Abstract
Cardiovascular-related pathologies are the single leading cause of death in patients with chronic kidney disease (CKD). Previously, we found that a 5/6th nephrectomy model of CKD leads to an upregulation of miR-21-5p in the left ventricle, targeting peroxisome proliferator-activated receptor-α and altering the expression of numerous transcripts involved with fatty acid oxidation and glycolysis. In the present study, we evaluated the potential for knockdown or overexpression of miR-21-5p to regulate lipid content, lipid peroxidation, and mitochondrial respiration in H9C2 cells. Cells were transfected with anti-miR-21-5p (40 nM), pre-miR-21-5p (20 nM), or the appropriate scrambled oligonucleotide controls before lipid treatment in culture or as part of the Agilent Seahorse XF fatty acid oxidation assay. Overexpression of miR-21-5p attenuated the lipid-induced increase in cellular lipid content, whereas suppression of miR-21-5p augmented it. The abundance of malondialdehyde, a product of lipid peroxidation, was significantly increased with lipid treatment in control cells but attenuated in pre-miR-21-5p-transfected cells. This suggests that miR-21-5p reduces oxidative stress. The cellular oxygen consumption rate (OCR) was increased in both pre-miR-21-5p- and anti-miR-21-5p-transfected cells. Levels of intracellular ATP were significantly higher in anti-mR-21-5p-transfected cells. Pre-miR-21-5p blocked additional increases in OCR in response to etomoxir and palmitic acid. Conversely, anti-miR-21-5p-transfected cells exhibited reduced OCR with both etomoxir and palmitic acid, and the glycolytic capacity was concomitantly reduced. Together, these results indicate that overexpression of miR-21-5p attenuates both lipid content and lipid peroxidation in H9C2 cells. This likely occurs by reducing cellular lipid uptake and utilization, shifting cellular metabolism toward reliance on the glycolytic pathway.
NEW & NOTEWORTHY Both overexpression and suppression of miR-21-5p augment basal and maximal mitochondrial respiration. Our data suggest that reliance on glycolytic and fatty acid oxidation pathways can be modulated by the abundance of miR-21-5p within the cell. miR-21-5p regulation of mitochondrial respiration can be modulated by extracellular lipids.
Keywords: fatty acid oxidation, microRNA, miR-21-5p, mitochondria
INTRODUCTION
Cardiovascular events are the leading cause of death in patients with chronic kidney diseases (CKD). Pathological cardiac changes secondary to CKD include left ventricular hypertrophy, diastolic dysfunction, reduced cardiac function, and/or an increased risk of adverse cardiovascular events (4, 8, 12, 23, 35). Relatively little is known about the mechanisms that mediate the associated cardiac pathology. Previous studies in our laboratory have shown that miR-21-5p abundance is increased within the left ventricle 7 wk after renal insufficiency was induced by 5/6th nephrectomy surgery (7). The increase in miR-21-5p abundance occurs during a time period where several other pathological change are observed in the heart, including chamber dilation, reduced fractional shortening, and lipid accumulation in cardiomyocytes (7). We found that upregulation of miR-21-5p corresponded with a suppression of peroxisome proliferator-activated receptor (PPAR)-α protein expression, which was reversed by suppression of miR-21-5p (7).
PPAR-α is a major regulator of genes associated with mitochondrial fatty acid oxidation (2, 16, 40, 41), the primary pathway for ATP production in a healthy heart. Impaired mitochondrial fatty acid oxidation is observed in heart failure (21, 25, 32). Because we observed a reduction in left ventricular function and increased lipid content in cardiomyoctes when miR-21-5p was elevated, we hypothesized that changes in miR-21-5p expression may regulate mitochondrial fatty acid oxidation in cardiomyocytes. The goal of the present study was to evaluate the effect of miR-21-5p knockdown and overexpression on lipid content, lipid peroxidation, and mitochondrial respiration in H9C2 rat ventricular cardiac myoblasts, a stable cell line used as a model to study cardiomyocyte metabolism.
MATERIALS AND METHODS
Lipid content and lipid peroxidation assessment.
H9C2 cells (American Type Culture Collection), a cardiomyoblast subclone of cells derived from embryonic rat ventricular heart tissue, were cultured in normal-glucose DMEM (Gibco/ThermoFisher Scientific) with 10% FBS (GIBCO/ThermoFisher Scientific) and subcultured onto coverslips for oil red O experiments or 96-well plates for lipid content and lipid peroxidation assays at <25 passages. Cells were transfected with 40 nM anti-miR-21-5p (Exiqon), 20 nM pre-miR-21-5p (Ambion/ThermoFisher Scientific), or the appropriate scrambled (Scr) oligonucleotide controls using Lipofectamine 2000 (Life Technologies/ThermoFisher Scientific) at ~80% confluency. Medium was replaced after 7 h and again 24 h after the initiation of transfection. Half of the cells were treated with lipid [0.66 mM oleic acid and 0.33 mM palmitic acid (OA/PA), Sigma-Aldrich] (42) for 48 h (n = 6/treatment group). For visualization of lipid by microscopy, cells were fixed in 10% formalin and oil red O staining (Sigma-Aldrich) performed as previously described (7). The red lipid signal was visualized, and images were captured using a Nikon E-400 microscope (Nikon Instruments) and acquired using a SPOT Insight digital camera (Diagnostic Instruments). Cells were then analyzed for lipid content by AdipoRed Assay (Lonza) or Lipid Peroxidation Assay (Sigma-Aldrich) for measurement of malondialdehyde (MDA), a product of lipid peroxidation. At the end of each experiment, total protein was evaluated in each well using a DC protein assay. This value was used to normalize the detected signal.
Cell culture and treatments for mitochondrial respiration assessment, glucose utilization assay, ATP content, and lactate production.
H9C2 cells (subcultured at ≤16 passages) were seeded at a density of 7,000 cells/well in a XF96 Seahorse plate. Cells were cultured in normal-glucose DMEM (GIBCO/ThermoFisher Scientific) with 10% FBS (GIBCO/ThermoFisher Scientific) overnight. The following day, cells were then transfected with 40 nM (LNA)-anti-miR-21-5p (Exiqon), 20 nM pre-miR-21-5p, or equimolar concentrations of the appropriate Scr controls for 7 h using Lipofectamine 2000 (Life Technologies). Medium was then replaced. The following day, culture medium was replaced with DMEM with 1 g/l d-glucose containing 10% FBS, and cells were cultured for an additional 24 h. This allowed cells to grow for a full 48 h after transfection of the oligonucleotides before measurements of mitochondrial respiration were performed with a Seahorse XF Analyzer with the Palmitate-BSA FAO Substrate Kit (Agilent). Cells used for glucose consumption (Glucose-Glo Assay, Promega), cellular ATP content (Luminescent ATP Detection Assay Kit, Abcam), or lactate production (Lactate-Glo Assay, Promega) were at passages 19–23 at the time of the experiment and were also treated as described above. On the day of the assay, medium was replaced with Seahorse assay medium, and assays were performed according to the manufacturer’s guidelines.
Mitochondrial respiration analysis using the Seahorse XF Analyzer.
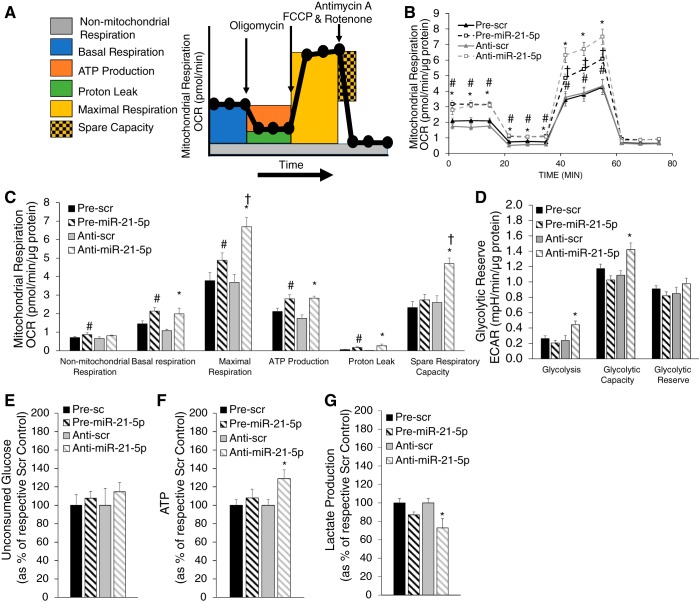
The Seahorse XF mitochondrial respiration analysis was performed at the Medical College of Wisconsin Redox and Bioenergetics Shared Resource Center. The day of the Seahorse assay, medium in the cell culture plates was exchanged for substrate limited medium for fatty acid oxidation medium and incubated for 30 min. Etomoxir (ETO; 40 μM final, Agilent) was added to half of the wells from each transfection group and allowed to incubate for 15 min. Some cells were also treated with bis-2-(5-phenylacetamido-1,3,4-thiadiazol-2-yl)ethyl sulfide (BPTES; 20 μM), UK5099 (1 μM), or clofibrate (200 μM), as indicated in the figures. Palmitate-BSA or BSA control were then added, and analysis using the XF assay was initiated. Components of the Cell Mito Stress Test (Agilent) were used to evaluate mitochondrial function (see Fig. 3A for a description) at the following final concentrations: 1.25 μM oligomycin, 3 mM FCCP, and 1 μM/1 μM rotenone/antimycin A. After analysis, cellular protein levels in each well were evaluated by DC protein assay analysis, and this value was used to normalize readings from the Seahorse XF Analyzer.
