Abstract
Tobacco smoking is a major risk factor for cardiovascular disease and hypertension. It is associated with the oxidative stress and induces metabolic reprogramming, altering mitochondrial function. We hypothesized that cigarette smoke induces cardiovascular mitochondrial oxidative stress, which contributes to endothelial dysfunction and hypertension. To test this hypothesis, we studied whether the scavenging of mitochondrial H2O2 in transgenic mice expressing mitochondria-targeted catalase (mCAT) attenuates the development of cigarette smoke/angiotensin II-induced mitochondrial oxidative stress and hypertension compared with wild-type mice. Two weeks of exposure of wild-type mice with cigarette smoke increased systolic blood pressure by 17 mmHg, which was similar to the effect of a subpresssor dose of angiotensin II (0.2 mg·kg−1·day−1), leading to a moderate increase to the prehypertensive level. Cigarette smoke exposure and a low dose of angiotensin II cooperatively induced severe hypertension in wild-type mice, but the scavenging of mitochondrial H2O2 in mCAT mice completely prevented the development of hypertension. Cigarette smoke and angiotensin II cooperatively induced oxidation of cardiolipin (a specific biomarker of mitochondrial oxidative stress) in wild-type mice, which was abolished in mCAT mice. Cigarette smoke and angiotensin II impaired endothelium-dependent relaxation and induced superoxide overproduction, which was diminished in mCAT mice. To mimic the tobacco smoke exposure, we used cigarette smoke condensate, which induced mitochondrial superoxide overproduction and reduced endothelial nitric oxide (a hallmark of endothelial dysfunction in hypertension). Western blot experiments indicated that tobacco smoke and angiotensin II reduce the mitochondrial deacetylase sirtuin-3 level and cause hyperacetylation of a key mitochondrial antioxidant, SOD2, which promotes mitochondrial oxidative stress.
NEW & NOTEWORTHY This work demonstrates tobacco smoking-induced mitochondrial oxidative stress, which contributes to endothelial dysfunction and development of hypertension. We suggest that the targeting of mitochondrial oxidative stress can be beneficial for treatment of pathological conditions associated with tobacco smoking, such as endothelial dysfunction, hypertension, and cardiovascular diseases.
Listen to this article’s corresponding podcast at https://ajpheart.podbean.com/e/mitochondrial-oxidative-stress-in-smoking-and-hypertension/.
Keywords: catalase, cigarette smoke, hypertension, mitochondria, oxidative stress, superoxide dismutase
INTRODUCTION
Hypertension represents a major risk factor for stroke, myocardial infarction, and heart failure, which cause one-third of deaths worldwide (58, 59). Thirty percent of adults have hypertension (21), and hypertension has been diagnosed in 46% men and 27% women among the Department of Veterans Affairs health care users (41); however, despite treatment with multiple drugs, 37% of patients remain hypertensive (7). Hypertension is a multifactorial disorder (23), and smoking is one of the major risk factors for the development of hypertension (38). Smoking increases blood pressure in both normotensive and hypertensive individuals (22, 46); however, smoking cessation success is very limited (7%) (7a, 8), and the risk for cardiovascular diseases remains elevated long after individuals quit smoking (29a, 35). Furthermore, patients with hypertension who smoke have significantly reduced responses to common classes of antihypertensive drugs, due to metabolic interference between cigarette smoking and drugs (38).
Cigarette smoke exposure results in a dose-dependent inhibition of mitochondrial complex I and complex II, which attenuates mitochondrial respiration and diminishes ATP production (69). Cigarette smoke increases cardiomyocyte ceramide accumulation (67), which increases the production of mitochondrial superoxide (O2·−) and H2O2 (19), and alters flow-induced vasodilation (20). Furthermore, cigarette smoke exposure causes metabolic alterations (3) and leads to metabolic reprogramming of the epithelium (54). The deleterious effects of cigarette smoke, however, are not limited to the airway epithelium and spread with circulation to multiple organs, leading to increased central nervous system sympathetic activity (45), endothelial dysfunction (1), and inflammation (42). These harmful effects are mediated by multiple cigarette smoke constituents, such as nicotine and other stable compounds accumulated in cigarette smoke condensate. Although the exact mechanism of cigarette smoke-mediated cardiovascular impairment is not fully elucidated, oxidative stress (47) is likely to play an important role in smoking-associated vascular dysfunction and hypertension.
In the past decade, it has become clear that oxidative stress contributes to hypertension by vascular constriction, kidney dysfunction, and inflammation (24). Mitochondria are an important source of O2·− radicals, and we showed that the mitochondria become dysfunctional in hypertension and defined the novel role of mitochondrial O2·− in this disease (12, 16). We showed that endothelial dysfunction and hypertension are associated with inactivation of the key mitochondrial antioxidant, SOD2, due to SOD2 hyperacetylation associated with impaired activity of mitochondrial deacetylase sirtuin (Sirt)3 (17). Hypertension is associated with a profound reduction in Sirt3 expression and activity in animal models and humans with essential hypertension (17).
Sirt3 is a key node in the regulation of mitochondrial function (25). It activates mitochondrial metabolism by deacetylation of tricarboxylic acid cycle enzymes (65), complex I (5, 50), and fatty acid β-oxidation enzymes (6, 28), maintains mitochondrial NADPH-GSH redox status by deacetylation of isocitrate dehydrogenase 2 (76), and activates SOD2 by deacetylation of specific lysine residues (66). Interestingly, cigarette smoke promotes both metabolic and redox alterations (4, 51), and nicotine can reduce Sirt3 expression (39). Meanwhile, Sirt3 depletion promotes vascular oxidative stress and hypertension (17).
We hypothesized that cigarette smoke induces mitochondrial oxidative stress, which contributes to endothelial dysfunction and development of hypertension. To test this hypothesis, we examined whether scavenging of mitochondrial H2O2 in mitochondria-targeted catalase (mCAT) mice prevents mitochondrial oxidative stress and attenuates hypertension in response to cigarette smoke, investigated the interplay between cigarette smoke and angiotensin II, and defined the potential role of Sirt3 impairment and SOD2 hyperacetylation.
EXPERIMENTAL PROCEDURES
Reagents.
The hydroxylamine spin probes 1-hydroxy-4-methoxy-2,2,6,6-tetramethylpiperidine hydrochloride and MitoTEMPO-H were purchased from Enzo Life Sciences (San Diego, CA). DMEM was from ThermoFisher Scientific (Waltham, MA). Sirt3 antibodies (catalog no. D22A3) were obtained from Cell Signaling Technology (Danvers, MA). SOD2 and SOD2-K68 acetyl antibodies (catalog nos. 169560 and 137037) were received from Abcam (San Francisco, CA). All antibodies were validated on knockout and mutant cells. Cigarette smoke condensate was purchased from Murty Pharmaceuticals (Lexington, KY). All other reagents were from Sigma (St. Louis, MO).
Animal experiments.
