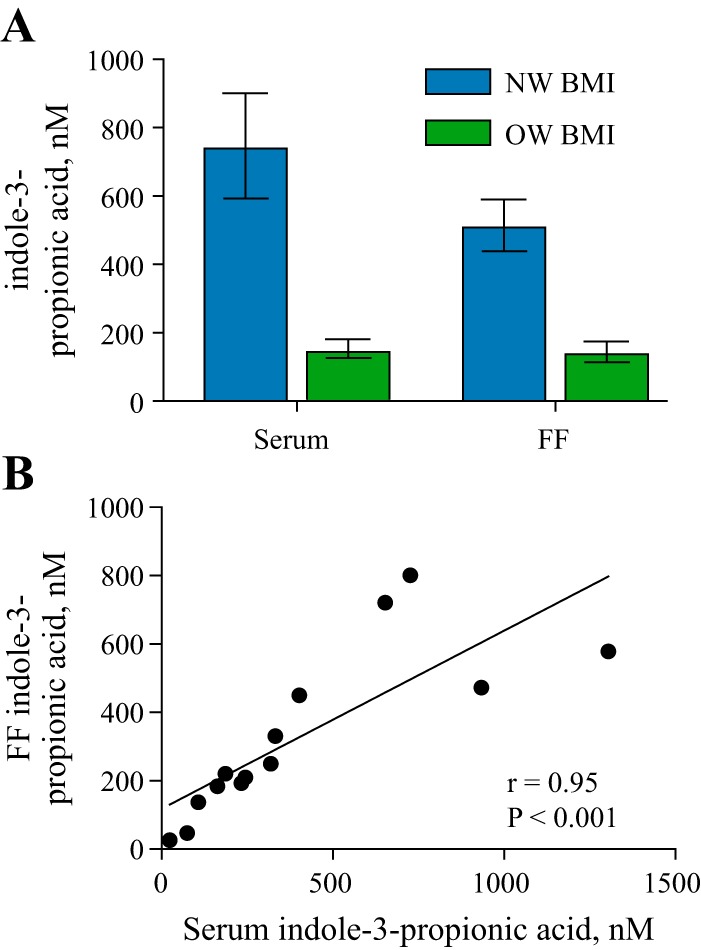
Serum indole-3-propionic acid (IPA) concentrations correlate with follicular fluid (FF) IPA concentrations. A: concentrations of IPA were measured by ultrahigh-performance liquid chromatography-high-resolution accurate mass analysis for both serum [normal weight (NW) = 9, overweight/obese (OW) = 8] and FF (NW = 7, OW = 7). Data are represented as means ± SE. Significant differences between groups was set at P < 0.05 by Mann-Whitney nonparametric tests. B: correlation analysis of serum peak ion height and serum IPA concentration using Spearman coefficients.
Associations between FF and serum metabolite levels and clinical parameters.
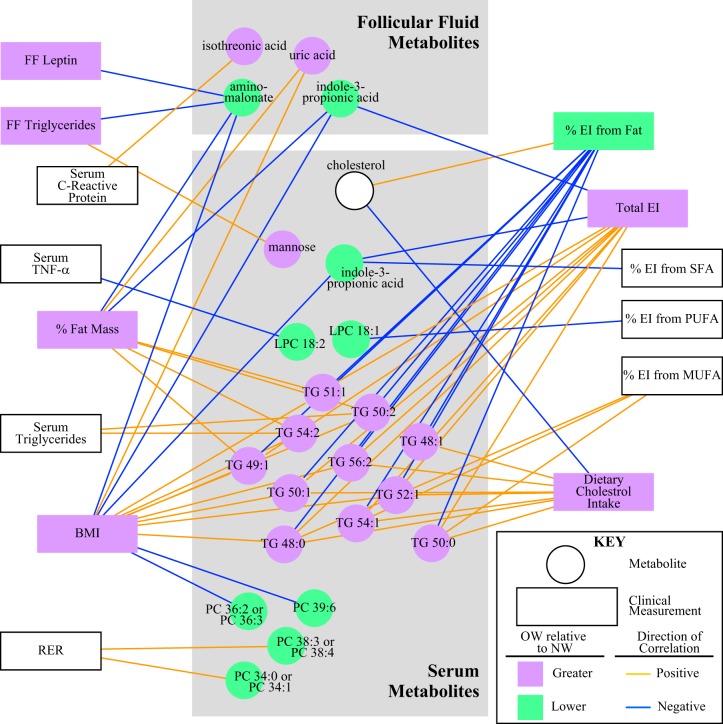
To better understand the relationships between clinical parameters, serum and FF analytes, and metabolomics results (ion abundance), a metabolic network was generated with metabolites identified from both BMI and BF analyses (Fig. 6). Variables presented in Fig. 6 are only shown if they share a significant correlation after false discovery rate correction. In line with the BMI modeling analyses, serum and FF IPA abundances were negatively correlated with the continuous BMI data and total energy intake. FF IPA was also negatively correlated with BF, whereas serum IPA negatively correlated with percent energy intake from saturated fatty acids, BMI, and total energy intake. A few FF metabolites were associated with other clinical markers. FF isothreonic acid (an oxidized metabolite of ascorbic acid) levels were positively associated with serum CRP levels. FF aminomalonate (a marker of oxidative stress) levels were negatively associated with FF leptin, FF triglycerides, BF, and BMI. Finally, FF uric acid (a product of the metabolic breakdown of purine nucleotides) was positively associated with BMI and total body fat mass.
