Immediate intervention by percutaneous coronary intervention or coronary artery bypass grafting can limit infarct size in myocardial infarction. However, the damage is not limited to the initial infarct, as ischemia-reperfusion (I/R) injury results from processes after reperfusion, which can cause other pathological conditions including myocardial stunning, ventricular arrhythmia, hemodynamic abnormalities, and, in the long term, development of heart failure. Ubiquitin is an important protein for regulating protein turnover via the ubiquitin-proteasome system (UPS). The UPS has been well studied in the context of cell and cancer biology, but accumulating evidence suggests that the UPS has an important role in the physiological condition of the heart and UPS dysfunction plays a role in cardiac pathologies. Additionally, both endogenous and exogenous ubiquitin play key roles in cardiovascular disease, including I/R injury. In a recent article published in the American Journal of Physiology-Heart and Circulatory Physiology, Scofield et al. (8) reported that in I/R models, ubiquitin treatment prevented left ventricular remodeling and preserved cardiac function, accompanied by suppression of the inflammatory response that typically occurs after I/R injury. It has already been reported that exogenous ubiquitin attenuates the inflammatory reaction, but Scofield et al. (8) are the first to report that exogenous ubiquitin protects the heart against I/R injury.
THE UPS IN THE HEART
The UPS is the major pathway for intracellular protein degradation in many organs, including the heart (5). It is now obvious that all parts of the cell involve the UPS, including the nucleus, cytoplasm, membrane proteins, and lumen of the endoplasmic reticulum. The UPS controls many vital processes such as the cell cycle, cell signaling, apoptosis, immune response, antigen presentation via the immunoproteasome, and protein turnover under pathophysiological conditions. It is known that the UPS has two main general steps: step 1 is ubiquitination, which enzymatically and covalently attaches multiple ubiquitin proteins to label target proteins, and step 2 is proteasome-mediated degradation of the ubiquitinated protein molecule. In step 1, there are three types of ubiquitination enzymes: ubiquitin activating enzyme (E1), which activates ubiquitin in an ATP-dependent manner to form a high-energy intermediate; ubiquitin carrier protein (E2), which generates a poly-ubiquitin chain by transferring ubiquitin from E1 to itself; and ubiquitin protein ligase (E3), which transfers ubiquitin from E2 enzymes to the substrate, typically via attachment of ubiquitin to a lysine residue. In step 2, the ubiquitinated proteins are degraded by a multienzymatic complex called the 26S proteasome, which is composed of 19S regulatory and 20S core particles. The 20S subpopulations have several proteolytic subunits that differ in their incorporation and position in proteasomes. In cardiac tissue, six proteolytic subunits are coexpressed by six genes. Two-dimensional electrophoresis analysis of cardiac 20S proteasomes revealed that not all of these subunits are integrated equally and that proteolytic subunits β1, β2, and β5 are integrated at much higher levels than β1i, β2i, and β5i. Those subunits determine where assembled proteasomes cleave polypeptides, such as after acidic (β1), alkaline (β2), and hydrophobic (β5) amino acid residues, which are known as having caspase-, trypsin-, and chymotrypsin-like activity, respectively.
In the heart, several cardiac ubiquitin ligases (E3 enzymes), such as atrogin-1, the COOH-terminus of heart shock protein 70-interacting protein, the muscle RING finger family, and murine double minute 2 protein, are involved in cardiac physiology (5). The UPS also strictly regulates the β2-adrenergic signal pathway in the heart. Activation of β2-adrenergic receptors (β2-ARs) stimulates their immediate ubiquitination, inhibition of proteasomes that degrade β2-ARs, and a reduction of β2-AR internalization. Consequently, the UPS regulates cardiac signal transduction pathways and transcription factors such as calcineurin, which is associated with regulation or promotion of pathologic hypertrophic growth of cardiomyocytes. Additionally, the UPS regulates glycogen synthase kinase-3β, which inhibits cardiac hypertrophy in pathophysiological conditions.