All experimental procedures were approved by the Vanderbilt University Institutional Animal Care and Use Committee. Transgenic mice expressing mCAT and their wild-type littermates (C57BL/6J, The Jackson Laboratory, Bar Harbor, ME) were used. Hypertension was induced by a low-suppressor dose of angiotensin II using osmotic pumps (15) or cigarette smoke exposure (57). Blood pressure was monitored by the tail-cuff method, as previously described (36, 73). Mice (3–4 mo old) were divided into the following four groups: sham, cigarette smoke exposure, angiotensin II infusion, and cigarette smoke exposure plus angiotensin II. Mice were exposed to cigarette smoke for 2 wk using the nose-only exposure setup of the inExpose system (Scireq, Montreal, QC, Canada) by one 10-s puff/min followed by ambient air at 2 l/min for 50 s (4 cigarettes/day). Animals were housed in soft restraints for the duration of cigarette smoke treatment and after treatment were immediately returned to their cages. Sham mice were housed in soft restraints for the duration of cigarette smoke treatment. The potential interplay between the renin-angiotensin system and smoking was tested in cigarette smoke-exposed mice coinfused with a subpressor low dose of angiotensin II (0.2 mg·kg−1·day−1), which alone does not cause hypertension (61, 64).
O2·− measurements by electron spin resonance.
Five aortic sections (2 mm) were incubated for 30 min at 37°C in Krebs/HEPES buffer containing 10 µM diethylenetriamene pentaacetate and then placed in a 1-ml syringe and frozen in liquid nitrogen, as previously described (13). Electron spin resonance (ESR) spectra were recorded using the quartz finger Dewar flask. The amplitude of the signal was measured, and the concentration of the detected O2·− was to be determined by accumulation of the corresponding stable compound nitroxide radical (11), determined from the 4-hydroxy-2,2,6,6-tetramethylpiperidinyloxy calibration curve.
Nitric oxide measurements by ESR.
Nitric oxide (NO) production in endothelial cells and vessels was quantified by ESR and colloid ferrous-diethyldithiocarbamate 2 [Fe(DETC)2], as we have previously described (9). All ESR samples were placed in a quartz Dewar (Corning, New York, NY) filled with liquid nitrogen. ESR spectra were recorded using an EMX ESR spectrometer (Bruker Biospin, Billerica, MA) and a superhigh-Q microwave cavity. The ESR settings were as follows: field sweep, 160 G; microwave frequency, 9.42 GHz; microwave power, 10 mW; modulation amplitude, 3 G; scan time, 150 ms; time constant, 5.2 s; and receiver gain, 60 dB (n = 4 scans).
Vasorelaxation experiments.
Isometric tension experiments were performed on 2-mm mouse aortic rings dissected free of perivascular fat. Experiments were performed in a horizontal wire myograph (models 610M and 620M, DMT, Aarhus, Denmark) containing physiological salt solution with the composition of 118 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 25 mM NaHCO3, 11 mM glucose, and 1.8 mM CaCl2. The isometric tone of each vessel was recorded using LabChart Pro v7.3.7 (AD Instruments). Aortic rings were equilibrated over a 2-h period by heating and stretching the vessels to an optimal baseline tension of 36 mN before contracting them with three cycles of 60 mM KCl physiological saline solution. Endothelium-dependent and -independent vascular relaxation was tested after preconstriction with 1 µM phenylephrine. Once the vessels reached steady-state contraction, increasing concentrations of acetylcholine were administered, and the response to each concentration of the drug was recorded.
Measurements of cardiolipin oxidation.
Cardiolipin oxidation was measured by liquid chromatography/mass spectrometry (LC/MS), as previously described (75). The extracted lipid fraction was separated online by ultraperformance LC, using a Waters Acquity UPLC system (Waters, Milford, MA). MS analysis was performed on a Quantum Ultra triple-quadrupole mass spectrometer (ThermoFisher Scientific).
Statistics.
Experiments were analyzed using a Student’s Newman-Keuls post hoc test and ANOVA. P values of <0.05 were considered significant.
RESULTS
Cigarette smoke and angiotensin II cooperatively induced oxidative stress and hypertension.
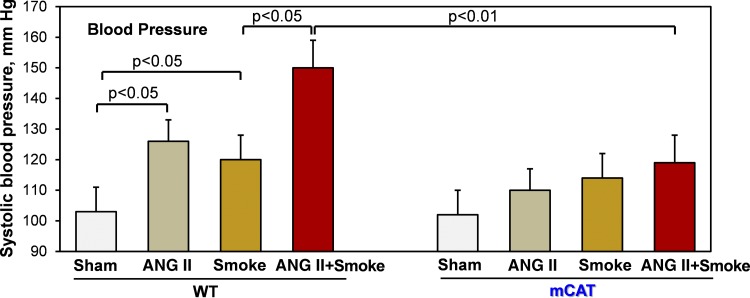
Hypertension is a multifactorial disorder, and exacerbated angiotensin II signaling contributes to the development of endothelial dysfunction and hypertension. We investigated the potential interplay between cigarette smoke and angiotensin II in the development of hypertension. Two-week exposure of wild-type mice to cigarette smoke increased systolic blood pressure by 17 mmHg, which was similar to the effect of a subpressor dose of angiotensin II (0.2 mg⋅kg−1⋅day−1) (64), leading to a moderate increase to the prehypertensive level (Fig. 1). The combined treatment with cigarette smoke and a low dose of angiotensin II cooperatively induced severe hypertension in wild-type mice, whereas the scavenging of mitochondrial H2O2 in mCAT mice completely prevented smoke-induced hypertension (Fig. 1). These data strongly support the role of mitochondrial oxidative stress in cigarette smoke-induced hypertension.
Fig. 1.
Cigarette smoke and angiotensin II (ANG II) cooperatively induce hypertension in C57BL/6 mice but not in mice expressing mitochondria-targeted catalase (mCAT). Mice had cigarette smoke nose-only exposure for 2 wk (4 cigarettes/day). Sham mice were housed in soft restraints for the duration of cigarette smoke treatment. Some mice received a subpressor low dose of ANG II (0.2 mg kg−1⋅day−1), which does not cause hypertension (61, 64). Data are expressed as means ± SD; n = 6. P values were calculated by two-way ANOVA. WT, wild type.
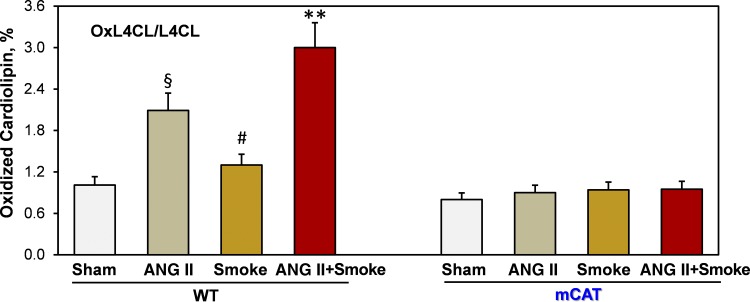
The specific role of cigarette smoke exposure in mitochondrial oxidative stress was confirmed by LC/MS analysis of oxidized cardiolipin. Cardiolipin selectively localizes at the matrix side of the mitochondrial inner membrane, and cardiolipin oxidation is a specific marker of mitochondrial oxidative stress (34). Two-week exposure of wild-type mice to cigarette smoke increased cardiolipin oxidation by 30%, whereas angiotensin II infusion doubled cardiolipin oxidation. Interestingly, combined treatment with cigarette smoke and a low dose of angiotensin II cooperatively enhanced cardiolipin oxidation by threefold compared with wild-type sham mice, but the scavenging of mitochondrial H2O2 in mCAT mice completely prevented cardiolipin oxidation (Fig. 2).
Fig. 2.