Fig. 6.
Network analysis display of metabolites that were identified by partial least squares-discriminant analysis modeling within both body mass index (BMI) and body fat (BF) analyses for both serum and follicular fluid separated into 2 gray boxes. Square nodes depict different clinical variables, and circular nodes are featured metabolites. Nodes in green indicate that metabolites or clinical variables are significantly lower in overweight/obese (OW) relative to normal weight (NW) women, and purple indicates significantly higher in OW relative to NW women. This analysis correlated all the metabolites and clinical variables with Spearman’s correlations filtered by P < 0.01. The directions of the correlations are depicted in yellow to show positive correlations and in blue to show negative correlations between metabolites and clinical variables. Only complete data were used for this analyses, so any sample with missing data was dropped from this analysis, resulting in disparate outcomes for serum C-reactive protein [i.e., serum C-reactive protein was statistically significant when comparing BMI in all women (Table 3) but was not statistically significant in the correlation network]. EI, energy intake; LPC, lysophosphatidylcholine; MUFA, monounsaturated fatty acid; PC, phosphatidylcholine; PUFA, polyunsaturated fatty acids; SFA, saturated fat; TG, triglyceride; RER, respiratory exchange ratio.
DISCUSSION
The results of this study demonstrate for the first time that excess weight and/or adiposity influence the metabolic signature of human FF as profiled by untargeted metabolomics. Perhaps most novel, we identified a potential circulating biomarker, IPA, that reflects FF differences between otherwise healthy OW and NW women undergoing fertility treatments. Although the functional relevance of IPA to oocyte development is currently unknown, one intriguing possibility is that the differences in IPA concentrations in the blood and in FF reflect changes in the production or disposition of the gut microbial metabolites between NW and OW women. We also identified several metabolites that discriminated FF between OW and NW women, including uric acid, aminomalonate, 2-ketoglucose dimethylacetal, isothreonic acid, and several currently unknown metabolites. As FF content reflects the follicle’s metabolism as well as molecules and metabolites needed for the bidirectional somatic cell-oocyte signaling (13, 61), our findings suggest that obesity is influencing the microenvironment that controls folliculogenesis, meiotic maturation, and spindle formation in the developing oocyte (46). Other studies have utilized metabolomics to investigate fertility success and pregnancy outcomes (40, 61), but only a few have investigated the effect of obesity on the FF metabolome (5, 52, 71).
In this study, we choose to classify women by BMI for two reasons: 1) feasibility within the clinic to classify/recruit participants and 2) consistency among our previous publications investigating oocyte gene expression in this cohort (50). Although BMI is widely used, it is derived from total weight and does not account for total adiposity. Therefore, we additionally assessed the untargeted metabolomics data using BF cutoffs to identify metabolites that may be more representative of fat accumulation. Our analyses identified several discriminant metabolites in the FF of OW compared with NW women when using both BMI and BF cutoffs. To our knowledge this is one of the first studies to show aminomalonate, 2-ketoglucose dimethylacetal, and IPA to be decreased whereas levels of isothreonic acid were increased in FF of OW women.
A major strength of this study was that we paired FF samples with serum samples to determine potential circulating biomarkers of FF metabolism. We identified and validated IPA, which is a bacterial derivative of tryptophan, in both fluids. After the deamination of tryptophan, Clostridium sporogenes can further metabolize tryptophan first into indole and then into IPA (11). IPA is present in human serum (68) and is known for its free radical scavenger and antioxidant properties (22). Animal studies have shown that IPA can lead to a reduction in markers of lipid peroxidation and DNA damage (22, 45). It also possesses antioxidant activity (45), which intersects with our recent findings of increased oocyte expression of proinflammatory genes and elevated proinflammatory markers in FF of OW compared with NW women (50). Recently, higher serum IPA was associated with reduced development of type 2 diabetes and was negatively correlated with serum CRP in the Finnish Diabetes Prevention Study (10, 48). We did not find the same significant correlation between FF or serum IPA and CRP; however, FF and serum IPA levels were lower in OW women and negatively associated with BMI, fat mass, and total energy intake in our network analysis. IPA supplemented in the diet of rats was shown to improve serum homeostatic model assessment-insulin resistance index (HOMA-IR), glucose, and insulin levels compared with control-fed animals (1). This could suggest a possible alternative mechanism for IPA, in addition to its role in the gut microbiome, as we see higher serum levels for insulin and HOMA-IR, although not significant, and reduced levels of IPA in OW compared with NW women in this study. Finally, synthetic derivatives of IPA can act as agonists to sphingosine-1-phosphate receptors, which are known to be important for oocyte maturation (48). Previous research in both animal models and clinical studies has shown that obesity can alter bacterial species within the gut microbiome compared with normal-weight controls (28, 29, 56). Taking this into account, we hypothesize that obesity modulates bacteria species to profiles that will decrease the production of IPA. Alternatively, it is possible that obesity’s systematic inflammation may lead to intestinal inflammation, which would modulate the gut microbiome, resulting in reduced levels of IPA. Future studies in germ-free models will be required to confirm this hypothesis and also determine FF IPA’s role in oocyte maturation and development.