THE ROLE OF UBIQUITIN IN INFLAMMATION
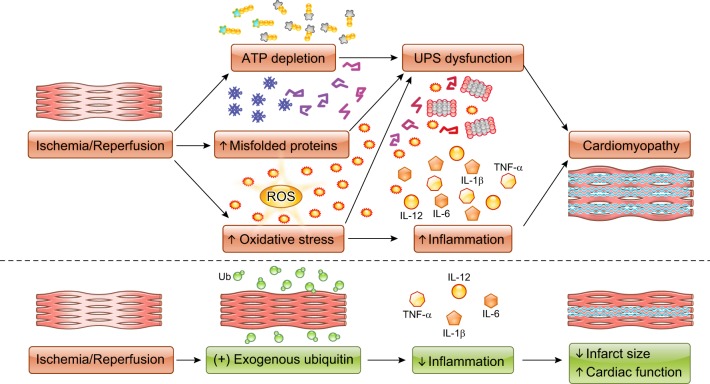
The UPS is not only important in protein degradation but is also involved in multiple inflammatory processes such as NF-κB activation (4). In addition to its intracellular functions, extracellular ubiquitin is also involved in inflammation (Fig. 1) (9). Extracellular ubiquitin has been identified as an endogenous agonist of chemokine (C-X-C motif) receptor 4 (CXCR4). Extracellular ubiquitin binds to CXCR4 on various hematopoietic cells, such as monocytes, B cells, and T cells, and induces Ca2+ influx into those cells (7). In the heart, extracellular ubiquitin interacts with CXCR4 and affects proliferation of cardiac fibroblasts via the ERK1/2 pathway (9). Extracellular ubiquitin also increases angiogenic factors in cardiac endothelial cells, such as vascular endothelial growth factor-A and matrix metalloproteinase (MMP)-2 (10). In the present article, Scofield et al. (8) reported that exogenous ubiquitin can attenuate cardiac inflammation and protect cardiac function after I/R injury. After I/R injury, infiltrating immune cells cause prolonged and/or excessive cytokine/chemokine release, resulting in immune-mediated destruction of cardiomyocytes. Scofield et al. also revealed that exogenous ubiquitin strikingly decreased infarct size after I/R and attenuated inflammatory responses in the heart, such as infiltration of leukocytes and macrophages. Moreover, exogenous ubiquitin increased the expression of MMP-2 and MMP-9, which might increase degradation of the extracellular matrix and contribute to the reduction of infarct size. These findings suggest that extracellular ubiquitin has the potential for new therapeutic applications to limit I/R injury via suppression of cardiac inflammation and fibrosis, and future studies that further detail and link ubiquitin receptor activity to its effects on inflammatory cells and cytokines will help to further refine these approaches.
Fig. 1.
Schematic view depicting the role of ubiquitin in ischemia-reperfusion injury. UPS, ubiquitin-proteasome system; ROS, reactive oxygen species; TNF-α, tumor necrosis factor-α; IL, interleukin; Ub, ubiquitin; ATP, adenosine triphosphate.
UBIQUITIN IN CARDIAC I/R INJURY
The involvement of proteasome dysfunction in myocardial I/R injury was first reported by Bulteau et al. (1). They demonstrated that trypsin-like activity was decreased after left anterior descending coronary artery occlusion in vivo. After a decade, it was reported that ATP-dependent proteasome activity decreases in the isolated perfused heart, which suggested 26S-proteasome dysfunction consistent with increases in myocardial ubiquitinated proteins (Fig. 1) (6). Consistent with that finding, genetic manipulation resulting in proteasome functional insufficiency was found to worsen I/R injury. Recently, Hu et al. (3) reported that cardiomyocyte-restricted knockout of ubiquilin 1, an extraproteasomal ubiquitin receptor, inhibited myocardial ubiquitination-proteasome coupling and resulted in exacerbated cardiac dysfunction and expanded post-I/R infarct size. These insights suggest that the UPS has a strong possibility of being a therapeutic target in preventing I/R injury. However, several conflicting findings exist. The experimental use of proteasome inhibitors attenuates I/R injury, resulting in reduced infarct size. In contrast, it has been described that I/R injury inhibits proteasome function, and, conversely, improving proteasome function results in protection against I/R injury in mice (5). There are several possible mechanisms for proteasome dysfunction in I/R injury, such as ATP depletion, direct inhibition by protein aggregates, and oxidative damage to proteasome and/or regulatory subunits. Further investigation will be needed to elucidate more detailed mechanisms that will hopefully reconcile seemingly contradictory findings and untangle the current complex relationship between ubiquitin and proteasome activity in I/R injury (Fig. 1).