Cigarette smoke and angiotensin II (ANG II) cooperatively induce oxidation of mitochondrial cardiolipin in C57BL/6 mice but not in mice expressing mitochondria-targeted catalase (mCAT). Mice had cigarette smoke nose-only exposure (4 cigarettes/day), a subpressor low dose of ANG II infusion (0.2 mg⋅kg−1⋅day−1), or combined ANG II + smoke treatment. After 2 wk of treatment, mice were euthanized for liquid chromatography/mass spectrometry analysis of oxidized cardiolipin in the heart. Data are expressed as means ± SD; n = 6. §P < 0.01 vs. sham mice; #P < 0.05 vs. sham mice; **P < 0.01 vs. ANG II. L4CL, tetralinoleoylcardiolipin; oxL4CL, monohydroxy L4CL; WT, wild type.
Cigarette smoke and angiotensin II induced endothelial dysfunction in wild-type but not in mCAT mice.
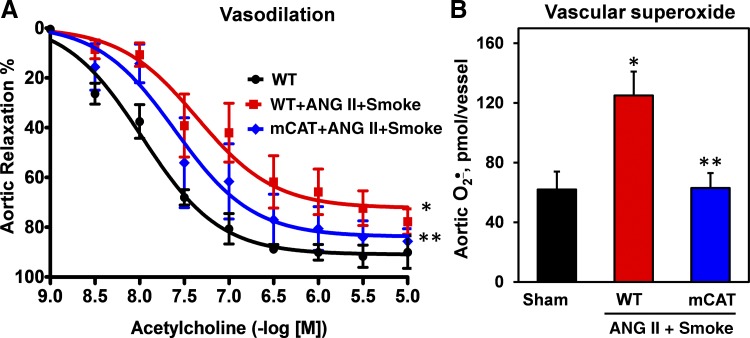
Impaired endothelium-dependent relaxation is a hallmark of endothelial dysfunction in hypertension, due to NO oxidation by O2·− (37). We tested whether mitochondrial oxidative stress, associated in response to cigarette smoke plus angiotensin II treatment, contributes to impaired vascular relaxation and overproduction of vascular O2·−. We did not see the alteration of endothelium-independent relaxation to the NO donor sodium nitroprusside (data not shown), but cigarette smoke plus angiotensin II led to severe impairment of endothelium-dependent vasorelaxation to acetylcholine, and expression of mitochondria-targeted catalase significantly attenuated the impairment of vasorelaxation (Fig. 3A). ESR analysis of aortic sections isolated from wild-type mice exposed to cigarette smoke plus angiotensin II revealed significant O2·− overproduction (Fig. 3B), as measured by ESR and the 1-hydroxy-4-methoxy-2,2,6,6-tetramethylpiperidine hydrochloride spin probe (11). Interestingly, expression of mitochondrial catalase in mCAT mice completely prevented the O2·− overproduction in cigarette smoke and angiotensin II treatment (Fig. 3B).
Fig. 3.
Vasodilatation (A) and vascular superoxide (O2·−) production (B) in aortas isolated from mice treated with a combination of cigarette smoke and angiotensin II (ANG II; 0.2 mg⋅kg−1⋅day−1) for 2 wk. Vascular O2·− was measured by the 1-hydroxy-4-methoxy-2,2,6,6-tetramethylpiperidine hydrochloride spin probe and electron spin resonance, as we have previously described (11). Results are means ± SD; n = 6. *P < 0.01 vs. wild-type (WT) mice; **P < 0.05 vs. WT + ANG II/smoke. mCAT, mice expressing mitochondria-targeted catalase.
Cigarette smoke and angiotensin II reduced Sirt3 expression and induced mitochondrial hyperacetylation.
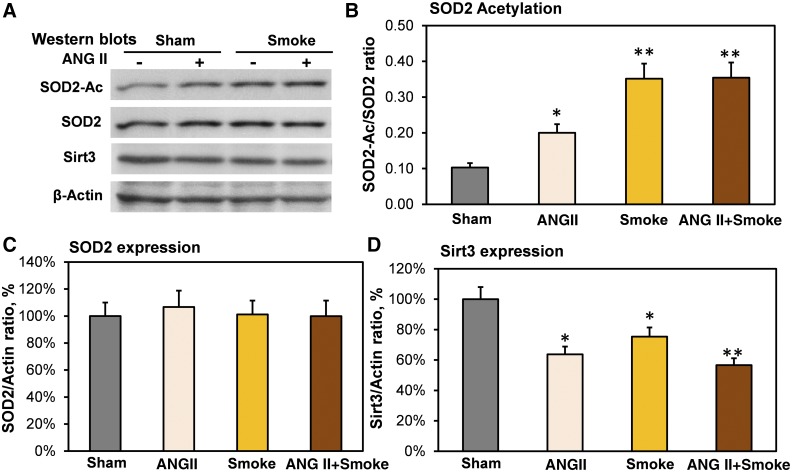
Cigarette smoke induces metabolic reprogramming in mitochondria (48, 54), and these metabolic alterations are associated with increased mitochondrial O2·− and reduced SOD2 activity (43). We have recently shown that hypertension is associated with SOD2 inactivation due to SOD2 hyperacetylation and reduced expression of mitochondrial deacetylase Sirt3 (17). We tested whether cigarette smoke reduces the Sirt3 level and induces SOD2 hyperacetylation, which contribute to hypertension and endothelial dysfunction. To test this hypothesis, we performed Western blot analysis of whole kidneys isolated from mice after 2 wk of treatment with cigarette smoke exposure, angiotensin II infusion, and cigarette smoke exposure plus angiotensin II compared with sham mice (Fig. 4A). We previously reported that hypertension is associated with diminished Sirt3 levels both in kidney and vasculature (17), and in this work, we analyzed Sirt3 expression in the kidney, due to the tissue availability. It was found that cigarette smoke exposure and angiotensin II infusion induced SOD2 hyperacetylation (Fig. 4B), which is linked to diminished SOD2 activity (17). Interestingly, SOD2 expression was not changed significantly (Fig. 4C). SOD2 acetylation was accompanied by a moderate reduction of the mitochondrial deacetylase Sirt3 level (Fig. 4D). Of note, the extent of SOD2 hyperacetylation does not match the loss of Sirt3 expression. For example, combined cigarette smoke exposure plus angiotensin II infusion reduced Sirt3 by 43% and increased SOD2 acetylation by 3.5-fold. These data suggest a potential role of increased acetylation rate and redox Sirt3 inactivation (17) in the imbalance of SOD2 acetylation-deacetylation, leading to SOD2 hyperacetylation in response to cigarette smoke.
Fig. 4.
Effect of cigarette smoke and angiotensin II (ANG II) on acetylation of mitochondrial antioxidant SOD2 (SOD2-Ac), expression of SOD2, and sirtuin 3 (Sirt3). Mice had cigarette smoke exposure, ANG II infusion, or combined ANG II + smoke treatment. After 2 wk of treatment, mice were euthanized for kidney Western blot experiments (A). Cigarette smoke exposure and ANG II infusion induced SOD2 hyperacetylation (B), which diminished SOD2 activity (17). SOD2 expression was not significantly changed (C). SOD2 acetylation was accompanied by a moderate reduction of mitochondrial deacetylase sirtuin 3 (Sirt3; D). Data are normalized by β-actin level and expressed as means ± SE; n = 6. *P < 0.01 vs. sham mice; **P < 0.001 vs. ANG II.
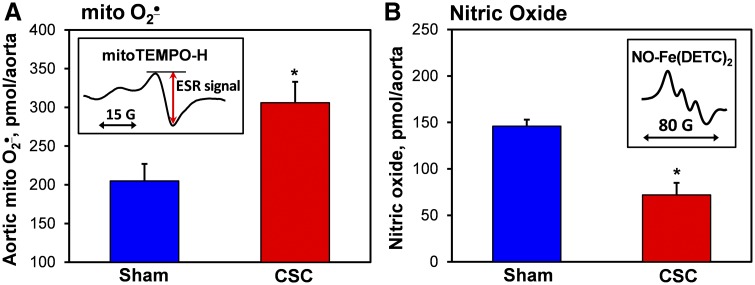
Endothelial dysfunction induced by cigarette smoke condensate.