Alterations to abundances of aminomalonate, 2-ketoglucose dimethylacetal, IPA, and isothreonic acid in FF of OW women point to higher levels of oxidative stress and decreased antioxidant capacity in FF of OW compared with NW women, which are in line with our previous results showing changes in oocyte gene expression related to oxidative stress (50). Aminomalonate, an amino dicarboxylic acid and a potential marker of oxidative stress (51), was significantly decreased in FF of OW compared with NW women and negatively associated with BMI and FF leptin levels. Aminomalonate is not well characterized but has been negatively associated with insulin resistance and cardiovascular disease risks in metabolomics studies (44, 51). The abundance of two antioxidant capacity markers, 2-ketoglucose dimethylacetal and isothreonic acid, were also differentially present in FF of OW compared with NW women. 2-Ketoglucose dimethylacetal, a marker of reduced antioxidant capacity, was decreased in OW compared with NW women (24, 41). 2-Ketoglucose dimethylacetal is involved in ascorbic acid metabolism, although the fact that humans are deficient for gulonolactone oxidase, which is the key enzyme for biosynthesis of ascorbic acid, suggests that this metabolite would likely be formed in the gut (39). This further reinforces our findings that the gut microbiome may play a significant role in regulating metabolite levels in FF. Levels of isothreonic acid, an oxidized metabolite of ascorbic acid, increase in conjunction with oxidative stress as previously demonstrated in the brain of mice (2). In our study, FF isothreonic acid levels were increased and positively associated with body fat mass percentages and serum CRP levels. No other studies have identified a distinct role of aminomalonate, 2-ketoglucose dimethylacetal, or isothreonic acid in oocyte biology or reproduction; however, it is recognized that ascorbic acid is beneficial for oocyte developmental competence and embryo quality (25) whereas increased levels of oxidative stress can be harmful to oocyte growth and development (13). Uric acid, antioxidant and scavenger of free radicals, was elevated in FF of OW women in this study. In both clinical studies and animal models, elevated uric acid has been associated with obesity, insulin resistance, abnormal glucose metabolism, and cardiovascular disease (17, 33, 69). Uric acid has previously been identified in FF and was positively correlated with BMI and associated with cumulus-oocyte degradation in water buffalo (5, 9, 17). Taken together, these results suggest that ovarian follicles from OW women have increased oxidative stress and reduced antioxidant capacity compared with those from NW women. This synergizes with our previous publication that found modulation of expression levels of genes related to oxidative stress in oocytes from overweight women compared with normal women (50). Additional studies are needed to further evaluate these hypotheses, such as measuring lipid peroxidation or glutathione redox ratio in the FF to confirm whether there is elevated oxidative stress in OW women.
Untargeted assessment of complex lipids further identified eight unknown complex lipids between BMI and %BF analyses. Within our BMI analyses two annotated complex lipids, GlcCer 42:1 and LPC 20:4, were identified to be lower in FF of OW compared with NW women. GlcCer is the precursor to glycosphingolipid production, and when upregulated it offers cellular protection and promotes proliferation (36). In response to high-fat feeding or diabetes, total GlcCer levels increase in plasma and liver tissues (7, 70). These results are not in line with our findings. This may be due to specificity of the GlcCer: we detected a difference in GlcCer 42:1 levels, which may have different properties/functions compared with total GlcCer. LPCs are produced from PC, considered one of the major lipid species in human serum, and thought to be linked to inflammation (19, 20). Similar to our results in the FF, LPC levels were decreased in serum of OW compared with NW subject (20) and have previously been measured in FF and metaphase II oocytes (27, 38). Interestingly, serum LPC 18:2 level negatively correlated with serum tumor necrosis factor-α levels, suggesting a potential association of LPC serum levels with systemic inflammation.
We further identified two other annotated complex lipids when analyzing by BF, DG (DG 34:1) and PE (PE p-36:4). DGs are the building blocks for glycerol(phospho)lipids, and triglycerides are one of the precursors (15). DG concentration has been shown to be increased with obesity in both animal models and clinical studies (23, 55, 62, 63). PE is considered an ether-linked phospholipid and known to have antioxidant properties (12, 16, 60). Ether-linked PE has also been shown to be elevated in the plasma of morbidly obese versus control patients, showing results similar to the increase we found within the FF of women with elevated BF. In a mouse model of aging or H2O2 exposures both DG (34:1) and PE (p-36:4) were shown to have increased levels in metaphase II oocytes compared with controls (54), which could suggest possible similar effects in oocytes exposed to an obese environment. In combination, these data demonstrate that obesity leads to changes in some complex lipids within the FF, which may have a partial role in oocyte developmental potential. These data support current research that has shown lipid metabolism to be important for oocyte maturation and development; however, excess levels above normal in oocytes and the follicular microenvironment can have detrimental effects on the oocyte. In both mouse and bovine models, elevated levels of fatty acids added to FF impacted the development of the oocyte through alterations in metabolism, endoplasmic reticulum stress, mitochondrial function, and nuclear maturation (14, 59, 65), thus suggesting that these changes in complex lipids within FF could have negative effects on the health of the oocytes from OW compared with NW women.