Scofield et al. (8) demonstrated the protective effects of exogenous ubiquitin against I/R injury in mouse models. The authors’ group has previously shown that treatment with ubiquitin prevented myocardial remodeling induced by chronic adrenergic receptor stimulation, at least in part by Akt activation (2). In the present article, the authors demonstrated that exogenous ubiquitin prevented left ventricular remodeling and preserved cardiac function after I/R injury. The cardioprotective effects of ubiquitin treatment presented itself relatively fast (16 h after treatment). Although the mechanisms behind the effects of ubiquitin on remodeling and preservation of cardiac function need to be investigated, exogenous ubiquitin seems to be a potential therapeutic approach for I/R injury. In addition to the antiapoptotic effects that were examined in the study and the signaling pathways detailed in previous studies, exogenous ubiquitin may have effects on other cell death systems, receptors, or signaling pathways that mediate I/R injury.
CONCLUSIONS
The UPS is a quality control system in the mammalian body that excludes harmful and useless aggregated proteins. In the heart, the UPS acts in an essential role in intracellular pathways, including apoptosis, transcriptional regulation, and inflammation. In the present article, Scofield et al. (8) demonstrated the role of exogenous ubiquitin in cardioprotection against I/R injury. This discovery not only demonstrates a new role for ubiquitin outside the cell but also a potential future therapeutic use of ubiquitin in treating patients with acute myocardial infarction.
GRANTS
This work was supported in part by National Institute of General Medical Sciences Grant P20-GM-113134 (to T. Matsui).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.K., J.K.H., and T.M. prepared figures; M.K., J.K.H., and T.M. drafted manuscript; M.K., J.K.H., and T.M. edited and revised manuscript; M.K., J.K.H., and T.M. approved final version of manuscript.
REFERENCES
- 1.Bulteau AL, Lundberg KC, Humphries KM, Sadek HA, Szweda PA, Friguet B, Szweda LI. Oxidative modification and inactivation of the proteasome during coronary occlusion/reperfusion. J Biol Chem 276: 30057–30063, 2001. doi: 10.1074/jbc.M100142200. [DOI] [PubMed] [Google Scholar]
- 2.Daniels CR, Foster CR, Yakoob S, Dalal S, Joyner WL, Singh M, Singh K. Exogenous ubiquitin modulates chronic β-adrenergic receptor-stimulated myocardial remodeling: role in Akt activity and matrix metalloproteinase expression. Am J Physiol Heart Circ Physiol 303: H1459–H1468, 2012. doi: 10.1152/ajpheart.00401.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu C, Tian Y, Xu H, Pan B, Terpstra EM, Wu P, Wang H, Li F, Liu J, Wang X. Inadequate ubiquitination-proteasome coupling contributes to myocardial ischemia-reperfusion injury. J Clin Invest 128: 5294–5306, 2018. doi: 10.1172/JCI98287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwai K. Diverse roles of the ubiquitin system in NF-κB activation. Biochim Biophys Acta 1843: 129–136, 2014. doi: 10.1016/j.bbamcr.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Pagan J, Seto T, Pagano M, Cittadini A. Role of the ubiquitin proteasome system in the heart. Circ Res 112: 1046–1058, 2013. doi: 10.1161/CIRCRESAHA.112.300521. [DOI] [PubMed] [Google Scholar]
- 6.Powell SR, Divald A. The ubiquitin-proteasome system in myocardial ischaemia and preconditioning. Cardiovasc Res 85: 303–311, 2010. doi: 10.1093/cvr/cvp321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saini V, Marchese A, Majetschak M. CXC chemokine receptor 4 is a cell surface receptor for extracellular ubiquitin. J Biol Chem 285: 15566–15576, 2010. doi: 10.1074/jbc.M110.103408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scofield SLC, Dalal S, Lim KA, Thrasher PR, Daniels CR, Peterson JM, Singh M, Singh K. Exogenous ubiquitin reduces inflammatory response and preserves myocardial function 3 days post ischemia/reperfusion injury. Am J Physiol Heart Circ Physiol. doi: 10.1152/ajpheart.00654.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scofield SLC, Daniels CR, Dalal S, Millard JA, Singh M, Singh K. Extracellular ubiquitin modulates cardiac fibroblast phenotype and function via its interaction with CXCR4. Life Sci 211: 8–16, 2018. doi: 10.1016/j.lfs.2018.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steagall RJ, Daniels CR, Dalal S, Joyner WL, Singh M, Singh K. Extracellular ubiquitin increases expression of angiogenic molecules and stimulates angiogenesis in cardiac microvascular endothelial cells. Microcirculation 21: 324–332, 2014. doi: 10.1111/micc.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]