Our data show that cigarette smoke induces SOD2 hyperacetylation and mitochondrial oxidative stress. This can be mediated by increased mitochondrial O2·−, which oxidizes and inactivates endothelial NO, leading to impaired vascular relaxation. To test this hypothesis, we studied whether cigarette smoke can directly affect vascular function. It was found that incubation of mouse aortic sections with cigarette smoke condensate increases the production of mitochondrial O2·− by 50% and reduces the endothelial NO by 51%, as measured by the mitochondria-targeted spin probe MitoTEMPO-H (11) and specific NO spin trap Fe(DETC)2 (9, 10) by ESR (Fig. 5). These data support that cigarette smoke alters mitochondrial function, leading to an imbalance of mitochondrial SOD2 acetylation and increased O2·− production, which contributes to endothelial oxidative stress and hypertension.
Fig. 5.
Effect of cigarette smoke condensate (CSC) on mitochondrial superoxide (mito O2·−) production (A) and endothelial nitric oxide (NO; B) in the mouse aorta. Aortic sections isolated from sham wild-type mice were treated in DMEM tissue culture medium with CSC (40 µg/ml) or DMSO as vehicle (sham) for 24 h before electron spin resonance (ESR) measurements (insets) (10). *P < 0.01 vs. sham mice (n = 6). Fe(DETC)2, ferrous-diethyldithiocarbamate.
DISCUSSION
This study provides the first evidence that cigarette smoke induces SOD2 hyperacetylation and enhances endothelial dysfunction and, cooperatively with angiotensin II, induces mitochondrial oxidative stress, which promotes the development of hypertension. Our data indicate that acute cigarette smoke exposure for 2 wk reduces expression of mitochondrial deacetylase Sirt3 and increases SOD2 hyperacetylation, which increases mitochondrial O2·−, leads to cardiolipin oxidation, and contributes to impaired vascular relaxation (Figs. 2–5). It is interesting that the scavenging of mitochondrial H2O2 in transgenic mice expressing mitochondria-targeted catalase completely prevents overproduction of mitochondrial O2·− and inhibits cigarette smoke-induced oxidative stress and hypertension (Figs. 1 and 2).
Tobacco smoking is strongly associated with oxidative stress (47). Clinical studies have shown that accumulation of the lipid peroxidation product malondialdehyde in blood plasma of smokers was increased by 2.5-fold, whereas activity of the major antioxidant enzymes catalase, SOD, and glutathione peroxidase was reduced significantly (44). This leads to oxidation of cysteine and glutathione, and the level of reduced glutathione is diminished in the kidney by twofold in mice exposed to cigarette smoke for 4 days (56). The resultant alteration in the thiol redox status impairs cellular redox signaling and can cause cellular dysfunction. Indeed, the smoking of a single cigarette rapidly reduces endothelial NO production and significantly diminishes blood plasma antioxidants (68). It has been proposed that cigarette smoke induces O2·− production in endothelial cells, leading to NO inactivation and NO synthase (NOS) uncoupling (53). Treatment of cultured endothelial cells with cigarette smoke condensate or smokers’ blood plasma increases cellular O2·− production and reduces NOS activity (31, 49). The specific mechanisms of smoking-mediated endothelial dysfunction and hypertension, however, remain unclear. Our data demonstrate that cigarette smoke directly induces mitochondrial O2·− and diminishes endothelial NO, which promote hypertension.
We have previously shown that cigarette smoke condensate induces metabolic reprogramming in mitochondria, which contributes to cancer development (48, 54). These metabolic alterations are associated with reduced SOD2 activity (43) and increased NADPH/NADH accumulation (54). The increased NADPH level can partially compensate for the mitochondrial oxidative stress, due to NADPH-dependent maintenance of the thiol redox status by glutathione reductase and thioredoxin reductase (32) as well as the scavenging of H2O2 and lipid peroxide by glutathione peroxidases (63). Our data show increased cardiolipin oxidation and blood pressure in wild-type mice exposed to cigarette smoke, which was prevented by the mitochondrial catalase, indicating that cigarette smoke-induced metabolic reprogramming does not completely compensate for the mitochondrial oxidative stress.
Our data indicate that expression of mitochondria-targeted catalase protects from cigarette smoke-induced mitochondrial oxidative stress measured by cardiolipin oxidation and attenuates endothelial dysfunction and hypertension (Figs. 1–3). Meanwhile, mCAT mice do not induce mitochondrial O2·− overproduction (30), and these mice have higher SOD2 activity (17), due to SOD2 deacetylation compared with wild-type littermates. This provides mCAT mice with enhanced activities of catalase and SOD2 in mitochondria, which protect mitochondria from both O2·− and H2O2. These data, therefore, do not provide definitive information as to whether O2·− or H2O2 is critical in the mediation of the effect of smoking and angiotensin II on blood pressure. We have previously shown that SOD2-overexpressing mice are protected from hypertension and endothelial dysfunction; however, mCAT mice have normal SOD2 expression, similar to wild-type mice. The scavenging of mitochondrial H2O2 is important in redox homeostasis of mitochondria (63), and it improves Sirt3-mediated SOD2 deacetylation (17). Indeed, the maintenance of thiol redox balance plays a key role in the regulation of mitochondrial function, and disruption of this redox organization is a common basis for disease (32, 33). Sirt3 is a key node in the regulation of mitochondrial function (25), and we have recently shown that Sirt3 S-glutathionylation contributes to Sirt3 inactivation in hypertension (17). The scavenging of mitochondrial H2O2 in transgenic mCAT mice prevents Sirt3 S-glutathionylation, reduces SOD2 hyperacetylation, and attenuates angiotensin II-induced hypertension (17), whereas treatment with the mitochondria-targeted H2O2 scavenger MitoEbselen, after the onset of hypertension, improved Sirt3 deacetylase activity and reduced blood pressure in wild-type mice (17). Furthermore, mitochondrial H2O2 plays an important role in redox cell signaling and contributes to ROS-induced ROS production by NADPH oxidases, xanthine oxidase, and other sources (12, 78). It is conceivable that Sirt3 redox inactivation and redox-dependent stimulation of ROS production contribute to cigarette smoke-induced mitochondrial alterations and endothelial dysfunction.
The diminished endothelial NO level is a hallmark of endothelial dysfunction in hypertension, and our data show that cigarette smoke and cigarette smoke condensate reduce NO and impair endothelial-dependent relaxation (Figs. 2 and 4). This can be associated with a diminished NOS activity and/or NO oxidation by O2·−. Indeed, cigarette smoke increases O2·− production, which contributes to impairment of endothelial-dependent relaxation corrected by SOD (62), whereas endothelial-independent relaxation is preserved in acute cigarette smoke exposure (18), suggesting that cigarette smoke-induced O2·− in endothelium contributes to a reduction of vascular NO levels. The precise mechanism of a smoke-induced reduction of NO, however, remains unclear. It can include the uncoupling and inhibition of NOS. l-Arginine depletion or O2·−/peroxynitrite-mediated tetrahydrobiopterin oxidation results in the uncoupling of NOS, leading to O2·− production rather than NO release (70). Indeed, cigarette smoke extract increases O2·− production and depletes the essential NOS cofactor tetrahydrobiopterin (1), and tetrahydrobiopterin supplementation improves endothelium-dependent relaxation in chronic smokers (26). Furthermore, supplementation l-arginine improves endothelium-dependent relaxation in a smoke exposure model (29). Cigarette smoke decreases expression of arginine transporter cationic amino acid transporter 1 and increases accumulation of an endogenous inhibitor of endothelial NOS, asymmetric dimethylarginine (77). It is conceivable that metabolic reprogramming and oxidative stress, in response to tobacco smoking, contribute to NO inactivation, NOS inhibition, and uncoupling. Further studies are warranted to define potential supplements that can improve the endothelium-independent relaxation in individuals with a history of tobacco smoking.