Unique to this study was the identification of two novel metabolites that could be potential serum microbial derived biomarkers of FF metabolism: IPA, identified by both BMI and BF as discussed above and shikimic acid identified by BF only. Similar to IPA, shikimic acid must be hydrolyzed by intestinal enzymes in the gut (42) and has no known role in the oocyte, but a derivate of shikimic acid, 3,4-oxo-isopropylidene-shikimic acid, has been shown to have anti-inflammatory effects (66). Another interesting observation is that shikimic acid, which is part of the shikimate pathway, divides and gives rise to tryptophan and further indole derivatives, such as IPA (4). IPA production has also been suggested to be dependent on the colonization of bacterium C. sporogenes within the gut microbiome and may influence metabolism (64). These findings warrant further investigation to determine whether obesity-induced effects on the gut microbiome are not only influencing the gut bacteria but further leading to alternations within the FF. It also important to note that although these metabolites are typically found within the gut, they could also be derived from other origins, such as bacteria within the vagina, so further studies would be needed to determine the origins of these bacterium-derived metabolites.
This study is not without limitations. Participants in this study were hormonally stimulated before their oocyte retrieval and had documented infertility. However, both groups (NW and OW) were exposed to the same procedures, so our results are likely to reflect differences in adiposity. There was also no significant difference in gonadotropin treatment amount between NW and OW women for FSH (NW: 2,424 ± 314.8 vs. OW: 2,276 ± 256 mIU/ml) or estradiol (NW: 2,271 ± 327.3 vs. OW: 2,629 ± 414.7 pg/ml) received during the fertility procedures. It is also important to note that we collected pooled FF from multiple follicles, as it is the standardized procedure of the fertility clinic. Another limitation is the sample volume of FF that was obtained from each participant, which limited our ability to validate multiple metabolites. Future studies are needed to confirm the antioxidant properties of IPA in the FF of overweight women. Although our study population was well phenotyped and considered healthy, results from this study are derived from a limited sample size. Results from these analyses identified potential pathways that may be ameliorated by lifestyle interventions and improved gut health, which is a novel finding. Finally, untargeted metabolomic profiling is powerful and a reliable tool that has enhanced our understanding of the metabolites that surround the oocyte, but the resulting data are semiquantitative and should be approached as hypothesis generating. Moreover, with a small sample size we were still able to validate our untargeted metabolomics data by validating the levels of IPA concentrations in both the serum and the FF.
In conclusion, this untargeted metabolomics approach is one of the first to identify obesity-associated differences in the human FF metabolome related to oxidative stress/antioxidant capacity, metabolism, and xenometabolism/amino acid biosynthesis. A novel finding is the presence in both serum and FF of IPA, which has been suggested to have antioxidant properties in other fluids and tissues and was highly correlated in both fluids, suggesting either a potential biomarker of FF antioxidant capacity in serum samples or a reflection of bacterial metabolism interactions with the follicular environment. In combination with previous findings of differential oocyte gene expression related to oxidative stress, inflammation, and lipid metabolism in NW versus OW women from this cohort, these results suggest that obesity significantly affects the follicular environment during the preconception period, a window of time that may be important for lifestyle interventions.
GRANTS
This work was funded by Arkansas Children’s Hospital Research Institute CUMG 035716 (M. L. Ruebel), US Department of Agriculture Agricultural Research Service Project 6026-51000-010-05S (A. Andres), and National Institutes of Health (NIH) Grants TRI 1RR-029884 (A. Andres) and 1P20 GM-121293 (B. D. Piccolo). M. L. Ruebel was also partially supported by NIH Grant T32 HD-087166.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.A. conceived and designed research; M.L.R., K.E.M., and L.P. performed experiments; M.L.R. and B.D.P. analyzed data; M.L.R., B.D.P., and K.S. interpreted results of experiments; M.L.R. and B.D.P. prepared figures; M.L.R. drafted manuscript; M.L.R., B.D.P., K.E.M., D.M., K.S., and A.A. edited and revised manuscript; M.L.R., B.D.P., K.E.M., L.P., D.M., K.S., and A.A. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Oliver Fiehn and the West Coast Metabolomics Center at the University of California-Davis for support of the untargeted metabolomics and lipidomics analysis. We also thank the staff of Arkansas Fertility and Gynecology Associates for support with subject recruitment and sample collection.