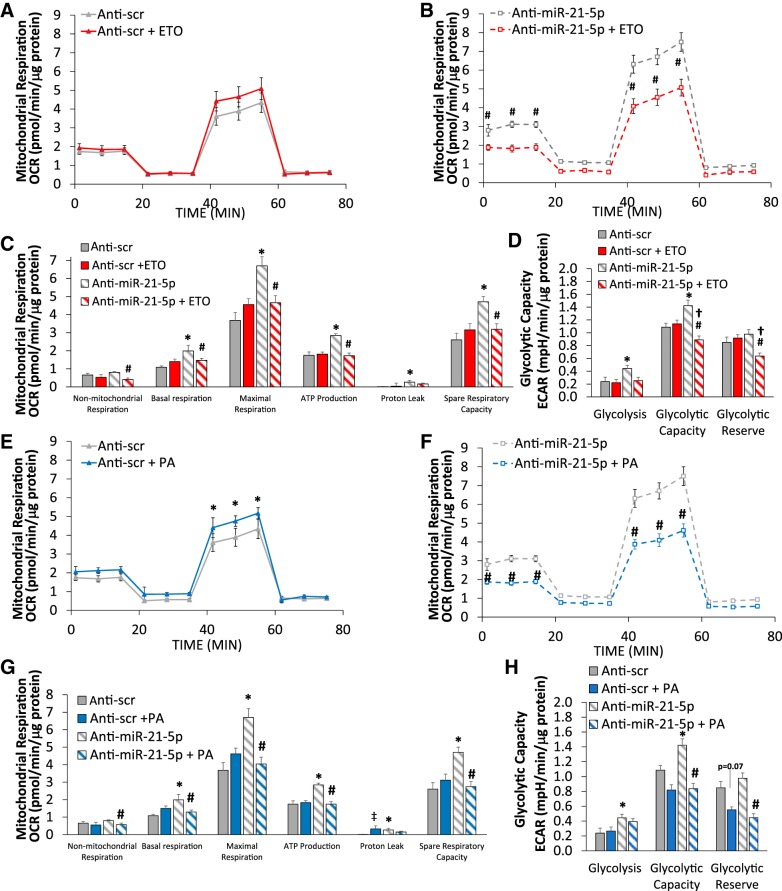
Fig. 3.
Baseline assessment of mitochondrial respiration with miR-21-5p overexpression and suppression. H9C2 cells were transfected with either pre-miR-21-5p (20 nM), anti-miR-21-5p (40 nM), or the appropriate scrambled (Scr) controls at the same concentration and then analyzed by the Seahorse XF FAO assay. A: protocol used in data collection and calculations for evaluation of mitochondrial respiration. All measured oxygen consumption rate (OCR) levels were normalized by total cellular protein. B: normalized OCR in pre-miR-21-5p and anti-miR-21-5p-transfected cells compared with their respective scrambled (Scr) control groups. C: measures of mitochondrial respiration calculated from the OCR trace in B. Both pre-miR-21-5p and anti-miR-21-5p-transfected cells exhibited increased mitochondrial respiration by several measures, with anti-miR-21-5p inducing large increases in both maximal respiration and spare respiratory capacity. D: glycolysis and glycolytic capacity, as indicated by extracellular acidification rate (ECAR), were significantly elevated in anti-miR-21-5p but not pre-miR-21-5p. E: residual glucose after 48 h of incubation and normalized by total cellular protein. F: cellular ATP (normalized by total protein) was significantly elevated in anti-miR-21-5p-treated cells, consistent with the elevated spare respiratory capacity in C and glycolytic capacity in D. Data are reported as means ± SE; n = 6–12. *P < 0.05 anti-miR-21-5p vs. anti-Scr; #P < 0.05 pre-miR-21-5p vs. pre-Scr; †P < 0.05 anti-miR-21-5p vs. pre-miR-21-5p by repeated-measures ANOVA (B) or one-way ANOVA (C−F).
Western blot analysis.
Protein was isolated from cells treated as described in Lipid content and lipid peroxidation assessment, as previously described (7). Extracted protein was quantified by the DC assay to standardize 10 μg protein loaded per lane. Western blots were preformed to probe for hexokinase II (ab214938, Abcam), pyruvate dehydrogenase E1 (ODPA; ab110330, Abcam), Ser293 phosphorylated pyruvate dehydrogenase (Ser293 p-ODPA; ab177461, Abcam), pyruvate dehydrogenase 2 (ab68164, Abcam), pyruvate dehydrogenase 4 (ab214938, Abcam), acetyl coA transferase 1 (ab168342, Abcam), and carnitine palmitoyltransferase (CPT; ab53432, Abcam). After the antibody-based blot, membranes were stained with Coomassie blue and imaged. The protein signal intensity was quantified to again normalize the Western blot signal by the amount of protein loaded (7, 22).
RESULTS
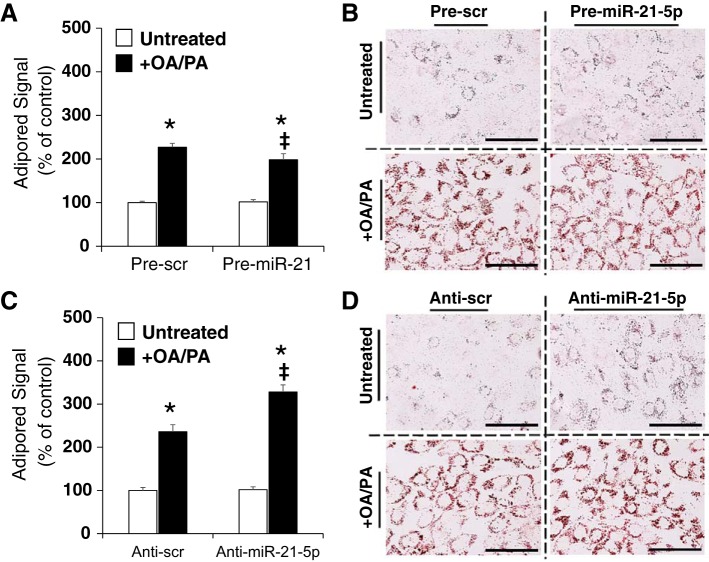
miR-21-5p regulates intracellular lipid content with OA/PA treatment.
H9C2 cells were transfected with oligonucleotides followed by lipid treatment as described in materials and methods. Lipid content was quantified by AdipoRed assay and visualized by microscopic imaging of oil red O-stained cells. Lipid content was not impacted by pre-miR-21-5p transfection in untreated cells, but the increase in lipid content in response to OA/PA treatment was attenuated in pre-miR-21-transfected cells (to 87% of pre-Scr levels; Fig. 1A). This was also apparent in oil red O-stained cells that went through the pre-miR-21-5p transfection and treatment (Fig. 1B). Anti-miR-21-5p transfection had no effect on lipid content in untreated cells (Fig. 1C). However, cells that received OA/PA lipid treatment exhibited an increase in cellular lipid content that was further augmented in cells that had been transfected with anti-miR-21-5p (Fig. 1C). Oil red O staining of cells that completed the same transfection and treatment protocol showed similar changes (Fig. 1D). Together, these data suggest that overexpression of miR-21-5p inhibits lipid accumulation in H9C2 cells.
Fig. 1.
miR-21-5p attenuates the increase in intracellular lipid content after oleic acid and palmitic acid (OA/PA) treatment. H9C2 cells were transfected with anti-miR-21-5p, pre-miR-21-5p, or the appropriate scrambled (Scr) oligonucleotide controls for 24 h followed by 48 h of OA/PA or vehicle treatment. Lipid content was quantified by AdipoRed assay and visualized by oil red O staining. A: anti-miR-21-5p transfection had no effect on lipid content in untreated cells; however, anti-miR-21-5p augmented the increase in lipid content in response to OA/PA treatment. B: representative oil red O staining from anti-miR-21-5p-transfected groups. C: pre-miR-21 transfection had no effect on lipid content in untreated cells, but the increase in lipid content in response to OA/PA treatment was attenuated in pre-miR-21-transfected cells. D: representative oil red O staining from pre-miR-21-5p-transfected groups. Bar = 100 μm. Data are reported as means ± SE. *P < 0.05 vs. untreated control, within transfection; ‡P < 0.05 vs. anti-Scr, same lipid treatment by two-way ANOVA.