Endothelial dysfunction is critically contributing to the development of hypertension, which is associated with impaired relaxation in both resistance and conductance vessels (72). In this work, we studied the effect of tobacco smoking on oxidative stress and impaired endothelial-dependent relaxation in the aorta; however, blood pressure is regulated by microcirculation in the resistance vessels, such as mesenteric arteries (40). In patients with hypertension, relaxation of resistant arteries to acetylcholine is blunted, and it is not affected by NOS inhibition but is improved by the NADPH inhibitor apocynin (71), indicating an impaired NO pathway in patients with hypertension and the potential role of oxidative stress. Endothelium-dependent relaxation of both the aorta and mesenteric arteries is redox dependent (27, 74); however, the lack of NO-dependent relaxation in resistance vessels can be partially compensated by NOS-independent relaxation (60). Endothelial dysfunction in both resistance and conductance vessels contributes to the end-organ damage in hypertension; however, additional studies are required to establish the specific effect of tobacco smoking on the function of resistance vessels, such as mesenteric arteries.
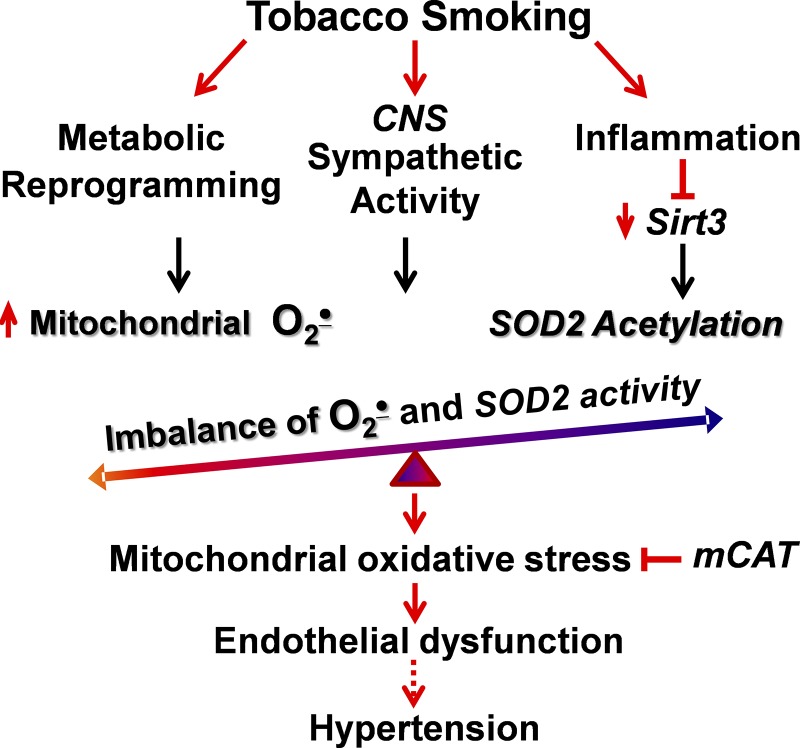
Hypertension is a multifactorial phenomenon that is mediated by a series of central, inflammatory, and metabolic pathways (23). The cross-talk among these multiple pathways increases oxidative stress in multiple organs, and ROS is critical in the pathophysiology of hypertension (14). Interestingly, tobacco smoking increases inflammation (42), stimulates central nervous system sympathetic activity (45), and causes metabolic alterations (54), which are all important risk factors of endothelial dysfunction and hypertension. We propose that tobacco smoking, by acting on these multiple pathways, leads to the development of mitochondrial oxidative stress, which contributes to the development of hypertension (Fig. 6). Indeed, in this work, we found that cigarette smoke exposure promotes mitochondrial dysfunction by Sirt3 depletion, SOD2 hyperacetylation, and cardiolipin oxidation. It is conceivable that mitochondria-targeted interventions may correct the metabolic, central, vascular, and inflammatory alterations that contribute to the increased risk for cardiovascular diseases, even long after individuals quit smoking (29a, 35).
Fig. 6.
Proposed role of mitochondrial oxidative stress in tobacco smoking-mediated endothelial dysfunction and hypertension. CNS, central nervous system; mCAT, mitochondria-targeted catalase; O2·−, superoxide; Sirt3, sirtuin 3.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01-HL-124116 and American Heart Association Grant 16GRNT31230017.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.D. conceived and designed research; S.D., H.I., B.R., L.A., A.V., and A.D. performed experiments; S.D., H.I., B.R., L.A., A.V., and A.D. analyzed data; S.D., L.A., and A.D. interpreted results of experiments; S.D., L.A., and A.D. prepared figures; A.D. drafted manuscript; S.D., S.M.J.R., O.B., T.B., P.P.M., D.G.H., and A.D. edited and revised manuscript; S.D., H.I., B.R., L.A., A.V., S.M.J.R., O.B., T.B., P.P.M., D.G.H., and A.D. approved final version of manuscript.