REFERENCES
- 1.Abildgaard A, Elfving B, Hokland M, Wegener G, Lund S. The microbial metabolite indole-3-propionic acid improves glucose metabolism in rats, but does not affect behaviour. Arch Physiol Biochem 124: 306–312, 2018. doi: 10.1080/13813455.2017.1398262. [DOI] [PubMed] [Google Scholar]
- 2.Arun P, Rittase WB, Wilder DM, Wang Y, Gist ID, Long JB. Defective methionine metabolism in the brain after repeated blast exposures might contribute to increased oxidative stress. Neurochem Int 112: 234–238, 2018. doi: 10.1016/j.neuint.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 3.Benjamini Y, Hochberg H. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 57: 289–300, 1995. [Google Scholar]
- 4.Bochkov DV, Sysolyatin SV, Kalashnikov AI, Surmacheva IA. Shikimic acid: review of its analytical, isolation, and purification techniques from plant and microbial sources. J Chem Biol 5: 5–17, 2012. doi: 10.1007/s12154-011-0064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bou Nemer L, Shi H, Carr BR, Word RA, Bukulmez O. Effect of body weight on metabolic hormones and fatty acid metabolism in follicular fluid of women undergoing in vitro fertilization: a pilot study. Reprod Sci 2018: 1933719118776787, 2018. doi: 10.1177/1933719118776787. [DOI] [PubMed] [Google Scholar]
- 6.Broughton DE, Moley KH. Obesity and female infertility: potential mediators of obesity’s impact. Fertil Steril 107: 840–847, 2017. doi: 10.1016/j.fertnstert.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 7.Brozinick JT, Hawkins E, Hoang Bui H, Kuo MS, Tan B, Kievit P, Grove K. Plasma sphingolipids are biomarkers of metabolic syndrome in non-human primates maintained on a Western-style diet. Int J Obes 37: 1064–1070, 2013. doi: 10.1038/ijo.2012.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cajka T, Fiehn O. Comprehensive analysis of lipids in biological systems by liquid chromatography-mass spectrometry. Trends Analyt Chem 61: 192–206, 2014. doi: 10.1016/j.trac.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cassano E, Tosto L, Balestrieri M, Zicarelli L, Abrescia P. Antioxidant defense in the follicular fluid of water buffalo. Cell Physiol Biochem 9: 106–116, 1999. doi: 10.1159/000016307. [DOI] [PubMed] [Google Scholar]
- 10.de Mello VD, Paananen J, Lindström J, Lankinen MA, Shi L, Kuusisto J, Pihlajamäki J, Auriola S, Lehtonen M, Rolandsson O, Bergdahl IA, Nordin E, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Landberg R, Eriksson JG, Tuomilehto J, Hanhineva K, Uusitupa M. Indolepropionic acid and novel lipid metabolites are associated with a lower risk of type 2 diabetes in the Finnish Diabetes Prevention Study. Sci Rep 7: 46337, 2017. doi: 10.1038/srep46337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dodd D, Spitzer MH, Van Treuren W, Merrill BD, Hryckowian AJ, Higginbottom SK, Le A, Cowan TM, Nolan GP, Fischbach MA, Sonnenburg JL. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature 551: 648–652, 2017. doi: 10.1038/nature24661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donovan EL, Pettine SM, Hickey MS, Hamilton KL, Miller BF. Lipidomic analysis of human plasma reveals ether-linked lipids that are elevated in morbidly obese humans compared to lean. Diabetol Metab Syndr 5: 24, 2013. doi: 10.1186/1758-5996-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dumesic DA, Meldrum DR, Katz-Jaffe MG, Krisher RL, Schoolcraft WB. Oocyte environment: follicular fluid and cumulus cells are critical for oocyte health. Fertil Steril 103: 303–316, 2015. doi: 10.1016/j.fertnstert.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Dunning KR, Russell DL, Robker RL. Lipids and oocyte developmental competence: the role of fatty acids and β-oxidation. Reproduction 148: R15–R27, 2014. doi: 10.1530/REP-13-0251. [DOI] [PubMed] [Google Scholar]
- 15.Eichmann TO, Lass A. DAG tales: the multiple faces of diacylglycerol—stereochemistry, metabolism, and signaling. Cell Mol Life Sci 72: 3931–3952, 2015. doi: 10.1007/s00018-015-1982-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engelmann B, Bräutigam C, Thiery J. Plasmalogen phospholipids as potential protectors against lipid peroxidation of low density lipoproteins. Biochem Biophys Res Commun 204: 1235–1242, 1994. doi: 10.1006/bbrc.1994.2595. [DOI] [PubMed] [Google Scholar]