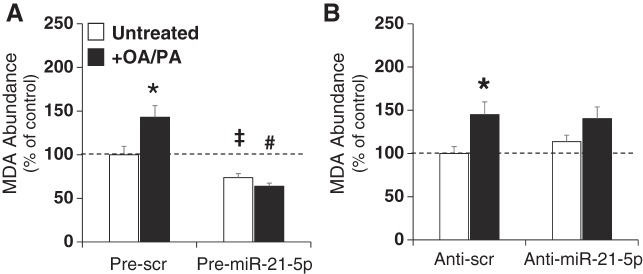
Overexpression of miR-21-5p attenuates lipid peroxidation.
H9C2 cells were treated as described above and then analyzed for MDA content, a byproduct of lipid peroxidation. Treatment with lipid (OA/PA) induced an increase in MDA in both Scr control groups (Fig. 2, A and B). Remarkably, transfection with pre-miR-21 significantly reduced lipid peroxidation both with and without OA/PA treatment (Fig. 2A), indicating that increased lipid peroxidation induced by exogenous lipid treatment is prevented by overexpression of miR-21-5p. This suggests that fatty acid oxidation may be reduced even in the absence of additional exogenous lipid. Cells transfected with anti-miR-21-5p responded similarly to anti-Scr cells with OA/PA, although the increase was not statistically different (Fig. 2B).
Fig. 2.
Alterations in lipid peroxidation with manipulation of miR-21-5p abundance. Lipid peroxidation was evaluated by malondialdehyde (MDA) assay following the transfection and lipid treatment protocol outline shown in Fig. 1 and described in materials and methods. A and B: pre-miR-21-5p treatment attenuated MDA abundance in both oleic acid and palmitic acid (OA/PA)-treated and untreated cells (A); however, anti-miR-21-5p transfection had no effect on MDA with or without lipid treatment (B). Bar = 100 μm. Data are reported as means ± SE. *P < 0.05 vs. control, treatment same transfection; #P < 0.05 vs. anti-scrambled (Scr) untreated; ‡P < 0.05 vs. anti-Scr + OA/PA by two-way ANOVA.
miR-21-5p levels modulate mitochondrial respiration.
Because manipulation of miR-21-5p levels clearly impacted the way in which H9C2 cells handled lipid, we wanted to better understand how manipulation of miR-21-5p would alter mitochondrial respiration in response to addition of exogenous PA. To accomplish this, we evaluated exogenous and endogenous fatty acid oxidation using the Seahorse XF Analyzer in conjunction with the XF Palmitate-BSA FAO Substrate Kit. The addition of 1.25 μM oligomycin, 3 mM FCCP, and 1 μM/1 μM rotenone/antimycin A allowed us to evaluate the contribution and sources of both mitochondrial and nonmitochondrial oxygen consumption to our measured signal. Figure 3A shows the calculations that can be made with the addition of each substrate. Pretreatment of a subset of cells with ETO allowed us to evaluate changes in mitochondrial respiration resulting from blockade of uptake of fatty acid through CPT1.
H9C2 cells were plated into 96-well Seahorse assay plates and transfected with anti-miR-21-5p, pre-miR-21-5p, or the appropriate Scr controls, as described in materials and methods. After 48 h of incubation, cells were prepared for Seahorse analysis. Medium was changed and half of the cells from each transfection group were treated with ETO for 30 min followed by the addition of PA to half of the cells of each ETO-treated group. When we compared mitochondrial respiration rate [oxygen consumption rate (OCR) normalized by total cellular protein in that sample], we found that both anti-miR-21-5p and pre-miR-21-5p increased both basal and maximal respiration rates (Fig. 3B). Further calculation of the implications of these differences on mitochondrial function is shown in Fig. 3C. We found that nonmitochondrial respiration, basal respiration, maximal respiration, ATP production, and proton leak were all significantly increased in response to either pre-miR-21-5p or anti-miR-21-5p compared with their respective control treatment. It should be noted that maximal respiration was significantly higher with anti-miR-21-5p than with pre-miR-21-5p. Interestingly, spare respiratory capacity was unchanged with pre-miR-21-5p but significantly increased with anti-miR-21-5p. Similarly, cellular ATP content was significantly higher in anti-miR-21-5p-treated cells (Fig. 3F).
Extracellular acidification rate (ECAR) was measured in these cells before and immediately after the addition of oligomycin (relative glycolysis and glycolytic capacity respectively, Fig. 3E) allowing us to calculate glycolytic reserve. ECAR measurements suggested that glycolysis and glycolytic capacity were significantly increased with anti-miR-21-5p, and there was a slight but nonsignificant reduction with pre-miR-21-5p (Fig. 3D). Because ECAR measurements are an indirect assessment of glycolysis, we also measured unconsumed glucose and lactate production in the culture medium. Residual glucose 48 h after transfection was not significantly different between groups (Fig. 3E); however, lactate was significantly reduced in anti-miR-21-5p-treated cells (Fig. 3G), indicating that anti-miR-21-5p-treated cells are using less glucose than other groups.
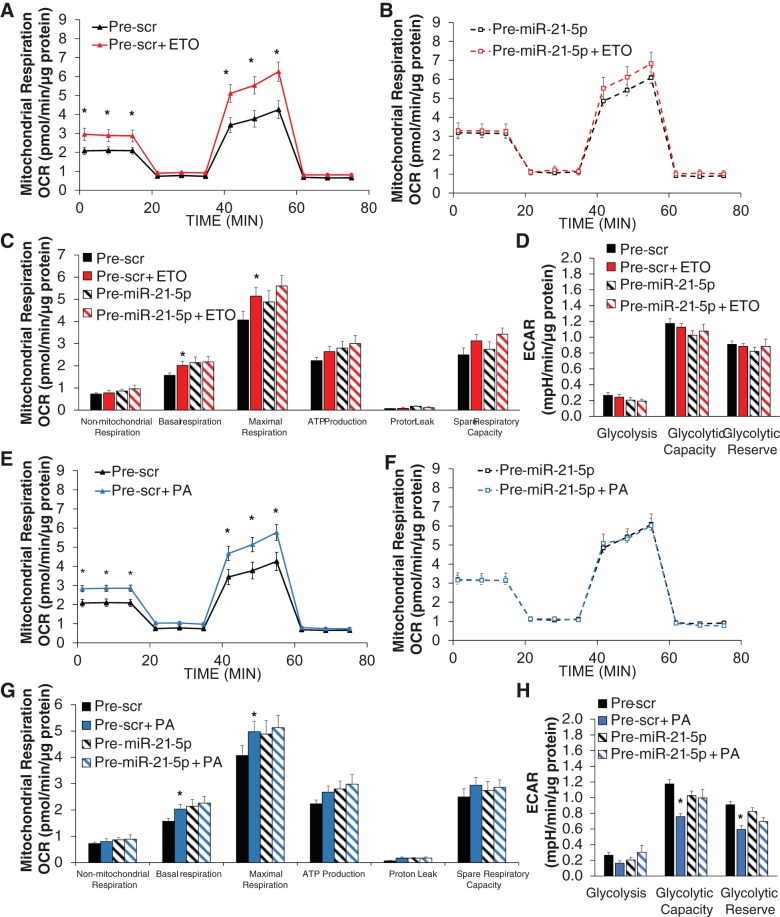
We then examined the effect of overexpression of miR-21-5p on mitochondrial respiration with the addition of ETO or exogenous PA. ETO increased both baseline mitochondrial respiration and maximal respiration in response to FCCP in pre-Scr control cells (Fig. 4A). While mitochondrial respiration was generally higher with pre-miR-21-5p (Fig. 4B) compared with control cells (Fig. 4A), ETO had no effect with elevated miR-21-5p levels (Fig. 4B). These effects were reflected in the functional measurements of mitochondrial respiration (Fig. 4C). ECAR values were not significantly altered in any of these treatments (Fig. 4D).
Fig. 4.
Pre-miR-21-5p-treated cells continue to rely on glycolysis with the addition of palmitic acid (PA). A and B: mitochondrial respiration was increased in pre-scrambled (Scr) cells treated with the carnitine palmitoyltransferase I (CPT1) inhibitor etomoxir (ETO) at baseline and after FCCP treatment (A), whereas no ETO effect was observed in pre-miR-21-5p-transfected cells (B). C and D: these findings were reflected in calculations of mitochondrial respiration (C) with no alteration in extracellular acidification rate (ECAR; D). E–G: mitochondrial respiration was increased in pre-Scr cells treated with PA at baseline and after FCCP treatment (E) but not in pre-miR-21-5p cells (F), resulting in no PA-induced change in basal respiration or maximal respiration (G). H: glycolytic capacity and glycolytic reserve were significantly reduced with PA treatment in pre-Scr, as the availability of PA substrate was increased; however, pre-miR-21-5p cells did not experience this change. Together, this suggests that pre-miR-21-5p-treated cells are unable to undergo a characteristic switch from glycolysis to fatty acid oxidation in the presence of excess lipid. Data are reported as means ± SE; n = 7–12. *P < 0.05, pre-Scr vs. pre-Scr + ETO (A–D) or pre-Scr + PA (E–H) by two-way repeated-measures ANOVA (A, B, E, and F) or two-way ANOVA with the Holm-Sidak method (C, D, G, and H).