REFERENCES
- 1.Abdelghany TM, Ismail RS, Mansoor FA, Zweier JR, Lowe F, Zweier JL. Cigarette smoke constituents cause endothelial nitric oxide synthase dysfunction and uncoupling due to depletion of tetrahydrobiopterin with degradation of GTP cyclohydrolase. Nitric Oxide 76: 113–121, 2018. doi: 10.1016/j.niox.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal AR, Yin F, Cadenas E. Short-term cigarette smoke exposure leads to metabolic alterations in lung alveolar cells. Am J Respir Cell Mol Biol 51: 284–293, 2014. doi: 10.1165/rcmb.2013-0523OC. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal AR, Zhao L, Sancheti H, Sundar IK, Rahman I, Cadenas E. Short-term cigarette smoke exposure induces reversible changes in energy metabolism and cellular redox status independent of inflammatory responses in mouse lungs. Am J Physiol Lung Cell Mol Physiol 303: L889–L898, 2012. doi: 10.1152/ajplung.00219.2012. [DOI] [PubMed] [Google Scholar]
- 5.Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci USA 105: 14447–14452, 2008. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bharathi SS, Zhang Y, Mohsen AW, Uppala R, Balasubramani M, Schreiber E, Uechi G, Beck ME, Rardin MJ, Vockley J, Verdin E, Gibson BW, Hirschey MD, Goetzman ES. Sirtuin 3 (SIRT3) protein regulates long-chain acyl-CoA dehydrogenase by deacetylating conserved lysines near the active site. J Biol Chem 288: 33837–33847, 2013. doi: 10.1074/jbc.M113.510354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byrd JB, Zeng C, Tavel HM, Magid DJ, O’Connor PJ, Margolis KL, Selby JV, Ho PM. Combination therapy as initial treatment for newly diagnosed hypertension. Am Heart J 162: 340–346, 2011. doi: 10.1016/j.ahj.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7a.Centers for Disease Control and Prevention (CDC) Quitting smoking among adults—United States, 2001–2010. MMWR Morb Mortal Wkly Rep 60: 1513–1519, 2011. [PubMed] [Google Scholar]
- 8.Deppen SA, Grogan EL, Aldrich MC, Massion PP. Lung cancer screening and smoking cessation: a teachable moment? J Natl Cancer Inst 106: dju122, 2014. doi: 10.1093/jnci/dju122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dikalov S, Fink B. ESR techniques for the detection of nitric oxide in vivo and in tissues. Methods Enzymol 396: 597–610, 2005. doi: 10.1016/S0076-6879(05)96052-7. [DOI] [PubMed] [Google Scholar]
- 10.Dikalov S, Griendling KK, Harrison DG. Measurement of reactive oxygen species in cardiovascular studies. Hypertension 49: 717–727, 2007. doi: 10.1161/01.HYP.0000258594.87211.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dikalov SI, Kirilyuk IA, Voinov M, Grigor’ev IA. EPR detection of cellular and mitochondrial superoxide using cyclic hydroxylamines. Free Radic Res 45: 417–430, 2011. doi: 10.3109/10715762.2010.540242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dikalov SI, Nazarewicz RR, Bikineyeva A, Hilenski L, Lassègue B, Griendling KK, Harrison DG, Dikalova AE. Nox2-induced production of mitochondrial superoxide in angiotensin II-mediated endothelial oxidative stress and hypertension. Antioxid Redox Signal 20: 281–294, 2014. doi: 10.1089/ars.2012.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dikalov SI, Polienko YF, Kirilyuk I. Electron paramagnetic resonance measurements of reactive oxygen species by cyclic hydroxylamine spin probes. Antioxid Redox Signal 28: 1433–1443, 2018. doi: 10.1089/ars.2017.7396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dikalov SI, Ungvari Z. Role of mitochondrial oxidative stress in hypertension. Am J Physiol Heart Circ Physiol 305: H1417–H1427, 2013. doi: 10.1152/ajpheart.00089.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dikalova A, Clempus R, Lassègue B, Cheng G, McCoy J, Dikalov S, San Martin A, Lyle A, Weber DS, Weiss D, Taylor WR, Schmidt HH, Owens GK, Lambeth JD, Griendling KK. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation 112: 2668–2676, 2005. doi: 10.1161/CIRCULATIONAHA.105.538934. [DOI] [PubMed] [Google Scholar]
- 16.Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, Dikalov SI. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res 107: 106–116, 2010. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dikalova AE, Itani HA, Nazarewicz RR, McMaster WG, Flynn CR, Uzhachenko R, Fessel JP, Gamboa JL, Harrison DG, Dikalov SI. Sirt3 impairment and SOD2 hyperacetylation in vascular oxidative stress and hypertension. Circ Res 121: 564–574, 2017. doi: 10.1161/CIRCRESAHA.117.310933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fira-Mladinescu O, Mihaicuţa S, Noveanu L, Fira-Mladinescu C, Muntean D, Tudorache V, Mihalaş G. Cigarette smoke extract induces endothelium dysfunction in isolated rabbit pulmonary arteries via an oxidative mechanism [in Romanian]. Rev Med Chir Soc Med Nat Iasi 110: 955–961, 2006. [PubMed] [Google Scholar]
- 19.Freed JK, Beyer AM, LoGiudice JA, Hockenberry JC, Gutterman DD. Ceramide changes the mediator of flow-induced vasodilation from nitric oxide to hydrogen peroxide in the human microcirculation. Circ Res 115: 525–532, 2014. doi: 10.1161/CIRCRESAHA.115.303881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freed JK, Durand MJ, Hoffmann BR, Densmore JC, Greene AS, Gutterman DD. Mitochondria-regulated formation of endothelium-derived extracellular vesicles shifts the mediator of flow-induced vasodilation. Am J Physiol Heart Circ Physiol 312: H1096–H1104, 2017. doi: 10.1152/ajpheart.00680.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fryar CD, Ostchega Y, Hales CM, Zhang G, Kruszon-Moran D. Hypertension prevalence and control among adults: United States, 2015-2016. NCHS Data Brief 2017: 1–8, 2017. [PubMed] [Google Scholar]
- 22.Groppelli A, Giorgi DM, Omboni S, Parati G, Mancia G. Persistent blood pressure increase induced by heavy smoking. J Hypertens 10: 495–499, 1992. doi: 10.1097/00004872-199205000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Harrison DG. The mosaic theory revisited: common molecular mechanisms coordinating diverse organ and cellular events in hypertension. J Am Soc Hypertens 7: 68–74, 2013. doi: 10.1016/j.jash.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrison DG, Marvar PJ, Titze JM. Vascular inflammatory cells in hypertension. Front Physiol 3: 128, 2012. doi: 10.3389/fphys.2012.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He W, Newman JC, Wang MZ, Ho L, Verdin E. Mitochondrial sirtuins: regulators of protein acylation and metabolism. Trends Endocrinol Metab 23: 467–476, 2012. doi: 10.1016/j.tem.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Heitzer T, Brockhoff C, Mayer B, Warnholtz A, Mollnau H, Henne S, Meinertz T, Münzel T. Tetrahydrobiopterin improves endothelium-dependent vasodilation in chronic smokers: evidence for a dysfunctional nitric oxide synthase. Circ Res 86: E36–E41, 2000. doi: 10.1161/01.RES.86.2.e36. [DOI] [PubMed] [Google Scholar]