- 17.Fabbrini E, Serafini M, Colic Baric I, Hazen SL, Klein S. Effect of plasma uric acid on antioxidant capacity, oxidative stress, and insulin sensitivity in obese subjects. Diabetes 63: 976–981, 2014. doi: 10.2337/db13-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiehn O, Wohlgemuth G, Scholz M, Kind T, Lee DY, Lu Y, Moon S, Nikolau B. Quality control for plant metabolomics: reporting MSI-compliant studies. Plant J 53: 691–704, 2008. doi: 10.1111/j.1365-313X.2007.03387.x. [DOI] [PubMed] [Google Scholar]
- 19.Gwak GY, Yoon JH. Lysophosphatidylcholine. In: Encyclopedia of Cancer, edited by Schwab M. Berlin: Springer, 2011, p. 2127–2128. [Google Scholar]
- 20.Heimerl S, Fischer M, Baessler A, Liebisch G, Sigruener A, Wallner S, Schmitz G. Alterations of plasma lysophosphatidylcholine species in obesity and weight loss. PLoS One 9: e111348, 2014. doi: 10.1371/journal.pone.0111348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heitmann BL, Lissner L, Osler M. Do we eat less fat, or just report so? Int J Obes Relat Metab Disord 24: 435–442, 2000. doi: 10.1038/sj.ijo.0801176. [DOI] [PubMed] [Google Scholar]
- 22.Hwang IK, Yoo KY, Li H, Park OK, Lee CH, Choi JH, Jeong YG, Lee YL, Kim YM, Kwon YG, Won MH. Indole-3-propionic acid attenuates neuronal damage and oxidative stress in the ischemic hippocampus. J Neurosci Res 87: 2126–2137, 2009. doi: 10.1002/jnr.22030. [DOI] [PubMed] [Google Scholar]
- 23.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes 51: 2005–2011, 2002. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 24.Kanzler C, Haase PT, Kroh LW. Antioxidant capacity of 1-deoxy-d-erythro-hexo-2,3-diulose and d-arabino-hexo-2-ulose. J Agric Food Chem 62: 2837–2844, 2014. doi: 10.1021/jf404322r. [DOI] [PubMed] [Google Scholar]
- 25.Kere M, Siriboon C, Lo NW, Nguyen NT, Ju JC. Ascorbic acid improves the developmental competence of porcine oocytes after parthenogenetic activation and somatic cell nuclear transplantation. J Reprod Dev 59: 78–84, 2013. doi: 10.1262/jrd.2012-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lavie CJ, Milani RV, Ventura HO, Romero-Corral A. Body composition and heart failure prevalence and prognosis: getting to the fat of the matter in the “obesity paradox”. Mayo Clin Proc 85: 605–608, 2010. doi: 10.4065/mcp.2010.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lepage N, Miron P, Hemmings R, Roberts KD, Langlais J. Distribution of lysophospholipids and metabolism of platelet-activating factor in human follicular and peritoneal fluids. J Reprod Fertil 98: 349–356, 1993. doi: 10.1530/jrf.0.0980349. [DOI] [PubMed] [Google Scholar]
- 28.Ley RE. Obesity and the human microbiome. Curr Opin Gastroenterol 26: 5–11, 2010. doi: 10.1097/MOG.0b013e328333d751. [DOI] [PubMed] [Google Scholar]
- 29.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA 102: 11070–11075, 2005. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lissner L. Measuring food intake in studies of obesity. Public Health Nutr 5: 889–892, 2002. doi: 10.1079/PHN2002388. [DOI] [PubMed] [Google Scholar]
- 31.Livingstone MB, Black AE. Markers of the validity of reported energy intake. J Nutr 133, Suppl 3: 895S–920S, 2003. doi: 10.1093/jn/133.3.895S. [DOI] [PubMed] [Google Scholar]
- 32.Machtinger R, Combelles CM, Missmer SA, Correia KF, Fox JH, Racowsky C. The association between severe obesity and characteristics of failed fertilized oocytes. Hum Reprod 27: 3198–3207, 2012. doi: 10.1093/humrep/des308. [DOI] [PubMed] [Google Scholar]
- 33.Maiuolo J, Oppedisano F, Gratteri S, Muscoli C, Mollace V. Regulation of uric acid metabolism and excretion. Int J Cardiol 213: 8–14, 2016. doi: 10.1016/j.ijcard.2015.08.109. [DOI] [PubMed] [Google Scholar]
- 34.Mehmood T, Hovde KH, Snipen L, Saebo S. A review of variable selection methods in partial least squares regression. Chemometr Intell Lab Syst 118: 62–69, 2012. doi: 10.1016/j.chemolab.2012.07.010. [DOI] [Google Scholar]
- 35.Mendez MA, Popkin BM, Buckland G, Schroder H, Amiano P, Barricarte A, Huerta JM, Quirós JR, Sánchez MJ, González CA. Alternative methods of accounting for underreporting and overreporting when measuring dietary intake-obesity relations. Am J Epidemiol 173: 448–458, 2011. doi: 10.1093/aje/kwq380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Messner MC, Cabot MC. Glucosylceramide in humans. Adv Exp Med Biol 688: 156–164, 2010. doi: 10.1007/978-1-4419-6741-1_11. [DOI] [PubMed] [Google Scholar]
- 37.Mevik BH, Wehrens R, Hovde KL. pls: Partial Least Squares and Principal Component Regression. R package version 2.7–0, 2015. https://cran.r-project.org/web/packages/pls/index.html.