The addition of exogenous PA generally increased mitochondrial respiration in pre-Scr controls (Fig. 4E) but had no effect on cells transfected with pre-miR-21-5p (Fig. 4F). Further analysis revealed that pre-miR-21-5p transfection had no effect on evaluated functional measures of mitochondrial respiration (Fig. 4G). Glycolytic capacity and glycolytic reserve were significantly reduced with the addition of PA in pre-Scr control cells, indicating that utilization of glucose was reduced with an increase in the lipid substrate (Fig. 4H). This effect was absent in pre-miR-21-5p-treated cells (Fig. 4H).
We also measured the response of anti-miR-21-5p-transfected cells to ETO and PA (Fig. 5). ETO again tended to increase mitochondrial respiration in anti-Scr-transfected cells (maximal respiration, P = 0.05–0.07; Fig. 5A). In contrast to what was observed in pre-miR-21-5p-transfected cells, ETO reduced baseline and FCCP induced mitochondrial respiration in anti-miR-21-5p-transfected cells (Fig. 5B). In fact, ETO normalized increases in functional measures of mitochondrial respiration induced by anti-miR-21-5p transfection (Fig. 5C). This normalization also caused a sharp reduction in glycolytic capacity and glycolytic reserve (Fig. 5D), suggesting that the large increase in mitochondrial respiration with suppression of miR-21-5p is the result of enhanced fatty acid oxidation rather than glycolysis. Furthermore, this indicates that anti-miR-21-5p-treated cells are unable to switch to a glycolytic fuel source when fatty acid oxidation is inhibited.
Fig. 5.
Etomoxir (ETO) and palmitic acid (PA) reduced mitochondrial respiration in anti-miR-21-5p-transfected cells. A and B: mitochondrial respiration tended to be increased in anti-scrambled (Scr) cells treated with ETO after FCCP treatment (A); however, ETO treatment led to a large reduction in mitochondrial respiration in the anti-miR-21-5p-treated group (B). C: calculated values of the sources of cellular respiration showing that ETO reduced the oxygen consumption rate (OCR) in anti-miR-21-5p-treated cells to the level of anti-Scr controls. D: anti-miR-21-5p-treated cells treated with ETO exhibited a significant reduction in the relative glycolytic capacity and glycolytic reserve, suggesting that with inhibition of carnitine palmitoyltransferase I (CPT1), they were unable undergo a compensatory increase in glycolysis. ECAR, extracellular acidification rate. E and F: mitochondrial respiration tended to be increased in anti-Scr-treated cells treated with PA (E); however, mitochondrial respiration was reduced in PA-treated anti-miR-21-5p-treated cells (F). G: the increase in mitochondrial respiration with anti-miR-21-5p transfection was completely lost when cells were treated with PA. H: anti-miR-21-5p-treated cells treated with PA appropriately reduced their relative glycolytic capacity and glycolytic reserve, similar to anti-Scr. Data are reported as means ± SE; n = 7–8 for pre-Scr. *P < 0.05, anti-Scr vs. anti-miR-21-5p; #P < 0.05, anti-miR-21-5p vs. anti-miR-21-5p + ETO (B−D) or anti-miR-21-5p + PA (F−H); †P < 0.05, anti-Scr + ETO vs. anti-miR-21-5p + ETO; ‡P < 0.05, anti-Scr vs. anti-Scr + PA by two-way repeated-measures ANOVA (A, B, E, and F) or two-way ANOVA with the Holm-Sidak method (C, D, G, and H).
A similar response was observed with the addition of PA, with a slight increase in anti-Scr cells (Fig. 5E) and a significant reduction with anti-miR-21-5p (Fig. 5F). Again, PA normalized increases in functional measures of mitochondrial respiration in anti-miR-21-5p controls. Anti-miR-21-5p-treated cells also exhibited reduced ECAR levels in response to PA treatment (Fig. 5H).
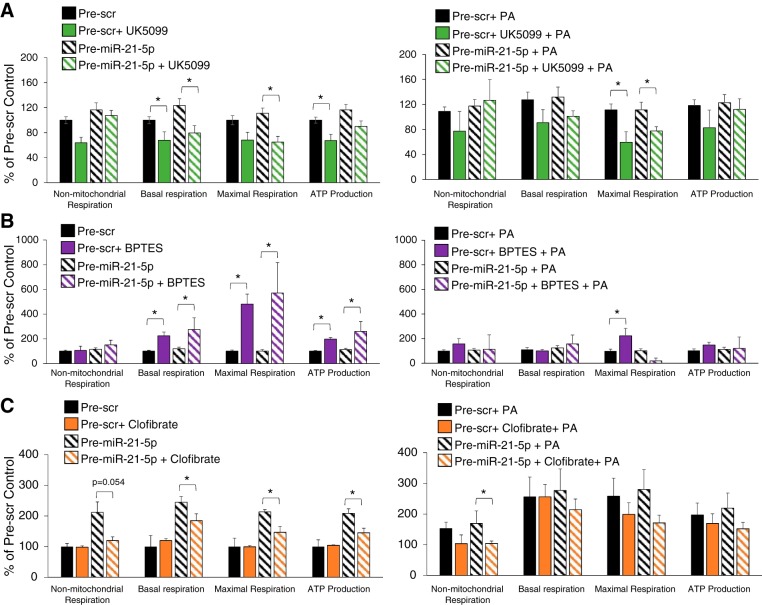
To evaluate the contribution of pyruvate and glutamine to OCR in H9C2 cells, pre-Scr or pre-miR-21-5p-transfected cells were treated with UK5099 or BPTES, respectively, and subjected to the same analysis described above in the presence or absence of PA (Fig. 6, A and B). Treatment with the pyruvate inhibitor UK5099 attenuated measures of mitochondrial respiration and ATP production in pre-Scr and pre-miR-21-5p-treated cells (Fig. 6A, left). Treatment with PA under the same conditions resulted in a similar pattern, with increased variability limiting significant changes to calculations of maximal respiration (Fig. 6A, right). In contrast with UK5099 treatment, treatment with BPTES augmented measures of mitochondrial respiration and ATP production (Fig. 6B, left). The addition of PA largely prevented BPTES-induced increases (Fig. 6B, right). Treatment with the PPAR-α agonist clofibrate reduced nonmitochondrial respiration, mitochondrial respiration, and ATP production in pre-miR-21-5p-treated cells but had no effect on pre-Scr-treated cells, indicating that overexpression of miR-21-5p made them less responsive to PPAR-α stimulation.
Fig. 6.
Cellular respiratory response to inhibition of the mitochondrial pyruvate transporter, glutaminase, or treatment with a peroxisome proliferator-activated receptor (PPAR)-α agonist. Cells were transfected with pre-Scr or pre-miR-21-5p and then treated with the indicated substrates in the presence or absence of palmitic acid (PA). Data are expressed as percentages of the average pre-scrambled (Scr) control value for each measurement. A: mitochondrial respiration and ATP production were reduced by treatment with UK5099 regardless of miR-21-5p abundance (left). This effect was similar in cells treated with PA (right); however, this only reached significance in the measurement of maximal respiration. B: treatment with bis-2-(5-phenylacetamido-1,3,4-thiadiazol-2-yl)ethyl sulfide (BPTES) caused the opposite effect, increasing mitochondrial respiration and ATP production (left). Lipid treatment prevented the BPTES-induced increase, with the exception of a small increase in maximal respiration in the pre-Scr group and a significant reduction in the pre-miR-21-5p-treated group (right). C: clofibrate was added to some cell wells 24 h before the oxygen consumption rate was measured. Clofibrate had no effect on mitochondrial respiration and ATP production in pre-Scr cells (left); however, it was significantly lower in pre-miR-21-5p-treated cells (right). This suggests that overexpression of miR-21-5p limited the ability of the cells to respond to PPAR-α signaling. The addition of PA normalized this response; n = 3-15/group. *P < 0.05, indicated treatment vs. untreated control by two-way ANOVA testing the effect of inhibitor or agonist with the Holm-Sidak method.