- 27.Hilgers RH, Kundumani-Sridharan V, Subramani J, Chen LC, Cuello LG, Rusch NJ, Das KC. Thioredoxin reverses age-related hypertension by chronically improving vascular redox and restoring eNOS function. Sci Transl Med 9: eaaf6094, 2017. doi: 10.1126/scitranslmed.aaf6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirschey MD, Shimazu T, Huang JY, Schwer B, Verdin E. SIRT3 regulates mitochondrial protein acetylation and intermediary metabolism. Cold Spring Harb Symp Quant Biol 76: 267–277, 2011. doi: 10.1101/sqb.2011.76.010850. [DOI] [PubMed] [Google Scholar]
- 29.Hutchison SJ, Sievers RE, Zhu BQ, Sun YP, Stewart DJ, Parmley WW, Chatterjee K. Secondhand tobacco smoke impairs rabbit pulmonary artery endothelium-dependent relaxation. Chest 120: 2004–2012, 2001. doi: 10.1378/chest.120.6.2004. [DOI] [PubMed] [Google Scholar]
- 29a.International Agency for Research on Cancer; World Health Organization . Risk of cardiovascular diseases after smoking cessation. In: IARC Handbooks of Cancer Prevention: Tobacco Control: Reversal of Risk after Quitting Smoking. Lyon, France: World Health Organization, 2007, vol. 11, p. 227–268. [Google Scholar]
- 30.Itani HA, Dikalova AE, McMaster WG, Nazarewicz RR, Bikineyeva AT, Harrison DG, Dikalov SI. Mitochondrial cyclophilin D in vascular oxidative stress and hypertension. Hypertension 67: 1218–1227, 2016. doi: 10.1161/HYPERTENSIONAHA.115.07085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaimes EA, DeMaster EG, Tian RX, Raij L. Stable compounds of cigarette smoke induce endothelial superoxide anion production via NADPH oxidase activation. Arterioscler Thromb Vasc Biol 24: 1031–1036, 2004. doi: 10.1161/01.ATV.0000127083.88549.58. [DOI] [PubMed] [Google Scholar]
- 32.Jones DP, Go YM. Redox compartmentalization and cellular stress. Diabetes Obes Metab 12, Suppl 2: 116–125, 2010. doi: 10.1111/j.1463-1326.2010.01266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones DP, Sies H. The redox code. Antioxid Redox Signal 23: 734–746, 2015. doi: 10.1089/ars.2015.6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kagan VE, Chu CT, Tyurina YY, Cheikhi A, Bayir H. Cardiolipin asymmetry, oxidation and signaling. Chem Phys Lipids 179: 64–69, 2014. doi: 10.1016/j.chemphyslip.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawachi I, Colditz GA, Stampfer MJ, Willett WC, Manson JE, Rosner B, Speizer FE, Hennekens CH. Smoking cessation and time course of decreased risks of coronary heart disease in middle-aged women. Arch Intern Med 154: 169–175, 1994. doi: 10.1001/archinte.1994.00420020075009. [DOI] [PubMed] [Google Scholar]
- 36.Krege JH, Hodgin JB, Hagaman JR, Smithies O. A noninvasive computerized tail-cuff system for measuring blood pressure in mice. Hypertension 25: 1111–1115, 1995. doi: 10.1161/01.HYP.25.5.1111. [DOI] [PubMed] [Google Scholar]
- 37.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem 278: 22546–22554, 2003. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- 38.Leone A. Does smoking act as a friend or enemy of blood pressure? Let release Pandora’s box. Cardiol Res Pract 2011: 264894, 2011. doi: 10.4061/2011/264894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Yu C, Shen G, Li G, Shen J, Xu Y, Gong J. Sirt3-MnSOD axis represses nicotine-induced mitochondrial oxidative stress and mtDNA damage in osteoblasts. Acta Biochim Biophys Sin (Shanghai) 47: 306–312, 2015. doi: 10.1093/abbs/gmv013. [DOI] [PubMed] [Google Scholar]
- 40.Lüscher TF, Dohi Y, Tschudi M. Endothelium-dependent regulation of resistance arteries: alterations with aging and hypertension. J Cardiovasc Pharmacol 19, Suppl 5: S34–S42, 1992. [PubMed] [Google Scholar]
- 41.McDonald M, Hertz RP. Pfizer Facts: Utilization of Veterans Affairs Healthcare Services by United States Veterans. New York: Pfizer U.S. Pharmaceuticals, 2003. http://citeseerx.ist.psu.edu/viewdoc/download;jsessionid=C2AA907798E6E212B04BD6FC06451317?doi=10.1.1.389.1296&rep=rep1&type=pdf. [Google Scholar]
- 42.Madani A, Alack K, Richter MJ, Krüger K. Immune-regulating effects of exercise on cigarette smoke-induced inflammation. J Inflamm Res 11: 155–167, 2018. doi: 10.2147/JIR.S141149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Margaret AL, Syahruddin E, Wanandi SI. Low activity of manganese superoxide dismutase (MnSOD) in blood of lung cancer patients with smoking history: relationship to oxidative stress. Asian Pac J Cancer Prev 12: 3049–3053, 2011. [PubMed] [Google Scholar]
- 44.Metta S, Basalingappa DR, Uppala S, Mitta G. Erythrocyte antioxidant defenses against cigarette smoking in ischemic heart disease. J Clin Diagn Res 9: BC08–BC11, 2015. doi: 10.7860/JCDR/2015/12237.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Middlekauff HR, Park J, Agrawal H, Gornbein JA. Abnormal sympathetic nerve activity in women exposed to cigarette smoke: a potential mechanism to explain increased cardiac risk. Am J Physiol Heart Circ Physiol 305: H1560–H1567, 2013. doi: 10.1152/ajpheart.00502.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Minami J, Ishimitsu T, Matsuoka H. Effects of smoking cessation on blood pressure and heart rate variability in habitual smokers. Hypertension 33: 586–590, 1999. doi: 10.1161/01.HYP.33.1.586. [DOI] [PubMed] [Google Scholar]
- 47.Morrow JD, Frei B, Longmire AW, Gaziano JM, Lynch SM, Shyr Y, Strauss WE, Oates JA, Roberts LJ II. Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N Engl J Med 332: 1198–1203, 1995. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- 48.Nagathihalli NS, Massion PP, Gonzalez AL, Lu P, Datta PK. Smoking induces epithelial-to-mesenchymal transition in non-small cell lung cancer through HDAC-mediated downregulation of E-cadherin. Mol Cancer Ther 11: 2362–2372, 2012. doi: 10.1158/1535-7163.MCT-12-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peluffo G, Calcerrada P, Piacenza L, Pizzano N, Radi R. Superoxide-mediated inactivation of nitric oxide and peroxynitrite formation by tobacco smoke in vascular endothelium: studies in cultured cells and smokers. Am J Physiol Heart Circ Physiol 296: H1781–H1792, 2009. doi: 10.1152/ajpheart.00930.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Porter GA, Urciuoli WR, Brookes PS, Nadtochiy SM. SIRT3 deficiency exacerbates ischemia-reperfusion injury: implication for aged hearts. Am J Physiol Heart Circ Physiol 306: H1602–H1609, 2014. doi: 10.1152/ajpheart.00027.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prasad S, Sajja RK, Kaisar MA, Park JH, Villalba H, Liles T, Abbruscato T, Cucullo L. Role of Nrf2 and protective effects of Metformin against tobacco smoke-induced cerebrovascular toxicity. Redox Biol 12: 58–69, 2017. doi: 10.1016/j.redox.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rahman MM, Laher I. Structural and functional alteration of blood vessels caused by cigarette smoking: an overview of molecular mechanisms. Curr Vasc Pharmacol 5: 276–292, 2007. doi: 10.2174/157016107782023406. [DOI] [PubMed] [Google Scholar]
- 54.Rahman SM, Ji X, Zimmerman LJ, Li M, Harris BK, Hoeksema MD, Trenary IA, Zou Y, Qian J, Slebos RJ, Beane J, Spira A, Shyr Y, Eisenberg R, Liebler DC, Young JD, Massion PP. The airway epithelium undergoes metabolic reprogramming in individuals at high risk for lung cancer. JCI Insight 1: e88814, 2016. doi: 10.1172/jci.insight.88814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raza H, John A, Nemmar A. Short-term effects of nose-only cigarette smoke exposure on glutathione redox homeostasis, cytochrome P450 1A1/2 and respiratory enzyme activities in mice tissues. Cell Physiol Biochem 31: 683–692, 2013. doi: 10.1159/000350087. [DOI] [PubMed] [Google Scholar]