- 38.Mok HJ, Shin H, Lee JW, Lee GK, Suh CS, Kim KP, Lim HJ. Age-associated lipidome changes in metaphase II mouse oocytes. PLoS One 11: e0148577, 2016. doi: 10.1371/journal.pone.0148577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishikimi M, Yagi K. Molecular basis for the deficiency in humans of gulonolactone oxidase, a key enzyme for ascorbic acid biosynthesis. Am J Clin Nutr 54, Suppl: 1203S–1208S, 1991. doi: 10.1093/ajcn/54.6.1203s. [DOI] [PubMed] [Google Scholar]
- 40.O’Gorman A, Wallace M, Cottell E, Gibney MJ, McAuliffe FM, Wingfield M, Brennan L. Metabolic profiling of human follicular fluid identifies potential biomarkers of oocyte developmental competence. Reproduction 146: 389–395, 2013. doi: 10.1530/REP-13-0184. [DOI] [PubMed] [Google Scholar]
- 41.Pallanca JE, Smirnoff N. Ascorbic acid metabolism in pea seedlings. A comparison of d-glucosone, l-sorbosone, and l-galactono-1,4-lactone as ascorbate precursors. Plant Physiol 120: 453–462, 1999. doi: 10.1104/pp.120.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev 2: 270–278, 2009. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pandey S, Pandey S, Maheshwari A, Bhattacharya S. The impact of female obesity on the outcome of fertility treatment. J Hum Reprod Sci 3: 62–67, 2010. doi: 10.4103/0974-1208.69332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piccolo BD, Graham JL, Stanhope KL, Fiehn O, Havel PJ, Adams SH. Plasma amino acid and metabolite signatures tracking diabetes progression in the UCD-T2DM rat model. Am J Physiol Endocrinol Metab 310: E958–E969, 2016. doi: 10.1152/ajpendo.00052.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poeggeler B, Pappolla MA, Hardeland R, Rassoulpour A, Hodgkins PS, Guidetti P, Schwarcz R. Indole-3-propionate: a potent hydroxyl radical scavenger in rat brain. Brain Res 815: 382–388, 1999. doi: 10.1016/S0006-8993(98)01027-0. [DOI] [PubMed] [Google Scholar]
- 46.Revelli A, Delle Piane L, Casano S, Molinari E, Massobrio M, Rinaudo P. Follicular fluid content and oocyte quality: from single biochemical markers to metabolomics. Reprod Biol Endocrinol 7: 40, 2009. doi: 10.1186/1477-7827-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Romero-Corral A, Somers VK, Sierra-Johnson J, Korenfeld Y, Boarin S, Korinek J, Jensen MD, Parati G, Lopez-Jimenez F. Normal weight obesity: a risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur Heart J 31: 737–746, 2010. doi: 10.1093/eurheartj/ehp487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roth Z, Hansen PJ. Sphingosine 1-phosphate protects bovine oocytes from heat shock during maturation. Biol Reprod 71: 2072–2078, 2004. doi: 10.1095/biolreprod.104.031989. [DOI] [PubMed] [Google Scholar]
- 49.Ruebel M, Shankar K, Gaddy D, Lindsey F, Badger T, Andres A. Maternal obesity is associated with ovarian inflammation and upregulation of early growth response factor 1. Am J Physiol Endocrinol Metab 311: E269–E277, 2016. doi: 10.1152/ajpendo.00524.2015. [DOI] [PubMed] [Google Scholar]
- 50.Ruebel ML, Cotter M, Sims CR, Moutos DM, Badger TM, Cleves MA, Shankar K, Andres A. Obesity modulates inflammation and lipid metabolism oocyte gene expression: a single-cell transcriptome perspective. J Clin Endocrinol Metab 102: 2029–2038, 2017. doi: 10.1210/jc.2016-3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rupérez FJ, Ramos-Mozo P, Teul J, Martinez-Pinna R, Garcia A, Malet-Martino M, Camafeita E, Lopez JA, Pastor-Vargas C, Egido J, Balayssac S, Gilard V, Barbas C, Martin-Ventura JL. Metabolomic study of plasma of patients with abdominal aortic aneurysm. Anal Bioanal Chem 403: 1651–1660, 2012. doi: 10.1007/s00216-012-5982-y. [DOI] [PubMed] [Google Scholar]
- 52.Sessions-Bresnahan DR, Schauer KL, Heuberger AL, Carnevale EM. Effect of obesity on the preovulatory follicle and lipid fingerprint of equine oocytes. Biol Reprod 94: 15, 2016. doi: 10.1095/biolreprod.115.130187. [DOI] [PubMed] [Google Scholar]
- 53.Snitker S. Use of body fatness cutoff points. Mayo Clin Proc 85: 1057, 2010. doi: 10.4065/mcp.2010.0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Troyanskaya O, Cantor M, Sherlock G, Brown P, Hastie T, Tibshirani R, Botstein D, Altman RB. Missing value estimation methods for DNA microarrays. Bioinformatics 17: 520–525, 2001. doi: 10.1093/bioinformatics/17.6.520. [DOI] [PubMed] [Google Scholar]
- 55.Turinsky J, O’Sullivan DM, Bayly BP. 1,2-Diacylglycerol and ceramide levels in insulin-resistant tissues of the rat in vivo. J Biol Chem 265: 16880–16885, 1990. [PubMed] [Google Scholar]