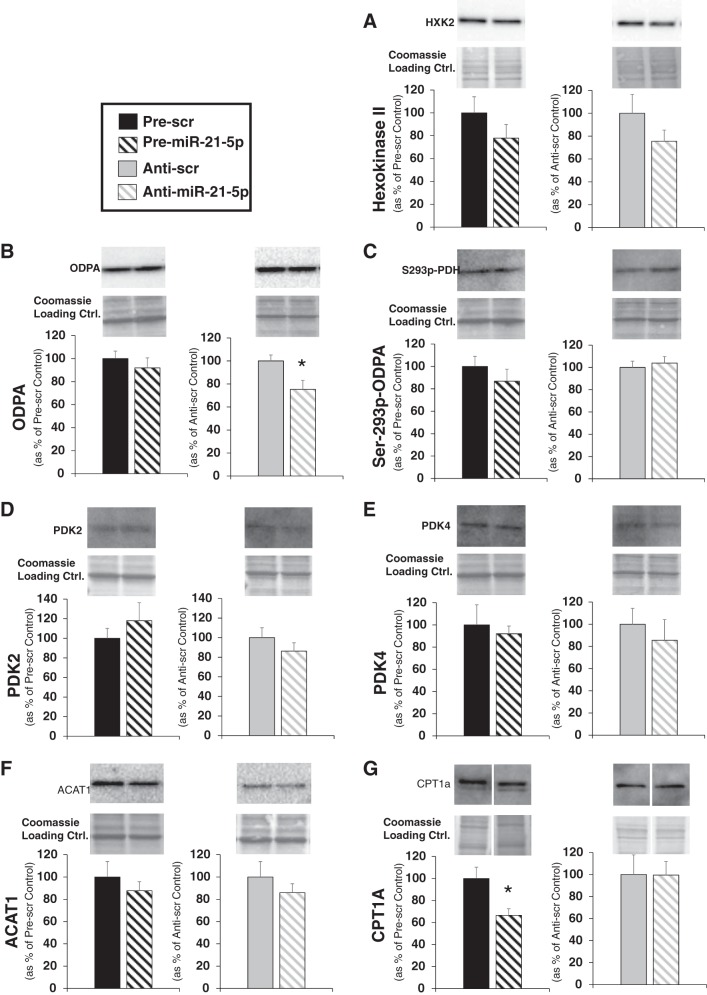
To determine if miR-21-5p may be regulating expression of proteins involved with a shift between fatty acid oxidation and glycolysis, we performed Western blot analysis on several relevant proteins on H9C2 cells that had been transfected with pre-miR-21-5p, anti-miR-21-5p, or their appropriate controls. Hexokinase 2 (Fig. 7A) converts glucose to glucose 6-phosphate, a key step in glycolysis. Pyruvate dehydrogenase E1 (ODPR; Fig. 7B), an enzyme that controls whether pyruvate is converted to acetyl CoA, can be inhibited when Ser293 phosphorylated (Ser293 p-ODPR; Fig. 7C) primarily by pyruvate dehydrogenase kinase 2 (PDK2; Fig. 7D) or pyruvate dehydrogenase 4 (PDK4; Fig. 7E). We observed that pyruvate dehydrogenase E1 (ODPA) expression was significantly reduced with anti-miR-21-5p treatment (Fig. 7B), but none of the other related proteins were significantly altered by miR-21-5p levels. We also measured the abundance of proteins that would indicate whether fatty acid uptake may be altered. Acetyl-CoA acetyltransferase (ACAT1; Fig. 7F) inhibits pyruvate dehydrogenase, reducing the ability to use substrates produced by the glycolytic pathway, but expression was unchanged by alterations in miR-21-5p abundance. CPT1 (Fig. 7G), an enzyme that brings long-chain fatty acids into the mitochondrial membrane for utilization in fatty acid oxidation, was significantly reduced by overexpression of miR-21-5p and pre-miR-21-5p significantly reduced the CPT1a levels in cells (Fig. 7G).
Fig. 7.
Western blot analysis of proteins regulating utilization of substrates derived from glucose oxidation or fatty acid oxidation. Western blot analysis was performed on protein extracted from H9C2 cells transfected with pre-miR-21-5p, anti-miR-21-5p, or scrambled (Scr) oligo controls for 48 h. A–E: primary antibodies suitable for the detection of rat hexokinase II (A), pyruvate dehydrogenase E1 (ODPA; B), Ser293 phosphorylated pyruvate dehydrogenase (p-ODPA; C), pyruvate dehydrogenase kinase 2 (PDK2; D), pyruvate dehydrogenase kinase (PDK4; E), acetyl-CoA acetyltransferase (ACAT1; F), and carnitine palmitoyltransferase I (CPT1a; G) were used to probe for these proteins. Quantification of protein loaded was performed on Coomassie-stained membranes and used to normalize protein expression. Representative bands from the blots, as well as the corresponding region of the Coomassie-stained membrane, are shown above the average expression value for each protein. Data are reported as means ± SE; n = 4/group. *P < 0.05 vs. the appropriate Scr control by a Student’s t-test.
DISCUSSION
Under physiological conditions, adult cardiac tissue is heavily reliant on fatty acid oxidation for ATP production, with up to 70% of ATP being generated through utilization of fatty acid substrates (1, 29, 33, 37, 52). Previous studies have shown that there are dramatic shifts in metabolic pathways with cardiac dysfunction and failure, with a reduced reliance on fatty acid oxidation and a compensatory increased reliance on glycolysis (reviewed extensively elsewhere; Refs. 10 and 13). As heart failure progresses, overall ATP production is reduced (9, 47, 51).
We have previously shown that miR-21-5p suppresses cardiac PPAR-α in the left ventricle of rats with renal insufficiency (7). Increased miR-21-5p levels have been observed in cardiac tissue in numerous models of cardiovascular disease, cardiac dysfunction, and heart failure (7, 38, 46, 48, 50). It is well known that PPAR-α protein abundance is reduced in heart failure (20), and reduced PPAR-α regulates the expression of numerous genes important for fatty acid oxidation-related cellular machinery (2, 16, 40, 41).
A major challenge in studying the regulation of metabolic pathways in cardiomyocytes is the availability of appropriate cell models. Neonatal rodent cardiomyocytes can be isolated, cultured, and transfected (36); however, neonatal cardiomyocytes are glycolytic compared with adult cardiomyocytes. Cardiomyocytes have been isolated from adult rats and cultured; however, they rapidly change their phenotype after isolation, making them incompatible with transfection protocols (34). While the H9C2 cells used in this study are not adult cardiomyocytes, they have been determined to be a more appropriate model of adult cardiomyocyte than the other rodent line available (HL-1, mouse) and they are stable over many passages (24). Furthermore, H9C2 cells have been used to evaluate fatty acid oxidation, as well as OCR, in numerous studies (5, 14, 15, 18, 19, 24, 27, 28, 39, 43, 44, 49).
Very little work has been done to elucidate the role that miR-21-5p plays in cardiac metabolism and cellular respiration. A recent publication by Li et al. (26) indicated that miR-21-5p upregulated the translation of mitochondrial proteins, in what was described as a compensatory mechanism in the spontaneously hypertensive rat model of hypertension-related cardiac pathology. Our observations support their finding that overexpression of miR-21-5p alters mitochondrial respiration; however, we also found that suppression of miR-21-5p can also dramatically impact mitochondrial respiration. The direct measurements of both mitochondrial respiration and ECAR, with the incorporation of exogenous PA and ETO treatments, provide novel insights into changes in mitochondrial substrate utilization induced by alterations in miR-21-5p in H9C2 cells. We find that the impact of miR-21-5p abundance upon cellular respiration can be modulated by available lipid substrates due to apparent differences in the ability of the cells to use that substrate.
ETO is a CPT1 inhibitor. CPT1 is an enzyme essential for shuttling long-chain fatty acids, such as PA, to the mitochondrial membrane for fatty acid oxidation. Thus, ETO is understood to inhibit the major pathway for by which of exogenous long chain fatty acids are used in metabolism. However, due to the substrate flexibility in healthy cardiomyocytes, ETO is unlikely to reduce OCR unless the glycolytic capacity is somehow also limited. It was previously shown that etomoxir treatment increased glucose oxidation in cardiomyocyes (30, 31). In fact, etomoxir use has been explored as a therapeutic treatment for heart failure because, by reducing fatty acid utilization by the mitochondria, cells are forced to become reliant upon glycolysis, which is a more oxygen-efficient metabolic pathway (3, 17, 45). ETO has also been reported to be a PPAR-α agonist as well as a CPT1 inhibitor (53). Since cardiomyocytes are heavily reliant on both PPAR-α signaling and fatty acid transport through CPT1 for normal function, these complex interactions likely explain why ETO has mixed effects in H9C2 cells depending on changes induced by miR-21-5p levels and substrate availability. Future studies under highly controlled cell culture conditions with isolated substrates will be important.