- 57.Rinaldi M, Maes K, De Vleeschauwer S, Thomas D, Verbeken EK, Decramer M, Janssens W, Gayan-Ramirez GN. Long-term nose-only cigarette smoke exposure induces emphysema and mild skeletal muscle dysfunction in mice. Dis Model Mech 5: 333–341, 2012. doi: 10.1242/dmm.008508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, Ahmed M, Aksut B, Alam T, Alam K, Alla F, Alvis-Guzman N, Amrock S, Ansari H, Ärnlöv J, Asayesh H, Atey TM, Avila-Burgos L, Awasthi A, Banerjee A, Barac A, Bärnighausen T, Barregard L, Bedi N, Belay Ketema E, Bennett D, Berhe G, Bhutta Z, Bitew S, Carapetis J, Carrero JJ, Malta DC, Castañeda-Orjuela CA, Castillo-Rivas J, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol 70: 1–25, 2017. doi: 10.1016/j.jacc.2017.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sacco RL, Benjamin EJ, Broderick JP, Dyken M, Easton JD, Feinberg WM, Goldstein LB, Gorelick PB, Howard G, Kittner SJ, Manolio TA, Whisnant JP, Wolf PA. American Heart Association Prevention Conference. IV. Prevention and rehabilitation of stroke. Risk factors. Stroke 28: 1507–1517, 1997. doi: 10.1161/01.STR.28.7.1507. [DOI] [PubMed] [Google Scholar]
- 60.Sainsbury CA, Coleman J, Brady AJ, Connell JM, Hillier C, Petrie JR. Endothelium-dependent relaxation is resistant to inhibition of nitric oxide synthesis, but sensitive to blockade of calcium-activated potassium channels in essential hypertension. J Hum Hypertens 21: 808–814, 2007. doi: 10.1038/sj.jhh.1002226. [DOI] [PubMed] [Google Scholar]
- 61.Saleh MA, McMaster WG, Wu J, Norlander AE, Funt SA, Thabet SR, Kirabo A, Xiao L, Chen W, Itani HA, Michell D, Huan T, Zhang Y, Takaki S, Titze J, Levy D, Harrison DG, Madhur MS. Lymphocyte adaptor protein LNK deficiency exacerbates hypertension and end-organ inflammation. J Clin Invest 125: 1189–1202, 2015. doi: 10.1172/JCI76327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shimosato T, Geddawy A, Tawa M, Imamura T, Okamura T. Chronic administration of nicotine-free cigarette smoke extract impaired endothelium-dependent vascular relaxation in rats via increased vascular oxidative stress. J Pharmacol Sci 118: 206–214, 2012. [Erratum in J Pharmacol Sci 128: 58, 2015.] doi: 10.1254/jphs.11187FP. [DOI] [PubMed] [Google Scholar]
- 63.Sies H. Role of metabolic H2O2 generation: redox signaling and oxidative stress. J Biol Chem 289: 8735–8741, 2014. doi: 10.1074/jbc.R113.544635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Simon G, Abraham G, Cserep G. Pressor and subpressor angiotensin II administration. Two experimental models of hypertension. Am J Hypertens 8: 645–650, 1995. doi: 10.1016/0895-7061(95)00047-S. [DOI] [PubMed] [Google Scholar]
- 65.Sol EM, Wagner SA, Weinert BT, Kumar A, Kim HS, Deng CX, Choudhary C. Proteomic investigations of lysine acetylation identify diverse substrates of mitochondrial deacetylase SIRT3. PLoS One 7: e50545, 2012. doi: 10.1371/journal.pone.0050545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tao R, Vassilopoulos A, Parisiadou L, Yan Y, Gius D. Regulation of MnSOD enzymatic activity by SIRT3 connects the mitochondrial acetylome signaling networks to aging and carcinogenesis. Antioxid Redox Signal 20: 1646–1654, 2014. doi: 10.1089/ars.2013.5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tippetts TS, Winden DR, Swensen AC, Nelson MB, Thatcher MO, Saito RR, Condie TB, Simmons KJ, Judd AM, Reynolds PR, Bikman BT. Cigarette smoke increases cardiomyocyte ceramide accumulation and inhibits mitochondrial respiration. BMC Cardiovasc Disord 14: 165, 2014. doi: 10.1186/1471-2261-14-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsuchiya M, Asada A, Kasahara E, Sato EF, Shindo M, Inoue M. Smoking a single cigarette rapidly reduces combined concentrations of nitrate and nitrite and concentrations of antioxidants in plasma. Circulation 105: 1155–1157, 2002. doi: 10.1161/hc1002.105935. [DOI] [PubMed] [Google Scholar]
- 69.van der Toorn M, Slebos DJ, de Bruin HG, Leuvenink HG, Bakker SJ, Gans RO, Koëter GH, van Oosterhout AJ, Kauffman HF. Cigarette smoke-induced blockade of the mitochondrial respiratory chain switches lung epithelial cell apoptosis into necrosis. Am J Physiol Lung Cell Mol Physiol 292: L1211–L1218, 2007. doi: 10.1152/ajplung.00291.2006. [DOI] [PubMed] [Google Scholar]
- 70.Vásquez-Vivar J, Hogg N, Martásek P, Karoui H, Pritchard KA Jr, Kalyanaraman B. Tetrahydrobiopterin-dependent inhibition of superoxide generation from neuronal nitric oxide synthase. J Biol Chem 274: 26736–26742, 1999. doi: 10.1074/jbc.274.38.26736. [DOI] [PubMed] [Google Scholar]
- 71.Virdis A, Bacca A, Colucci R, Duranti E, Fornai M, Materazzi G, Ippolito C, Bernardini N, Blandizzi C, Bernini G, Taddei S. Endothelial dysfunction in small arteries of essential hypertensive patients: role of cyclooxygenase-2 in oxidative stress generation. Hypertension 62: 337–344, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00995. [DOI] [PubMed] [Google Scholar]
- 72.Warnholtz A, Wendt M, August M, Münzel T. Clinical aspects of reactive oxygen and nitrogen species. Biochem Soc Symp 71: 121–133, 2004. doi: 10.1042/bss0710121. [DOI] [PubMed] [Google Scholar]
- 73.Widder JD, Guzik TJ, Mueller CF, Clempus RE, Schmidt HH, Dikalov SI, Griendling KK, Jones DP, Harrison DG. Role of the multidrug resistance protein-1 in hypertension and vascular dysfunction caused by angiotensin II. Arterioscler Thromb Vasc Biol 27: 762–768, 2007. doi: 10.1161/01.ATV.0000259298.11129.a2. [DOI] [PubMed] [Google Scholar]
- 74.Wind S, Beuerlein K, Armitage ME, Taye A, Kumar AH, Janowitz D, Neff C, Shah AM, Wingler K, Schmidt HH. Oxidative stress and endothelial dysfunction in aortas of aged spontaneously hypertensive rats by NOX1/2 is reversed by NADPH oxidase inhibition. Hypertension 56: 490–497, 2010. doi: 10.1161/HYPERTENSIONAHA.109.149187. [DOI] [PubMed] [Google Scholar]
- 75.Yin H, Vergeade A, Shi Q, Zackert WE, Gruenberg KC, Bokiej M, Amin T, Ying W, Masterson TS, Zinkel SS, Oates JA, Boutaud O, Roberts LJ II. Acetaminophen inhibits cytochrome c redox cycling induced lipid peroxidation. Biochem Biophys Res Commun 423: 224–228, 2012. doi: 10.1016/j.bbrc.2012.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yu W, Dittenhafer-Reed KE, Denu JM. SIRT3 protein deacetylates isocitrate dehydrogenase 2 (IDH2) and regulates mitochondrial redox status. J Biol Chem 287: 14078–14086, 2012. doi: 10.1074/jbc.M112.355206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang WZ, Venardos K, Chin-Dusting J, Kaye DM. Adverse effects of cigarette smoke on NO bioavailability: role of arginine metabolism and oxidative stress. Hypertension 48: 278–285, 2006. doi: 10.1161/01.HYP.0000231509.27406.42. [DOI] [PubMed] [Google Scholar]
- 78.Zinkevich NS, Gutterman DD. ROS-induced ROS release in vascular biology: redox-redox signaling. Am J Physiol Heart Circ Physiol 301: H647–H653, 2011. doi: 10.1152/ajpheart.01271.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]