- 56.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444: 1027–1031, 2006. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 57.Vahratian A. Prevalence of overweight and obesity among women of childbearing age: results from the 2002 National Survey of Family Growth. Matern Child Health J 13: 268–273, 2009. doi: 10.1007/s10995-008-0340-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Valckx SD, De Pauw I, De Neubourg D, Inion I, Berth M, Fransen E, Bols PE, Leroy JL. BMI-related metabolic composition of the follicular fluid of women undergoing assisted reproductive treatment and the consequences for oocyte and embryo quality. Hum Reprod 27: 3531–3539, 2012. doi: 10.1093/humrep/des350. [DOI] [PubMed] [Google Scholar]
- 59.Van Hoeck V, Leroy JL, Arias Alvarez M, Rizos D, Gutierrez-Adan A, Schnorbusch K, Bols PE, Leese HJ, Sturmey RG. Oocyte developmental failure in response to elevated nonesterified fatty acid concentrations: mechanistic insights. Reproduction 145: 33–44, 2013. doi: 10.1530/REP-12-0174. [DOI] [PubMed] [Google Scholar]
- 60.Vance JE. Lipoproteins secreted by cultured rat hepatocytes contain the antioxidant 1-alk-1-enyl-2-acylglycerophosphoethanolamine. Biochim Biophys Acta 1045: 128–134, 1990. doi: 10.1016/0005-2760(90)90141-J. [DOI] [PubMed] [Google Scholar]
- 61.Wallace M, Cottell E, Gibney MJ, McAuliffe FM, Wingfield M, Brennan L. An investigation into the relationship between the metabolic profile of follicular fluid, oocyte developmental potential, and implantation outcome. Fertil Steril 97: 1078–1084.e8, 2012. doi: 10.1016/j.fertnstert.2012.01.122. [DOI] [PubMed] [Google Scholar]
- 62.Watt MJ. Storing up trouble: does accumulation of intramyocellular triglyceride protect skeletal muscle from insulin resistance? Clin Exp Pharmacol Physiol 36: 5–11, 2009. doi: 10.1111/j.1440-1681.2008.05075.x. [DOI] [PubMed] [Google Scholar]
- 63.Watt MJ, Dzamko N, Thomas WG, Rose-John S, Ernst M, Carling D, Kemp BE, Febbraio MA, Steinberg GR. CNTF reverses obesity-induced insulin resistance by activating skeletal muscle AMPK. Nat Med 12: 541–548, 2006. doi: 10.1038/nm1383. [DOI] [PubMed] [Google Scholar]
- 64.Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA 106: 3698–3703, 2009. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu LL, Russell DL, Norman RJ, Robker RL. Endoplasmic reticulum (ER) stress in cumulus-oocyte complexes impairs pentraxin-3 secretion, mitochondrial membrane potential (ΔΨm), and embryo development. Mol Endocrinol 26: 562–573, 2012. doi: 10.1210/me.2011-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xing J, Sun J, You H, Lv J, Sun J, Dong Y. Anti-inflammatory effect of 3,4-oxo-isopropylidene-shikimic acid on acetic acid-induced colitis in rats. Inflammation 35: 1872–1879, 2012. doi: 10.1007/s10753-012-9509-7. [DOI] [PubMed] [Google Scholar]
- 67.Yang YJ, Kim MK, Hwang SH, Ahn Y, Shim JE, Kim DH. Relative validities of 3-day food records and the food frequency questionnaire. Nutr Res Pract 4: 142–148, 2010. doi: 10.4162/nrp.2010.4.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Young SN, Anderson GM, Gauthier S, Purdy WC. The origin of indoleacetic acid and indolepropionic acid in rat and human cerebrospinal fluid. J Neurochem 34: 1087–1092, 1980. doi: 10.1111/j.1471-4159.1980.tb09944.x. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y, Yamamoto T, Hisatome I, Li Y, Cheng W, Sun N, Cai B, Huang T, Zhu Y, Li Z, Jing X, Zhou R, Cheng J. Uric acid induces oxidative stress and growth inhibition by activating adenosine monophosphate-activated protein kinase and extracellular signal-regulated kinase signal pathways in pancreatic β cells. Mol Cell Endocrinol 375: 89–96, 2013. doi: 10.1016/j.mce.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 70.Zhao H, Przybylska M, Wu IH, Zhang J, Siegel C, Komarnitsky S, Yew NS, Cheng SH. Inhibiting glycosphingolipid synthesis improves glycemic control and insulin sensitivity in animal models of type 2 diabetes. Diabetes 56: 1210–1218, 2007. doi: 10.2337/db06-0719. [DOI] [PubMed] [Google Scholar]
- 71.Zhao H, Zhao Y, Li T, Li M, Li J, Li R, Liu P, Yu Y, Qiao J. Metabolism alteration in follicular niche: the nexus among intermediary metabolism, mitochondrial function, and classic polycystic ovary syndrome. Free Radic Biol Med 86: 295–307, 2015. doi: 10.1016/j.freeradbiomed.2015.05.013. [DOI] [PubMed] [Google Scholar]