We also learned that H9C2 cells are flexible in the type of substrate that they can use. When we inhibited mitochondrial pyruvate transporter using UK5099, mitochondrial respiration and ATP production was reduced, but when glutaminase was inhibited by BPTES, these values increased. These observations are consistent with what might be expected in cells that do not rely on glutamine oxidation. Here, the metabolic shifts caused by changes in miR-21-5p expression seem to occur as a result of altered molecular machinery related to glycolytic and fatty acid oxidation pathways. This is demonstrated by the way in which cells respond to an acute treatment with relevant metabolic pathway inhibitors, such as ETO or UK5099. Our data indicate that overexpression of miR-21-5p increased mitochondrial respiration, but this increase is not impacted by inhibition of exogenous fatty acid utilization (by ETO treatment) or in response to additional PA (Fig. 4, A and G). Furthermore, overexpression of miR-21-5p reduced the abundance of CPT1 protein. Together, this suggests that the increase in mitochondrial respiration observed by overexpression of miR-21-5p may be through an enhanced ability to use the glycolytic pathway. The reduced lipid peroxidation in untreated and lipid-treated pre-miR-21-5p cells also suggests an overall reduction in cellular fatty acid uptake and/or a reduction in oxidative stress (see summary in Fig. 8B).
Fig. 8.
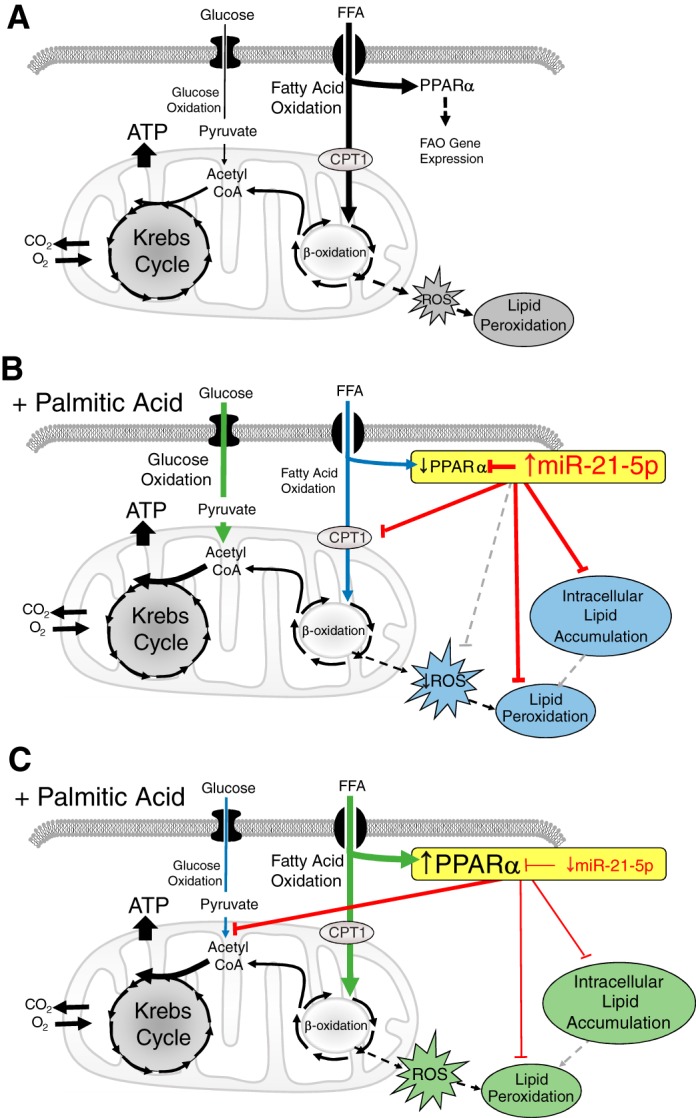
Summary of cellular effects and proposed mechanism of the miR-21-5p effect in response to lipid. A: a simplified diagram of components of glucose and fatty acid oxidation pathways relating to this study. B: increased miR-21-5p levels would be expected to suppress of peroxisome proliferator-activated receptor (PPAR)-α, and possible other genes, when palmitic acid (PA) is added. This would reduce fatty acid oxidation (FAO) and increase glucose oxidation. Attenuated lipid uptake would reduce intracellular lipid levels, and lower β-oxidation could reduce reactive oxygen species (ROS) and result in the observed decrease in lipid peroxidation. It is also possible that miR-21-5p may inhibit oxidative stress (gray hatched line). In this scenario, increasing extracellular lipid or treatment with etomoxir (ETO) would not reduce the oxygen consumption rate because the FAO and uptake machinery regulated by PPAR-α would already be attenuated and the cells more reliant on the glycolytic pathway. C: reduced miR-21-5p levels in the presence of elevated extracellular lipid would be expected to relieve suppression of PPAR-α, leading to an increase in cellular lipid uptake and fatty acid oxidation and reduced reliance on glucose oxidation. Increased lipid content was observed in anti-miR-21-5p-treated cells. The increased β-oxidation may augment ROS production, leading to enhanced peroxidation of the intracellular lipid. In this situation, ETO treatment would impact the oxygen consumption rate because the cells have made FAO the predominant metabolic pathway. The reduction of the oxygen consumption rate in response to exogenous PA may be caused by lipotoxicity related to the increased uptake. Our results indicate that increases or decreases in miR-21-5p modulate mitochondrial respiration by shifting the balance of fatty acid and glucose oxidation. In total, these findings suggest that there is an optimal level of miR-21-5p. CPT1a, carnitine palmitoyltransferase I; FFA, free fatty acid.
We found that suppression of miR-21-5p could also increase mitochondrial respiration through a mechanism that is heavily reliant on exogenous lipid levels. ETO treatment inhibited the anti-miR-21-5p-related increase in mitochondrial respiration (Fig. 5C) and revealed a reduction in ECAR (Fig. 5D), suggesting an impaired ability to undergo glycolysis in these cells (see summary in Fig. 8C). This anti-miR-21-5p-related increase in mitochondrial respiration was also inhibited by PA treatment, which may indicate that elevated lipid content resulting from exogenous fatty acid addition (Fig. 1B) induces lipotoxicity. Cardiomyocyte-specific overexpression of PPAR-α has been previously shown to enhance accumulation of triglycerides and worsen cardiac function in mice fed a high-triglyceride diet (11). We observed something similar where PA treatment normalized any clofibrate-induced effect on mitochondrial respiration and ATP production (Fig. 6C). Additionally, suppression of miR-21-5p resulted in a reduced abundance of the catalytic subunit of pyruvate dehydrogenase (Fig. 7B) for reasons that are not clear. Anti-miR-21-5p-treated cells respond to PA by reducing glycolysis, indicating that the cells are capable of responding to increased PA availability with a typical reduction in glycolysis; however, it also suggests that the majority of the anti-miR-21-5p-associated increase in OCR under baseline conditions (e.g., in the absence of excess PA) appears to be due to glycolysis.
This study provides direct evidence that modulation of miR-21-5p levels can impact substrate utilization and mitochondrial respiration in H9C2 cells and that this effect can be further modulated by the availability of lipid substrate. Our data indicate that in the absence of additional lipid, overexpression of miR-21-5p in H9C2 cells increases OCR without impacting glycolysis. However, additional treatment of pre-miR-21-5p-treated cells with PA does not increase OCR or inhibit glycolysis (Fig. 5A). Even when fatty acids are available in abundance, changes resulting from the overexpression of miR-21-5p cause cells to favor glycolysis rather than using fatty acid. As further evidence, cells that overexpress miR-21-5p had blunted mitochondrial respiration and ATP production when treated with clofibrate (Fig. 6C). Together, this suggests that cells that overexpress miR-21-5p are unable to use the additional fatty acid substrate when it is available and that this may be due to changes beyond those induced by targeting of PPAR-α alone. This is further supported by a lower lipid content (Fig. 1) and MDA abundance (Fig. 2) in lipid-treated pre-miR-21 cells compared with pre-Scr cells. Among the most remarkable findings in this study were the large increases in baseline OCR, ECAR, and ATP content in anti-miR-21-5p-treated cells compared with anti-Scr controls (Fig. 3), which the data suggest occurs primarily through increasing fatty acid oxidation. This effect is completely lost with inhibition of CPT1 by ETO or with PA treatment, indicating that substrate-induced metabolic shifts may also be dysregulated when miR-21-5p levels are suppressed. These studies highlight the complexity of factors regulating metabolic pathways in cardiac cells and the importance of assessing alterations in mitochondrial respiration in addition to gene expression changes. Furthermore, they point to the importance of maintaining physiologically optimal levels of miR-21-5p; either too much or too little may be pathological. To fully elucidate the complex processes that regulate metabolism in these cells, additional studies are needed to comprehensively evaluate changes in metabolic mRNA transcripts, proteins, and metabolites to determine the mechanism(s) by which cellular miR-21-5p levels impact metabolism in response to different substrates.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant 1-R01-HL-128332-01A1 (to V. L. Nasci, S. Chuppa, K. Goodreau, and A. J. Kriegel) and American Heart Association Grants 100000968 and 18PRE34000045 (to V. L. Nasci).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.J.K. conceived and designed research; V.L.N., S.C., K.G., and A.J.K. performed experiments; V.L.N., S.C., L.G., and A.J.K. analyzed data; L.G., R.K.D., and A.J.K. interpreted results of experiments; V.L.N., S.C., K.G., and A.J.K. prepared figures; A.J.K. drafted manuscript; V.L.N., K.G., R.K.D., and A.J.K. edited and revised manuscript; V.L.N., S.C., L.G., K.G., R.K.D., and A.J.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Steven Komas for performing the Seahorse XF analysis for this study through the Medical College of Wisconsin Redox and Bioenergetics Shared Resource and for offering expertise as we optimized cell culture conditions.
REFERENCES
- 1.Bing RJ, Siegel A, Ungar I, Gilbert M. Metabolism of the human heart. II. Studies on fat, ketone and amino acid metabolism. Am J Med 16: 504–515, 1954. doi: 10.1016/0002-9343(54)90365-4. [DOI] [PubMed] [Google Scholar]
- 2.Brandt JM, Djouadi F, Kelly DP. Fatty acids activate transcription of the muscle carnitine palmitoyltransferase I gene in cardiac myocytes via the peroxisome proliferator-activated receptor alpha. J Biol Chem 273: 23786–23792, 1998. doi: 10.1074/jbc.273.37.23786. [DOI] [PubMed] [Google Scholar]
- 3.Bristow M. Etomoxir: a new approach to treatment of chronic heart failure. Lancet 356: 1621–1622, 2000. doi: 10.1016/S0140-6736(00)03149-4. [DOI] [PubMed] [Google Scholar]
- 4.Butler J, Forman DE, Abraham WT, Gottlieb SS, Loh E, Massie BM, O’Connor CM, Rich MW, Stevenson LW, Wang Y, Young JB, Krumholz HM. Relationship between heart failure treatment and development of worsening renal function among hospitalized patients. Am Heart J 147: 331–338, 2004. doi: 10.1016/j.ahj.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Li X, Zhang W, He J, Xu B, Lei B, Wang Z, Cates C, Rousselle T, Li J. Activation of AMPK inhibits inflammatory response during hypoxia and reoxygenation through modulating JNK-mediated NF-κB pathway. Metabolism 83: 256–270, 2018. doi: 10.1016/j.metabol.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chuppa S, Liang M, Liu P, Liu Y, Casati MC, Cowley AW, Patullo L, Kriegel AJ. MicroRNA-21 regulates peroxisome proliferator-activated receptor alpha, a molecular mechanism of cardiac pathology in cardiorenal syndrome type 4. Kidney Int 93: 375–389, 2018. doi: 10.1016/j.kint.2017.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clementi A, Virzì GM, Goh CY, Cruz DN, Granata A, Vescovo G, Ronco C. Cardiorenal syndrome type 4: a review. Cardiorenal Med 3: 63–70, 2013. doi: 10.1159/000350397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conway MA, Allis J, Ouwerkerk R, Niioka T, Rajagopalan B, Radda GK. Detection of low phosphocreatine to ATP ratio in failing hypertrophied human myocardium by 31P magnetic resonance spectroscopy. Lancet 338: 973–976, 1991. doi: 10.1016/0140-6736(91)91838-L. [DOI] [PubMed] [Google Scholar]
- 10.Doenst T, Nguyen TD, Abel ED. Cardiac metabolism in heart failure: implications beyond ATP production. Circ Res 113: 709–724, 2013. doi: 10.1161/CIRCRESAHA.113.300376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finck BN, Han X, Courtois M, Aimond F, Nerbonne JM, Kovacs A, Gross RW, Kelly DP. A critical role for PPARα-mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: modulation by dietary fat content. Proc Natl Acad Sci USA 100: 1226–1231, 2003. doi: 10.1073/pnas.0336724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forman DE, Butler J, Wang Y, Abraham WT, O’Connor CM, Gottlieb SS, Loh E, Massie BM, Rich MW, Stevenson LW, Young JB, Krumholz HM. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol 43: 61–67, 2004. doi: 10.1016/j.jacc.2003.07.031. [DOI] [PubMed] [Google Scholar]
- 13.Fragasso G. Deranged cardiac metabolism and the pathogenesis of heart failure. Card Fail Rev 2: 8–13, 2016. doi: 10.15420/cfr.2016:5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukushima A, Alrob OA, Zhang L, Wagg CS, Altamimi T, Rawat S, Rebeyka IM, Kantor PF, Lopaschuk GD. Acetylation and succinylation contribute to maturational alterations in energy metabolism in the newborn heart. Am J Physiol Heart Circ Physiol 311: H347–H363, 2016. doi: 10.1152/ajpheart.00900.2015. [DOI] [PubMed] [Google Scholar]
- 15.Gilde AJ, van der Lee KA, Willemsen PH, Chinetti G, van der Leij FR, van der Vusse GJ, Staels B, van Bilsen M. Peroxisome proliferator-activated receptor (PPAR) α and PPARβ/δ, but not PPARγ, modulate the expression of genes involved in cardiac lipid metabolism. Circ Res 92: 518–524, 2003. doi: 10.1161/01.RES.0000060700.55247.7C. [DOI] [PubMed] [Google Scholar]
- 16.Guo L, Fang H, Collins J, Fan XH, Dial S, Wong A, Mehta K, Blann E, Shi L, Tong W, Dragan YP. Differential gene expression in mouse primary hepatocytes exposed to the peroxisome proliferator-activated receptor alpha agonists. BMC Bioinformatics 7, Suppl 2: S18, 2006. doi: 10.1186/1471-2105-7-S2-S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holubarsch CJ, Rohrbach M, Karrasch M, Boehm E, Polonski L, Ponikowski P, Rhein S. A double-blind randomized multicentre clinical trial to evaluate the efficacy and safety of two doses of etomoxir in comparison with placebo in patients with moderate congestive heart failure: the ERGO (etomoxir for the recovery of glucose oxidation) study. Clin Sci (Lond) 113: 205–212, 2007. doi: 10.1042/CS20060307. [DOI] [PubMed] [Google Scholar]
- 18.Jiang YJ, Lu B, Xu FY, Gartshore J, Taylor WA, Halayko AJ, Gonzalez FJ, Takasaki J, Choy PC, Hatch GM. Stimulation of cardiac cardiolipin biosynthesis by PPARα activation. J Lipid Res 45: 244–252, 2004. doi: 10.1194/jlr.M300314-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Johnson R, Dludla P, Joubert E, February F, Mazibuko S, Ghoor S, Muller C, Louw J. Aspalathin, a dihydrochalcone C-glucoside, protects H9c2 cardiomyocytes against high glucose induced shifts in substrate preference and apoptosis. Mol Nutr Food Res 60: 922–934, 2016. doi: 10.1002/mnfr.201500656. [DOI] [PubMed] [Google Scholar]
- 20.Karbowska J, Kochan Z, Smoleński RT. Peroxisome proliferator-activated receptor alpha is downregulated in the failing human heart. Cell Mol Biol Lett 8: 49–53, 2003. [PubMed] [Google Scholar]
- 21.Kato T, Niizuma S, Inuzuka Y, Kawashima T, Okuda J, Tamaki Y, Iwanaga Y, Narazaki M, Matsuda T, Soga T, Kita T, Kimura T, Shioi T. Analysis of metabolic remodeling in compensated left ventricular hypertrophy and heart failure. Circ Heart Fail 3: 420–430, 2010. doi: 10.1161/CIRCHEARTFAILURE.109.888479. [DOI] [PubMed] [Google Scholar]
- 22.Kriegel AJ, Terhune SS, Greene AS, Noon KR, Pereckas MS, Liang M. Isomer-specific effect of microRNA miR-29b on nuclear morphology. J Biol Chem 293: 14080–14088, 2018. doi: 10.1074/jbc.RA117.001705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krumholz HM, Chen YT, Vaccarino V, Wang Y, Radford MJ, Bradford WD, Horwitz RI. Correlates and impact on outcomes of worsening renal function in patients ≥65 years of age with heart failure. Am J Cardiol 85: 1110–1113, 2000. doi: 10.1016/S0002-9149(00)00705-0. [DOI] [PubMed] [Google Scholar]
- 24.Kuznetsov AV, Javadov S, Sickinger S, Frotschnig S, Grimm M. H9c2 and HL-1 cells demonstrate distinct features of energy metabolism, mitochondrial function and sensitivity to hypoxia-reoxygenation. Biochim Biophys Acta 1853: 276–284, 2015. doi: 10.1016/j.bbamcr.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lei B, Lionetti V, Young ME, Chandler MP, d’Agostino C, Kang E, Altarejos M, Matsuo K, Hintze TH, Stanley WC, Recchia FA. Paradoxical downregulation of the glucose oxidation pathway despite enhanced flux in severe heart failure. J Mol Cell Cardiol 36: 567–576, 2004. doi: 10.1016/j.yjmcc.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Li H, Zhang X, Wang F, Zhou L, Yin Z, Fan J, Nie X, Wang P, Fu XD, Chen C, Wang DW. MicroRNA-21 lowers blood pressure in spontaneous hypertensive rats by upregulating mitochondrial translation. Circulation 134: 734–751, 2016. doi: 10.1161/CIRCULATIONAHA.116.023926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Nguyen P, Baris TZ, Poirier MC. Molecular analysis of mitochondrial compromise in rodent cardiomyocytes exposed long term to nucleoside reverse transcriptase inhibitors (NRTIs). Cardiovasc Toxicol 12: 123–134, 2012. doi: 10.1007/s12012-011-9148-5. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Shim E, Crespo-Mejias Y, Nguyen P, Gibbons A, Liu D, Shide E, Poirier MC. Cardiomyocytes are protected from antiretroviral nucleoside analog-induced mitochondrial toxicity by overexpression of PGC-1α. Cardiovasc Toxicol 15: 224–231, 2015. doi: 10.1007/s12012-014-9288-5. [DOI] [PubMed] [Google Scholar]
- 29.Lopaschuk GD, Belke DD, Gamble J, Itoi T, Schönekess BO. Regulation of fatty acid oxidation in the mammalian heart in health and disease. Biochim Biophys Acta 1213: 263–276, 1994. doi: 10.1016/0005-2760(94)00082-4. [DOI] [PubMed] [Google Scholar]
- 30.Lopaschuk GD, McNeil GF, McVeigh JJ. Glucose oxidation is stimulated in reperfused ischemic hearts with the carnitine palmitoyltransferase 1 inhibitor, Etomoxir. Mol Cell Biochem 88: 175–179, 1989. doi: 10.1007/BF00223440. [DOI] [PubMed] [Google Scholar]
- 31.Lopaschuk GD, Spafford M. Response of isolated working hearts to fatty acids and carnitine palmitoyltransferase I inhibition during reduction of coronary flow in acutely and chronically diabetic rats. Circ Res 65: 378–387, 1989. doi: 10.1161/01.RES.65.2.378. [DOI] [PubMed] [Google Scholar]
- 32.Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev 90: 207–258, 2010. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 33.Neely JR, Morgan HE. Relationship between carbohydrate and lipid metabolism and the energy balance of heart muscle. Annu Rev Physiol 36: 413–459, 1974. doi: 10.1146/annurev.ph.36.030174.002213. [DOI] [PubMed] [Google Scholar]
- 34.Nippert F, Schreckenberg R, Schlüter KD. Isolation and cultivation of adult rat cardiomyocytes. J Vis Exp. In press. doi: 10.3791/56634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nohria A, Hasselblad V, Stebbins A, Pauly DF, Fonarow GC, Shah M, Yancy CW, Califf RM, Stevenson LW, Hill JA. Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol 51: 1268–1274, 2008. doi: 10.1016/j.jacc.2007.08.072. [DOI] [PubMed] [Google Scholar]
- 36.Olson JM, Yan Y, Bai X, Ge ZD, Liang M, Kriegel AJ, Twaroski DM, Bosnjak ZJ. Up-regulation of microRNA-21 mediates isoflurane-induced protection of cardiomyocytes. Anesthesiology 122: 795–805, 2015. doi: 10.1097/ALN.0000000000000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Opie LH. Metabolism of the heart in health and disease. I. Am Heart J 76: 685–698, 1968. doi: 10.1016/0002-8703(68)90168-3. [DOI] [PubMed] [Google Scholar]
- 38.Patrick DM, Montgomery RL, Qi X, Obad S, Kauppinen S, Hill JA, van Rooij E, Olson EN. Stress-dependent cardiac remodeling occurs in the absence of microRNA-21 in mice. J Clin Invest 120: 3912–3916, 2010. doi: 10.1172/JCI43604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Purushothaman S, Nair RR. Mitoprotective antioxidant EUK-134 stimulates fatty acid oxidation and prevents hypertrophy in H9C2 cells. Mol Cell Biochem 420: 185–194, 2016. doi: 10.1007/s11010-016-2788-9. [DOI] [PubMed] [Google Scholar]
- 40.Rakhshandehroo M, Hooiveld G, Müller M, Kersten S. Comparative analysis of gene regulation by the transcription factor PPARalpha between mouse and human. PLoS One 4: e6796, 2009. doi: 10.1371/journal.pone.0006796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rakhshandehroo M, Sanderson LM, Matilainen M, Stienstra R, Carlberg C, de Groot PJ, Müller M, Kersten S. Comprehensive analysis of PPARα-dependent regulation of hepatic lipid metabolism by expression profiling. PPAR Res 2007: 26839, 2007. doi: 10.1155/2007/26839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ricchi M, Odoardi MR, Carulli L, Anzivino C, Ballestri S, Pinetti A, Fantoni LI, Marra F, Bertolotti M, Banni S, Lonardo A, Carulli N, Loria P. Differential effect of oleic and palmitic acid on lipid accumulation and apoptosis in cultured hepatocytes. J Gastroenterol Hepatol 24: 830–840, 2009. doi: 10.1111/j.1440-1746.2008.05733.x. [DOI] [PubMed] [Google Scholar]
- 43.Saeedi R, Parsons HL, Wambolt RB, Paulson K, Sharma V, Dyck JR, Brownsey RW, Allard MF. Metabolic actions of metformin in the heart can occur by AMPK-independent mechanisms. Am J Physiol Heart Circ Physiol 294: H2497–H2506, 2008. doi: 10.1152/ajpheart.00873.2007. [DOI] [PubMed] [Google Scholar]
- 44.Saeedi R, Saran VV, Wu SS, Kume ES, Paulson K, Chan AP, Parsons HL, Wambolt RB, Dyck JR, Brownsey RW, Allard MF. AMP-activated protein kinase influences metabolic remodeling in H9c2 cells hypertrophied by arginine vasopressin. Am J Physiol Heart Circ Physiol 296: H1822–H1832, 2009. doi: 10.1152/ajpheart.00396.2008. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt-Schweda S, Holubarsch C. First clinical trial with etomoxir in patients with chronic congestive heart failure. Clin Sci (Lond) 99: 27–35, 2000. doi: 10.1042/cs0990027. [DOI] [PubMed] [Google Scholar]
- 46.Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JT, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, Engelhardt S. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 456: 980–984, 2008. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 47.Tian R, Nascimben L, Kaddurah-Daouk R, Ingwall JS. Depletion of energy reserve via the creatine kinase reaction during the evolution of heart failure in cardiomyopathic hamsters. J Mol Cell Cardiol 28: 755–765, 1996. doi: 10.1006/jmcc.1996.0070. [DOI] [PubMed] [Google Scholar]
- 48.Toischer K, Rokita AG, Unsöld B, Zhu W, Kararigas G, Sossalla S, Reuter SP, Becker A, Teucher N, Seidler T, Grebe C, Preuss L, Gupta SN, Schmidt K, Lehnart SE, Krüger M, Linke WA, Backs J, Regitz-Zagrosek V, Schäfer K, Field LJ, Maier LS, Hasenfuss G. Differential cardiac remodeling in preload versus afterload. Circulation 122: 993–1003, 2010. doi: 10.1161/CIRCULATIONAHA.110.943431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vadvalkar SS, Matsuzaki S, Eyster CA, Giorgione JR, Bockus LB, Kinter CS, Kinter M, Humphries KM. Decreased mitochondrial pyruvate transport activity in the diabetic heart: role of mitochondrial pyruvate carrier 2 (MPC2) acetylation. J Biol Chem 292: 4423–4433, 2017. doi: 10.1074/jbc.M116.753509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, Richardson JA, Olson EN. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci USA 103: 18255–18260, 2006. doi: 10.1073/pnas.0608791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ventura-Clapier R, Garnier A, Veksler V. Energy metabolism in heart failure. J Physiol 555: 1–13, 2004. doi: 10.1113/jphysiol.2003.055095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wisneski JA, Stanley WC, Neese RA, Gertz EW. Effects of acute hyperglycemia on myocardial glycolytic activity in humans. J Clin Invest 85: 1648–1656, 1990. doi: 10.1172/JCI114616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zarain-Herzberg A, Rupp H. Therapeutic potential of CPT I inhibitors: cardiac gene transcription as a target. Expert Opin Investig Drugs 11: 345–356, 2002. doi: 10.1517/13543784.11.3.345. [DOI] [PubMed] [Google Scholar]